Abstract
OBJECTIVE
Individuals at high risk for chronic cardiometabolic disease (cardiovascular disease [CVD], type 2 diabetes, and chronic kidney disease [CKD]) share many risk factors and would benefit from early intervention. We developed a nonlaboratory-based risk-assessment tool for identification of people at high cardiometabolic disease risk.
RESEARCH DESIGN AND METHODS
Data of three population-based cohorts from different regions of the Netherlands were merged. Participants were 2,840 men and 3,940 women, white, aged 28–85 years, free from CVD, type 2 diabetes, and CKD diagnosis at baseline. The outcome was developing cardiometabolic disease during 7 years follow-up.
RESULTS
Age, BMI, waist circumference, antihypertensive treatment, smoking, family history of myocardial infarction or stroke, and family history of diabetes were significant predictors, whereas former smoking, history of gestational diabetes, and use of lipid-lowering medication were not. The models showed acceptable calibration (Hosmer and Lemeshow statistics, P > 0.05) and discrimination (area under the receiver operating characteristic [ROC] curve 0.82 [95% CI 0.81–0.83] for women and 0.80 [0.78–0.82] for men). Discrimination of individual outcomes was lowest for diabetes (area under the ROC curve 0.70 for men and 0.73 for women) and highest for CVD mortality (0.83 for men and 0.85 for women).
CONCLUSIONS
We demonstrate that a single risk stratification tool can identify people at high risk for future CVD, type 2 diabetes, and/or CKD. The present risk-assessment tool can be used for referring the highest risk individuals to health care for further (multivariable) risk assessment and may as such serve as an important part of prevention programs targeting chronic cardiometabolic disease.
Chronic cardiometabolic diseases, including cardiovascular disease (CVD), type 2 diabetes, and chronic kidney disease (CKD), are leading causes of comorbidity and premature death (1). Moreover, these diseases have a heavy impact on the quality of life (1) and generate high health care costs. Another shared aspect of these chronic cardiometabolic diseases is that early treatment of people at high risk reduces disease burden and is cost-effective (2–4). In addition, the three chronic cardiometabolic diseases—CVD, type 2 diabetes, and CKD—do share many risk factors. Therefore, common opportunities for prevention have been acknowledged (5). A joint prevention program may be more effective because it stresses the importance of multiple risk factor assessment and control in those at high risk. Furthermore, a joint program will reduce time and financial burden.
A risk score is a helpful tool to identify individuals at high risk. A growing number of single outcome risk scores for identification of people at risk for either future CVD (6,7), type 2 diabetes (8,9), or CKD (10) have been developed. Next to these evidence-based identification methods, an increasing amount of self-assessment health checks are available, especially on the Internet (11). We considered that the use of several risk-assessment tools for separate related diseases can be inefficient and confusing. A single risk score that predicts risk for a combination of chronic metabolic diseases is still lacking.
Furthermore, simple risk scores comprising information that do not require blood testing are especially useful for primary prevention and public health initiatives. Untrained people can therefore assess their risk, and only those at high risk can then be referred to health care for more extensive risk factor measurement. For the single outcome of CVD, a nonlaboratory-based risk score has been published (7), and for type 2 diabetes, such a risk questionnaire also exists (8). However, to date, no risk score that predicts the combined end points of these diseases has been developed. In light of this, we sought to develop a simple risk stratification tool for identification of people at high risk of CVD morbidity and/or mortality, type 2 diabetes, and/or CKD.
RESEARCH DESIGN AND METHODS
Study population
The study population consisted of merged data of three population-based cohort studies from different regions of the Netherlands.
The Rotterdam Study commenced in 1990 by invitation of randomly selected inhabitants of 55 years old or older of whom 7,983 agreed to participate (78%) (12). The 1997–1999 follow-up examination included all relevant information for the present aim and was therefore used for the present analysis.
The Hoorn study started in 1989 by invitation of randomly selected individuals aged 50–75 years, and 72% agreed to participate. In 2000, a glucose-stratified subsample (n = 1,074) of the original study population was reinvited for a follow-up examination (13).
