Abstract
OBJECTIVE
To examine the performance of current screening recommendations for detecting dysglycemia in children and adolescents with obesity.
RESEARCH DESIGN AND METHODS
In a cross-sectional study, an oral glucose tolerance test and demographic (age, sex, family history of diabetes, and ethnicity), clinical (BMI z score, waist circumference, and pubertal stage), and laboratory variables used in current pediatric screening criteria for type 2 diabetes mellitus were measured in 259 overweight or obese youth aged 5–17 years. Glycemic status was based on American Diabetes Association (ADA) thresholds. The performance (sensitivity and specificity) of current screening criteria and newly developed models to identify isolated IGT were compared.
RESULTS
Dysglycemia was present in 20.8% of the cohort. Of the 54 participants with dysglycemia, 68% had a normal fasting glucose and were identified with the 2-h glucose test. Current ADA criteria had low sensitivity (41.7% [95% CI 25.6–57.8]) and moderate specificity (69.5% [63.5–75.6]) to identify IGT. In receiver operating characteristic (ROC) analysis, the addition of hemoglobin A1c (HbA1c) or FPG did not improve the ROC area under the curve (AUC) (HbA1c: 0.64 vs. 0.63; P = 0.54; HbA1c + FPG: 0.66; P = 0.42), but adding triglyceride level did (AUC 0.72 vs. 0.63; P = 0.03). A simple model with fasting triglyceride level >1.17 mmol/L improved AUC compared with ADA screening criteria (0.68 vs. 0.57; P = 0.04).
CONCLUSIONS
The prevalence of IGT is high among obese children and youth. Current screening criteria have low sensitivity to detect isolated IGT. Although adding nonfasting laboratory values to history and physical measures does not improve diagnostic accuracy, adding fasting lipid profile improves predictive value.
Obesity-related metabolic abnormalities are common in children and adolescents with obesity. Impaired glucose tolerance (IGT), an important predictor of progression to type 2 diabetes mellitus (T2DM) in youth (1), is identified in overweight and obese children, although the prevalence varies considerably with the population studied. Although 20–25% of overweight youth presenting to a weight management program in the northeastern U.S. were diagnosed with IGT (2), clinical cohorts in other countries have had much lower prevalence (5–17%) (3–5). In adults, IGT is a strong predictor for progression to T2DM (6) and increased risk of cardiovascular disease, independent of the development of T2DM (7). Randomized controlled trials of lifestyle or medication interventions in adults with IGT have demonstrated that T2DM can be prevented (8,9). Because the detection of IGT requires the performance of a cumbersome oral glucose tolerance test (OGTT), strategies to minimize the number of people requiring such a test have been studied in adults (10,11). Few such studies have been done in children and adolescents.
Current American Diabetes Association (ADA) guidelines recommend screening high-risk populations with a fasting plasma glucose (FPG) test (12,13), although they acknowledge that the best screening test and the population of obese children and youth that should be screened require further investigation (14). The majority of children with IGT have a normal fasting glucose (2), suggesting that FPG alone may be inadequate to identify prediabetes and that an OGTT be considered for screening in at-risk youth. Because the OGTT is costly, it should be performed on those at highest risk only, but little evidence evaluating the risk prediction properties of current screening criteria is available.
This study examines the clinical usefulness of current screening recommendations in identifying dysglycemia (T2DM, impaired fasting glucose [IFG], or IGT) in a cohort of 259 children and youth (aged 5–17 years) presenting to a weight management program and identifies a potential new screening tool for identification of obese youth with dysglycemia.
RESEARCH DESIGN AND METHODS
The study population was composed of youth at the time of entry into a weight management program who enrolled in the DEterminants of Change in Childhood Obesity (DECCO) study, a prospective, observational study examining determinants of health indicators at baseline and during weight management in an established weight management program. All subjects aged 5–17, with no untreated endocrine disorder, were eligible for study participation. Participants in the DECCO study had four study visits—baseline (enrollment in weight management program), 6 months, 1 year, and 2 years (1 year after completion of monthly program)—whereas this analysis considers only the baseline data in a cross-sectional manner. Written informed consent was provided by the legal guardian and the child provided signed assent. The study was approved by the institutional review board at the Hamilton Health Sciences Corporation (Hamilton, ON, Canada).
