Abstract
OBJECTIVE
To test the hypothesis that hepatic steatosis is associated with risk factors for type 2 diabetes in overweight and obese youth, mediated by cardiorespiratory fitness.
RESEARCH DESIGN AND METHODS
This was a cross-sectional study comparing insulin sensitivity between 30 overweight and obese adolescents with hepatic steatosis, 68 overweight and obese adolescents without hepatic steatosis, and 11 healthy weight adolescents without hepatic steatosis. Cardiorespiratory fitness was determined by a graded maximal exercise test on a cycle ergometer. Secondary outcomes included presence of metabolic syndrome and glucose response to a 75-g oral glucose challenge.
RESULTS
The presence of hepatic steatosis was associated with 55% lower insulin sensitivity (P = 0.02) and a twofold greater prevalence of metabolic syndrome (P = 0.001). Differences in insulin sensitivity (3.5 vs. 4.5 mU ⋅ kg−1 ⋅ min−1, P = 0.03), prevalence of metabolic syndrome (48 vs. 20%, P = 0.03), and glucose area under the curve (816 vs. 710, P = 0.04) remained between groups after matching for age, sex, and visceral fat. The association between hepatic steatosis and insulin sensitivity (β = −0.24, t = −2.29, P < 0.025), metabolic syndrome (β = −0.54, t = −5.8, P < 0.001), and glucose area under the curve (β = 0.33, t = 3.3, P < 0.001) was independent of visceral and whole-body adiposity. Cardiorespiratory fitness was not associated with hepatic steatosis, insulin sensitivity, or presence of metabolic syndrome.
CONCLUSIONS
Hepatic steatosis is associated with type 2 diabetes risk factors independent of cardiorespiratory fitness, whole-body adiposity, and visceral fat mass.
Type 2 diabetes is one of the fastest growing chronic diseases in childhood (1–3). Despite increasing awareness of pediatric type 2 diabetes, little data exist delineating the pathophysiological factors associated with the loss of glucose tolerance. Two particular biomarkers implicated in the development of glucose intolerance include excessive intra-abdominal fat (1,2) and, more recent, excessive hepatic triglyceride independent of visceral adipose mass (3–5).
Delineating the value of these biomarkers for type 2 diabetes risk in overweight youth remains challenging because of their significant collinearity (6). Recent studies have used reciprocal matching strategies to dissect the independent role of each biomarker as a predictor of insulin resistance and type 2 diabetes risk in obese adults (7,8). In these studies, cardiorespiratory fitness and hepatic steatosis emerged as important predictors of insulin sensitivity. Similar matching strategies have not been performed in studies of overweight adolescents. The independent roles of hepatic triglyceride, visceral fat, and cardiorespiratory fitness as predictors of metabolic risk among overweight youth remain undetermined.
The primary aim of this study was to determine the independent associations of visceral fat mass and hepatic steatosis on type 2 diabetes risk factors in a cohort of overweight and obese adolescents. A secondary aim was to assess whether cardiorespiratory fitness levels mediated these associations. We designed two complementary cross-sectional studies to test the hypothesis that hepatic steatosis is associated with cardiometabolic and type 2 diabetes risk, independent of visceral fat among overweight and obese adolescents.
RESEARCH DESIGN AND METHODS
Study design and study population
This cross-sectional study compared risk factors associated with type 2 diabetes between overweight and obese adolescents stratified by the presence or absence of hepatic steatosis and healthy weight adolescents without hepatic steatosis. Through local advertising, we recruited 108 adolescents between age 13 and 18 years considered overweight or obese according to age- and sex-specific BMI cut points established by the International Obesity Task Force (9). We also recruited 11 healthy weight normoglycemic adolescents to provide an estimate of expected values for insulin sensitivity and hepatic triglyceride in the absence of obesity. Exclusion criteria included diagnosis of type 2 diabetes or impaired glucose tolerance (IGT), treatment with antipsychotics or corticosteroids, orthopedic injury or illness preventing participation in the exercise test, and enrolment in a weight loss program in the previous 6 months. A standard 2-h 75-g oral glucose tolerance test was used to screen for type 2 diabetes. Youth with type 2 diabetes or IGT (10) were excluded (n = 8). Youth unable to complete the magnetic resonance imaging (MRI) as a result of size restriction were subsequently excluded from the study (n = 2). Because type 2 diabetes is more prevalent in the First Nations population in our region (11), ethnicity was self-reported for inclusion as a confounding variable. All participants and parents provided written informed consent and assent. The study was approved by the University of Manitoba Biomedical Research Ethics Board and performed according to the Declaration of Helsinki.
