Abstract
OBJECTIVE
To test the hypothesis that the risk of persistent glucose impairment after gestational diabetes mellitus (GDM) is increased in patients with polycystic ovary syndrome (PCOS).
RESEARCH DESIGN AND METHODS
The prospective case-control study included 42 pregnant patients with PCOS and GDM and 84 pregnant control patients with GDM but without clinical and biochemical hyperandrogenism, polycystic ovaries, and oligo-anovulation. The case and control subjects were matched one to two for age and BMI. The glycemic profiles were studied in all subjects 6 weeks, 12 weeks, and 18 months after delivery. The incidence and the relative risk (RR) were calculated for overall persistence of an abnormal glycemic pattern and for each specific alteration, i.e., impaired glucose tolerance (IGT), impaired fasting glucose (IFG), and diabetes mellitus (DM).
RESULTS
At 18 months after delivery, the incidences of IFG, IGT, and IFG-IGT were significantly (P < 0.05) higher in the cases than in the controls. At the 18-month follow-up, the RR for the composite outcome of glucose metabolism impairment in PCOS women was 3.45 (95% CI 1.82–6.58).
CONCLUSIONS
Patients with PCOS are at increased risk for a persistent impaired glucose metabolism after GDM.
Polycystic ovary syndrome (PCOS) is a common multifaceted disease that is characterized by ovulatory disorders, hyperandrogenism, and polycystic ovaries (PCOs) on ultrasonography (1). Several other features, such as obesity and insulin resistance, are related to PCOS and represent risk factors for alterations in glucose metabolism (2). In fact, patients with PCOS have an increased incidence of impaired glucose tolerance (IGT), impaired fasting glucose (IFG), and diabetes mellitus (DM) (3).
This PCOS-related metabolic impairment has an additive effect on the natural state of insulin resistance observed during pregnancy (4) by worsening the baseline insulin resistance and leading to an increased risk for several complications of pregnancy, particularly gestational DM (GDM).
Preliminary meta-analytic data (5,6) have given discordant results regarding the increased risk for GDM in PCOS subjects. Furthermore, a more recent meta-analysis (7), including 18 studies that had the GDM risk in PCOS as an end point for a total of 2,385 PCOS and 89,669 control patients, confirmed that the risk of GDM is approximately three times higher in pregnant patients with PCOS compared with the non-PCOS population.
Moreover, GDM is not only important because of the obstetrical complications but also because of the long-term metabolic sequelae from the persistence of glucose metabolism alterations. In fact, the incidence of DM and IGT in the population with prior GDM is ∼14 and 40%, respectively (8).
After pregnancy, the presence of PCOS-related features, such as hyperandrogenism and/or insulin resistance, could increase the incidence of a prediabetic or diabetic state, which would impair the normal improvement of the insulin-glucose metabolism. To our knowledge, no studies in the literature have assessed the incidence of glucose metabolism alterations in the PCOS patients with GDM. The aim of the current study was to test the hypothesis that PCOS increases the risk of persistent glucose impairment in patients with previous GDM.
RESEARCH DESIGN AND METHODS
A more complete and detailed research design and methods has been provided in the Supplementary Appendix.
Design
The current report is a prospective case-control study.
Ethics
The procedures used in the study protocol were in accordance with the Helsinki Declaration on human experimentation guidelines. The study was approved by the Ethical Committee of the Department of Obstetrics and Gynecology of the Magna Graecia University of Catanzaro. The purpose of the protocol was carefully explained to all women, and their written consent was obtained before entering the study.
Subjects
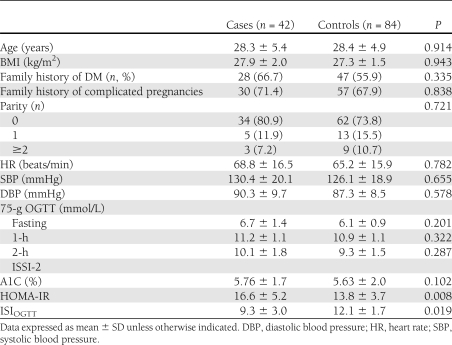
Between February 2003 and October 2009, 243 pregnant patients were screened for GDM and PCOS from a large population of women who were suffering from hyperandrogenism and/or ovulatory disorders and seeking pregnancy, and 42 subjects were included in this study protocol as cases. In addition, other 84 non-PCOS, pregnant patients with GDM were screened in our GDM ambulatory and enrolled, after matching procedure for age and BMI with cases, as controls.