The Prevention of Renal and Vascular End-stage Disease (PREVEND) study commenced in 1997 by invitation of all inhabitants of the city of Groningen aged 28–75 years of whom 48% responded. The original PREVEND cohort (n = 8,592) consisted of all respondents with albuminuria (morning urinary albumin concentration >10 mg/L) and a random sample of respondents without albuminuria. For the present analysis, we used a subgroup of 3,432 participants, including all PREVEND participants without albuminuria and a random sample of those with albuminuria, as such being representative for the general population (14). In 2005, all surviving participants were reinvited for follow-up examination. All participants provided written informed consent prior to study commencement, and the study protocols were approved by local medical ethics committees.
Exclusion criteria
Initially, the merged dataset consisted of 12,489 individuals. For the present analyses, people were eligible if they were white, aged 28–85 years, and had no prevalent diagnosed CVD (myocardial infarction, percutaneous transluminal coronary angioplasty, coronary artery bypass graft, angina pectoris, stroke, claudication intermittent, peripheral intervention, or heart failure), type 2 diabetes (self-reported type 2 diabetes and/or use of antidiabetic medication), or CKD (self-report or estimated glomerular filtration rate <15 mL/min/1.73 m2). After exclusion, 9,462 eligible individuals remained, of whom 7,418 had follow-up information on the three diseases of interest. Of these, 638 individuals died of causes other than cardiovascular and were therefore excluded, resulting in 6,780 individuals in the present analyses.
Definition of outcome variable
The outcome was the diagnosis of one of the following diseases during the follow-up period: fatal or nonfatal CVD (myocardial infarction, percutaneous transluminal coronary angioplasty, coronary artery bypass graft, angina pectoris, stroke, claudication intermittent, peripheral intervention, or heart failure), sudden death, type 2 diabetes, and/or CKD.
Among all cohorts, vital status was obtained from the municipal health service. In case of mortality, causes of death were obtained from medical records of local hospitals and general practitioners (Rotterdam, Hoorn) or the Dutch Central Bureau of Statistics (PREVEND). On a yearly basis, participants’ medical records were queried for cardiovascular morbidity information (15–17). Information on angina pectoris and intermittent claudication was derived from the Rose questionnaire (18). Data on heart failure were not available from the PREVEND study; however, heart failure incidence is low in this age range (<75 years) and often secondary to coronary heart disease. Cardiovascular death was defined as death due to diseases of the cardiovascular system (ICD-10: I00–I99) and sudden death (ICD-10: R96).
Type 2 diabetes and CKD diagnosis was based on measurements performed at the follow-up examination. Type 2 diabetes was defined using an oral glucose tolerance test or by use of antidiabetic medication (19). In PREVEND, postload plasma glucose measurements were not available, so definition of type 2 diabetes was based on fasting glucose levels only.
CKD was defined by estimated glomerular filtration rate <60 mL/min/1.73 m2 (20), which corresponds to stage 3 or higher (21). Creatinine values were calibrated using regression to age- and sex-adjusted mean values from a nationally representative U.S. survey as described previously (22).
Model development
Easily obtainable risk factors were identified from available risk scores that detect or predict CVD, type 2 diabetes, or CKD. As such, we excluded variables that required physical testing (blood pressure) or laboratory measurement. The following candidate predictors are used: age, smoking (former or current), use of antihypertensives, use of lipid-lowering medication, BMI, waist circumference, parent or sibling with myocardial infarction or stroke (before the age of 65 years), parent or sibling with diabetes, and history of gestational diabetes.
Selected variables were entered into sex-specific multivariable logistic regression models. Nonsignificant predictors (P > 0.05) were removed. To evaluate potential additional value of socioeconomic status (level of education) and coffee and alcohol consumption, we assessed whether adding these variables improved discrimination.
To handle missing data, single imputation was applied by using the expectation-maximization algorithm. Missing values were estimated by using multivariable regression models that were conditional on relevant predictors. Single imputation has been described as a superior method to complete case analysis and equal to multiple imputation if the percentage of missing variables is low, which is the case in this study (0–6.9% as a maximum) (23).
Model performance
Model performance was evaluated in terms of discrimination (area under the receiver operating characteristic [ROC] curve) for the composite and separate disease outcomes. Hosmer-Lemeshow goodness-of-fit test was performed to assess calibration of the risk model.
