Abstract
OBJECTIVE
The Diabetes Prevention Program (DPP) and its Outcomes Study (DPPOS) demonstrated that either intensive lifestyle intervention or metformin could prevent type 2 diabetes in high-risk adults for at least 10 years after randomization. We report the 10-year within-trial cost-effectiveness of the interventions.
RESEARCH DESIGN AND METHODS
Data on resource utilization, cost, and quality of life were collected prospectively. Economic analyses were performed from health system and societal perspectives.
RESULTS
Over 10 years, the cumulative, undiscounted per capita direct medical costs of the interventions, as implemented during the DPP, were greater for lifestyle ($4,601) than metformin ($2,300) or placebo ($769). The cumulative direct medical costs of care outside the DPP/DPPOS were least for lifestyle ($24,563 lifestyle vs. $25,616 metformin vs. $27,468 placebo). The cumulative, combined total direct medical costs were greatest for lifestyle and least for metformin ($29,164 lifestyle vs. $27,915 metformin vs. $28,236 placebo). The cumulative quality-adjusted life-years (QALYs) accrued over 10 years were greater for lifestyle (6.81) than metformin (6.69) or placebo (6.67). When costs and outcomes were discounted at 3%, lifestyle cost $10,037 per QALY, and metformin had slightly lower costs and nearly the same QALYs as placebo.
CONCLUSIONS
Over 10 years, from a payer perspective, lifestyle was cost-effective and metformin was marginally cost-saving compared with placebo. Investment in lifestyle and metformin interventions for diabetes prevention in high-risk adults provides good value for the money spent.
Intensive lifestyle and metformin interventions can delay or prevent progression from impaired glucose tolerance (IGT) to type 2 diabetes (1–3). The Diabetes Prevention Program (DPP), a randomized controlled clinical trial, demonstrated that compared with the placebo intervention (placebo), the intensive lifestyle intervention (lifestyle) reduced the incidence of type 2 diabetes by 58%, and the metformin intervention (metformin) reduced the incidence of type 2 diabetes by 31% over 2.8 years (3). The Diabetes Prevention Program Outcomes Study (DPPOS) is a long-term follow-up of the DPP participants to investigate whether the delay in the development of diabetes observed during the DPP is sustained and to assess the long-term effects of the interventions on health.
The DPPOS has followed participants for an additional 7 years during which time participants in lifestyle and metformin were encouraged to continue those interventions, and all participants were offered a group lifestyle intervention (4). The incidence of diabetes during the 10-year average follow-up after randomization was reduced by 34% in those initially randomized to lifestyle and 18% in those initially randomized to metformin compared with placebo (4). Previously, we reported the resource utilization and costs of care in the DPP (5), and the cost per quality-adjusted life-year (QALY) gained over the 3-year timeframe of the randomized controlled clinical trial (6). We also used 3-year DPP data and a computer model to simulate the longer-term cost-effectiveness of the interventions. Although we (7) and others (8,9) suggested that lifestyle would be cost-effective or even cost-saving over the long term, one analysis suggested that it might be too expensive for health plans or a national program to implement (10). In this report, we present a within trial intent-to-treat analysis spanning the combined 10-year DPP/DPPOS timeframe to assess the longer-term cost-effectiveness of lifestyle and metformin for diabetes prevention. All of the DPPOS clinical centers as well as the DPP Coordinating Center had institutional review board approvals. All participants gave written informed consent.
RESEARCH DESIGN AND METHODS
Interventions
DPP.
The DPP enrolled 3,234 participants with IGT and fasting hyperglycemia who were at least 25 years of age and had BMI of 24 kg/m2 or higher (22 kg/m2 in Asian Americans) (3). Enrollment began in July 1996 and ended in May 1999. Mean age was 51 years of age and mean BMI was 34.0 kg/m2 (3). Sixty-eight percent were women, and forty-five percent were members of minority groups (3).
The goals for participants randomized to lifestyle were to achieve and maintain a weight reduction of at least 7% of initial body weight through diet and physical activity of moderate intensity, such as brisk walking, for at least 150-min per week. A 16-session core curriculum (given approximately weekly in individual participant sessions) and subsequent individual sessions (usually monthly) and group sessions with case managers were designed to reinforce the behavioral changes. The medication interventions (metformin and placebo) were initiated at a dose of 850 mg taken orally once a day. At 1 month, the dose was increased to 850-mg twice daily. Adherence was reinforced during individual quarterly visits with case managers. Standard lifestyle recommendations were provided to all groups through written information and an annual 20- to 30-min individual session that emphasized the importance of a healthy lifestyle. Mean follow-up at the end of the DPP was 3.2 years. For the purposes of this analysis, we assumed that all subjects were enrolled in the DPP for exactly 3 years.
