Abstract
OBJECTIVE
The current study aimed to investigate whether microalbuminuria or moderately decreased glomerular filtration rate (GFR) is a better predictor for the development and progression of retinopathy in type 2 diabetic patients.
RESEARCH DESIGN AND METHODS
Type 2 diabetic patients without cardiovascular diseases, malignancy, pregnancy, and acute intercurrent illness were enrolled between 1 August 2001 and 31 December 2002. All participants provided their detailed medical history and underwent an eye fundus examination. They were followed up in outpatient clinics, and serum creatinine, urinary albumin-to-creatinine ratio (UACR), and retinal photographs were followed up annually until 31 December 2009. The primary outcomes were development and progression of diabetic retinopathy and nephropathy. The secondary outcomes were cardiovascular events and all-cause mortality.
RESULTS
Among 487 participants, 81 subjects had normoalbuminuria and moderate renal impairment (baseline eGFR 30–59.9 mL/min/1.73 m2), and 106 subjects had microalbuminuria and baseline eGFR ≥60 mL/min/1.73 m2. Patients with microalbuminuria and eGFR ≥60 mL/min/1.73 m2 had a significantly greater risk for development and progression of diabetic retinopathy (HR 3.34 [95% CI 1.04–10.70]) compared with those with moderate renal impairment and normoalbuminuria after multivariate adjustment. Risks for renal outcome, cardiovascular events, and all-cause mortality were not significantly different between the two groups.
CONCLUSIONS
Microalbuminuria has a greater impact on predicting the development and progression of diabetic retinopathy compared with moderate decline in GFR among type 2 diabetic patients.
Diabetic retinopathy is a highly specific vascular complication and a sight-threatening problem related to diabetes. Diabetic retinopathy is characterized by gradually progressive alterations in the retinal microvasculature, leading to retinal nonperfusion, increased vascular permeability, and pathologically intraocular proliferation of retinal vessels. Both diabetic retinopathy and nephropathy are microvascular complications of diabetes. With the retina and glomerulus, diabetes-specific microvascular disease is characterized by similar pathophysiologic features. Chronic hyperglycemia is the central initiating factor for all types of diabetic microvascular disease. In exposure to high plasma glucose, hyperglycemic damage is limited to some cell types. Endothelial cells develop intracellular hyperglycemia, since they cannot downregulate glucose transport (1). The common pathologic trait of diabetic microvascular disease is progressive narrowing and eventual occlusion of vascular lumina, subsequently leading to inadequate perfusion of the affected tissues. In the retina, diabetes induces programmed cell death of Muller and ganglion cells (2) and pericytes and endothelial cells (3). In the glomerulus, urinary protein loss and renal function decline are associated with widespread capillary occlusion and podocyte loss.
Elevated urinary albumin excretion has been found to increase the risk of proliferative diabetic retinopathy (PDR) and is associated with a higher prevalence of PDR (4–8). Diabetic retinopathy is present in virtually all type 1 diabetic patients with nephropathy, whereas only 50–60% of type 2 diabetic patients with nephropathy have retinopathy (5). Accordingly, nephropathy and retinopathy are not concurrent in type 2 diabetes. Although the prevalence and risk factors for diabetic retinopathy in type 2 diabetes have been extensively investigated (6–8), there are little data addressing the issue of whether microalbuminuria or moderate decline of estimated glomerular filtration rate (eGFR) (30–59.9 mL/min/1.73 m2) is a competing risk factor for the development and progression of diabetic retinopathy. Therefore, the current study strived to investigate whether microalbuminuria or moderately decreased eGFR is a better predictor for the retinal outcome in a type 2 diabetic cohort.
