Abstract
OBJECTIVE
Soluble preadipocyte factor 1 (Pref-1) inhibits adipocyte differentiation. We tested whether circulating levels of soluble Pref-1 are higher in smaller fetuses.
RESEARCH DESIGN AND METHODS
We performed longitudinal assessments of circulating Pref-1 in infants born appropriate for gestational age (AGA) or small for gestational age (SGA) and also in late-gestational women and in newborns on days 2 and 3.
RESULTS
At birth, Pref-1 levels were ~100-fold higher than in adults, being in SGA fetuses ~50% higher than in AGA fetuses. By age 4 months, Pref-1 had reached near-adult levels and the original AGA versus SGA difference had disappeared. Pref-1 levels were low in late-gestational women and were still elevated in newborns.
CONCLUSIONS
Pref-1 is abundantly present in the fetus, is higher in SGA than in AGA fetuses, and is likely to be of fetal origin. We speculate that Pref-1 in early life contributes to variation in postnatal adipocyte numbers, in the subsequent expandability of adipose tissue, and thus in the susceptibility to diabetes in later life.
The number of subcutaneous adipocytes wherein fat can be stored during adulthood is essentially determined in two windows of adipogenesis, namely, fetal life and puberty (1,2). For example, in monozygotic twins discordant for growth before birth, the smaller twin continues to have a lower number of subcutaneous adipocytes after birth (3). It is still poorly understood how a transient restraint of fetal growth can be linked to a persistent lowering of adipocyte number. We propounded that one of the potential links is a downregulation of adipogenesis in a critical window of growth restraint before birth. In a first test of this concept, we studied at birth whether circulating preadipocyte factor 1 (Pref-1) is higher in smaller fetuses. Pref-1 is a transmembrane protein that is encoded by an imprinted (paternally expressed) gene on chromosome 14q32 and that contains an extracellular domain with epidermal growth factor–like repeats; juxtamembrane cleavage generates a soluble 50-kDa form, which is the Pref-1 form that inhibits adipocyte differentiation by upregulating Sox9 in preadipocytes (4,5). Thus far, the circulating levels of Pref-1 have not been studied in human fetuses, newborns, infants, or pregnant women.
RESEARCH DESIGN AND METHODS
Pref-1 was measured longitudinally (at birth and at 4 months) in serum from 72 infants (42 born appropriate for gestational age [AGA] [19 girls and 23 boys] and 30 born small for gestational age [SGA] [16 girls and 14 boys]) who had been recruited into a study that was initiated in 2007 and that assesses longitudinally the endocrine-metabolic state and the body composition of SGA infants compared with breastfed AGA control subjects across the first postnatal years (6,7). For the present substudy focusing on Pref-1, specific inclusion criteria were as follows: 1) birth at Hospital Sant Joan de Déu after an uncomplicated, term (38–40 weeks), and singleton pregnancy (no maternal hypertension and no gestational diabetes); 2) birth weight between 3.0 and 3.8 kg for AGA control subjects (birth weight range between −1 and +1 SD), and birth weight <2.6 kg for SGA infants (below −2 SD); 3) cord serum available to measure Pref-1; and 4) written informed consent in the Catalan language. Specific exclusion criteria were complications at birth (need for resuscitation or for parenteral nutrition), congenital malformations, or an extremely low birth weight (below −3.0 SD).
Gestational age was calculated according to the last menses and confirmed by first-trimester ultrasound. The prevalence of delivery by caesarean section was 12%. A total of 17 mothers smoked during pregnancy; they delivered 7 AGA and 10 SGA infants.
Pref-1 was also measured in 11 healthy AGA newborns (mean birth weight 3.3 kg) sampled on postnatal day 2 or 3 (mean age 44 h [range 30–57]) and from 18 women in late gestation (mean 35 weeks [32–38]) who subsequently delivered healthy infants (mean birth weight 3.3 kg [2.7–4.0]) after uncomplicated singleton pregnancies (mean duration 39 weeks [37–41]).
Body weight was measured at birth with a beam balance (Seca, Hamburg, Germany). Blood from the longitudinally studied infants was sampled at birth (from the umbilical cord before placental separation) and in the prefeeding state at age 4 months. All samples were centrifuged and frozen at −80°C until analysis. Body composition was assessed by absorptiometry at age 2 weeks (mean ± SEM 17 ± 1 days) with a Lunar Prodigy, coupled to Lunar software (version 3.4/3.5; Lunar, Madison, WI) adapted for infants (6,7). Soluble 50-kDa Pref-1 was assessed by ELISA (R&D Systems, Minneapolis, MN) (intra- and interassay coefficients of variation 3.6 and 6.2%, respectively; detection limit 0.01 μg/L).
Statistical analyses were performed using SPSS 12.0 (SPSS, Chicago, IL). Results are expressed as means ± SEM. Comparisons between groups were performed by t test. Skewed data were log transformed prior to comparison. P < 0.05 was considered significant.
All assessments were approved by the institutional review board of Barcelona University Hospital. Written (parental) consent was an inclusion criterion.