DPP/DPPOS bridge.
At the end of the DPP in July 2001, masked treatment was discontinued and each participant had a 1-h debriefing and closure visit during which he or she was informed of the main DPP results. In light of the proven benefits of lifestyle, all participants were offered a group-implemented 16-session lifestyle intervention between January and July 2002. Forty percent of lifestyle, fifty-eight percent of metformin, and fifty-seven percent of placebo participants attended at least one session (11). Each session lasted 1 h and was taught by one staff member. Participants received reminders for the sessions and the full packet of course materials. The original lifestyle group was offered additional lifestyle support and was not encouraged to take metformin. The original metformin group was encouraged to continue metformin and to participate in the group lifestyle intervention. Those randomized to placebo stopped placebo and were encouraged to participate in the group lifestyle intervention. For the purposes of this analysis, we assumed that year 4 represented the DPP/DPPOS bridge.
DPPOS maintenance.
The DPPOS maintenance phase started in September 2002. All active participants were eligible for continued follow-up, and 2,766 of 3,150 (88%) enrolled (4). These included 910 from lifestyle, 924 from metformin, and 932 from placebo. During the DPPOS, the group lifestyle intervention was implemented as the Healthy Lifestyle Program (HELP) for all participants. HELP reinforced the original weight loss and physical activity goals and focused on current topics in nutrition, physical activity, stress management, and diabetes prevention. HELP consisted of four quarterly 1-h group visits. All participants received a reminder for HELP sessions. Although all participants were invited to attend all HELP sessions, many chose to attend fewer.
The DPP participants initially randomized to lifestyle were also eligible to receive two additional sessions, referred to as BOOST sessions, per year to reinvigorate their self-management behaviors for weight loss. Those randomized to metformin and placebo were excluded from BOOST sessions. The sessions were more intensive than HELP sessions and reinforced specific behavioral self-management activities (e.g., self-monitoring of fat, calories, and/or physical activity, as well as weight checks) important for weight loss and physical activity adherence and/or maintenance. In addition, the sessions promoted home-based behavioral self-management of weight and physical activity through the use of motivational campaigns. Lifestyle participants received reminders for each BOOST session.
During the DPPOS, metformin participants taking study-provided metformin received an annual complete blood count and serum creatinine for drug safety monitoring. Participants were encouraged to see their personal physicians for treatment of emergent adverse events. Only metformin participants were encouraged to take metformin, and only 1% of nondiabetic participants in lifestyle and 3% of nondiabetic participants in placebo took metformin prescribed outside the study (4). For the purposes of this analysis, we assumed that years 5–10 represented DPPOS follow-up.
Interventions for participants who developed diabetes.
Participants identified with glucose levels diagnostic of diabetes at their 6-monthly visits were seen within 6 weeks for glucose testing to confirm the diagnosis. Participants with confirmed newly diagnosed diabetes received 1 h of individual counseling focused on self-monitoring of blood glucose, were provided with meters and test strips and encouraged to monitor their glucose levels once daily, and were maintained in their randomized intervention groups. Treatment for diabetes and surveillance for complications and comorbidities were performed by the participants’ own health care providers. Medications used by the DPP participants for management of diabetes were recorded every 6 months on a drug summary form.
Costs
We calculated the total direct medical costs associated with the DPP/DPPOS interventions over each of the 10 years after randomization (Supplementary Table 1). Direct medical costs of the interventions were estimated from the resources used and unit costs adjusted to 2010 U.S. dollars (Supplementary Table 2). As a sensitivity analysis, we estimated what the cost of lifestyle might have been during the DPP if it had been administered in a group format rather than individually (DPP group lifestyle intervention). We recalculated the costs of lifestyle assuming that the core curriculum and monthly follow-up visits with the lifestyle case managers, which were conducted individually during the 3 years of the DPP, were conducted as group sessions with 10 participants. Studies have shown that group intervention programs can be as effective as individual programs (12,13). Although metformin was implemented with brand name metformin (Glucophage), we assumed that it was implemented with generically priced metformin throughout the 10 years of the DPP/DPPOS.
As previously described, we also estimated the direct medical cost of care outside the study (5). The direct medical cost of care outside the study included the costs of hospital, emergency room, urgent care, outpatient services, and telephone calls to health care providers. These were determined annually from patient self-report. They also included the cost of prescription medications. This was estimated from the number of prescription medications that participants reported taking at semiannual visits and the mean average wholesale price of a prescription medication dispensed by a large U.S. pharmacy benefit manager in 2009.
