Abstract
OBJECTIVE
To compare the effect of the prepubertal duration of diabetes on the occurrence of complications in two groups of patients after the same number of years of the disease.
RESEARCH DESIGN AND METHODS
This multicenter study enrolled 105 patients aged 16–40.3 years; 53 were prepubertal at diagnosis (aged 0–3) and 52 were pubertal (Tanner stage) and aged 9–14.9. The mean duration of disease was 19.8 and 19.5 years for prepubertal and pubertal patients, respectively. In all patients, retinal photographs were taken and centrally graded. Urinary albumin excretion (UAE; 86 case subjects), blood pressure (BP; 89 case subjects), and lifetime HbA1c (72 case subjects) were also evaluated.
RESULTS
The prevalence of diabetic retinopathy (DR) was higher in pubertal than in prepubertal patients, both for any grade DR (71 vs. 40%, P = 0.002) and for mild or more severe DR (P = 0.005). The prevalence of abnormal UAE was not different in the two groups. Hypertension was found only in three patients, all pubertal at diagnosis. In the small group with moderate-to-severe DR, lifetime HbA1c levels, as percentages above the upper normal reference value, were higher (P < 0.01) in prepubertal than in pubertal patients.
CONCLUSIONS
If diabetes is diagnosed in infants or toddlers and the prepubertal duration of diabetes is very long, the patients seem to be protected against DR. This protection disappears if lifetime metabolic control is bad. Instead, when onset is at puberty, the DR risk is higher and less dependent on metabolic control and may be influenced by age-related factors, such as BP.
Whether diabetes onset before puberty fully contributes to the development of chronic complications remains a point of debate. Previous studies of pre- versus postpubertal duration effects on diabetes complications have yielded conflicting results. According to some reports (1–5), hyperglycemia has little or no effect on the development of complications before puberty, whereas others (6–8) suggest that prepubertal years also contribute significantly to diabetes complications. Differences in diabetes duration and definitions of pediatric age and puberty may account for these inconsistencies. Some reports that focus on prepubertal children that were very young at diagnosis (4,6), set an upper age limit of 5 years for recruitment, which cannot ensure that the prepubertal stage was maintained at least during the first 5 years of diabetes duration.
It is important to clarify whether prepubertal years are significant contributors to microangiopathy because, in theory, one could treat young children with type 1 diabetes less aggressively (9). If, on the other hand, those who develop type 1 diabetes during puberty are at higher risk for late complications, then special attention should be paid to them (10).
To help disentangle the role of puberty years on diabetes complications and overcome some of the methodological problems of previous studies, we assessed diabetic retinopathy (DR) and diabetic nephropathy in two pediatric cohorts who were well separated by pubertal status at diagnosis, as defined by Tanner stage, but developed diabetes in the same calendar years and had similar durations. The first cohort includes patients with diabetes onset as toddlers aged 0–3 years, therefore with a prepubertal diabetes duration of at least 5 years before the onset of puberty. The other cohort includes children who developed diabetes after the beginning of puberty.
RESEARCH DESIGN AND METHODS
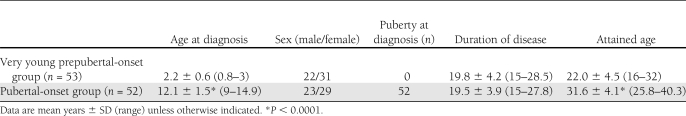
This was a multicenter study involving 11 pediatric units in Italy. The centers recruited, among the patients who had been initially diagnosed and treated between 1981 and 1992, those who were aged 0–3 years and those who were in puberty or postpubertal at the onset of type 1 diabetes. Because most patients had been transferred to adult care at the time of this study, each center recruited only from years in which at least 60% of the original cohort could be traced. A total of 105 patients were enrolled (45 males/60 females), of which 53 were aged <3 years at onset (prepubertal group) and 52 were pubescent (n = 37) or pubertal (n = 15) at onset (pubertal group). Diabetes duration was at least 15 years (range 15–28.5, mean 19.7 ± 4.0): in 69 case subjects, it was <20 years and in 36 case subjects was ≥20 years. Because in the two groups of patients, very young and pubertal, duration of disease was superimposable, attained age was consequently higher (P < 0.0001) in pubertal than in prepubertal. Pubertal status at onset was defined in all patients by Tanner stage: stages 2 and 5, respectively, were the thresholds for the definition of pubescent and pubertal.