A clinical evaluation, a complete hormonal and metabolic pattern assessment, and a 2-h, 75-g oral glucose tolerance test (OGTT) were preliminarily evaluated in each subject before pregnancy to confirm or exclude the diagnosis of PCOS (for cases and controls, respectively) and of pre-existing DM.
PCOS was diagnosed before pregnancy according to the international criteria (1), whereas pregnant patients were included as controls if they had regular menstrual cycles before pregnancy, no signs of clinical hyperandrogenism, normal ranges of serum androgen levels, and no PCO morphologies on transvaginal ultrasonography.
In each subject, GDM was defined as any degree of glucose intolerance with onset or first recognition during pregnancy using well-recognized criteria (9). In particular, glucose concentrations were measured at basal and after a 2-h, 75-g OGTT in women who were not previously diagnosed with overt DM between the 24th and 28th weeks of gestation (9).
For all patients, the exclusion criteria consisted of the following: age >35 years, severe obesity, multiple pregnancies, a gestational age at the GDM diagnosis that was >28 or <24 weeks, medical conditions or other concurrent medical illnesses, a previous diagnosis of DM, cigarette smoking, drug/alcohol abuse, noncompliance with our study protocol, and a previous use of any antidiabetic drugs.
Protocol
At baseline and throughout the study period, each subject was monitored with clinical, biochemical, and ultrasonographic assessments according to a specific schedule. Upon entering the study, the socio/economic, work, and educational statuses and associated medical conditions were carefully assessed for each subject. In addition, the women were asked to complete a questionnaire on their family history of DM or complicated pregnancies.
Clinical and ultrasonographic assessments were performed at study entry and every 4 weeks until delivery. The clinical evaluation consisted of an obstetric examination, anthropometric measurements, and heart rate and blood pressure assessments. During the same visits, the daily diet and physical activity were monitored in all patients using two well-validated questionnaires. Biochemical assessments were performed for each subject monthly.
At study entry, a 2-h, 75-g OGTT (see above), A1C levels, insulin resistance, and β-cell function were evaluated. In particular, insulin resistance was assessed with the use of two surrogate indexes, i.e., the insulin sensitivity index (ISIOGTT) and the homeostasis model assessment of insulin resistance (HOMA-IR), whereas insulin secretion–sensitivity index-2 (ISSI-2) was used as measure of β-cell function.
The monitoring and ongoing care were given by the attending obstetrical team with a physician’s support. Interventions included individualized medical nutrition therapy (MNT), self-monitoring of blood glucose (SMBG), and insulin therapy, with the dose adjusted on the basis of glucose levels. The goals of GDM therapy were to maintain the maternal capillary glucose concentrations at <96 mg/dL (5.3 mmol/L) in the fasting state and <140 mg/dL (7.8 mmol/L) at 1 h and <120 mg/dL (6.7 mmol/L) 2 h after initiation of a meal.
Treatment compliance was evaluated by an examination of the self-reported daily diary that was performed at each follow-up visit. The adherence to the interventions (MNT, SMBG, and insulin therapy) was defined as high, moderate, or low according to multidisciplinary parameters.
During the study, all subjects underwent trans-abdominal ultrasonographic evaluations, which consisted of measurements of fetal growth and abdominal circumference, the placenta’s location and grade, and an amniotic fluid index. An umbilical artery pulsatility index and cardiotocography were performed when required. Each subject was advised to monitor fetal movements during the last 8–10 weeks of pregnancy and to immediately report any reduction in their perception.
At hospital discharge, persistent hyperglycemia was evaluated by measuring fasting glucose levels and MNT, whenever it was confirmed. If necessary, pharmacological therapy was continued. The maternal, perinatal, and neonatal outcomes were noted for each patient.
Each patient underwent three follow-up visits at 6 weeks, 12 weeks, and 18 months after delivery. In particular, each patient underwent anthropometric measurements and biochemical assessments, which consisted of a complete blood count, serum glucose, insulin, and A1C levels at baseline, and glucose concentrations during a 2-h, 75-g OGTT.
The presence of DM or prediabetes was evaluated at each follow-up visit. In particular, DM was diagnosed when the fasting plasma glucose levels were ≥126 mg/dL (7.0 mmol/L) and/or the 2-h plasma glucose levels were ≥200 mg/dL (11.1 mmol/L) during a 75-g OGTT. Conversely, hyperglycemia, which was not sufficient to meet the diagnostic criteria for DM, was defined as prediabetes and categorized as either IFG or IGT depending on whether it was identified through the fasting plasma glucose levels or the OGTT. At each follow-up visit, the rate of breast-feeding and its frequency and duration were also evaluated.