Direct nonmedical costs were assessed twice, once during the DPP and once during the DPPOS, and costs were annualized. In estimating the direct nonmedical costs of the interventions, we considered the costs of food, food preparation items (blenders, cookbooks, food scales, freezers, microwave ovens, mixers, popcorn poppers, steamers, and woks), exercise classes, gym memberships, personal trainers, and exercise equipment (bicycles, exercise videos, free weights, golf clubs, home gyms, shoes, stationary bicycles, steps and treadmills) (5). We also considered the costs of transportation to study visits and to medical visits (5). The value of the time that participants spent shopping, cooking, exercising, and traveling to and attending appointments was also assessed (5). The costs of exercise were valued according to whether participants “disliked,” were “neutral,” or “liked” leisure time physical activity (5,14) (Supplementary Table 2). Although direct nonmedical costs are not usually paid by private insurers or government health programs, we included them in our cost calculations from a societal perspective.
Outcomes
We assessed outcomes in terms of QALYs (15). QALYs measure length of life adjusted for quality of life as assessed by the health utility score. By convention, health utility scores are placed on a continuum where perfect health is assigned a value of 1.0 and health judged equivalent to death is assigned a value of 0.0. We assessed health utilities annually using the Self-Administered Quality of Well-Being Index (QWB-SA) (6). The QWB-SA is a widely used, validated, multiattribute utility model that combines preference-weighted values for symptoms and functioning to provide a health utility score. The numerical value assigned by the QWB-SA to quality of life reflects the public's judgment of the desirability of the health state. Mathematically, QALYs are calculated as the sum of the product of the number of years of life and the quality of life, measured in health utilities, in each of those years.
Perspective
For the base-case analysis, we followed the recommendations of the Panel on Cost-Effectiveness in Health in Medicine (15) and took the perspective of a health system. Thus, we included only direct medical costs in our base-case analysis. We included direct nonmedical costs excluding participant time in a sensitivity analysis from a modified societal perspective and direct nonmedical costs including participant time in a sensitivity analysis from a full societal perspective. These sensitivity analyses assessed the impact of covering the cost of the behavioral interventions implemented by the study participants on society as a whole.
Analyses
The analyses of lifestyle, metformin, and placebo were based on the design, cost, and clinical effectiveness of the interventions as implemented in the 3 years of the DPP and the 7 years of the DPPOS. For the DPP group lifestyle sensitivity analysis, we estimated what the costs of lifestyle would have been during the 3 years of DPP if the 16-session core curriculum and monthly follow-up visits with the case managers had been conducted as group sessions with 10 participants. We further assumed that outcomes would have been the same as observed for lifestyle. We excluded from the analyses the costs of the research component of the DPP/DPPOS. All costs were expressed as year 2010 U.S. dollars (Supplementary Table 2). Analyses were performed with a 10-year time horizon. Initial analyses were performed without discounting. Subsequently, both cost and health outcomes were converted to net present value using a 3% discount rate, and incremental cost-effectiveness ratios were calculated using the discounted costs and QALYs.