As the apparent performance of a model is usually better in the derivation dataset than in another dataset, we estimated the amount of overfitting in the regression coefficients and the area under the ROC curves by bootstrapping techniques (24). These bootstrap-adjusted measures represent the values that can be expected when the model is applied to future similar populations. The regression coefficients were adjusted for overfitting by multiplying the coefficients by the slope index (25). Bootstrap-adjusted regression coefficients were then multiplied and rounded; as such, the maximum scores for both men and women were 100. For each individual, the total risk score can be calculated by totaling the scores of each item. Bootstrapping was performed in R 2.9.0 for Windows.
The performance of the currently developed risk score with respect to the separate outcomes CVD and type 2 diabetes was compared with the performance of the Gaziano et al. (7) risk score including age, systolic blood pressure, current smoking, BMI, use of antihypertensive medication, and reported diabetes and the Finnish diabetes risk score including age, BMI, waist circumference, use of antihypertensives, history of gestational diabetes, and family history of diabetes (8) by applying these scores in the present dataset.
RESULTS
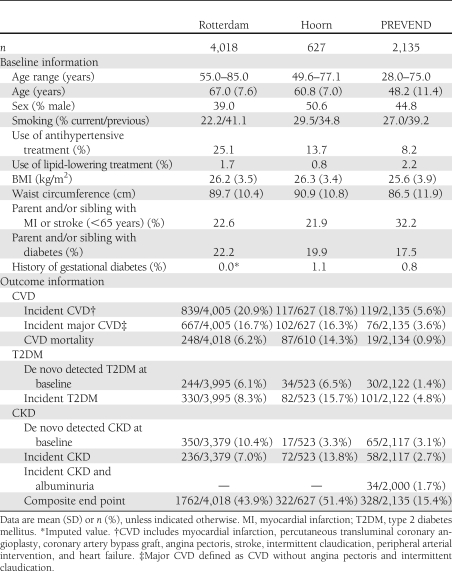
Baseline characteristics and incidence data for study participants are presented in Table 1. The percentage of the population that developed chronic cardiometabolic disease was 36.4% for males and 34.3% for females. Of those who developed the composite outcome, 82% developed one of the diseases, 38% developed fatal or nonfatal CVD, 22% developed type 2 diabetes, and 22% developed CKD. The other 18% had two or more incident disease outcomes. Of all type 2 diabetes patients, 37.5% had de novo–detected type 2 diabetes at baseline. Of all patients with CKD, 54% had de novo CKD at baseline (Table 1). Mean follow-up was 6.9 (SD 1.1) years.
Table 1.
Baseline characteristics and incidence data for each cohort
Individuals who participated in the baseline examination but who were lost to follow-up were significantly older and more often smokers than individuals included in the current study. Individuals lost to follow-up did not differ from individuals in the study with respect to BMI, waist circumference, or use of antihypertensive medication.
Model development
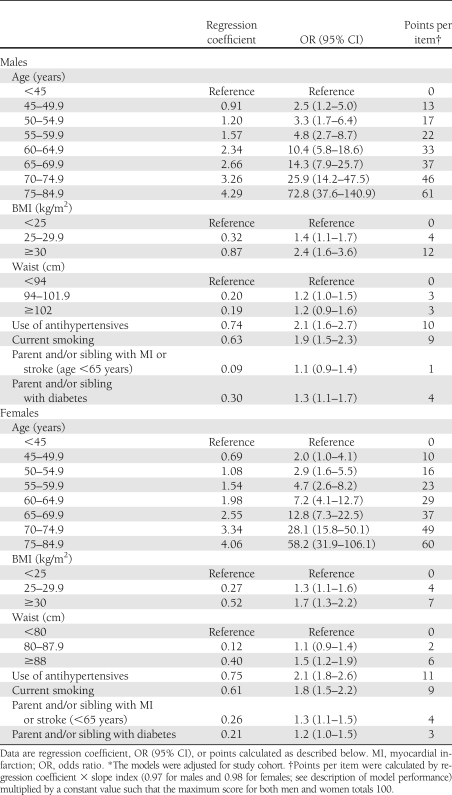
All candidate predictor variables were significantly associated with the outcome, with the exception of former smoking, history of gestational diabetes, and use of lipid-lowering medication. Consequently, these variables were not included in the model. The sex-specific multivariable logistic regression models are shown in Table 2.