Statistical analysis
Categorical variables were compared by using Pearson χ2 or Fisher exact test. The normal distribution of the continuous variables data was evaluated with the use of a Kolmogorov-Smirnov test, and a log transformation was used for the nonnormal data. Thus, our data were expressed as the mean ± SD. Continuous variables were analyzed with a one-way ANOVA and an ANOVA for repeated measures with a Bonferroni test for the post hoc analysis. The relative risk (RR) was calculated with a 95% CI for composite outcomes and for each alteration of glucose homeostasis. Forward, stepwise, multivariate, logistic regression analysis was performed to identify independent predictors of a persistent glucose metabolism impairment. Statistical significance was set at P < 0.05.
RESULTS
Before pregnancy, the cases and controls were significantly (P < 0.05) different by the Ferriman-Gallwey score and for testosterone, androstenedione, dehydroepiandrostenedione, sex-hormone binding globulin, free androgen index, and fasting insulin levels and HOMA-IR (data not shown). No other significant differences in any clinical and biochemical data were detected between the cases and controls (data not shown).
At the same time, the distribution between cases and controls of obese (15 [35.7%] vs. 33 [39.3%], respectively), overweight (19 [45.2%] vs. 38 [45.2%], respectively), normal weight (6 [14.3%] vs. 9 [10.7%], respectively), and lean (2 [4.8%] vs. 4 [4.8%], respectively) subjects was not significantly (P = 0.865) different. Similarly, no significant (P = 0.776) difference between the cases and controls was detected in the incidence of patients with IGT (6 [14.3%] vs. 14 [16.7%], respectively) and IFG (5 [11.9%] vs. 15 [17.9%], respectively).
Spontaneous conception occurred in 22 out of 42 (52.4%) cases and 71 out of 84 (84.5%) controls (P = 0.0002), whereas the others had conceived under ovulation inductors, such as clomiphene citrate (11 [26.2%] cases and 4 [4.8%] controls), aromatase inhibitors (2 [4.8%] cases and 0 [0.0%] controls), or gonadotropins (7 [16.7%] cases and 9 [10.7%] controls). No difference between cases and controls was observed in the proportion of pregnancies achieved with the use of in vitro fertilization techniques (5 [11.9%] vs. 7 [8.3%], respectively; P = 0.532). Patients had full-blown, non-PCO, nonhyperandrogenic, and ovulatory phenotypes in 19 (45.2%), 8 (19.0%), 5 (11.9%), and 10 (23.8%) out of the 42 PCOS women, respectively. The data recorded at study entry for both groups are shown in Table 1.
Table 1.
Clinical data in cases and controls at baseline
No differences between groups were observed in socio/economic status, daily diet, and physical activity (data not shown). All case and control subjects underwent MNT and SMBG, whereas the rate of patients who needed insulin therapy was higher for the cases than the controls (20 out of 42 cases [47.6%] and 22 out of 84 controls [26.2%]; P = 0.027).
No significant (P = 0.234) difference between cases and controls was detected for adherence to the treatment assigned, and, specifically, it was high in 20 out of 42 (47.6%) and 39 out of 84 (46.4%), moderate in 16 out of 42 (38.1%) and 35 out of 84 (41.7%), and low in 6 out of 42 (14.3%) and 10 out of 84 (11.9%) cases and controls, respectively.
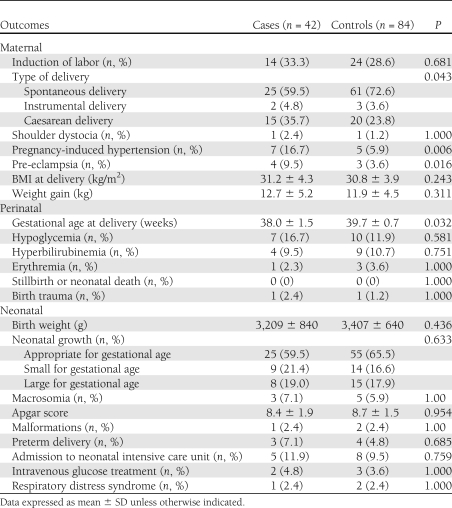
The main maternal, perinatal, and neonatal outcomes recorded in the cases and controls are detailed in Table 2. A total of 16 out of 42 cases (38.1%) and 15 out of 84 controls (17.9%) had adverse maternal/perinatal/neonatal outcomes (P = 0.017). No differences between groups were recorded in the placental location and grade distribution, amniotic fluid index, and mean umbilical artery pulsatility index (data not shown).
Table 2.