RESULTS
Costs
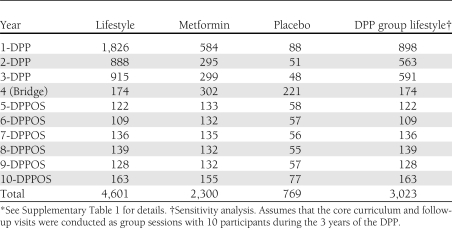
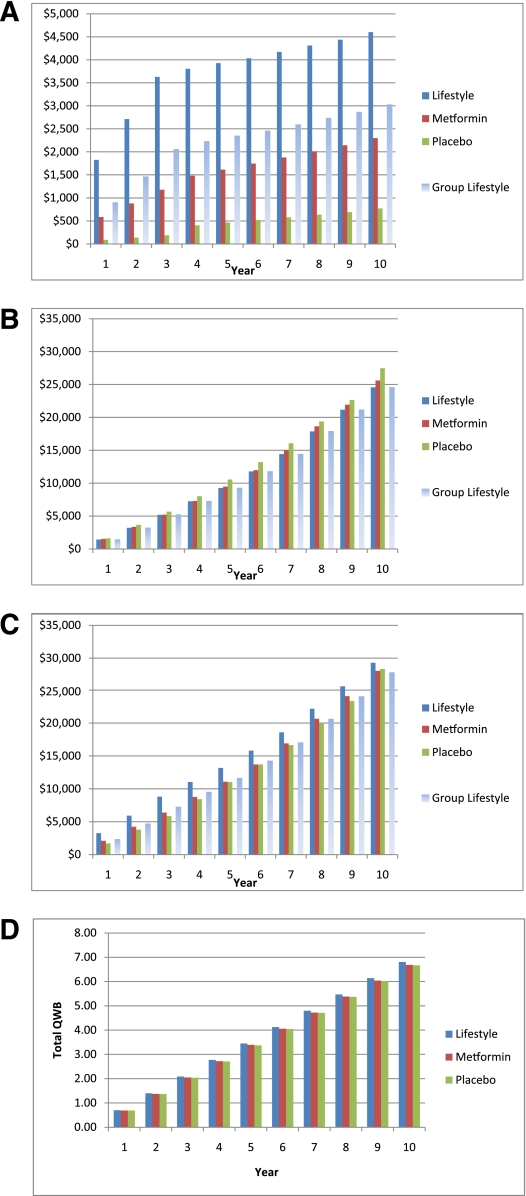
The annual undiscounted, per capita, direct medical costs of lifestyle, metformin, and placebo over 10 years are summarized in Table 1 as are the costs of the DPP group lifestyle sensitivity analysis. Figure 1A illustrates the cumulative, undiscounted, per participant intervention costs. The cumulative, undiscounted per participant cost of the lifestyle intervention as implemented in the DPP ($4,601) was substantially greater than the metformin intervention ($2,300) or the placebo intervention ($769). The estimated cost of the DPP group lifestyle intervention ($3,023) was approximately one-third less than that of the lifestyle intervention. The costs of lifestyle were substantially less during the DPPOS than during the DPP because of the change from an individual- to a group-implemented intervention and because fewer visits took place. The costs of placebo were higher during the DPPOS than during the DPP because placebo participants engaged in the group lifestyle intervention. During the DPPOS, lifestyle and metformin each cost approximately $140 per participant per year. The costs of the interventions during the DPP differ somewhat from those reported previously as we have added the costs of fasting glucose and glucose tolerance testing and incorporated generic pricing for metformin (5). Detailed cost calculations are presented in Supplementary Table 1.
Table 1.
Undiscounted, per capita, direct medical costs of the DPP/DPPOS interventions by intervention group and study year ($)*
Figure 1.
—A: Cumulative, undiscounted, per participant, direct medical costs of the DPP/DPPOS interventions by intervention group and study year. B: Cumulative, undiscounted, per participant, direct medical costs of medical care received outside the DPP/DPPOS by intervention group and study year. C: Cumulative, undiscounted, per participant, total direct medical costs of the DPP/DPPOS interventions and medical care received outside the DPP/DPPOS by intervention group and study year. D: Cumulative, undiscounted, per participant, total Quality of Well-Being Index by intervention group and year.
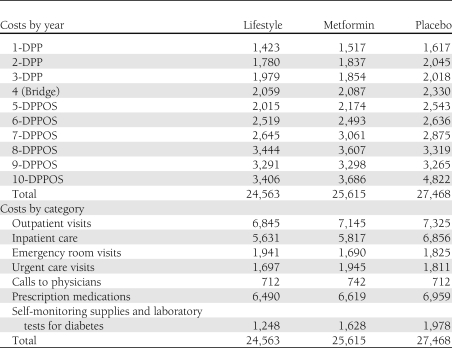
The cumulative, undiscounted per capita direct medical costs of nonintervention-related medical care by intervention group and year following randomization are shown in Table 2 and Fig. 1B. These are the costs of medical care received outside the DPP/DPPOS. The direct medical costs of nonintervention-related medical care were substantially greater than the costs of the interventions, and within 3 years, the cumulative costs of nonintervention-related medical care exceeded the 10-year cumulative direct medical costs of the interventions. The cumulative per-participant direct medical costs of nonintervention-related medical care increased substantially over time. From the outset of the DPP, the per-participant costs of placebo exceeded those of lifestyle and metformin. The greater cost of nonintervention-related medical care for placebo was largely driven by greater use of outpatient and inpatient services, prescription medications, and by the greater rate of conversion to diabetes with the attendant costs of self-monitoring and laboratory tests (Table 2). Across treatment groups, the direct medical costs of nonintervention-related medical care were 34–44% higher among diabetic participants compared with nondiabetic participants (Supplementary Table 3). Over 10 years, cumulative, per capita nonintervention-related direct medical costs were greater by $1,853 and $2,905 for placebo compared with metformin and lifestyle, respectively.