The patients were recalled between 2007 and 2009 to the pediatric care centers where diabetes diagnoses had been established, and retinal photographs were taken through dilated pupils of two 50° fields of each eye, centered temporal to the macula and nasal to the disc, according to the EURODIAB (the Epidemiology and Prevention of Diabetes) protocol (11). The digital pictures were centrally graded in the Diabetic Retinopathy Centre of the Department of Internal Medicine at Turin University by a trained reader. The pictures were graded according to a 5-degree severity scale based on the American Academy of Ophthalmology simplified classification (12): no DR (grade 1), mild nonproliferative DR (grade 2), moderate nonproliferative DR (grade 3), severe nonproliferative DR (grade 4), and proliferative DR (grade 5). For statistical purposes, all patients with DR grades 3, 4, and 5 were grouped together as moderate-to-severe DR. At the time of retinal photographs, the following were evaluated: urinary albumin excretion (UAE) in 86 patients and BMI and arterial blood pressure (BP) in 89 case subjects. BMI was expressed according to the international classification of the Word Health Organization: patients with BMI >30 kg/m2 were considered obese, and patients with BMI between 25 and 29.9 kg/m2 were considered overweight. BP was measured with the patients seated using a standard mercury sphygmomanometer and was calculated as the mean of two measurements. For the definition of hypertension, the classification of the World Health Organization was followed.
Microalbuminuria was defined as the presence of UAE between 30 and 300 mg/day or as an albumin excretion rate ≥20 μg/min, and macroalbuminuria was defined as the presence of UAE >300 mg/day or albumin excretion rate >150 μg/min.
Repeated measurements of HbA1c were available in 72 patients. They were retrieved from medical records and laboratory reports from the departments of pediatrics and internal medicine where the patients had been followed.
HbA1c had been measured by different methods (minicolumns Bio-Rad column method, high-performance liquid chromatography, or DCA 2000 analyzer); thus, to compare results obtained in different laboratories, the values were transformed into percentages of the HbA1c levels above the upper normal reference value of each center. HbA1c values were averaged throughout the entire disease duration (except the value at diagnosis) and separately for the years 0–5, 5–10, 10–15, 15–20, and 20–25.
This study was performed in accordance with the Declaration of Helsinki as revised in the year 2000 and was approved in the participating centers by an institutional review board. Written informed consent was obtained from each patient or parent.
Statistical methods
All calculations were carried out using the commercially available program SPSS 14.0.1 (Chicago, IL). Data distribution was analyzed by means of skewness and kurtosis coefficients and Kolmogorov-Smirnov test. In normally distributed variables, comparison between groups was performed by unpaired Student t test, and in nonnormally distributed variables, by Mann-Whitney U test. Cohort differences for continuous data were assessed using a one-way ANOVA (with Bonferroni corrections for multiple comparisons).
Categorical variables were analyzed using Pearson χ2 test. Data are presented as mean ± SD or as indicated. P < 0.05 was considered significant for each test.