Study visit and parameters
The baseline study visit occurred in the morning, after an 8- to 12-h fast (allowing water), and comprised an evaluation of cardiometabolic risk factors (including an OGTT), anthropometric evaluation, and completion of questionnaires as described below. Standing height was measured using a Harpenden Stadiometer (London, UK). BMI (kg/m2) and BMI z score were calculated based on Centers for Disease Control and Prevention normative data, using NUTSTAT, a component of the Epi Info program (15). Waist circumference (WC) was measured half-way between the iliac crest and lower rib (16) using a nonstretching tape with attached spring balance pulled to a tension of 250 g. WC z scores were calculated based on age- and sex-specific Canadian normative data (17,18). Blood pressure (BP) was measured three times on the right arm, using an oscillometric method (Omron Healthcare, Inc., Lake Forest, IL) with appropriate-sized cuff after the children had been sitting at rest for ∼10 min (19). The average of the three measures was used for further analysis. Age, sex, and height cutoffs for defining hypertension were based on the 95th centile using U.S. normative data as recommended (19).
Parents reported the family history of premature coronary heart disease (defined as events in first- or second-degree male relatives <55 years old and/or female relatives <65 years old), T2DM, and obesity. Children aged ≥8 years completed a confidential questionnaire identifying their cigarette smoking history and self-assessed pubertal stage (20,21). Boys and girls aged <8 years were assumed to be prepubertal, based on normative data for pubertal onset.
An OGTT was conducted as follows: a baseline venous blood sample was taken and the patient consumed 1.75 g/kg glucose solution up to a maximum of 75 g orally. Venous blood samples were drawn 30, 60, and 120 min after baseline. Prediabetes was defined as IFG, IGT, or IFG + IGT using ADA criteria (IFG: fasting glucose level ≥5.6 mmol/L; IGT: 2-h glucose level ≥7.8 mmol/L). Dysglycemia included prediabetes or T2DM. Baseline laboratory analyses were conducted in the laboratory of Hamilton Health Sciences and included plasma glucose, total cholesterol (TC), HDL cholesterol (HDL-C), and triglyceride (TG); LDL cholesterol (LDL-C) was calculated using the equation of Friedewald (22). Glucose was measured using an enzymatic reference method with hexokinase, and cholesterol levels were measured with an enzymatic colorimetric method on a Roche INTEGRA analyzer. Dyslipidemia was defined as fasting TG >1.7 mmol/L and/or HDL-C <1.03 mmol/L and/or LDL-C >3.3 mmol/L, according to current recommendations (23).
Statistical analysis
Study participants with dysglycemia were compared with those without dysglycemia using an unpaired t test for continuous variables and χ2 test for discrete variables. Laboratory variables (TC, HDL-C, LDL-C, TG, glucose, hemoglobin A1c (HbA1c) alanine aminotransferase [ALT], and aspartate aminotransferase [AST]) were log transformed to improve normality and are presented in Table 1 as geometric means and 95% CIs. All other continuous variables are presented as mean ± SD and discrete variables as n (%).
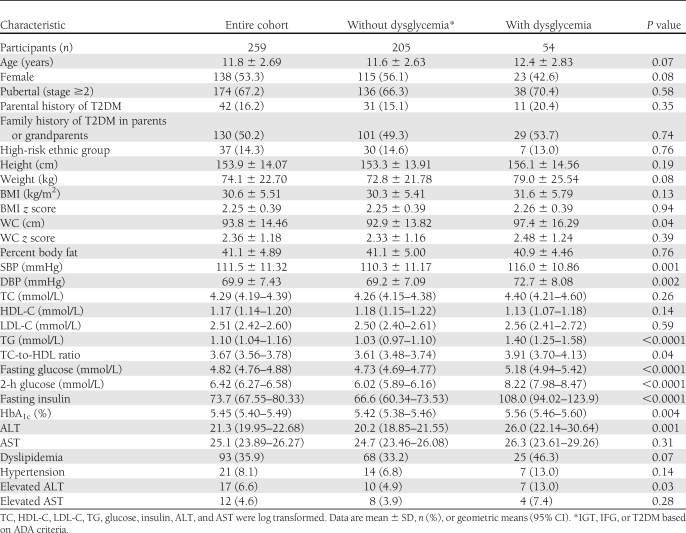
Table 1.
