Abstract
OBJECTIVE
To determine the effect of niacin on fasting glucose (FG) and new-onset diabetes in statin/ezetimibe-treated patients.
RESEARCH DESIGN AND METHODS
This was a prespecified secondary analysis among 942 hyperlipidemic patients randomized to ezetimibe/simvastatin (E/S; 10/20 mg) or E/S + extended-release niacin (N; titrated to 2 g) over 64 weeks.
RESULTS
FG levels peaked by 8–12 weeks, then declined even without antidiabetic medication. At 64 weeks, 3.5% taking E/S+N versus 2.6% taking E/S met criteria for new-onset diabetes (P = 0.66). An additional 1.4% taking E/S+N versus 0.4% taking E/S transiently met criteria for diabetes and then remitted (P = 0.46). Of 28 new-diabetes diagnoses in the E/S+N group, 25 occurred by 24 weeks. Among patients with baseline diabetes, 13.9% taking E/S+N and 11.6% taking E/S underwent antidiabetic treatment modification.
CONCLUSIONS
Increased FG and new-onset diabetes with E/S+N occurred mainly around the time of initial uptitration of N and often improved or remitted without specific treatment.
Niacin, an effective agent for treating dyslipidemia, increases fasting glucose (FG) and reduces insulin sensitivity via mechanisms that remain unclear (1,2). This analysis of a large 64-week randomized, double-blind trial evaluated the effect of combination ezetimibe/simvastatin (E/S) + extended-release niacin (N; Niaspan; Abbott Laboratories) on FG and new-onset diabetes in patients with hyperlipidemia (3,4).
RESEARCH DESIGN AND METHODS
This prespecified secondary analysis assessed the effect of E/S+N versus N and E/S (24 weeks) and versus E/S (64 weeks) on new-onset diabetes and FG in type IIa/IIb hyperlipidemic patients (3,4) (see Supplementary Fig. 2 for N-arm results). Men and women (aged 18–79 years) with LDL cholesterol 3.36–4.92 mmol/L and triglycerides <5.65 mmol/L were enrolled. Patients with hemoglobin A1c ≥8.0% or with repeated hypoglycemia/unstable glycemic control were excluded. Changes in dosage or new antidiabetic pharmacotherapy were permitted except within 2 months of initial screening. After a 4-week washout, patients were treated with E/S (10/20 mg) + N (to 2 g) or E/S (10/20 mg). N was initiated at 0.5 g/day and increased by 0.5 g every 4 weeks up to 2 g/day.
Absence of diabetes at baseline was defined as FG <7.0 mmol/L, not on antidiabetic medication, and no medical history of diabetes. Criteria for new-onset diabetes included an adverse event specifying a diabetes diagnosis, initiation of antidiabetic medication, or two consecutive FG measurements ≥7.0 mmol/L (2). FG was measured every 4 weeks during 24 weeks and at weeks 32, 42, 52, and 64.