RESEARCH DESIGN AND METHODS
Approximately 1,000 type 2 diabetic patients who were at least 18 years of age receiving regular follow-up at the outpatient clinics of Taipei Veterans General Hospital were invited to participate in this study. Patients with cardiovascular disease, malignancy, pregnancy, and acute intercurrent illness were excluded. Seven hundred and seventy-seven participants provided written informed consent and subsequently received examinations for serum creatinine, urine albumin excretion, blood pressure, fasting blood glucose, HbA1c, serum cholesterol, triglyceride, and eye fundus photographs (Supplementary Fig. 1). Baseline data were collected between August 2001 and December 2002. After exclusion of subjects with baseline advanced diabetic retinopathy that meant proliferative diabetic retinopathy, postlaser photocoagulation, or vitrectomy and those who had missing eGFR or urine albumin-to-creatinine ratio (UACR) data, 579 subjects had data for both eGFR and UACR. Among the 579 subjects, 92 patients with eGFR <30 mL/min/1.73 m2 and/or UACR >300 mg/g Cr were excluded. Moderate renal impairment was defined as baseline eGFR 30–59.9 mL/min/1.73 m2, as well as normoalbuminuria as baseline UACR <30 mg/g Cr and microalbuminuria as baseline UACR 30–299.9 mg/g Cr. Finally, a total of 487 subjects were enrolled in the study and categorized into four groups, including eGFR ≥60 mL/min/1.73 m2 and normoalbuminuria (group 1), moderate renal impairment and normoalbuminuria (group 2), eGFR ≥60 mL/min/1.73 m2 and microalbuminuria (group 3), and moderate renal impairment and microalbuminuria (group 4). The study was approved by the institutional review board of the Taipei Veterans General Hospital.
Baseline examination
Blood pressure was taken twice by using an electric blood pressure monitor in the sitting position after 10 min of rest. The mean of the measurements was used. Questions pertaining to ever smoking or not were recorded. Based on medical records, information about duration of diabetes and prescribed medication was obtained. Venipuncture was performed, and a fasting serum sample was collected for the measurement of diabetic profiles, serum creatinine, and other biochemical tests. eGFR was calculated by computer using the Modification of Diet in Renal Disease (MDRD) equation (9). Urinary albumin excretion was determined as the UACR from the mean of two spot urine samples on two consecutive mornings. Color photographs of the retinas were taken according to the methodology of the ACCORD (Action to Control Cardiovascular Risk in Diabetes) Eye Study (10), which was slightly modified from the Early Treatment Diabetic Retinopathy Study (ETDRS) (11). Macula- and disc-centered views were taken at an angle of 45° with a fundus camera after pharmacological mydriasis. The fundus photographs were evaluated by trained graders, who were unaware of the medical conditions, on the basis of the photographic standards defined for the ACCORD Eye Study (10). The ETDRS Severity Scale has 17 steps, ranging from no retinopathy in either eye (step 1) to high-risk proliferative retinopathy in both eyes (step 17). We combined the severity of retinopathy into four categories: absent (steps 1– 3), mild to moderate nonproliferative diabetic retinopathy (NPDR) (steps 4–7), severe NPDR (steps 8–11), and advanced diabetic retinopathy (PDR, postlaser photocoagulation, or vitrectomy) (step 12 or above). Subjects with baseline advanced diabetic retinopathy were excluded from the analysis.
Assays
HbA1c was measured using high-performance liquid chromatography (HPLC) instruments (HLC-723 GHB IIIs; Tosoh, Tokyo, Japan) with a reference range of 4.2–5.8%. The interassay between-batch coefficient of variation (CV) was <2.0% at A1C levels between 4.4 and 8.2%. Urinary albumin concentration was measured by rate nephelometry (IMMAGE Immunochemistry System; Beckman Coulter, Brea, CA). The interassay CV was 7.5% at urine albumin of 0.56 mg/dL and 2.0% at 3.19 mg/dL, respectively. Urinary creatinine was measured using a Hitachi 7600 automatic analyzer (Hitachi, Tokyo, Japan). The interassay CV was <2.0% at creatinine of 2.0 mg/dL.