Table 2.
Sex-specific multivariable logistic regression models for the prediction of chronic metabolic disease*
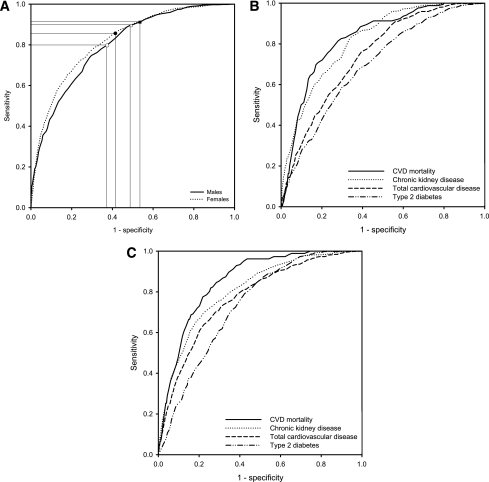
The Hosmer-Lemeshow test showed good calibration for the male and female model (χ2 = 7.6, P = 0.48; χ2 = 6.3, P = 0.62, respectively). Figure 1A shows that discrimination for the composite outcome was slightly better for women (area under the ROC curve 0.82 [95% CI 0.81–0.83]) as compared with men (0.80 [0.78–0.82]). Discrimination of the separate outcome measures ranged from 0.83 for CVD mortality to 0.70 for type 2 diabetes among men (Fig. 1B) and from 0.85 to 0.73 among women (Fig. 1C).
Figure 1.
A: ROC curves for the composite outcome in males (area under the ROC curve 0.80 [95% CI 0.78–0.82]) and females (0.82 [0.81–0.83]) with reference lines for cut point 40 in males (open circles) and females (black circles) and 35 in males (open squares) and females (black squares). ROC curve for the separate outcomes in males (area under the ROC curve 0.83 [0.80–0.86], 0.82 [0.80– 0.84], 0.75 [0.72–0.77], and 0.70 [0.67–0.73] for CVD mortality, CKD, total CVD, and type 2 diabetes, respectively) (B) and 0.85 [0.83 to 0.88], 0.81 [0.79–0.83], 0.77 [0.75–0.79] and 0.73 [0.71–0.75] for CVD mortality, CKD, total CVD, and type 2 diabetes, respectively, among females (C).
Level of education and alcohol intake were significant predictors of outcome for both men and women, but adding these variables to the model did not improve discrimination or calibration of the model. Coffee consumption was not a significant risk factor for outcome.
Model performance
Comparison of bootstrap-adjusted and original area under the ROC curve showed only a marginal difference, indicating a good internal validity. The area under the ROC curve decreased from 0.801 [0.784–0.817] to 0.795 [0.778–0.812] in males and from 0.820 [0.807–0.833] to 0.817 [0.804–0.830] in females.
Model application
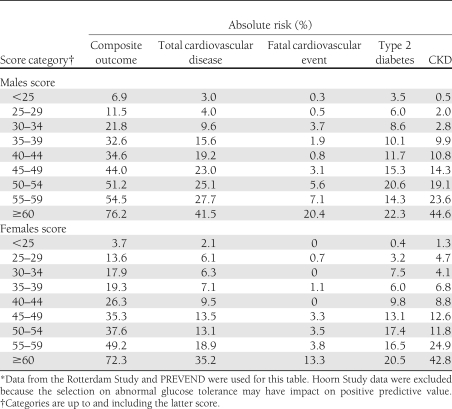
Cutoff values 35 and 40, as examples, are marked in the ROC curve (Fig. 1A). Table 3 presents absolute risks for the composite and separate outcomes per score category. A cutoff ≥35 corresponds to a 7-year risk on the composite outcome of 50% for men and 47% for women, as opposed to an 11 and 9% risk (men and women, respectively) for a score below this cutoff. Sensitivity and specificity for this ≥35 cutoff are 85 and 55% for men and 90 and 49% for women. A higher cutoff of ≥40 corresponds to a 7-year risk on the composite outcome of 54% for men and 52% for women, as opposed to a 15 and 11% risk (men and women, respectively) for a score below this cutoff. Sensitivity and specificity for the ≥40 cutoff are 75 and 66% for men and 83 and 62% for women.