RESULTS
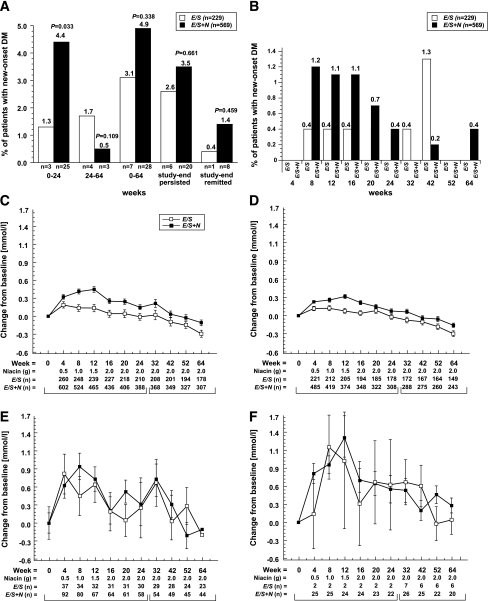
Of 942 patients who received E/S (n = 272) and E/S+N (n = 670) (3), 798 were nondiabetic at baseline (FG <7.0 mmol/L). Other baseline characteristics were previously reported (Supplementary Table 1) (3,4). During 64 weeks, new-onset diabetes occurred in 3.1% of patients on E/S vs. 4.9% on E/S+N (P = 0.34) (3,4) (Fig. 1A and Supplementary Table 2). In most cases, diagnosis of diabetes was based on consecutive FG measurements ≥7.0 mmol/L (E/S, 2.2%; E/S+N, 4.4%). Approximately 1% in each group initiated antidiabetic treatment; similar percentages had an adverse event specifying new type 2 diabetes. New-onset diabetes occurred more frequently in patients treated with E/S+N (4.4%) versus E/S (1.3%; P = 0.033) during 0–24 weeks (Fig. 1A and B). An additional 0.5% of patients on E/S+N and 1.7% on E/S developed diabetes during 24–64 weeks (P = 0.11) (3,4). At study end, new-onset diabetes persisted in 3.5% of patients on E/S+N vs. 2.6% on E/S (P = 0.66), whereas 1.4% taking E/S+N vs. 0.4% taking E/S transiently met criteria for diabetes and then remitted (P = 0.46) (2). Multivariate predictors of new-onset diabetes were consistent with results in the literature (5). The addition of niacin to E/S predicted new-onset diabetes in the first 24 weeks, but not over the entire 64-week period (Supplementary Table 3).
Figure 1.
Time course of new-onset diabetes and effects on FG. A: Summary of new-onset diabetes. Three pairs of bars on the left compare E/S versus E/S+N groups for patients without diabetes at baseline who developed new-onset diabetes during 0–24 weeks, 24–64 weeks, and cumulatively during 64 weeks. Two pairs of bars on the right enumerate those who developed diabetes and still met criteria for diabetes at study end or transiently met criteria for diabetes, then remitted by 64 weeks (2). Details of patients who met new-onset criteria are described in Supplementary Table 2. B: Frequency of patients who developed new-onset by treatment interval. Mean changes from baseline in FG were generated using an ANCOVA model with terms for treatment and baseline FG during 24 weeks and 24–64 weeks in the full cohort (C), in patients without diabetes at baseline (D) and with diabetes at baseline (E), and those who developed new-onset diabetes (F).
Fasting glucose levels peaked by 4–8 weeks for E/S and 8–12 weeks for E/S+N, then declined to baseline levels by 64 weeks (Fig. 1C–F). Increases were greatest in those with diabetes at baseline and new-onset diabetes and were higher with E/S+N versus E/S in patients without diabetes (Supplementary Table 4). Among patients with new-onset diabetes, most had peak FG ≥7.0 and ≤8.9 mmol/L (Fig. 1A and Supplementary Fig. 1). During 64 weeks, FG elevations to >8.9 mmol/L occurred in four patients receiving E/S+N and none receiving E/S. The return to baseline FG levels occurred mostly without antidiabetic medications, which were initiated by only 0.9% on E/S+N vs. 1.3% on E/S (P = 0.70). Among patients with baseline diabetes, 13.9% taking E/S+N and 11.6% taking E/S underwent changes in antidiabetic regimen.
CONCLUSIONS
The rate of new-onset diabetes with E/S+N therapy was higher compared with E/S during 24 weeks, but was only marginally higher over 64 weeks. Most cases of new-onset diabetes with E/S+N occurred within 24 weeks during niacin titration to the 2-g dose, with few additional occurrences during the remaining 40 weeks. Similarly, FG elevations occurred at early times with E/S+N, then declined to pretreatment levels by 64 weeks, largely without antidiabetic medications. No trend toward early-onset diabetes was observed with E/S. These effects were not attributed to study treatment adherence, which remained high in both groups through 64 weeks (4).