Study outcomes
The patients were followed up in our clinics until 31 December 2009. The primary outcomes were composite retinal outcome and progression of nephropathy (renal outcome). These subjects received repeated eye fundus photographs or were examined by ophthalmologists during follow-up. The composite retinal outcome was defined as an increase of at least three steps on the ETDRS Severity Scale or development of advanced diabetic retinopathy. Advanced diabetic retinopathy was defined as development of proliferative diabetic retinopathy, retinopathy treated with laser photocoagulation, or vitrectomy. Progressive loss of renal function (renal outcome) was defined as eGFR decrease ≥15 mL/min/1.73 m2 and final eGFR <60 mL/min/1.73 m2.
The secondary outcomes were cardiovascular events and all-cause mortality. Cardiovascular events were defined as admission owing to angina, myocardial infarction, heart failure, acute coronary syndrome, and cerebrovascular accident. Two research physicians who did not know the eGFR and UACR of these patients verified all information. Subsequently, a medical expert in the field reviewed all events coded by the research physicians and verified that all coding rules had been applied correctly. When discrepancies between the medical expert and research physicians occurred, the expert’s judgment was considered final.
Statistical analysis
Statistical analysis was performed using SPSS version 18.0 for Windows. The results were expressed as means ± SD unless otherwise indicated. The continuous variables were analyzed by one-way ANOVA. The noncontinuous data were presented as percentages and analyzed by Pearson χ2 test. eGFR and UACR were expressed as median (interquartile range) and analyzed by nonparametric test. Hazard ratios (HRs) of incidence and 95% CIs were calculated by multivariable Cox regression models. In comparison between eGFR 30–59.9 and ≥60 mL/min/1.73 m2 (reference), the results were adjusted for age, sex, systolic blood pressure, BMI, HbA1c, total cholesterol, triglyceride, duration of diabetes, history of hypertension, history of smoking, insulin use, ACE inhibitors or angiotensin II receptor blockers (ARBs), and UACR. In comparison between UACR 30–299.9 and <30 mg/g (reference), the results were adjusted for age, sex, systolic blood pressure, BMI, HbA1c, total cholesterol, triglyceride, duration of diabetes, history of hypertension, history of smoking, insulin use, ACE inhibitors or ARBs, and eGFR. For the comparison between patients of groups 2 and 3, the Cox model was adjusted for age, sex, systolic blood pressure, BMI, HbA1c, total cholesterol, triglyceride, duration of diabetes, history of hypertension, history of smoking, and use of insulin, ACE inhibitors, or ARBs. A P value of <0.05 was considered statistically significant.
RESULTS
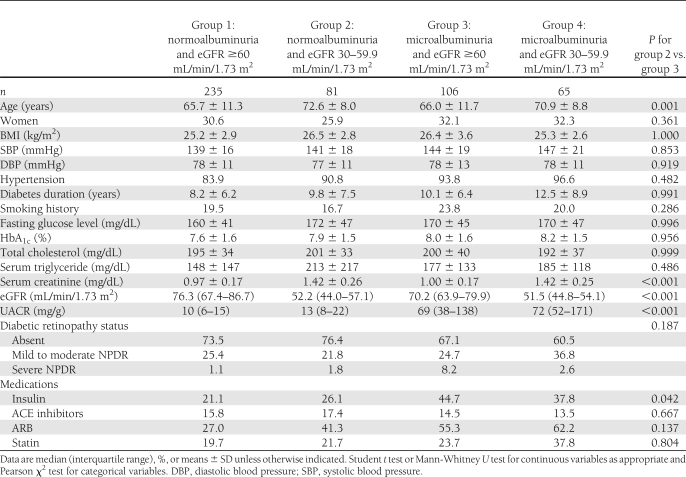
The clinical characteristics of 487 participants are summarized in Table 1 according to the eGFR and UACR at baseline. The enrolled subjects were 33–93 years old (mean age 68 years). Approximately 30% of the subjects were women. The mean duration of diabetes at enrollment was 9.4 years. Patients with moderate renal impairment and normoalbuminuria were older and had a lower proportion of receiving insulin therapy than those with microalbuminuria and eGFR ≥60 mL/min/1.73 m2.