Table 3.
Absolute 7-year risk for the composite outcome and each of the separate disease outcomes per score category*
The number of participants per score category partitioned by age categories is presented in Supplementary Table 1. This table shows that the ≥35 cutoff, for example, would imply that no males and only 0.2% of the females below the age of 45 years will be identified as high risk for development of cardiometabolic disease within 7 years. In contrast, individuals who are aged ≥65 years will all be identified as high risk. In the 5-year age intervals between 45 and 65 years, the percentage of males with a score of ≥35 is 4.5, 7.2, 33.6, and 87.8%, and the percentage of females per 5-year higher age starting with age 45–49.9 years is 4.2, 15.2, 44.8, and 80.3%.
Comparison with existing risk scores
The performance of the nonlaboratory-based risk score of Gaziano et al. (7) was highly similar to the present risk score. For CVD mortality, however, the Gaziano et al. (7) model performed significantly worse in males (area under the ROC curve 0.78 as compared with 0.83 for the present model; P < 0.001). For females, the models performed similar in predicting CVD mortality. For the prediction of type 2 diabetes, the Finnish diabetes risk score (8) performed slightly, but not significantly, worse than the present model (area under the ROC curve 0.69 as compared with 0.70 for the present model among males and 0.72 compared with 0.73 among females).
Sensitivity analysis
Restricting CVD outcome to major CVD events alone, we found similar discrimination for CVD in men, but higher discrimination in women (area under the ROC curve increased from 0.77 to 0.82).
Restricting CKD definition to having CKD and microalbuminuria (urinary albumin excretion >30 mg/24 h) or having macroalbuminuria (urinary albumin excretion >300 mg/24 h), discrimination was almost the same (information from PREVEND population only).
Excluding people with de novo detected type 2 diabetes and/or CKD at baseline decreased model discrimination for the composite outcome to area under the ROC curve 0.79 [0.77–0.81] in both males and females. Exclusion of these patients also resulted in slightly lower model discrimination for the outcome type 2 diabetes (0.69 compared with 0.70) for males, but better discrimination among females (0.76 compared with 0.73). Model discrimination especially decreased for CKD prediction from 0.82 to 0.79 among males and from 0.81 to 0.76 among females. Exclusion of these de novo patients at baseline had no effect on model discrimination of total CVD and even improved model discrimination for the prediction CVD mortality among females (from 0.85 to 0.88).
Excluding individuals using antihypertensive or lipid-lowering medication from the analysis, the model discrimination for the composite outcome marginally decreased (to 0.79 among males and 0.80 among females).
CONCLUSIONS
In the current study, we developed and validated a nonlaboratory-based risk prediction tool for primary prevention purposes that can identify people who are currently free from disease but at high risk of future CVD, type 2 diabetes, and/or CKD. The present score is the first predicting multiple chronic cardiometabolic disease outcomes and, as such, is a novelty in health care. The performance of the risk questionnaire was similar to existing nonlaboratory-based risk scores that predict the separate diseases.
The composite outcome
Prior to this study, no score was available that predicted the combined outcome of CVD, type 2 diabetes, and/or CKD. Some studies assessed whether existing scores for type 2 diabetes predicted CKD prevalence or whether the metabolic syndrome predicts type 2 diabetes or CVD but showed weak performance (26).
The definition that we used for the outcome was heterogeneous. However, we found that the separate outcome measures were well predicted also when comparing to existing risk scores. Furthermore, our model predicted a more restricted definition of CVD and CKD as well as the broader definition.
Current risk scores
CVD risk scores that are presently available require measurement of systolic blood pressure, including the nonlaboratory-based score of Gaziano et al. (7). Although systolic blood pressure measurement is easy and noninvasive, this information is considerably less suitable for self-report. The present model without systolic blood pressure performed at least as good as the model of Gaziano et al. (7).