Baseline characteristics of the study cohort
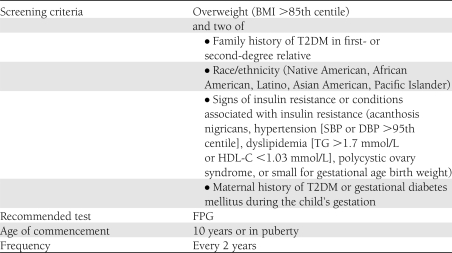
The ADA currently recommends criteria for screening for T2DM in children (Table 2). The sensitivity and specificity of these criteria for identifying dysglycemia (T2DM, IFG, and IGT) and isolated IGT in our population were calculated. To examine the association of risk variables with dysglycemia, logistic regression analysis was used and odds ratios (ORs) were calculated. The sensitivity and specificity of other risk strategies were also evaluated including 1) current Canadian Diabetes Association (CDA) guidelines (24), 2) a risk score developed by Reinehr et al. (4), and 3) fasting glucose >4.8 mmol/L (3). The CDA screening criteria are similar to the ADA criteria but somewhat less stringent. The CDA recommends screening all subjects in puberty or aged ≥10 years who have two of the following: BMI >95th centile, high-risk ethnic group or family history of T2DM, signs or symptoms of insulin resistance, and/or use of antipsychotic medications. Reinehr et al. (4) developed a simple risk score that includes parental history of T2DM (2 points), pubertal onset (1 point), and extreme obesity defined as BMI z score >2.58 (1 point) and recommended screening children or youth with ≥2 points. Maffeis et al. (3) recommended doing an OGTT to identify IGT in children or youth if the FPG exceeded 4.8 mmol/L.
Table 2.
ADA-recommended criteria for screening children and youth for T2DM
As we were primarily interested in reducing the number of OGTTs performed, while detecting patients with isolated IGT, several models for the prediction of isolated IGT were developed from least invasive to most complex. Variables that were significant in univariate analysis were included (age, sex, family history of T2DM, systolic BP [SBP], BMI z score, WC, HbA1c, FPG, elevated ALT, TC-to-HDL ratio, and TG). Model 1 included data available with history and clinical exam only; model 2 included data from history, clinical exam, and nonfasting blood work (HbA1c); model 3 included data from history, clinical exam, HbA1c and fasting glucose; and model 4 added in the influence of fasting TG. To evaluate the predictive properties of current ADA and CDA screening criteria and each model to identify IGT, we performed receiver operating characteristic (ROC) analysis on the entire population with complete data. To estimate the discriminative value of the predictive models, we calculated the ROC area under the curve (AUC) for the outcome IGT. The AUC for the ADA and CDA screening criteria and each of the models was compared using a χ2 test.
Although the models were developed using continuous variables, we sought to establish a user-friendly screening test using thresholds for each variable and to examine these in a logistic model. The applied thresholds considered were previously recommended, including BMI z score 2.58 (4) and HbA1c 5.7%, or were obtained using optimal discrimination on the ROC curve (TG >1.17 mmol/L).
RESULTS
The descriptive characteristics and risk variables of the cohort (n = 259) and of those with and without dysglycemia (prediabetes or T2DM) are presented in Table 1. Although the mean age was 11.8 years, participants ranged in age from 5 to 17 years. Of the participants, 1 (0.39%) had undiagnosed T2DM based on a 2-h glucose >11.1 mmol/L. Prediabetes was present in 20.5% of the cohort, and 4.2% had IFG, 13.9% isolated IGT, and 2.3% IFG + IGT. Thus, of the 53 participants with prediabetes identified using an OGTT, 36 (68.0%) had isolated IGT and would not have been identified if only fasting glucose had been measured. Furthermore, the 1 participant with T2DM also had a normal fasting glucose. We identified no difference in the prevalence of prediabetes in those <10 vs. ≥10 years (17.8 vs. 22.0%; P = 0.45).
The participants with dysglycemia (prediabetes or T2DM) did not differ in age, self-reported pubertal stage, ethnicity, BMI z score, WC z score, or percent body fat from those without dysglycemia (Table 1). A family history of T2DM was relatively common in the children in this cohort (50.2%), and the prevalence was not different in those with and without dysglycemia. Diagnosed T2DM in at least one parent was less common (16.2%) but also did not differ between groups. Children with dysglycemia had higher SBP and diastolic BP (DBP), fasting TG, HbA1c, and ALT. The prevalence of elevated ALT was also higher in the dysglycemia group (13.0 vs. 4.9%; P = 0.03), but no differences in the prevalence of dyslipidemia (P = 0.07) or hypertension (P = 0.14) were noted. The prevalence of HbA1c above the recommended threshold of 5.7% was not different in those with dysglycemia (P = 0.10). The strongest predictor of dysglycemia in univariate analysis was HbA1c (OR per 1-SD increment 3.4 [95% CI 1.24–9.35]; P = 0.02). Other cardiovascular risk factors that predicted dysglycemia included serum TG (1.87 [1.33–2.63]; P = 0.0003) and SBP (1.04 [1.01–1.07]; P = 0.01).