Table 1.
Characteristics of participants at baseline by eGFR and urine albumin excretion
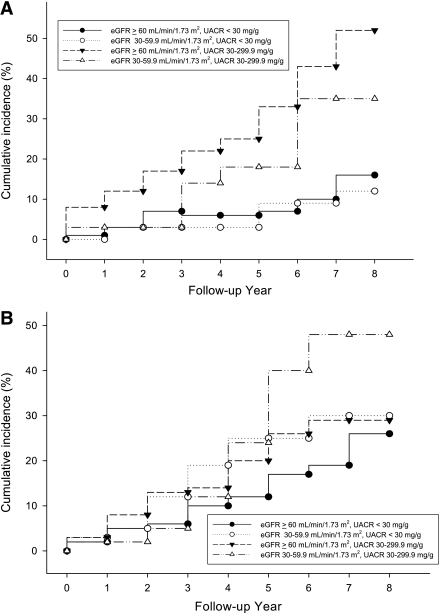
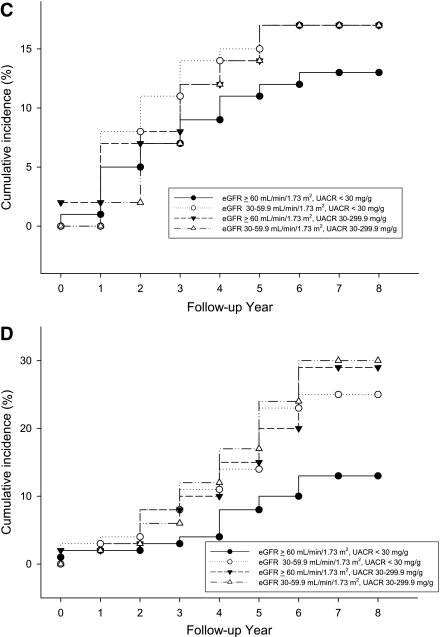
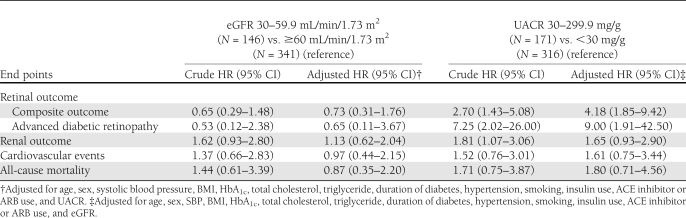
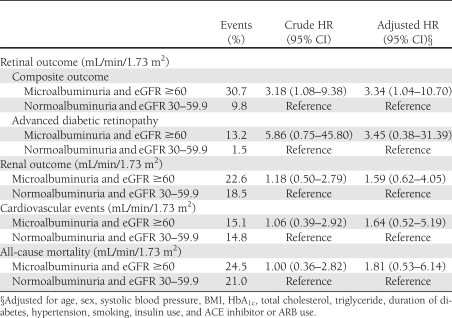
During the follow-up of 6.6 ± 1.2 years (median 7.6 years), 16.5% of the subjects noted the development or progression of diabetic retinopathy (composite retinal outcome), 5.4% of the subjects developed advanced diabetic retinopathy, 19.5% of subjects had progressive loss of renal function, 12.9% of subjects suffered from cardiovascular events, and 17.0% of subjects died. Kaplan-Meier analysis curves for end points of composite retinal outcome, renal outcome, cardiovascular events, and all-cause mortality (Fig. 1A–D) showed that patients with microalbuminuria (groups 3 and 4) were at a higher risk for development and progression of diabetic retinopathy than were those with normoalbuminuria (groups 1 and 2). Cox proportional hazards model was then used to examine the relative risks of retinal, renal outcome, cardiovascular events, and all-cause mortality in relation to microalbuminuria and moderate renal impairment, as seen in Tables 2 and 3. The HR of composite retinal outcome (Table 2) showed significant difference between normoalbuminuria and microalbuminuria after multivariate adjustment (HR 4.18 [95% CI 1.85–9.42], P = 0.001) but no difference between eGFR ≥60 mL/min/1.73 m2 and moderate renal impairment (0.73 [0.31–1.76], P = 0.487). The result was similar when the outcome changed to development of advanced diabetic retinopathy, even though this occurred little. As for the renal outcome, cardiovascular events, and all-cause mortality, the HRs were not statistically different between normoalbuminuria and microalbuminuria or between eGFR ≥60 mL/min/1.73 m2 and moderate renal impairment. The HR of composite retinal outcome (Table 3) was significantly higher in group 3 subjects than that of group 2 subjects after adjustment for multiple confounders (3.34 [1.04–10.70], P = 0.043). Such statistical significance did not present when outcome was development of advanced diabetic retinopathy. There was a trend that group 3 subjects had a higher risk for development of advanced diabetic retinopathy, progressive loss of renal function, cardiovascular events, and all-cause mortality compared with group 2 subjects (Table 3), but statistical significance was not reached. When adding 92 individuals with eGFR <30 mL/min/1.73 m2 and albumin-to-creatinine ratio >300 mg/g Cr in the Cox proportional hazards model, the data were quite similar to the previous results and showed that microalbuminuria had greater risk in the progression of retinopathy than moderately reduced eGFR (data not shown).