Noninvasive risk scores for incident type 2 diabetes are available (8,9), of which the Finnish score is most widely applied and validated. The performance of this score in a previous validation study appeared to be somewhat better than in the current study (27). This is potentially due to the exclusion of people with prevalent medical history of CVD, type 2 diabetes, or CKD in the current study.
For CKD, existing scores will largely detect people already diagnosed with CVD or type 2 diabetes, because prior CVD and type 2 diabetes are important predictors in these scores (10). The current risk tool is the first that predicts CKD among individuals free of CVD and type 2 diabetes history and is therefore not suitable for people already known with these diseases.
Methodological issues
Age appeared to be the strongest predictor of chronic cardiometabolic disease. However, a single predictor model with age alone performed less well than the present developed model (data not shown). Furthermore, prevention is to be targeted also at younger age-groups with existing, treatable risk factors, so sole reliance on age as risk predictor is undesirable.
Development of this risk score was done in all data available from three cohort studies. The reason for this approach was that we sought the best estimate of future cardiometabolic risk. A drawback of this approach was that we had no data left for external validation of the score.
The present risk score is the first to predict three chronic cardiometabolic diseases in one combined outcome. This gave rise to some specific methodological issues. First, we had continuous registration of CVD morbidity and mortality but intermittent registration for type 2 diabetes and CKD incidence. This may have resulted in an overrepresentation of fatal events, because people who died of CVD during follow-up were included in analyses, but people who survived and did not attend the follow-up examination could not be included. Second, in the present analyses, we excluded patients with known type 2 diabetes and CKD at baseline, but those identified by screening were considered as cases. We reasoned that by using the present risk-assessment tool in integrative prevention programs, individuals with prevalent but undiagnosed type 2 diabetes or CKD should not be missed. Our sensitivity analysis showed that true incident cases were only marginally worse predicted than de novo detected cases at baseline.
Study limitations
Some limitations with respect to the current study should be considered. First, external validation and calibration in other populations will importantly strengthen the usefulness and reliability of the present risk score. The white population was studied, and estimated risks may well be different for other populations.
Second, study individuals aged <50 years were all PREVEND study participants, whereas individuals of 77 years of age and older were Rotterdam study participants. This implies that we had fewer cases in these age-groups, which forced us to create larger age-groups in the highest and lowest age ranges. Furthermore, although we did not find effect modification by study population, reliance on one study cohort might have led to less representative risk estimates in these age-groups.
Third, the present risk score predicts risk within a relatively short time frame (7 years). So lifetime risk is not accounted for (28). This may imply that repeated risk assessment over time is required to optimally identify people at risk for chronic cardiometabolic disease.
Fourth, selective participation in the follow-up examination of a relatively healthier population has occurred, probably because of a relatively older age of the study population,. This may have led to underestimation of reported risks.
Fifth, parts of the data used were taken from earlier years when use of blood pressure medication and statins was not as common as it is nowadays. Effect estimates of medication use were, however, not different among the more recent PREVEND cohort as compared with the earlier cohorts.
Sixth, despite the relatively large sample size, the sample size seemed to limit the precision of the absolute risk estimates per score category for the separate disease outcomes as apparent from the variation in risk with increasing score (Table 3).
Recommendations for further study
Next to external validation of the score, which is an important recommendation for further study, we also emphasize that the agreement between the high-risk individuals identified by the present score and those identified by existing risk scores should be elaborated further. An advantage of the present score will be that the presence of multiple, slightly increased risk factors (age, overweight, smoking) together will identify a high-risk group that will not be identified by single disease risk scores because those risk scores rely on risk factors specific for one, but not another, disease. Exact numbers to underpin such hypothesis are clearly warranted.
Implementation
The current risk questionnaire has widespread potential public health applications. In The Netherlands, the questionnaire is implemented in a prevention guideline for general practitioners (29). The risk questionnaire serves as a risk-stratification tool to identify people who are most at risk for chronic cardiometabolic disease. High-risk individuals are referred to their general practitioner who will further examine relevant risk factors (laboratory as well as blood pressure). Treatment or lifestyle intervention can then be initiated upon the appropriate treatment goals (i.e., blood pressure, LDL cholesterol levels, and/or glucose levels) according to the current guidelines.