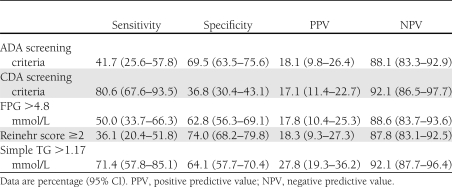
The ADA recommends screening children aged ≥10 years (or pubertal children) who are overweight or obese and have two associated risk factors as described in Table 2. A total of 83 children and youth (32%) met current screening criteria. Current screening criteria had a sensitivity of 38.9% (95% CI 25.9–51.9) and a specificity of 69.8% (63.5–76.0) to detect dysglycemia. In a similar manner, the sensitivity to detect isolated IGT was 41.7% (25.6–57.8) with specificity of 69.5% (63.5–75.6). Thus, in applying the current ADA screening criteria, less than half of those children with isolated IGT were identified. As noted in Table 3, Reinehr score and FPG >4.8 mol/L also had low sensitivity and comparable specificity to the ADA criteria. The CDA criteria had higher sensitivity to identify a child with IGT (81.8 vs. 43.2% using ADA) but lower specificity (37.7 vs. 70.2%).
Table 3.
Sensitivity and specificity of current screening guidelines, published scores, and simple TG model for detecting isolated IGT
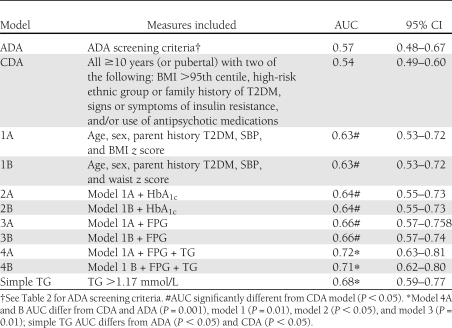
As described in Research Design and Methods, we proceeded to develop the models based on increasing complexity. Each model differed significantly from the reference line, suggesting some predictive value. The logistic models 1A to 4A are presented in Supplementary Table 1. SBP and fasting TG are the most important variables contributing to the model. We compared the models using the AUC of the ROC curves (Table 4). Adding HbA1c or elevated ALT values to data obtained from the clinical history and physical exam did not improve the prediction of IGT, but adding fasting blood work was beneficial. Adding fasting glucose alone demonstrated no increased predictive power, but adding fasting TGs did. Similar conclusions were reached if the anthropometric variable included was BMI z score or WC z score.
Table 4.
ROC analysis of models for prediction of isolated IGT using ADA criteria
Using thresholds for each variable in model 4, the potential predictors age >10 (P = 0.49), pubertal (P = 0.52), parental T2DM (P = 0.15), HbA1c >5.7% (P = 0.43), fasting blood glucose (P = 0.85), SBP >95th centile (P = 0.82), and BMI z score >2.58 were not significant and were not included in the final logistical model. Remaining in the model was TG >1.17 mmol/L (OR 4.3; P < 0.0001). This variable alone had clinical usefulness (Table 3), and AUC that exceeded the ADA and CDA screening criteria was somewhat lower than continuous models 4A and 4B (Table 4).
CONCLUSIONS
Current ADA screening criteria had low discriminatory capacity for identifying dysglycemia in our cohort of overweight children presenting to a weight management program. Using a specific multilevel approach to applying screening criteria, we have identified a screening algorithm requiring history and physical examination only, with comparable discriminatory capacity (AUC in ROC analysis) to ADA recommendations but requiring less information (age, sex, parent history of T2DM, SBP, and BMI z score). The addition of a nonfasting blood test to measure HbA1c did not improve the predictive properties, but adding a fasting TG improved the discriminatory capacity. In fact, a fasting TG >1.17 mmol/L had better discriminatory capacity and clinical usefulness than current ADA criteria in identifying isolated IGT.