Our incidence rates for diabetes are somewhat exaggerated due to frequent FG sampling (11 times in 64 weeks) compared with FG monitoring in clinical practice. In addition, 29% of patients meeting criteria for new-onset diabetes on E/S+N remitted by study end. Most cases had incremental FG elevations modestly exceeding 7.0 mmol/L. Nevertheless, the clinical impact of new-onset diabetes for individual patients can be profound. Four patients on E/S+N had destabilization of glucose homeostasis (FG ≥8.9 mmol/L), and 8 of 798 initially nondiabetic patients received antidiabetic medication among both treatment groups.
All new-onset diabetes in this study arose in patients with baseline impaired FG (≥5.6 to <7.0 mmol/L). Among such patients treated with E/S+N, the rate of new-onset diabetes persisting at 64 weeks was slightly higher (10.1% in 64 weeks) than that suggested for impaired FG in general (25% in 3–5 years) (6). It should be noted that in addition to niacin, treatment with statins has also been associated with increased development of diabetes (7).
The temporal pattern shown in this article may help explain apparently contradictory results in past studies. Niacin-induced hyperglycemia was prominent in studies of short duration (2–12 weeks) and/or at high niacin doses of 3–4.5 g/day (1,8), whereas longer-term studies at lower doses (≤3 g) showed only modest or statistically nonsignificant effects (9–11). Likewise, insulin resistance was found in 2-week niacin studies, whereas a 4-month study gave no such evidence (1,12,13). The time course of niacin-induced hyperglycemia is interestingly similar to that of skin flushing, which is mediated by a G-protein-coupled receptor, GPR109A, and is subject to desensitization via β-arrestin (14). In humans treated with MK-0354 (a GPR109A agonist that lacks effects on lipoproteins), FG and insulin trended higher (15). Either niacin or MK-0354 binding to GPR109A strongly inhibits adipocyte triglyceride lipolysis and fatty-acid release, and the resulting perturbation in fuel supply might be linked to increased FG and insulin levels. We speculate that the waning of niacin’s hyperglycemic effect, analogous to flushing, could be related to potential desensitization of GPR109A on adipocytes.
Our ability to define glycemic responses fully was limited by the study design, optimized to evaluate drug effects on lipids and safety. Characterization of incident diabetes was limited by the small number of cases; nonetheless, the low incidence is reassuring. As with any secondary analysis, these results are considered hypothesis-generating.
In summary, combination E/S+N produced small initial increases in FG and new diagnoses of diabetes that dissipated over time, largely without the use of antidiabetic medication. Consistent with guidelines, niacin is a reasonable option to treat dyslipidemia in patients with or at risk for diabetes, but risk/benefit should be weighed, and glucose should be checked periodically (2).
Acknowledgments
This study was funded by Merck/Schering-Plough Pharmaceuticals.
J.R.G. has received educational and research grants from Merck, Abbott, Genzyme, Amarin Pharma, and GlaxoSmithKline and consulting fees/honoraria from Abbott Laboratories and Merck and has served as a consultant/advisory board member for Acura/King Pharma. J.R.G. also holds an equity interest in Eli Lilly & Co. and has received Continuing Medical Education Grant support from sanofi-aventis. S.F. has served as an advisor to Merck, Pfizer, Takeda, Roche, Amrin, and Kowa. A.J.A., J.E.T., A.S., and A.M.T. are employees of Merck and own stock/stock options. E.J. is a Merck contract employee. No other potential conflicts of interest relevant to this article were reported.
J.R.G. and J.E.T. contributed to the conception and design of the study, analysis and interpretation of the data, drafting of the article, and critical revision of the article for important intellectual content and approved the final version of the manuscript. S.F., A.S., and A.M.T. contributed to the conception and design of the study, analysis and interpretation of the data, and critical revision of the article for important intellectual content and approved the final version of the manuscript. A.J.A. and E.J. provided statistical expertise, collection, analysis and interpretation of the data, and critical revision of the article for important intellectual content and approved the final version of the manuscript. J.R.G. and J.E.T. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009; the XV International Symposium on Atherosclerosis, Boston, Massachusetts, 14–18 June 2009; and the 6th IAS-Sponsored Workshop on HDL, Whistler, British Columbia, Canada, 17–20 May 2010.