Table 2.
Undiscounted, per capita, direct medical costs of care outside the DPP/DPPOS by intervention group and study year, and distribution of undiscounted, per capita, 10-year, direct medical costs of care outside the DPP/DPPOS by intervention group and type ($)
Over 10 years, the cumulative, undiscounted, per capita direct medical costs of the interventions were greater for participants randomized to lifestyle ($4,601) and metformin ($2,300) compared with placebo ($769). In contrast, the cumulative, undiscounted, per capita direct medical costs of nonintervention-related medical care (medical care received outside the DPP/DPPOS) were greater for placebo ($27,468) than for metformin ($25,615) or lifestyle ($24,563). By year 10, the cumulative, undiscounted, per capita, total direct medical costs of the interventions and nonintervention-related medical care were higher for lifestyle than for placebo but were lower for metformin than for placebo (Fig. 1C). They were also lower for the DPP group lifestyle (Fig. 1C).
Supplementary Table 4 summarizes the undiscounted, per capita, cumulative, 10-year direct nonmedical costs by intervention group and type defined as diet-related costs, physical activity–related costs, transportation-related costs, and participant time-related costs. Diet-related costs were substantial but did not differ among intervention groups. As might be expected, physical activity–related costs were greatest for lifestyle. Transportation-related costs were also substantially higher for lifestyle and metformin due to the greater number of study visits. Total diet-, physical activity-, and transportation-related costs were greatest for lifestyle but similar for metformin and placebo. Participant time contributed substantially to direct nonmedical costs. Participant time related to the interventions (time spent traveling to study visits, at study visits, and for intervention-related calls) was greater for lifestyle and metformin than for placebo. Participant time related to medical care outside of the interventions was generally greater for placebo than for metformin or lifestyle. Time spent shopping and cooking was the largest component of participant time but differed little across intervention groups. Although lifestyle subjects spent more time exercising, the adjusted value of the time they spent exercising was less than for either metformin or placebo because of their greater enjoyment of leisure time physical activity and the lower opportunity cost. The total, per capita, 10-year, direct nonmedical costs—including the costs of participant time—were lowest for metformin ($144,143) and similar for placebo and lifestyle ($147,043 and $147,493, respectively).
Health utilities
Every year after randomization, quality of life was better for lifestyle than for metformin or placebo (Supplementary Table 3). Across treatment groups, quality of life was worse among participants who developed diabetes (Supplementary Table 3). Since more placebo participants developed diabetes, the cumulative, undiscounted, per participant quality of well-being score gained over 10 years was greatest for lifestyle (6.81), intermediate for metformin (6.69), and least for placebo (6.67) (Fig. 1D).
Cost-effectiveness
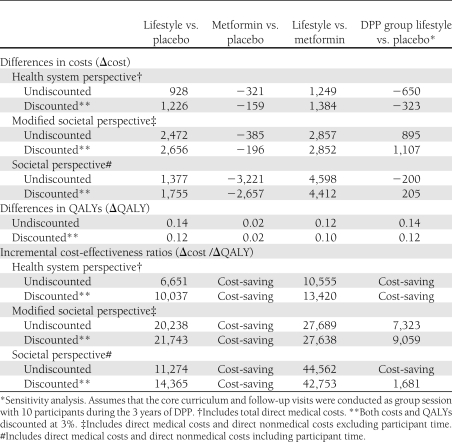
Table 3 summarizes the differences in total costs and QALYs among the treatment groups and the incremental cost-effectiveness ratios of lifestyle and metformin versus placebo, and lifestyle versus metformin. The incremental cost-effectiveness ratio is also shown for the DPP-group lifestyle versus placebo. From the health system perspective, from the modified societal perspective, and from the societal perspective, lifestyle cost more than placebo but was also more effective as assessed by the QALYs that were gained. From a health system perspective, with both costs and health outcomes discounted at 3% per year, the cost of lifestyle compared with placebo was approximately $10,000 per QALY gained; however, metformin had slightly lower costs and nearly the same outcome (as assessed by QALYs) as placebo. Compared with metformin, lifestyle cost more but produced better health outcomes. From a health system perspective, with both costs and health outcomes discounted at 3% per year, the cost of lifestyle compared with metformin was approximately $13,400 per QALY gained. DPP-group lifestyle, like metformin, was generally less expensive and more effective than placebo.
Table 3.