Figure 1.
Cumulative incidence of composite retinal outcome (A), renal outcome (B), cardiovascular events (C), and all-cause mortality (D) of four groups of subjects. ●, group 1; ○, group 2; ▼, group 3; ▵, group 4.
Table 2.
HRs for retinal and renal outcomes, cardiovascular events, and all-cause mortality in the Cox proportional hazards models
Table 3.
HRs for retinal and renal outcomes, cardiovascular events, and all-cause mortality in the Cox proportional hazards models
CONCLUSIONS
This is the first study, to our knowledge, to compare the effect of microalbuminuria and moderate renal dysfunction in diabetic retinopathy in type 2 diabetic patients. In the follow-up of 7.6 years, our data revealed that the risks for renal outcome, cardiovascular events, and all-cause mortality were not significantly different between the patients of groups 2 and 3. However, microalbuminuria had a more predictive impact on the composite retinal outcome compared with patients with moderate renal impairment.
Intriguingly, our study showed that microalbuminuria significantly increases the risk for development and progression of diabetic retinopathy in type 2 diabetic patients even after adjustment for duration of diabetes, one of the most important predictors of diabetic retinopathy, and other comorbid conditions (12). Diabetic retinopathy and nephropathy seem to progress in a parallel manner. This may be because diabetic retinopathy shares similar pathophysiologic features with diabetic nephropathy. The classic clinical course of nephropathy in type 1 diabetes is the development of microalbuminuria, followed by macroalbuminuria and then by loss of GFR (13). In contrast, kidney disease in type 2 diabetes is more heterogeneous than that in type 1 diabetes. Albuminuria was absent in one-third of type 2 diabetic patients with chronic renal insufficiency according to the Third National Health and Nutrition Survey (14). In these patients, normoalbuminuria may be due to using blockers of the renin-angiotensin system or aggressive antihypertensive therapy (14,15). In fact, patients with diabetes are also susceptible to nondiabetic renal disease (NDRD) (16–18). Since microalbuminuria is a pathophysiologic surrogate of the underlying diabetic nephropathy, a decrease in GFR with normoalbuminuria in such patients sometimes may be due to superimposed nondiabetic renal disease or accelerated aging of the kidney. Therefore, in the current study, there is a significantly increased risk of development and progression of diabetic retinopathy for subjects with microalbuminuria and presumably through its influence on alterations in the microvasculature of retina and kidneys.