Primary outcome measure—insulin sensitivity
Whole-body insulin sensitivity was determined from a modified frequently sampled intravenous glucose tolerance test (12). Fasting blood samples were collected prior to an intravenous bolus of a 25% glucose solution (0.3 g/kg body wt to a maximum dose of 34 g glucose) at time 0, followed by an intravenous bolus of regular human insulin (0.03 units/kg body wt) at 20 min. Serum glucose and insulin were collected at 1, 2, 3, 4, 5, 6, 8, 10, 14, 19, 22, 25, 30, 40, 50, 70, 100, 140, and 180 min. Glucose and insulin kinetics were modeled using the Bergman Minimal Model provided in customized software (MINMOD) to estimate whole-body insulin sensitivity (13). The acute insulin response and disposition index were also determined from this method as measures of glucose-stimulated insulin secretion.
Secondary outcome measures—metabolic syndrome and serum glucose levels in response to an oral glucose challenge
Metabolic syndrome was treated as a binary outcome using cut points for systolic blood pressure, serum triglycerides, waist circumference, fasting glucose, and HDL cholesterol (HDL-C) that were statistically derived to reflect cut points in adults (14). Adolescents were classified as having metabolic syndrome if they had three or more of five comorbidities (14). Automated blood pressure (Dynamap) was measured in triplicate on the left arm with participants seated. Waist circumference was measured at the level of the iliac crest in duplicate with a flexible tape measure. Waist circumference was expressed as z scores according to Canadian reference data for between-group comparisons (15). Glucose area under the curve (AUC) was determined by summing the area under serum glucose measures between each 15- or 30-min segment after a 75-g oral glucose challenge using the trapezium rule (16).
Biochemical measurements
Plasma glucose was measured on a Roche Modular P analyzer with an ultraviolet test principle (hexokinase method). Insulin was measured on an Immulite solid-phase, two-site chemiluminescent immunometric assay. Serum lipoproteins, triglycerides, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were measured on a Roche Modular P analyzer. LDL cholesterol (LDL-C) was calculated using the Friedewald equation (LDL-C = total cholesterol − [HDL-C − (triglyceride/2.2)]).
Primary exposure variables—hepatic triglyceride content and visceral fat mass
MRI and spectroscopy were performed using a 1.5 T whole-body magnet (GE Medical Systems, Milwaukee, WI) as previously described (17,18). High-resolution images of the liver were obtained in three planes using standard clinical techniques, and a voxel was placed in an area devoid of subcutaneous or visceral fat for the acquisition of proton spectra. A total of 64 spectra were averaged for the determination of intracellular water and lipid content. LCModel software (19) was used to isolate and quantify lipid and water peaks. Hepatic steatosis was defined as hepatic triglyceride content of >5.5% fat/water. Previous population-based studies show that 5.5% fat/water approximates the 95th percentile for healthy normoglycemic adults and is equivalent to biopsy-derived lipid concentration of 5.5 mg/g (20). Visceral fat mass was quantified from high-resolution magnetic resonance images at a level between the third and fifth lumbar vertebrae as previously described (17,21) using Slicer3 software (version 3.21; Boston, MA).
Confounding/exploratory variables—cardiorespiratory fitness, physical activity, adiposity, and adipocyte lipolysis
Cardiorespiratory fitness was determined using indirect calorimetry with a graded maximal exercise test to exhaustion on a cycle ergometer (22). Physical activity was measured as the average of step counts during 7 consecutive days using hip pedometers. Dual-energy X-ray absorptiometry (Hologic, Bedford, MA) was used to quantify fat mass (kilograms), fat-free mass (kilograms), and percent body fat. Nonesterified fatty acids (NEFAs) were measured at 30-min intervals during the oral glucose challenge to determine insulin-mediated suppression of lipolysis.