Differences in costs and QALYs and incremental cost-effectiveness ratios for lifestyle and metformin versus placebo and lifestyle versus metformin over 10 years from three alternative perspectives
CONCLUSIONS
Using 3 years of DPP data and computer simulation modeling, we and others suggested that screening for glucose intolerance in the overweight and obese population and implementing lifestyle and metformin interventions would have favorable cost-effectiveness ratios (7–9,16). However, one analysis suggested that lifestyle might be too expensive for health plans or a national program to implement (10). The current study, a 10-year, within-trial, intention-to-treat analysis of the DPP/DPPOS demonstrates that lifestyle is indeed cost-effective, and metformin is marginally cost-saving or at least cost-neutral compared with placebo. Even when the direct nonmedical costs of the interventions are considered, the interventions are cost-effective. Health and social policies should support the funding of intensive lifestyle and metformin interventions for diabetes prevention in high-risk adults.
When a new treatment is more effective and less costly than usual care, it should be widely adopted and used. Unfortunately, fewer than 1 in 5 new interventions in health and medicine are cost-saving compared with usual care (17). Published cost-effectiveness ratios—that is the cost in dollars per QALY gained for prevention and treatment—range from less than $10,000 per QALY to greater than $1 million per QALY with most falling between $10,000 and $50,000 per QALY. While influenza immunization has been demonstrated to be cost-saving in the Medicare population, interventions such as mammography, antihypertensive treatment, and cholesterol treatment for secondary prevention of cardiovascular disease have been estimated to cost between $10,000 and $60,000 per QALY (18). Widely implemented interventions such as dialysis for end-stage renal disease ($50,000 to $100,000 per QALY) and left ventricular assist devices ($500,000 to $1.4 million per QALY) are substantially more expensive.
From the perspective of a health system or society, what is the value of delaying or preventing the development of type 2 diabetes? From a health system perspective, it delays or prevents the direct medical costs of diabetes including the costs of diabetes education and nutritional counseling, glucose monitoring, antihyperglycemic treatments, and surveillance and treatment of complications (19–21). From a societal perspective, diabetes prevention also reduces health care–related costs to the individual not reimbursed by the health system and time lost from work and usual activities (19). It also improves quality of life.
The direct medical costs of diabetes are enormous. The American Diabetes Association has estimated that total per capita health care expenditures for people with diabetes are $11,744 per year, of which $6,649 is attributed to diabetes (19). This estimate likely overstates the costs of diabetes in DPP participants, since DPP participants who developed diabetes were screen-detected very early in their clinical course and had few complications. The costs of diabetes increase with duration of diabetes and with the presence of complications and comorbidities and would be expected to be lower for persons with short durations of diabetes and for those without complications (22). Patients with new clinically diagnosed diabetes have been reported to have costs 2.1 times those of individuals without diabetes, and the incremental cost of diabetes is apparent from the time of diagnosis (20). In 2000, using data from a single managed-care health plan, we estimated that the median, annual, direct medical cost of care for a man with diet-controlled type 2 diabetes with no microvascular, neuropathic, or cardiovascular risk factors or complications was approximately $1,700 (23). More recently, using data from approximately 7,100 type 2 diabetic patients enrolled in eight managed-care health plans participating in Translating Research Into Action for Diabetes (TRIAD), we demonstrated that median, annual, per capita, direct medical costs of care were approximately $2,500 for a man with recent-onset diabetes without complications or comorbidities (R. Li, personal communication). These costs of recent-onset type 2 diabetes are quite consistent with those observed during the DPP/DPPOS. Compared with the substantial costs of diabetes, the costs of lifestyle, metformin, and DPP group lifestyle seem quite small.
It is now clear that the use of a 3-year time horizon in our previous within-trial economic analysis resulted in a higher cost per QALY gained than the current analysis, which used a 10-year time horizon (6). With a 3-year time horizon, treatment costs were higher, and the benefits of lifestyle and metformin in terms of averted diabetes were less. The costs of both lifestyle and metformin were greatest in year 1, decreased substantially in years 2 and 3, and decreased further during years 4 through 10. In contrast, much of the benefit of both lifestyle and metformin, as assessed by both cumulative, nonintervention-related direct medical costs and quality of life, occurred after 3 years of follow-up.