RESULTS
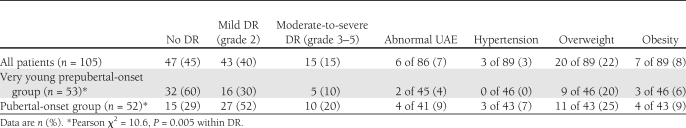
Clinical characteristics are summarized in Table 1. After an average of nearly 20 years’ duration, the prevalence of any DR in the whole group of patients was 55% (58 of 105): 40% (43 of 105) had grade 2 DR, 9% (9 of 105) had grade 3 DR, 4% (4 of 105) had grade 4 DR, and 2% (2 of 105) had grade 5 DR (Table 2). Among the more severe grades of DR, the distribution of males and females was not significantly different both in the prepubertal and in the pubertal group. In the more severe grades of DR, the prevalence of overweight (15%) and obese (8%) patients was not different from the group without DR (18 and 5%, respectively). Diabetes duration was longer in the patients with DR than in patients without DR (20.7 ± 4.3 vs. 18.4 ± 3.4 years, P = 0.004), whereas there were no differences between the durations of disease of the patients with mild and those with moderate-to-severe DR. The two patients with grade 5 DR both had 27 years of duration and were pubertal at onset.
Table 1.
Clinical characteristics of the patients
Table 2.
Prevalence of DR, nephropathy, hypertension, and BMI in the entire sample and in the two groups of patients
The prevalence of DR was higher in the patients who were pubertal at onset than in those who were prepubertal, both for any DR (71 vs. 40%, P = 0.002) and for mild or more severe DR (Pearson χ2 = 10.6, P = 0.005) (Table 2). These differences were confirmed only in the patients with <20 years’ duration (any DR 63 vs. 27%, P = 0.005), not in those with longer duration (any DR 88 vs. 63%, P = NS). The prevalence of overweight, obesity, and hypertension was not significantly different in the prepubertal and pubertal groups (Table 2) and in the various grades of RD. Only three patients were hypertensive, and they all belonged to the pubertal group. Of these patients, one had no DR, one had grade 3, and one had grade 5.
The prevalence of increased UAE in the entire sample was 7% (6 of 86): 2 out of the 45 prepubertal patients, both without DR, and 4 out of the 41 pubertal (Table 2), of whom 2 associated with grade 2, 1 with grade 3, and 1 with grade 5 DR. This difference was not statistically significant. Lifetime HbA1c values of the patients with abnormal UAE were not significantly different from those of the normoalbuminuric patients. None of the patients showing increased UAE were hypertensive, and 2 of them (1 prepubertal and 1 pubertal) reverted to normal after ACE-inhibition therapy (13).
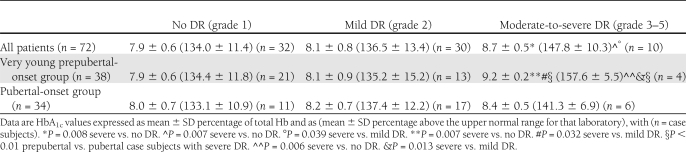
Lifetime HbA1c values did not differ between prepubertal and pubertal patients in the whole sample or in the patients without DR or with mild DR (Table 3). However, in the small group of patients with moderate-to-severe DR, lifetime HbA1c levels were higher in the prepubertal than in pubertal patients (157.6 ± 5.5 vs. 141.3 ± 6.9%, P < 0.01) (Table 3). None of the above subgroups had different HbA1c values in the first 5 years of disease.
Table 3.
Lifetime HbA1c values in the two groups of patients, prepubertal and pubertal, subdivided according to grade of DR
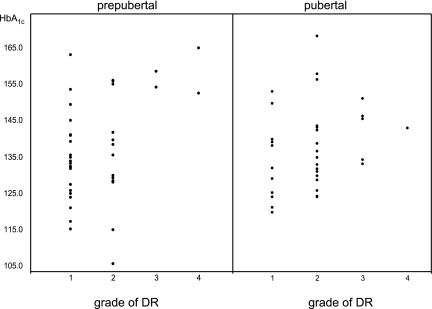
Lifetime levels of HbA1c were higher in the patients with moderate-to-severe DR than in those with mild or no DR in the whole patient sample and among the prepubertal patients, whereas no such difference was observed among the pubertal patients (Table 3 and Fig. 1). No significant differences regarding HbA1c values in the first 5 years of disease were found in any comparison.