Statistical analyses
All data are presented as means (95% CIs) unless otherwise stated. Data were tested for normal distribution by the Kolmogorov-Smirnov test. Adolescents were stratified into one of three categories according to weight status and hepatic triglyceride content: 1) healthy weight normoglycemic adolescents without hepatic steatosis (n = 11), 2) overweight adolescents without hepatic steatosis (triglyceride <5.5% fat/water, n = 68), and 3) overweight adolescents with hepatic steatosis (triglyceride ≥5.5% fat/water, n = 30). We controlled for potential confounding effects of age, sex, and visceral adiposity (within 20%) by pair matching overweight youth with hepatic steatosis to overweight youth without hepatic steatosis. To validate the associations between hepatic steatosis and type 2 diabetes risk factors, we conducted a reciprocal matching study with a separate cohort of overweight youth discrepant for visceral fat mass (<70.0 vs. >80.0 cm2) but matched for age, sex, and hepatic triglyceride content. For nonnormally distributed variables, Mann-Whitney U tests were used to examine differences between groups of pair-matched subjects. Comparisons of normally distributed variables between subjects within each pair-matched grouping were performed using Student t test. Levene test was used to assess the equality of group variances on the dependant variables. Correlations were assessed by Spearman rank correlation. Multiple stepwise linear regression analysis was used to determine if hepatic triglyceride was associated with various metabolic risk factors independent of age, ethnicity, sex, BMI z score, cardiorespiratory fitness, and visceral obesity. All analyses were performed with SPSS version 16.0 (SAS, Chicago, IL). P < 0.05 was considered statistically significant.
RESULTS
Participant characteristics
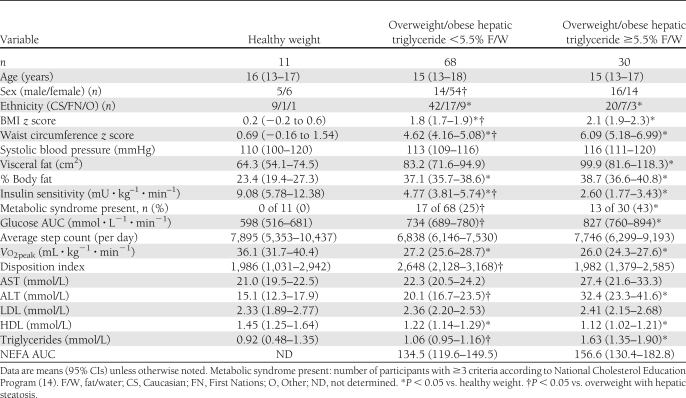
Hepatic steatosis was evident in 31% (n = 30) of overweight and obese adolescents and none of the healthy weight adolescents. Overweight and obese adolescents with or without hepatic steatosis displayed lower insulin sensitivity, higher rates of metabolic syndrome, and higher serum glucose after the oral glucose challenge compared with healthy weight adolescents (Table 1). BMI z score (2.1 vs. 1.8, P < 0.001) and waist circumference z score (6.09 vs. 4.62, P = 0.004) were higher among overweight and obese adolescents with hepatic steatosis compared with those without, despite similar visceral fat mass and percent body fat (Table 1). The proportion of male participants was higher in the cohort of youth with hepatic steatosis relative to overweight and obese youth without (43 vs. 25%, P = 0.04) (Table 1). A greater proportion of First Nations youth composed the overweight and obese groups, compared with the healthy weight group (24 vs. 9%, P < 0.01). A higher proportion of youth with hepatic steatosis met the criteria for obesity compared with youth without hepatic steatosis (90 vs. 65%, P < 0.05). Physical activity levels (steps per day) were not significantly different between groups of youth.
Table 1.
Characteristics of study participants
Metabolic characteristics of overweight adolescents with hepatic steatosis
Insulin sensitivity was ∼55% lower, the presence of metabolic syndrome was approximately twofold higher (50 vs. 25%, P = 0.001), and serum glucose during the oral glucose challenge was ∼10% higher among youth with hepatic steatosis compared with healthy weight, overweight, and obese youth without hepatic steatosis. None of the adolescents had clinically relevant elevations (greater than two times upper reference range) in liver aminotransferases (AST or ALT). Cardiorespiratory fitness was not different between adolescents with and without hepatic steatosis. The NEFA AUC after a 75-g oral glucose challenge was not different between youth with hepatic steatosis compared with overweight and obese youth without hepatic steatosis (Table 1).