There are a number of limitations to our analyses. First, the DPPOS was an observational follow-up of the DPP, a randomized controlled clinical trial. It is likely that during the DPPOS, when 57% of placebo participants also attended at least one group lifestyle intervention (HELP) session, placebo was more effective than the usual care that nonstudy participants might receive (11). Thus, if real-world usual care was used for comparison, the difference between lifestyle and placebo might have been greater. The fact that 58% of metformin participants also attended at least one HELP session may also have made metformin appear more effective and cost-effective compared with lifestyle (11). Second, the costs of medical care outside the interventions appear low compared with those reported in the literature for people with diabetes (19). This likely reflects the fact that trial participants were earlier in the natural history of diabetes and were healthier than patients with diabetes in the general population. It is important to note that resource utilization and cost were assessed in an identical fashion across intervention groups so the differences among groups, which determine the incremental cost-effectiveness ratios, should be reasonably accurate. Third, in our analysis of the DPP group lifestyle intervention, we assumed that lifestyle could be implemented in a group rather than in an individual format at one-third lower cost and achieve the same outcomes. Although group-implemented lifestyle interventions have been shown to be at least as effective as individual programs for weight loss, there has not been a direct comparison of individual and group lifestyle interventions for diabetes prevention (12,13). Finally, we recognize that in assessing the direct nonmedical costs of the interventions, we have overestimated diet-related costs. Clearly, people need to eat whether or not they participate in a randomized controlled clinical trial. We did not have a good method for distinguishing study-related and nonstudy-related food consumption. Nevertheless, the data are instructive in that they indicate that there were not substantial differences in diet-related costs across intervention groups.
This 10-year, within trial, intention-to-treat economic analysis of the DPP/DPPOS demonstrates that lifestyle, when compared with placebo, is cost-effective, and metformin is marginally cost-saving from a health system and societal perspective. If a DPP group lifestyle intervention could be delivered at one-third lower cost than the DPP lifestyle intervention and achieve the same outcomes, it would also be cost-saving or cost-effective compared with placebo. Even when compared with metformin, lifestyle was cost-effective from both a health system and societal perspective. These analyses should assist health plans and policy makers in comparing the benefit of diabetes prevention to other preventive and palliative interventions. The adoption of diabetes prevention programs by health plans and society will result in important health benefits over 10 years and represents a good value for the money spent.
Acknowledgments
During the DPPOS, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health provided funding to the clinical centers and the DPP Coordinating Center for the design and conduct of the study, and collection, management, analysis, and interpretation of the data. The Southwestern American Indian Centers were supported directly by the NIDDK, including its Intramural Research Program, and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, and the U.S. Department of Veterans Affairs supported data collection at many of the clinical centers. Funding was also provided by the National Institute of Child Health and Human Development; the National Institute on Aging; the National Eye Institute; the National Heart, Lung, and Blood Institute; the Office of Research on Women's Health; the National Institute on Minority Health and Health Disparities; the Centers for Disease Control and Prevention; and the American Diabetes Association.
Bristol-Myers Squibb and Parke-Davis provided additional funding and material support during the DPP. Lipha (Merck-Sante) provided medication and LifeScan Inc. donated materials during the DPP and the DPPOS. No other potential conflicts of interest relevant to this article were reported.
W.H.H. researched data, wrote the manuscript, and is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. S.L.E. and M.B.B. researched data, conducted analyses, reviewed and edited the manuscript, and contributed to discussion. R.E.R., M.G.M., R.T.A., T.J.O., M.A.F., P.Z., and C.D.S. researched data, reviewed and edited the manuscript, and contributed to discussion.
Parts of this study were presented in oral form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011, and in poster form at the 47th Annual Meeting of the European Association for the Study of Diabetes, Lisbon, Portugal, 12–16 September 2011.
The DPP Research Group gratefully acknowledges the commitment and dedication of the participants of the DPP and the DPPOS.
APPENDIX
Members of the writing group are William H. Herman, MD, MPH1; Sharon L. Edelstein, ScM2; Robert E. Ratner, MD3; Maria G. Montez, RN, MSHP4; Ronald T. Ackermann, MD, MPH5; Trevor J. Orchard, MBBCh, MMedSci6; Mary A. Foulkes, PhD2; Ping Zhang, PhD7; Christopher D. Saudek, MD8†; and Morton B. Brown, PhD1.
From the 1University of Michigan, Ann Arbor, Michigan; 2The Biostatistics Center, The George Washington University, Rockville, Maryland; the 3Medstar Health Research Institute, Washington, D.C.; the 4University of Texas Health Science Center at San Antonio, San Antonio, Texas; the 5Northwestern University Feinberg School of Medicine, Chicago, Illinois; the 6University of Pittsburgh, Pittsburgh, Pennsylvania; the 7Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, Georgia; and 8The Johns Hopkins University School of Medicine, Baltimore, Maryland.