Figure 1.
Distribution of HbA1c values, as percentage above the upper normal reference value, in the prepubertal-onset and pubertal-onset patients, subdivided according to grade of severity of DR.
CONCLUSIONS
In this case series of patients who developed type 1 diabetes after 1980, 55% had any DR and 15% had moderate-to-severe DR after almost 20 years’ duration. These prevalence data are not readily comparable with those of previous reports. Pambianco et al. (14) reported higher prevalence of proliferative DR and renal failure, but their patients had been diagnosed with diabetes in an earlier era, the 1950s to 1970s. Donaghue et al. (6) reported data more similar to ours (52% for any DR and 21% for more severe DR), but their patients were younger and had a shorter disease duration. The study by Romero et al. (15) is comparable to ours both for patient characteristics and DR classification and reports the same prevalence of any DR (55.4%) but a higher rate of more severe DR (27%). In disagreement with Harjutsalo et al. (16), we have not found differences between the sexes with regard to risk of severe DR; however, the differences observed by Harjutsalo et al. (16) were in patients with age at onset ≥15 years, whereas all our patients were age at onset <15 years. In the same series of patients, only 7% had micro-macroalbuminuria, a percentage eightfold lower than that of DR. Although it is well known that nephropathy is a complication less frequent and less related to glycemic control than DR, this low prevalence is not found in other studies, which, moreover, considered durations of disease shorter than ours, including 18% in Svensson et al. (4), 14% in Olsen et al. (8), and 41% in Romero et al. (15). Furthermore, this percentage would have been even lower if we had excluded the two patients who normalized, as occurs frequently among adolescents and young adults (13,17). We can speculate that the Italian diet may be protective toward renal structure either directly or indirectly by a positive influence on lipid profile (13).
The effects of pre- versus postpubertal duration of diabetes on the development of DR were examined in a number of studies, with somewhat conflicting results. According to Donaghue et al. (6), prepubertal duration improves the prediction for DR over postpubertal duration alone, and the results do not support the hypothesis that prepubertal children are protected against the development of diabetes complications. However, those authors were unable to separate clearly prepuberty from puberty because they considered subjects aged 12–13 years prepubertal and in whom hormonal changes may well be under way even before they become clinically manifested. Donaghue et al. (6) identified a group of smaller children, <5 years of age, with relative immunity to the development of complications. When they re-examined the same patients as young adults, Donaghue et al. (2) confirmed a smaller risk increment for clinical DR for each year of prepubertal duration compared with each year of postpubertal duration. Svensson et al. (4), using survival analysis, showed a protective effect of prepubertal duration, but possible confounders in their study were the high variability of diabetes duration and that pubertal stage was assumed but not documented. Our results suggest that patients who develop diabetes as toddlers are less prone to develop DR than those in whom onset occurs in pubertal years. The inclusion criteria adopted for this study is the strictest reported so far and should permit a rigorous assessment of the role of prepubertal duration on the development of diabetes complications. In fact, physiological puberty cannot start before age 8 years, and the prepubertal patients considered for this study were aged 0–3 years at diagnosis, which ensured that they had remained in prepubertal years for at least 5–7 years, which is the longest period possible. Porta et al. (3), in a different series of patients, confirmed a protective effect of prepubertal years, although only up to 20 years’ duration, after which, as reported also by Pambianco et al. (14), any favorable patterns seen at 20 years had largely disappeared with 25 years’ duration. Different results were found for nephropathy, since the few case subjects with abnormal UAE were equally inserted in prepubertal and pubertal groups. Also, Drummond et al. (9), using glomerular morphometry obtained from kidney biopsies, concluded that it is total duration rather than duration before or after puberty that affects the rate of progression of the renal lesions.