Matched comparisons
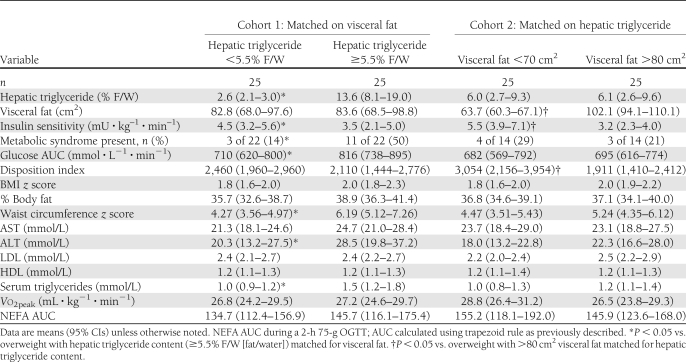
As hepatic triglyceride content and visceral fat mass are strongly associated, we used a reciprocal matching strategy similar to that used by Fabbrini et al. (7) to dissect the individual contribution of each measure of adiposity. This complementary research design permits the isolation of each aspect of adiposity on metabolic risk controlling for the collinearity in the exposure variables. After matching for age, sex, and visceral fat mass, we found that insulin sensitivity remained ∼25% lower (3.5 vs. 4.5 mU ⋅ kg–1 ⋅ min–1, P = 0.03), the presence of metabolic syndrome remained greater than twofold higher (48 vs. 20%, P = 0.03), and the glucose AUC was ∼20% higher in overweight and obese adolescents with hepatic steatosis compared with overweight and obese adolescents without hepatic steatosis (Table 2). In the reciprocal comparison, when youth were matched for hepatic triglyceride content, no differences in metabolic syndrome or glucose AUC were observed, despite significant differences in visceral fat mass (63.7 vs. 102.1 cm2) (Table 2). Insulin sensitivity remained significantly different between groups (3.2 vs. 5.5 mU ⋅ kg–1 ⋅ min–1, P = 0.02). No differences in physical activity or cardiorespiratory fitness were seen in either comparison.
Table 2.
Characteristics of pair-matched overweight and obese youth for age, sex, and visceral fat or hepatic triglyceride content
Associations.
Hepatic triglyceride content was positively associated with glucose AUC (ρ = 0.44, P < 0.001), BMI z score (ρ = 0.47, P < 0.001), waist circumference z score (ρ = 0.34, P < 0.001), systolic blood pressure (ρ = 0.16, P = 0.005), serum triglycerides (ρ = 0.43, P < 0.001), and ALT (ρ = 0.36, P < 0.001) and negatively associated with insulin sensitivity (ρ = −0.40, P < 0.001) and HDL-C (ρ = −0.31, P < 0.001). Cardiorespiratory fitness was not associated with insulin sensitivity or hepatic triglyceride content. Multiple regression analysis revealed that hepatic triglyceride content was associated with the presence of metabolic syndrome (r = 0.57, P < 0.001), insulin sensitivity (r = 0.36, P = 0.04), and glucose AUC (r = 0.24, P = 0.02) after adjusting for age, sex, BMI z score, waist circumference z score, visceral fat, whole-body fat mass, fitness level, and NEFA AUC.
CONCLUSIONS
This study reveals a novel combination of findings with clinical implications for predicting type 2 diabetes risk in youth. First, hepatic steatosis is associated with 1) increased prevalence of metabolic syndrome, 2) elevated serum glucose in response to an oral glucose challenge, and 3) dyslipidemia, independent of visceral fat mass and cardiorespiratory fitness. Second, several metabolic characteristics associated with visceral adiposity in adolescents, including metabolic syndrome and preclinical dysglycemia, can be attributed to the presence of elevated hepatic triglyceride levels. Finally, cardiorespiratory fitness was not associated with hepatic triglyceride content, nor did it mediate the association between hepatic steatosis and type 2 diabetes risk factors.
Our data support the concept that hepatic steatosis is an early precursor to glucose intolerance and cardiometabolic risk independent of fitness in overweight and obese youth. Previous cross-sectional studies show that both visceral fat mass and hepatic triglyceride content are associated with metabolic abnormalities in overweight adolescents (23,24). Recent studies showing hepatic steatosis as a determinant of insulin resistance in overweight youth failed to include a cohort matched for hepatic triglyceride content with disparate visceral fat mass as a positive control (4,7). By effectively isolating hepatic steatosis from visceral obesity, we demonstrated that hepatic steatosis is the dominant predictor of metabolic syndrome among overweight or obese adolescents.
Several case control studies show that metabolic risk factor clustering is associated with the presence of hepatic steatosis (4,5). Using biopsy-proven or MRI-derived measures of hepatic triglyceride, previous reports show that ∼50% of adolescents with hepatic steatosis meet diagnostic criteria for metabolic syndrome, a rate approximately two- to threefold higher than in adolescents without hepatic steatosis (5,25). Conventional wisdom suggests that the association between hepatic steatosis and metabolic risk factor clustering is mediated by whole-body insulin resistance (4,26). In contrast, we found no differences in the prevalence of metabolic syndrome in youth with discrepant visceral fat mass, matched for hepatic triglyceride content, despite significant differences in insulin sensitivity. Thus, insulin resistance may not be the sole factor linking hepatic steatosis to metabolic risk in overweight youth. These data imply that hepatic steatosis is a sensitive biomarker and potential mediating factor for metabolic risk factor clustering and glucose intolerance in overweight and obese adolescents independent of insulin resistance.