Dysglycemia was common (20.8%) and IGT was identified in 16.2% of the participants, comparable to the findings of several American studies (2,25). Silent T2DM was relatively rare in this population (1 of 259) but was identified on a 2-h blood glucose alone. As with previous studies, the prevalence of IFG was low, and 68% of children with elevated 2-h glucose levels had fasting glucose <5.6 mmol/L (ADA cutpoint for IFG). Among our population of obese children and adolescents, important risk predictors for the presence of IGT included higher HbA1c and fasting serum TG. SBP, parental T2DM, and higher TC-to-HDL ratio were also predictive variables. Important variables without evident influence include age, pubertal stage, sex, body size (BMI), and WC, suggesting that among obese youth, these variables have little predictive potential for dysglycemia.
Current ADA screening criteria had low sensitivity and only moderate specificity to identify isolated IGT. Other previously recommended screening tools also had low sensitivity. Using ROC analysis, we identified a model using history, physical examination, and fasting laboratory data (glucose and TG) with better discrimination of IGT than current ADA and CDA screening criteria. This may enable reasonable identification of children with prediabetes while avoiding excessive use of OGTTs.
HbA1c thresholds recently have been recommended for identifying adults at high risk for T2DM and for diagnosis of T2DM in adults (26). This approach also has been recommended recently for adolescents, although the clinical usefulness in this age-group has since been challenged (27,28). Using National Health and Nutrition Examination Survey data, HbA1c thresholds of 5.7 and 6.0% had poorer performance in identifying prediabetes in adolescents compared with adults. We found no added predictive benefit of including HbA1c with data from history and physical examination in identifying IGT. However, adding the fasting TG level improved the AUC.
When applying thresholds to develop a simple clinically applicable version of model 4, only fasting TG >1.17 mmol/L remained in the model, and this alone had sensitivity of 70% and specificity of 64% to identify IGT. Furthermore, the AUC exceeded that of current ADA and CDA screening methods.
Despite being one of very few studies to critically examine the usefulness of current screening recommendations to identify prediabetes in obese youth, our study does have some limitations. Even though no influence of pubertal stage was identified on prevalence of prediabetes, it is noteworthy that puberty was not assessed by a physician but was self-reported by the study participants using a validated methodology. Although we identified no difference in prevalence of prediabetes in those aged <10 compared with those ≥10 years, only 73 participants aged <10 were included. Thus, further observation of the prevalence of prediabetes and evaluation of appropriate screening criteria in this age group should occur. Our classification of the glycemic status of the study participants was based on a single OGTT, and given published concerns on the reliability of this measure, we may have misclassified some of the participants. However, our methodology is consistent with that used in most studies examining the risk of progression to T2DM and comparable studies identifying the prevalence of prediabetes in youth. Finally, our evaluation was within a select population: all children and youth were overweight, 93% were obese, and all were referred for weight management.
Balancing the costs and the consequences of more invasive testing with the potential health advantages of screening depends on how important one considers the identification of a disorder. Although in adults we have interventions that prevent the progression of prediabetes to T2DM, thus arguing for high importance of identification of prediabetes, questions remain on the natural history of prediabetes in youth. Very few longitudinal data are available in children or adolescents. In one study, 8 of 33 (24%) youth with IGT at baseline progressed to T2DM during a 2-year period (1), comparable to annual progression rates of 5–10% reported in adults (6). Should future research support the importance of intervening in children and youth with IGT, screening criteria with high sensitivity would be required. Although screening obese children and youth with a fasting TG provides moderate sensitivity and specificity and is better than currently recommended approaches, ∼30% of obese children with IGT will not be identified using this screening criteria, suggesting that an OGTT be done in all obese children and youth. Pending efficacy studies in youth, this approach would be questioned. Longitudinal studies examining the outcome of children and youth with prediabetes are urgently required.
Acknowledgments
This study was funded by the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Ontario.
No potential conflicts of interest relevant to this article were reported.
K.M.M. researched data and wrote the manuscript. L.X. analyzed data and reviewed and edited the manuscript. M.T., S.A.A., and S.Y. contributed to discussion and reviewed and edited the manuscript. Z.Y. researched data and contributed to discussion. K.M.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 3rd International Congress on Prediabetes and Metabolic Syndrome, Nice, France, 1–4 April 2009.
The conduct of this study would not have been possible without the research team, especially Vivian Vaughn-Williams, Elizabeth Helden, Susan Docherty-Skippen, and Cathy Wright, and the clinicians at the Children’s Exercise and Nutrition Centre. The investigators are particularly indebted to the families who participated in this project.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1659/-/DC1.