†Deceased.
Footnotes
References
- 1. Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 2. Tuomilehto J, Lindström J, Eriksson JG, et al. ; Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 3. Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knowler WC, Fowler SE, Hamman RF, et al. ; Diabetes Prevention Program Research Group 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hernan WH, Brandle M, Zhang P, et al. ; Diabetes Prevention Program Research Group Costs associated with the primary prevention of type 2 diabetes mellitus in the diabetes prevention program. Diabetes Care 2003;26:36–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Herman WH, Brandle M, Zhang P, et al. ; Diabetes Prevention Program Research Group Within-trial cost-effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care 2003;26:2518–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herman WH, Hoerger TJ, Brandle M, et al. ; Diabetes Prevention Program Research Group The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med 2005;142:323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Palmer AJ, Roze S, Valentine WJ, Spinas GA, Shaw JE, Zimmet PZ. Intensive lifestyle changes or metformin in patients with impaired glucose tolerance: modeling the long-term health economic implications of the diabetes prevention program in Australia, France, Germany, Switzerland, and the United Kingdom. Clin Ther 2004;26:304–321 [DOI] [PubMed] [Google Scholar]
- 9. Caro JJ, Getsios D, Caro I, Klittich WS, O'Brien JA. Economic evaluation of therapeutic interventions to prevent type 2 diabetes in Canada. Diabet Med 2004;21:1229–1236 [DOI] [PubMed] [Google Scholar]
- 10. Eddy DM, Schlessinger L, Kahn R. Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes. Ann Intern Med 2005;143:251–264 [DOI] [PubMed] [Google Scholar]
- 11. Venditti EM, Bray GA, Carrion-Petersen ML, et al. ; Diabetes Prevention Program Research Group First versus repeat treatment with a lifestyle intervention program: attendance and weight loss outcomes. Int J Obes (Lond) 2008;32:1537–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kingsley RG, Wilson GT. Behavior therapy for obesity: a comparative investigation of long-term efficacy. J Consult Clin Psychol 1977;45:288–298 [DOI] [PubMed] [Google Scholar]
- 13. Renjilian DA, Perri MG, Nezu AM, McKelvey WF, Shermer RL, Anton SD. Individual versus group therapy for obesity: effects of matching participants to their treatment preferences. J Consult Clin Psychol 2001;69:717–721 [PubMed] [Google Scholar]
- 14. Hatziandreu EI, Koplan JP, Weinstein MC, Caspersen CJ, Warner KE. A cost-effectiveness analysis of exercise as a health promotion activity. Am J Public Health 1988;78:1417–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. (Eds.). Cost-effectiveness in health and medicine. New York, Oxford University Press, 1996. [Google Scholar]
- 16. Hoerger TJ, Hicks KA, Sorensen SW, et al. Cost-effectiveness of screening for pre-diabetes among overweight and obese U.S. adults. Diabetes Care 2007;30:2874–2879 [DOI] [PubMed] [Google Scholar]
- 17. Cohen JT, Neumann PJ, Weinstein MC. Does preventive care save money? Health economics and the presidential candidates. N Engl J Med 2008;358:661–663 [DOI] [PubMed] [Google Scholar]
- 18. Neumann PJ, Rosen AB, Weinstein MC. Medicare and cost-effectiveness analysis. N Engl J Med 2005;353:1516–1522 [DOI] [PubMed] [Google Scholar]
- 19. American Diabetes Association Economic costs of diabetes in the U.S. in 2007. Diabetes Care 2008;31:596–615 [DOI] [PubMed] [Google Scholar]
- 20. Brown JB, Nichols GA, Glauber HS, Bakst AW. Type 2 diabetes: incremental medical care costs during the first 8 years after diagnosis. Diabetes Care 1999;22:1116–1124 [DOI] [PubMed] [Google Scholar]
- 21. Gilmer TP, O'Connor PJ, Manning WG, Rush WA. The cost to health plans of poor glycemic control. Diabetes Care 1997;20:1847–1853 [DOI] [PubMed] [Google Scholar]
- 22. Caro JJ, Ward AJ, O'Brien JA. Lifetime costs of complications resulting from type 2 diabetes in the U.S. Diabetes Care 2002;25:476–481 [DOI] [PubMed] [Google Scholar]
- 23. Brandle M, Zhou H, Smith BRK, et al. The direct medical cost of type 2 diabetes. Diabetes Care 2003;26:2300–2304 [DOI] [PubMed] [Google Scholar]