RESULTS
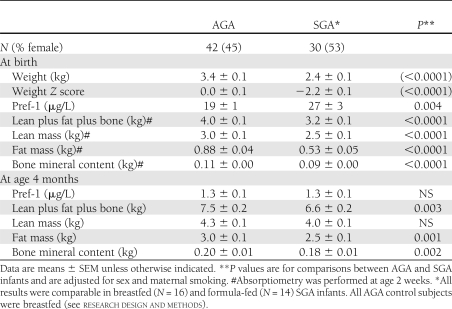
Pref-1 levels in late-gestational women were 0.7 ± 0.1 μg/L (range 0.02–1.6), which were comparable with those reported in nonpregnant women (8). Circulating Pref-1 was readily detectable in all infants. Pref-1 levels were comparable in girls and boys, and the results of both sexes were therefore pooled (Table 1).
Table 1.
Results from AGA versus SGA infants
At birth, Pref-1 levels ranged from 6 to 100 μg/L and were thus one to two orders of magnitude higher than in the maternal circulation. Pref-1 levels were ~50% higher in SGA than in AGA fetuses. By age 4 months, Pref-1 concentrations had reached a near-adult level and the original AGA versus SGA difference in Pref-1 levels had disappeared. No close associations were observed between Pref-1 concentrations and body composition either at birth or at age 4 months. On day 2 or 3, serum concentrations of Pref-1 in AGA newborns were still 14 ± 2 μg/L (range 8–26), suggesting that prenatal Pref-1 has a fetal rather than a placental or maternal origin.
CONCLUSIONS
In the first months after birth, SGA infants prioritize the recovery of lean mass to that of fat mass and of bone mineral content. Soluble Pref-1 is thought to reduce adipogenesis as well as bone formation by inhibiting the differentiation of multipotent mesenchymal cells into adipocytes, chondrocytes, and osteoblasts (5,9,10). Here, we show that Pref-1 is abundantly present in the circulation of the human fetus and that SGA fetuses have higher Pref-1 levels than do AGA control subjects.
Growth restraint in early life is a major risk factor for diabetes in later life (11,12). Our finding that fetal growth restraint is associated with markedly high Pref-1 levels suggests that Pref-1 may be among the mediators of a reduced adipocyte differentiation in growth-restrained fetuses, and thus of a reduction in their life-long lipid-storage capacity and also of their adult vulnerability to metabolic disease, once lipid storage becomes an issue.
In conclusion, Pref-1 is abundantly present in the circulation of the human fetus and is likely to be of fetal rather than maternal or placental origin. Circulating Pref-1 levels are higher in SGA than in AGA fetuses.
Acknowledgments
This study was supported by a grant from the Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III (PI08/0443). F.d.Z. is a clinical investigator supported by the Clinical Research Council of the Leuven University Hospitals and by the University of Leuven (OT-04-35). G.S. is a predoctoral investigator of Fondo de Investigación Sanitaria, Instituto de Salud Carlos III (FI06/00425). A.L.-B. is a clinical investigator of the I3 Fund for Scientific Research (Ministry of Science and Innovation, Madrid, Spain).
No potential conflicts of interest relevant to this article were reported.
F.d.Z. wrote the manuscript. M.D. researched data and reviewed and edited the manuscript. G.S. researched data and contributed to discussion. A.M.-A. and D.S.-I. researched data. A.L.-B. and L.I. contributed to discussion and reviewed and edited the manuscript. L.I. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Luis del Río, MD, and Silvana di Gregorio, MD, from Centro Médico CETIR, Barcelona, Spain, for performing the absorptiometry measurements.
References
- 1.Knittle JL, Timmers K, Ginsberg-Fellner F, Brown RE, Katz DP. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J Clin Invest 1979;63:239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature 2008;453:783–787 [DOI] [PubMed] [Google Scholar]
- 3.Ginsberg-Fellner F. Growth of adipose tissue in infants, children and adolescents: variations in growth disorders. Int J Obes 1981;5:605–611 [PubMed] [Google Scholar]
- 4.Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell 1993;73:725–734 [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Sul HS. Pref-1 regulates mesenchymal cell commitment and differentiation through Sox9. Cell Metab 2009;9:287–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.López-Bermejo A, Petry CJ, Diaz M, et al. The association between the FTO gene and fat mass in humans develops by the postnatal age of two weeks. J Clin Endocrinol Metab 2008;93:1501–1505 [DOI] [PubMed] [Google Scholar]
- 7.Ibáñez L, Sebastiani G, López-Bermejo A, Diaz M, Gómez-Roig MD, de Zegher F. Gender specificity of body adiposity and circulating adiponectin, visfatin, insulin, and insulin growth factor-I at term birth: relation to prenatal growth. J Clin Endocrinol Metab 2008;93:2774–2778 [DOI] [PubMed] [Google Scholar]
- 8.Aronis KN, Kilim H, Chamberland JP, Breggia A, Rosen C, Mantzoros CS. Preadipocyte factor-1 levels are higher in women with hypothalamic amenorrhea and are associated with bone mineral content and bone mineral density through a mechanism independent of leptin. J Clin Endocrinol Metab 2011;96:E1634–E1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Kim KA, Kim JH, Sul HS. Pref-1, a preadipocyte secreted factor that inhibits adipogenesis. J Nutr 2006;136:2953–2956 [DOI] [PubMed] [Google Scholar]
- 10.Lee K, Villena JA, Moon YS, et al. Inhibition of adipogenesis and development of glucose intolerance by soluble preadipocyte factor-1 (Pref-1). J Clin Invest 2003;111:453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cusi K. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Curr Diab Rep 2010;10:306–315 [DOI] [PubMed] [Google Scholar]
- 12.Whincup PH, Kaye SJ, Owen CG, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA 2008;300:2886–2897 [DOI] [PubMed] [Google Scholar]