Some authors have suggested that increased risk for complications in patients with diabetes onset in pubertal years may be due to the deterioration of glycemic control, but they could not support this claim with clinical data (6,9). Our results, which include lifetime HbA1c for approximately two-thirds of the population studied, do not support this hypothesis. Indeed, HbA1c did not differ in patients with prepubertal and pubertal onset, nor did it differ throughout the entire disease duration or during the first 5 years. If anything, patients with pubertal onset who developed more severe DR had lower HbA1c values than patients with prepubertal onset and similar to those with no or mild DR. This suggests that factors other than glycemic control, associated with chronological age per se rather than disease duration, may be major determinants for the risk of DR in pubertal-onset diabetes. Excess body weight, equally distributed between prepubertal and pubertal groups, seems an unlikely confounding factor, whereas we cannot exclude that hypertension may be one of these factors since all three case subjects with hypertension belonged to the pubertal group. The difference between the two groups was not statistically significant, but the number of cases was very low.
As shown in Fig. 1, poor control over the entire disease duration is associated with severe DR in prepubertal-onset diabetes, whereas control during the first 5 years did not differ in patients with and without DR and may not play a role. A possible consequence of these data is that maintaining good metabolic control should always be attempted irrespective of age (7), although maintenance of metabolic control does not need to be exceedingly strict while children are very young and prone to hypoglycemia. However, when interpreting these results, we need to bear in mind that the number of patients with severe DR in our study was especially small.
The strengths of this study are a good definition of pubertal age, pubertal characteristics clearly separate in the two cohorts (with longest and shortest prepubertal periods), a multicenter approach, the central grading of digital retinal pictures, sufficiently long duration, and lifetime glycemic control in most of the patients. The limited statistical power due to few patients with longer duration and the fact that this is not a population-based study are possible limits of this study.
In summary, our study suggests that 1) patients diagnosed with onset of diabetes at the youngest age are more protected against microvascular complications than patients diagnosed during puberty, but poor long-term glycemic control can delete this protection; and 2) the onset of diabetes during puberty is in itself an important independent risk factor, as may be an increase in BP as well, even when metabolic control is satisfactory.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
S.S. researched data and wrote the manuscript. M.P. researched data, contributed to discussion, and reviewed the manuscript. G.M. researched data and edited the manuscript. F.R., S.R., F.C., D.I., S.T., and V.C. researched data. S.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Stefano Gualandi, PhD, University of Bologna, for help with statistical analysis.
APPENDIX
Collaborators of the ISPED Diabetes Study Group who researched data include the following: Francesco Cadario, MD, Department of Pediatrics, Maggiore della Carità Hospital Novara, University of Piemonte Orientale, Italy; Giuseppe D’Annunzio, MD, Department of Pediatrics, IRCCS Gaslini Children’s Hospital, University of Genova, Genova, Italy; Alessandro Salvatoni, MD, Pediatric Clinic, Insubria University, Varese, Italy; Riccardo Schiaffini, MD, Endocrinology and Diabetes Palidoro Unit, University Department of Pediatric Medicine, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy; Sonia Toni, MD, Meyer Pediatric Institute, University of Firenze, Firenze, Italy; Maria Antonietta Zedda, MD, Pediatric Clinic, University of Cagliari, Cagliari, Italy; and Stefano Zucchini, MD, Department of Pediatrics, University of Bologna, Bologna, Italy.
Footnotes
*A list of collaborators of the Diabetes Study Group of the Italian Society of Paediatric Endocrinology and Diabetology can be found in the Appendix.