Increased urinary albumin excretion and reduced GFR both have been demonstrated to be an independent risk for progressive kidney failure, cardiovascular events, and mortality in patients with chronic kidney disease (CKD) (19–29). These findings are also consistent in patients with diabetic nephropathy (23,25,26,29). Therefore, it is pivotal to combine eGFR and albuminuria for more precise assessment of the clinical outcomes. Recently, the American Diabetes Association recommended that in addition to level of eGFR, the presence of abnormal urine albumin excretion may be useful in the staging of diabetic nephropathy (30). Previous data have shown the increased risks for renal and cardiovascular outcomes and mortality in patients with albuminuria and stage 1 or 2 CKD than in those with stage 3 CKD without proteinuria, although not all the studies have compared statistical significance (21,23,25,27). Our study compared the clinical outcomes among these two groups of patients directly. The results showed that patients with microalbuminuria and eGFR ≥60 mL/min/1.73 m2 have a tendency to increased risks for progressive loss of renal function, cardiovascular events, and all-cause mortality compared with those with moderate renal impairment and normoalbuminuria. Although statistical significance was not reached, the trend corroborates the previous findings.
Some limitations in the current study should be acknowledged. The overall sample size became rather small when we stratified these patients into four groups. The numbers of renal events were low because most subjects were in the early stage of diabetic nephropathy. For patients with early diabetic nephropathy, it may take more follow-up time for the events to occur. However, as our study had a longer observation period for the study end points, this may compensate for these two shortcomings.
In summary, our data show that microalbuminuria is a more useful biomarker than moderately decreased eGFR in predicting retinal outcome of type 2 diabetic patients. Patients with microalbuminuria have a higher risk of the development and progression of diabetic retinopathy even when eGFR is still >60 mL/min/1.73 m2. Accordingly, we emphasize the importance of regular follow-up of UACR besides eGFR in type 2 diabetic patients, and changes in UACR and eGFR should also be identified during follow-up. Once microalbuminuria occurs, diabetic retinopathy should be more aggressively assessed in these patients even if eGFR is >60 mL/min/1.73 m2.
Acknowledgments
This study was supported by grants from the National Science Council (NSC 100-2811-B-010-001-MY3), Taipei Veterans General Hospital (V100C-143 and V100E4-003), the Bureau of Health Promotion, the Department of Health (DOH98-HP-1110), and Ministry of Education Aim for the Top University Plan. H.-S.C. received a grant from Taipei Veterans General Hospital (V99C1-158) to support this work.
No potential conflicts of interest relevant to this article were reported.
Y.-H.C. wrote the manuscript, contributed to discussion, and researched data. H.-S.C. researched data, contributed to discussion, and reviewed and edited the manuscript. D.-C.T. contributed to study design and discussion and reviewed and edited the manuscript. H.-S.C. and D.-C.T. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1955/-/DC1.
References
- 1.Kaiser N, Sasson S, Feener EP, et al. Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes 1993;42:80–89 [DOI] [PubMed] [Google Scholar]
- 2.Hammes HP, Federoff HJ, Brownlee M. Nerve growth factor prevents both neuroretinal programmed cell death and capillary pathology in experimental diabetes. Mol Med 1995;1:527–534 [PMC free article] [PubMed] [Google Scholar]
- 3.Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest 1996;97:2883–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vigstrup J, Mogensen CE. Proliferative diabetic retinopathy: at risk patients identified by early detection of microalbuminuria. Acta Ophthalmol (Copenh) 1985;63:530–534 [DOI] [PubMed] [Google Scholar]
- 5.Gall MA, Rossing P, Skøtt P, et al. Prevalence of micro- and macroalbuminuria, arterial hypertension, retinopathy and large vessel disease in European type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1991;34:655–661 [DOI] [PubMed] [Google Scholar]