Prior evidence suggests hepatic steatosis contributes to the loss of glucose tolerance among overweight and obese adolescents. First, hepatic steatosis is a common feature of IGT and type 2 diabetes (24). Second, hepatic steatosis is associated with a loss of insulin-induced suppression of hepatic glucose output among overweight and obese individuals (7). Finally, glucose-stimulated insulin secretion is impaired among obese adolescents with hepatic steatosis (4,26). Our study extends these findings by showing that glucose excursion after an oral glucose challenge is higher among overweight and obese adolescents with hepatic steatosis. In contrast to others (4), we did not observe a difference in the acute insulin response to glucose expressed in absolute terms or relative to insulin sensitivity between adolescents with and without hepatic steatosis. However, considering that differences in serum glucose occurred later during the 2-h challenge, the earliest defects in β-cell function may occur in the second phase of insulin secretion.
Defects in mitochondrial oxidative capacity are purported to render individuals susceptible to ectopic lipid accumulation. Previous cross-sectional studies of hepatic steatosis in adolescents have failed to provide a measure of mitochondrial function or fitness levels. Because recent studies show that the association between cardiorespiratory fitness and insulin resistance appears to be mediated in part by hepatic triglyceride content, we felt it was important to assess fitness in this cohort (8). Furthermore, recent intervention studies in adults (27) suggest that increased physical activity or elevated cardiorespiratory fitness may attenuate triglyceride accumulation in the liver. In contrast to these studies, our data do not support a role for cardiorespiratory fitness in the clustering of cardiometabolic disease risk among overweight or obese adolescents. Specifically, cardiorespiratory fitness was not associated with any of the risk factors for type 2 diabetes, nor was it different between overweight and obese youth with and without hepatic steatosis. It is possible that hepatic steatosis in youth is predominantly a function of diet and/or factors related to fatty acid uptake and synthesis. Alternatively, fitness levels achieved in the convenience sample of youth studied here may have been too low to detect associations with cardiometabolic risk. Prospective intervention studies are needed to determine if increasing cardiorespiratory fitness can reduce hepatic triglyceride content in adolescents.
The cross-sectional design precludes determination of a causal nature for the associations described. However, the strategy of reciprocal matching permitted the dissection of the independent roles of hepatic steatosis and visceral adiposity in metabolic risk factor clustering in overweight and obese adolescents. Differences in sex and ethnicity existed between groups; however, after matching for sex and including ethnicity as a covariate in multiple linear regression models, group-wise differences in insulin sensitivity, presence of metabolic syndrome, or glucose AUC were unchanged. Finally, in the absence of data on dietary intake, we were unable to study this covariate.
Hepatic steatosis is a prevalent comorbidity in overweight and obese adolescents that exists prior to clinically elevated serum aminotransferases. The presence of hepatic steatosis is accompanied by a clustering of factors associated with type 2 diabetes and cardiovascular disease, independent of visceral fat mass, whole-body adiposity, and cardiorespiratory fitness. These results support the growing body of evidence that hepatic steatosis is a potentially modifiable biomarker of risk for type 2 diabetes and metabolic syndrome in overweight and obese adolescents.
Acknowledgments
B.A.W. received fellowship funding from the Manitoba Health Research Council and Manitoba Institute of Child Health. K.D.M.W. received fellowship funding from the Canadian Institutes of Health Research. J.M.M. received establishment grants from the Manitoba Institute of Child Health and the Manitoba Health Research Council, an operating grant from the Lawson Foundation, and a Scholar Award from the Canadian Diabetes Association.
No potential conflicts of interest relevant to this article were reported.
B.A.W. contributed to study design, researched data, and wrote the manuscript. K.D.M.W. researched data and edited the manuscript. A.C.M. researched data and contributed to discussion. E.A.C.S. and H.J.D. contributed to discussion and edited the manuscript. L.R. contributed to the development of local spectroscopy and to discussion. H.S. reviewed spectroscopy design in the study. J.M.M. contributed to the concept and study design, researched data, and edited the manuscript. B.A.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors are grateful for the clinical expertise provided by Barry Clabbert (Clinical Chemistry, University of Manitoba, Faculty of Medicine); Angella Griffith and Bria Sharkey (summer students, Manitoba Institute of Child Health); and Elaine Kalyta, Maureen McKay, and Amy Yakimowski (clinical research nurses, Manitoba Institute of Child Health); and are indebted to the parents and children who volunteered their time and effort for the completion of this study.