References
- 1.Weiss R, Taksali SE, Tamborlane WV, Burgert TS, Savoye M, Caprio S. Predictors of changes in glucose tolerance status in obese youth. Diabetes Care 2005;28:902–909 [DOI] [PubMed] [Google Scholar]
- 2.Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity[corrected in: N Engl J Med 2002;346:1756]. N Engl J Med 2002;346:802–810 [DOI] [PubMed] [Google Scholar]
- 3.Maffeis C, Pinelli L, Brambilla P, et al. Fasting plasma glucose (FPG) and the risk of impaired glucose tolerance in obese children and adolescents. Obesity (Silver Spring) 2010;18:1437–1442 [DOI] [PubMed] [Google Scholar]
- 4.Reinehr T, Wabitsch M, Kleber M, de Sousa G, Denzer C, Toschke AM. Parental diabetes, pubertal stage, and extreme obesity are the main risk factors for prediabetes in children and adolescents: a simple risk score to identify children at risk for prediabetes. Pediatr Diabetes 2009;10:395–400 [DOI] [PubMed] [Google Scholar]
- 5.Sabin MA, Hunt LP, Ford AL, Werther GA, Crowne EC, Shield JP. Elevated glucose concentrations during an oral glucose tolerance test are associated with the presence of metabolic syndrome in childhood obesity. Diabet Med 2008;25:289–295 [DOI] [PubMed] [Google Scholar]
- 6.Gerstein HC, Santaguida P, Raina P, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract 2007;78:305–312 [DOI] [PubMed] [Google Scholar]
- 7.Qiao Q, Jousilahti P, Eriksson J, Tuomilehto J. Predictive properties of impaired glucose tolerance for cardiovascular risk are not explained by the development of overt diabetes during follow-up. Diabetes Care 2003;26:2910–2914 [DOI] [PubMed] [Google Scholar]
- 8.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trial Research Group Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002;359:2072–2077 [DOI] [PubMed] [Google Scholar]
- 9.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindström J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 2003;26:725–731 [DOI] [PubMed] [Google Scholar]
- 11.Schulze MB, Hoffmann K, Boeing H, et al. An accurate risk score based on anthropometric, dietary, and lifestyle factors to predict the development of type 2 diabetes. Diabetes Care 2007;30:510–515 [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sellers EA, Panagiotopoulos C, Lawson ML; Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Type 2 diabetes in children and adolescents. Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 2008;32(Suppl. 1):S162–S167 [Google Scholar]
- 14.American Diabetes Association Type 2 diabetes in children and adolescents. Pediatrics 2000;105:671–680 [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Epi Info program [software tool online], 2008. Available from http://www.cdc.gov/epiinfo/ Accessed 13 June 2008
- 16.Fernández JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr 2004;145:439–444 [DOI] [PubMed] [Google Scholar]
- 17.Tremblay MS, Shields M, Laviolette M, Craig CL, Janssen I, Gorber SC. Fitness of Canadian children and youth: results from the 2007-2009 Canadian Health Measures Survey. Health Rep 2010;21:7–20 [PubMed] [Google Scholar]
- 18.Statistics Canada. 2007–2009 Canadian Health Measures Survey (Catalogue no. 82-003-X). Health Reports 2010
- 19.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004;114(Suppl. 2, 4th Report):555–576 [PubMed]
- 20.Brooks-Gunn J, Warren MP, Rosso J, Gargiulo J. Validity of self-report measures of girls’ pubertal status. Child Dev 1987;58:829–841 [PubMed] [Google Scholar]
- 21.Neinstein LS. Adolescent self-assessment of sexual maturation: reassessment and evaluation in a mixed ethnic urban population. Clin Pediatr (Phila) 1982;21:482–484 [DOI] [PubMed] [Google Scholar]
- 22.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 23.Kavey RE, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Taubert K; American Heart Association American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. J Pediatr 2003;142:368–372 [DOI] [PubMed] [Google Scholar]
- 24.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 2008;32(Suppl. 1):S168–S180 [DOI] [PubMed] [Google Scholar]
- 25.Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab 2008;93:4231–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JM, Wu EL, Tarini B, Herman WH, Yoon E. Diagnosis of diabetes using hemoglobin A1c: should recommendations in adults be extrapolated to adolescents? J Pediatr 2011;158:947–952 [DOI] [PMC free article] [PubMed]
- 28.Nowicka P, Santoro N, Liu H, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care 2011;34:1306–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]