Cutoff point selection is always a trade-off between the sensitivity and specificity. Cutpoint selection should therefore depend on the setting of implementation (study population, resources, costs, health care structure, etc.) and on test characteristics. The present data demonstrated that a cutoff between 35 and 40 was optimal in terms of test characteristics in the present population. This implies that individuals who are aged <45 years are unlikely to be identified as high risk for chronic cardiometabolic disease development within the time frame of 7 years. Further, such a cut point would highly impact the elderly population, as all individuals aged ≥65 years have a score >35 based on their age alone. So the present score will be especially distinguish between high and low cardiometabolic risk among individuals aged 45–65 years.
With the present instrument, primary health care can establish a more active approach in prevention by using this tool in public health campaigns and on the Internet to encourage individuals to assess risk and get treated if needed. It provides a framework for a joint strategy of disease prevention that is likely to be more efficient and cost-effective. It may serve as an evidence-based, primary health care-embedded alternative of the many self-assessment health checks available to date.
For the first time, a single nonlaboratory-based risk stratification tool was developed that can identify people at high risk for future CVD, type 2 diabetes, and/or CKD. This tool can be embedded in prevention programs to identify people in need of multiple risk factor measurement and subsequent treatment. It could simplify prevention of chronic cardiometabolic disease in primary care.
Acknowledgments
M.A. was supported financially by a grant from the Dutch Diabetes Research Foundation (Grant 2005.015.001). S.J.L.B. received support from the Netherlands Heart Foundation, Dutch Diabetes Research Foundation, and Dutch Kidney Foundation, together participating in the framework of the Center for Translational Molecular Medicine (http://www.ctmm.nl) project PREDICCt (Grant 01C-104-07).
No potential conflicts of interest relevant to this article were reported.
M.A. designed the research, performed statistical analyses, and drafted the manuscript. R.S.N. interpreted the data, drafted the manuscript, and contributed to the discussion. S.J.L.B., C.D.A.S., M.W.H., and H.L.H. contributed to the discussion. G.N., A.H., J.C.M.W., and R.T.G. acquired the data and contributed to the discussion. J.M.D. designed the research, handled funding and supervision, and contributed to the discussion. M.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this work were presented in oral form at the 46th Annual Meeting of the European Diabetes Epidemiology Group, Jerez de la Frontera, Spain, 15–18 May 2011.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1417/-/DC1.
References
- 1.World Health Organization The Global Burden of Disease: 2004 Update. Geneva, WHO Press, 2008 [Google Scholar]
- 2.Graham I, Atar D, Borch-Johnsen K, et al. ; European Society of Cardiology (ESC); European Association for Cardiovascular Prevention and Rehabilitation (EACPR); Council on Cardiovascular Nursing; European Association for Study of Diabetes (EASD); International Diabetes Federation Europe (IDF-Europe); European Stroke Initiative (EUSI); Society of Behavioural Medicine (ISBM); European Society of Hypertension (ESH); WONCA Europe (European Society of General Practice/Family Medicine); European Heart Network (EHN); European Atherosclerosis Society (EAS) European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur J Cardiovasc Prev Rehabil 2007;14(Suppl. 2):S1–S113 [DOI] [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 2001;135:73–87 [DOI] [PubMed] [Google Scholar]
- 5.Rosenzweig JL, Ferrannini E, Grundy SM, et al. ; Endocrine Society Primary prevention of cardiovascular disease and type 2 diabetes in patients at metabolic risk: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2008;93:3671–3689 [DOI] [PubMed] [Google Scholar]
- 6.D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–753 [DOI] [PubMed] [Google Scholar]