- 6.Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in type II diabetes over 6 years from diagnosis. Diabetologia 2001;44:156–163 [DOI] [PubMed] [Google Scholar]
- 7.Tapp RJ, Shaw JE, Harper CA, et al. ; AusDiab Study Group The prevalence of and factors associated with diabetic retinopathy in the Australian population. Diabetes Care 2003;26:1731–1737 [DOI] [PubMed] [Google Scholar]
- 8.Manaviat MR, Afkhami M, Shoja MR. Retinopathy and microalbuminuria in type II diabetic patients. BMC Ophthalmol 2004;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levey AS, Coresh J, Greene T, et al. ; Chronic Kidney Disease Epidemiology Collaboration Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247–254 [DOI] [PubMed] [Google Scholar]
- 10.Chew EY, Ambrosius WT, Davis MD, et al. ; ACCORD Study Group; ACCORD Eye Study Group Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 2010;363:233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Early Treatment Diabetic Retinopathy Study Research Group Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 1991;98(Suppl.):823–833 [PubMed] [Google Scholar]
- 12.Klein R, Klein BE, Moss SE. Epidemiology of proliferative diabetic retinopathy. Diabetes Care 1992;15:1875–1891 [DOI] [PubMed] [Google Scholar]
- 13.Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med 1984;311:89–93 [DOI] [PubMed] [Google Scholar]
- 14.Kramer HJ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 2003;289:3273–3277 [DOI] [PubMed] [Google Scholar]
- 15.MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, Smith TJ, McNeil KJ, Jerums G. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care 2004;27:195–200 [DOI] [PubMed] [Google Scholar]
- 16.Lee EY, Chung CH, Choi SO. Non-diabetic renal disease in patients with non-insulin dependent diabetes mellitus. Yonsei Med J 1999;40:321–326 [DOI] [PubMed] [Google Scholar]
- 17.Tone A, Shikata K, Matsuda M, et al. Clinical features of non-diabetic renal diseases in patients with type 2 diabetes. Diabetes Res Clin Pract 2005;69:237–242 [DOI] [PubMed] [Google Scholar]
- 18.Soni SS, Gowrishankar S, Kishan AG, Raman A. Non diabetic renal disease in type 2 diabetes mellitus. Nephrology (Carlton) 2006;11:533–537 [DOI] [PubMed] [Google Scholar]
- 19.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–1305 [DOI] [PubMed] [Google Scholar]
- 20.Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med 2004;351:1285–1295 [DOI] [PubMed] [Google Scholar]
- 21.Brantsma AH, Bakker SJ, Hillege HL, de Zeeuw D, de Jong PE, Gansevoort RT; PREVEND Study Group Cardiovascular and renal outcome in subjects with K/DOQI stage 1-3 chronic kidney disease: the importance of urinary albumin excretion. Nephrol Dial Transplant 2008;23:3851–3858 [DOI] [PubMed] [Google Scholar]
- 22.Wen CP, Cheng TY, Tsai MK, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet 2008;371:2173–2182 [DOI] [PubMed] [Google Scholar]
- 23.Ninomiya T, Perkovic V, de Galan BE, et al. ; ADVANCE Collaborative Group Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 2009;20:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol 2009;20:1069–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meguro S, Shigihara T, Kabeya Y, Tomita M, Atsumi Y. Increased risk of renal deterioration associated with low e-GFR in type 2 diabetes mellitus only in albuminuric subjects. Intern Med 2009;48:657–663 [DOI] [PubMed] [Google Scholar]
- 26.Jerums G, Panagiotopoulos S, Premaratne E, MacIsaac RJ. Integrating albuminuria and GFR in the assessment of diabetic nephropathy. Nat Rev Nephrol 2009;5:397–406 [DOI] [PubMed] [Google Scholar]
- 27.Hemmelgarn BR, Manns BJ, Lloyd A, et al. ; Alberta Kidney Disease Network Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010;303:423–429 [DOI] [PubMed] [Google Scholar]
- 28.Tonelli M, Muntner P, Lloyd A, et al. ; Alberta Kidney Disease Network Using proteinuria and estimated glomerular filtration rate to classify risk in patients with chronic kidney disease: a cohort study. Ann Intern Med 2011;154:12–21 [DOI] [PubMed] [Google Scholar]
- 29.Drury PL, Ting R, Zannino D, et al. Estimated glomerular filtration rate and albuminuria are independent predictors of cardiovascular events and death in type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia 2011;54:32–43 [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]