The authors acknowledge Stephen W. Gutkin, Rete Biomedical Communications Corporation (Wyckoff, NJ), with support from the study sponsor, and Martha Vollmer, MA, of Merck for the assistance in manuscript preparation.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1369/-/DC1.
Clinical trial reg. no. NCT00271817, clinicaltrials.gov.
References
- 1.Guyton JR. Niacin in cardiovascular prevention: mechanisms, efficacy, and safety. Curr Opin Lipidol 2007;18:415–420 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2011;34(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guyton JR, Brown BG, Fazio S, Polis A, Tomassini JE, Tershakovec AM. Lipid-altering efficacy and safety of ezetimibe/simvastatin coadministered with extended-release niacin in patients with type IIa or type IIb hyperlipidemia. J Am Coll Cardiol 2008;51:1564–1572 [DOI] [PubMed] [Google Scholar]
- 4.Fazio S, Guyton JR, Polis AB, et al. Long-term safety and efficacy of triple combination ezetimibe/simvastatin plus extended-release niacin in patients with hyperlipidemia. Am J Cardiol 2010;105:487–494 [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Li CY, Sattar N. Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care 2008;31:1898–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan DM, Davidson MB, DeFronzo RA, et al. ; American Diabetes Association Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 2007;30:753–759 [DOI] [PubMed] [Google Scholar]
- 7.Sampson UK, Linton MF, Fazio S. Are statins diabetogenic? Curr Opin Cardiol 2011;26:342–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garg A, Grundy SM. Nicotinic acid as therapy for dyslipidemia in non-insulin-dependent diabetes mellitus. JAMA 1990;264:723–726 [PubMed] [Google Scholar]
- 9.Coronary Drug Project Research Group Clofibrate and niacin in coronary heart disease. JAMA 1975;231:360–381 [PubMed] [Google Scholar]
- 10.Elam MB, Hunninghake DB, Davis KB, et al. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: the ADMIT study: A randomized trial. Arterial Disease Multiple Intervention Trial. JAMA 2000;284:1263–1270 [DOI] [PubMed] [Google Scholar]
- 11.Grundy SM, Vega GL, McGovern ME, et al. ; Diabetes Multicenter Research Group Efficacy, safety, and tolerability of once-daily niacin for the treatment of dyslipidemia associated with type 2 diabetes: results of the assessment of diabetes control and evaluation of the efficacy of niaspan trial. Arch Intern Med 2002;162:1568–1576 [DOI] [PubMed] [Google Scholar]
- 12.Vega GL, Cater NB, Meguro S, Grundy SM. Influence of extended-release nicotinic acid on nonesterified fatty acid flux in the metabolic syndrome with atherogenic dyslipidemia. Am J Cardiol 2005;95:1309–1313 [DOI] [PubMed] [Google Scholar]
- 13.Rasouli N, Hale T, Kahn SE, Spencer HJ, Elbein SC. Effects of short-term experimental insulin resistance and family history of diabetes on pancreatic beta-cell function in nondiabetic individuals. J Clin Endocrinol Metab 2005;90:5825–5833 [DOI] [PubMed] [Google Scholar]
- 14.Walters RW, Shukla AK, Kovacs JJ, et al. beta-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J Clin Invest 2009;119:1312–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai E, Waters MG, Tata JR, et al. Effects of a niacin receptor partial agonist, MK-0354, on plasma free fatty acids, lipids, and cutaneous flushing in humans. J Clin Lipidol 2008;2:375–383 [DOI] [PubMed] [Google Scholar]