References
- 1.Kostraba JN, Dorman JS, Orchard TJ, et al. Contribution of diabetes duration before puberty to development of microvascular complications in IDDM subjects. Diabetes Care 1989;12:686–693 [DOI] [PubMed] [Google Scholar]
- 2.Donaghue KC, Fairchild JM, Craig ME, et al. Do all prepubertal years of diabetes duration contribute equally to diabetes complications? Diabetes Care 2003;26:1224–1229 [DOI] [PubMed] [Google Scholar]
- 3.Porta M, Dalmasso P, Grassi G, et al. Pre-pubertal onset of type 1 diabetes and appearance of retinopathy. Diabetes Metab 2004;30:229–233 [DOI] [PubMed] [Google Scholar]
- 4.Svensson M, Eriksson JW, Dahlquist G. Early glycemic control, age at onset, and development of microvascular complications in childhood-onset type 1 diabetes: a population-based study in northern Sweden. Diabetes Care 2004;27:955–962 [DOI] [PubMed] [Google Scholar]
- 5.Svensson M, Nyström L, Schön S, Dahlquist G. Age at onset of childhood-onset type 1 diabetes and the development of end-stage renal disease: a nationwide population-based study. Diabetes Care 2006;29:538–542 [DOI] [PubMed] [Google Scholar]
- 6.Donaghue KC, Fung AT, Hing S, et al. The effect of prepubertal diabetes duration on diabetes. Microvascular complications in early and late adolescence. Diabetes Care 1997;20:77–80 [DOI] [PubMed] [Google Scholar]
- 7.Holl RW, Lang GE, Grabert M, Heinze E, Lang GK, Debatin KM. Diabetic retinopathy in pediatric patients with type-1 diabetes: effect of diabetes duration, prepubertal and pubertal onset of diabetes, and metabolic control. J Pediatr 1998;132:790–794 [DOI] [PubMed] [Google Scholar]
- 8.Olsen BS, Sjølie AK, Hougaard P, et al. ; Danish Study Group of Diabetes in Childhood The significance of the prepubertal diabetes duration for the development of retinopathy and nephropathy in patients with type 1 diabetes. J Diabetes Complications 2004;18:160–164 [DOI] [PubMed] [Google Scholar]
- 9.Drummond KN, Kramer MS, Suissa S, et al. ; International Diabetic Nephropathy Study Group Effects of duration and age at onset of type 1 diabetes on preclinical manifestations of nephropathy. Diabetes 2003;52:1818–1824 [DOI] [PubMed] [Google Scholar]
- 10.Morimoto A, Nishimura R, Matsudaira T, Sano H, Tajima N; Diabetes Epidemiology Research International Study Group Is pubertal onset a risk factor for blindness and renal replacement therapy in childhood-onset type 1 diabetes in Japan? Diabetes Care 2007;30:2338–2340 [DOI] [PubMed] [Google Scholar]
- 11.Aldington SJ, Kohner EM, Meuer S, Klein R, Sjølie AK. Methodology for retinal photography and assessment of diabetic retinopathy: the EURODIAB IDDM complications study. Diabetologia 1995;38:437–444 [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson CP, Ferris FL, 3rd, Klein RE, et al. ; Global Diabetic Retinopathy Project Group Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110:1677–1682 [DOI] [PubMed] [Google Scholar]
- 13.Salardi S, Balsamo C, Zucchini S, et al. High rate of regression from micro-macroalbuminuria to normoalbuminuria in children and adolescents with type 1 diabetes treated or not with enalapril: the influence of HDL cholesterol. Diabetes Care 2011;34:424–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes 2006;55:1463–1469 [DOI] [PubMed] [Google Scholar]
- 15.Romero P, Salvat M, Fernández J, Baget M, Martinez I. Renal and retinal microangiopathy after 15 years of follow-up study in a sample of type 1 diabetes mellitus patients. J Diabetes Complications 2007;21:93–100 [DOI] [PubMed] [Google Scholar]
- 16.Harjutsalo V, Maric C, Forsblom C, Thorn L, Wadén J, Groop PH; FinnDiane Study Group Sex-related differences in the long-term risk of microvascular complications by age at onset of type 1 diabetes. Diabetologia 2011;54:1992–1999 [DOI] [PubMed] [Google Scholar]
- 17.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003;348:2285–2293 [DOI] [PubMed] [Google Scholar]