- 7.Gaziano TA, Young CR, Fitzmaurice G, Atwood S, Gaziano JM. Laboratory-based versus non-laboratory-based method for assessment of cardiovascular disease risk: the NHANES I Follow-up Study cohort. Lancet 2008;371:923–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindström J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 2003;26:725–731 [DOI] [PubMed] [Google Scholar]
- 9.Schmidt MI, Duncan BB, Bang H, et al. ; Atherosclerosis Risk in Communities Investigators Identifying individuals at high risk for diabetes: The Atherosclerosis Risk in Communities study. Diabetes Care 2005;28:2013–2018 [DOI] [PubMed] [Google Scholar]
- 10.Kshirsagar AV, Bang H, Bomback AS, et al. A simple algorithm to predict incident kidney disease. Arch Intern Med 2008;168:2466–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronda G, Portegijs P, Dinant GJ, Buntinx F, Norg R, van der Weijden T. Use of diagnostic self-tests on body materials among Internet users in the Netherlands: prevalence and correlates of use. BMC Public Health 2009;9:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofman A, van Duijn CM, Franco OH, et al. The Rotterdam Study: 2012 objectives and design update. Eur J Epidemiol 2011;26:657–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snijder MB, Dekker JM, Visser M, et al. ; Hoorn study Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care 2004;27:372–377 [DOI] [PubMed] [Google Scholar]
- 14.Gansevoort RT, Verhave JC, Hillege HL, et al. ; for the PREVEND Study Group The validity of screening based on spot morning urine samples to detect subjects with microalbuminuria in the general population. Kidney Int Suppl 2005:S28–S35 [DOI] [PubMed] [Google Scholar]
- 15.Vliegenthart R, Oudkerk M, Hofman A, et al. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation 2005;112:572–577 [DOI] [PubMed] [Google Scholar]
- 16.Linssen GCM, Bakker SJL, Voors AA, et al. N-terminal pro-B-type natriuretic peptide is an independent predictor of cardiovascular morbidity and mortality in the general population. Eur Heart J 2010;31:120–127 [DOI] [PubMed] [Google Scholar]
- 17.van ‘t Riet E, Rijkelijkhuizen JM, Alssema M, Nijpels G, Stehouwer CD, Heine RJ, Dekker JM. HbA1c is an independent predictor of nonfatal cardiovascular disease in a Caucasian population without diabetes: a 10-year follow-up of the Hoorn Study. Eur J Cardiovasc Prev Rehabil 2012;19:23–31 [DOI] [PubMed] [Google Scholar]
- 18.Rose G, Blackburn H. Cardiovascular Survey Methods. Geneva, WHO Press, 1968 [PubMed] [Google Scholar]
- 19.World Health Organization Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia. Report of a WHO/IDF Consultation. Geneva, WHO Press, 2006 [Google Scholar]
- 20.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 21.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(Suppl. 1):S1–S266 [PubMed] [Google Scholar]
- 22.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis 2002;39:920–929 [DOI] [PubMed] [Google Scholar]
- 23.van der Heijden GJMG, Donders AR, Stijnen T, Moons KGM. Imputation of missing values is superior to complete case analysis and the missing-indicator method in multivariable diagnostic research: a clinical example. J Clin Epidemiol 2006;59:1102–1109 [DOI] [PubMed] [Google Scholar]
- 24.Efron B, Tibshirani R. An Introduction to the Bootstrap. Monographs on Statistics and Applied Probability. New York, Chapman & Hall, 1993 [Google Scholar]
- 25.Steyerberg EW, Borsboom GJ, van Houwelingen HC, Eijkemans MJ, Habbema JD. Validation and updating of predictive logistic regression models: a study on sample size and shrinkage. Stat Med 2004;23:2567–2586 [DOI] [PubMed] [Google Scholar]
- 26.Cameron A. The metabolic syndrome: validity and utility of clinical definitions for cardiovascular disease and diabetes risk prediction. Maturitas 2010;65:117–121 [DOI] [PubMed] [Google Scholar]
- 27.Alssema M, Vistisen D, Heymans MW, et al. ; DETECT-2 collaboration The Evaluation of Screening and Early Detection Strategies for Type 2 Diabetes and Impaired Glucose Tolerance (DETECT-2) update of the Finnish diabetes risk score for prediction of incident type 2 diabetes. Diabetologia 2011;54:1004–1012 [DOI] [PubMed] [Google Scholar]
- 28.Lloyd-Jones DM, Wilson PW, Larson MG, et al. Framingham risk score and prediction of lifetime risk for coronary heart disease. Am J Cardiol 2004;94:20–24 [DOI] [PubMed] [Google Scholar]
- 29.Dekker JM, Alssema M, Janssen PGH, et al. NHG Guideline Prevention of cardiometabolic diseases M96. Huisarts Wet 2011;54:138–155 [in Dutch] [Google Scholar]