References
- 1.Demerath EW, Reed D, Rogers N, et al. Visceral adiposity and its anatomical distribution as predictors of the metabolic syndrome and cardiometabolic risk factor levels. Am J Clin Nutr 2008;88:1263–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goran MI, Bergman RN, Gower BA. Influence of total vs. visceral fat on insulin action and secretion in African American and white children. Obes Res 2001;9:423–431 [DOI] [PubMed] [Google Scholar]
- 3.McGavock J, Sellers E, Dean H. Physical activity for the prevention and management of youth-onset type 2 diabetes mellitus: focus on cardiovascular complications. Diab Vasc Dis Res 2007;4:305–310 [DOI] [PubMed] [Google Scholar]
- 4.D’Adamo E, Cali AM, Weiss R, et al. Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care 2010;33:1817–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation 2008;118:277–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003;34:267–273 [DOI] [PubMed] [Google Scholar]
- 7.Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A 2009;106:15430–15435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haufe S, Engeli S, Budziarek P, et al. Cardiorespiratory fitness and insulin sensitivity in overweight or obese subjects may be linked through intrahepatic lipid content. Diabetes 2010;59:1640–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 2008;32:S1–S201 [DOI] [PubMed] [Google Scholar]
- 11.Young TK, Reading J, Elias B, O’Neil JD. Type 2 diabetes mellitus in Canada’s first nations: status of an epidemic in progress. CMAJ 2000;163:561–566 [PMC free article] [PubMed] [Google Scholar]
- 12.Ball GD, Shaibi GQ, Cruz ML, Watkins MP, Weigensberg MJ, Goran MI. Insulin sensitivity, cardiorespiratory fitness, and physical activity in overweight Hispanic youth. Obes Res 2004;12:77–85 [DOI] [PubMed] [Google Scholar]
- 13.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther 2003;5:1003–1015 [DOI] [PubMed] [Google Scholar]
- 14.Jolliffe CJ, Janssen I. Development of age-specific adolescent metabolic syndrome criteria that are linked to the Adult Treatment Panel III and International Diabetes Federation criteria. J Am Coll Cardiol 2007;49:891–898 [DOI] [PubMed] [Google Scholar]
- 15.Katzmarzyk PT. Waist circumference percentiles for Canadian youth 11-18y of age. Eur J Clin Nutr 2004;58:1011–1015 [DOI] [PubMed] [Google Scholar]
- 16.Altman D. Practical Statistics for Medical Research London, Chapman & Hall, 1991 [Google Scholar]
- 17.McGavock JM, Lingvay I, Zib I, et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation 2007;116:1170–1175 [DOI] [PubMed] [Google Scholar]
- 18.Szczepaniak LS, Babcock EE, Schick F, et al. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol 1999;276:E977–E989 [DOI] [PubMed] [Google Scholar]
- 19.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 2001;14:260–264 [DOI] [PubMed] [Google Scholar]
- 20.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387–1395 [DOI] [PubMed] [Google Scholar]
- 21.Zib I, Jacob AN, Lingvay I, et al. Effect of pioglitazone therapy on myocardial and hepatic steatosis in insulin-treated patients with type 2 diabetes. J Investig Med 2007;55:230–236 [DOI] [PubMed] [Google Scholar]
- 22.McGavock JM, Mandic S, Vonder Muhll I, et al. Low cardiorespiratory fitness is associated with elevated C-reactive protein levels in women with type 2 diabetes. Diabetes Care 2004;27:320–325 [DOI] [PubMed] [Google Scholar]
- 23.Taksali SE, Caprio S, Dziura J, et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes 2008;57:367–371 [DOI] [PubMed] [Google Scholar]
- 24.Weiss R, Dufour S, Taksali SE, et al. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet 2003;362:951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgert TS, Taksali SE, Dziura J, et al. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab 2006;91:4287–4294 [DOI] [PubMed] [Google Scholar]
- 26.Deivanayagam S, Mohammed BS, Vitola BE, et al. Nonalcoholic fatty liver disease is associated with hepatic and skeletal muscle insulin resistance in overweight adolescents. Am J Clin Nutr 2008;88:257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larson-Meyer DE, Heilbronn LK, Redman LM, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care 2006;29:1337–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]