Maternal, perinatal, and neonatal outcomes in cases and controls
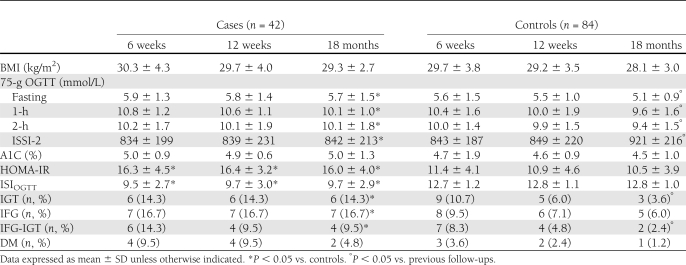
In Table 3, the BMI measurements and glucose metabolism parameters that were recorded at 6 weeks, 12 weeks, and 18 months after delivery for the cases and controls are reported. At each follow-up after delivery, a trend (0.05 < P < 0.07) toward a significantly higher BMI was observed in the cases compared with controls (Table 3). No significant differences were observed in other anthropometric measurements (data not shown). A significant (P < 0.05) difference between the cases and controls was detected in the HOMA-IR and ISIOGTT at each follow-up evaluation (Table 3). The OGTT parameters (including ISSI-2) were significantly (P < 0.05) different between cases and controls only after 18 months.
Table 3.
BMI measurements and glucose metabolism parameters recorded after 6 and 12 weeks and 18 months from delivery in PCOS (cases) and non-PCOS (controls) patients
At the 18-month follow-up, a significant (P < 0.05) improvement in the OGTT parameters and in the IGT and IFG-IGR incidence were observed in the control group (Table 3). At the same follow-up visit, a significant (P < 0.05) difference in the IGT, IFG, IFG-IGR, and DM incidence was detected between the cases and controls (Table 3).
The rate of patients who breast-fed was significantly (P = 0.019) lower in cases than in controls (27 of 42 [64.3%] vs. 71 of 84 [84.5%], respectively). Additionally, the duration (months) of breast-feeding was significantly lower in cases than in controls (2.1 ± 1.1 vs. 5.3 ± 2.6; P = 0.007), whereas its daily frequency was not different between groups (3.6 ± 1.2 vs. 4.8 ± 2.1, respectively; P = 0.122).
The RRs for IGT, IFG, IGT-IFG, and DM after GDM in PCOS women were 4.0 (95% CI 1.05–15.02; P = 0.042), 2.80 (95% CI 0.94–8.3; P = 0.063), 4.0 (95% CI 0.76–20.96; P = 0.101), and 4.0 (95% CI 0.37–42.86; P = 0.252), respectively. The RR for the composite outcome of glucose metabolism impairment after GDM in PCOS women was 3.45 (95% CI 1.82–6.58; P = 0.0002).
The composite outcome was significantly influenced by BMI (P = 0.010) and the presence of hyperandrogenism (P = 0.032), oligo-anovulation (P = 0.044), IGT (P = 0.011), and IFG (P = 0.007) before pregnancy. The need for insulin therapy during pregnancy (P = 0.036) and the breast-feeding (P = 0.024) also significantly influenced the composite outcome.
CONCLUSIONS
GDM is present in 0.6–15% of pregnancies, and it is associated with a potential recurrence of GDM (10) and an increased risk for the development of type 1 (11) or type 2 (12) DM or IGT, with a relevant impact on the later onset of cardiovascular disease (3,12). In particular, the incidence of developing DM after GDM may vary between 20 and 60% within 5 years of pregnancy (13). This wide variability depends on the characteristics of the population studied (14).
In the current study, we wanted to evaluate the hypothesis that PCOS is a pathologic condition with an increased risk for the persistence/development of an impairment in glucose metabolism after GDM. Our study hypothesis was based on recognition of the syndrome as a condition related to several risk factors for IGT and cardiovascular diseases (3), to an increased risk for GDM (7), and to an increased conversion rate form normal glucose tolerance to IGT and from IGT to DM (15,16).
To this aim, we prospectively enrolled 42 consecutive subjects who satisfied standardized criteria for both PCOS (1) and GDM (9), and who were representative of the PCOS population because the distributions of phenotypes, such as of IGT/IFG and BMI, reflected those described in the literature (3). A well-selected sample of non-PCOS women with GDM, who had similar characteristics and risk factors, were selected as controls. In addition, cases and controls were also similar in prepregnancy conditions, such as parity and incidence of IGT or IFG. All these points strengthen the findings obtained in the current study.
At 6 weeks, 12 weeks, and 18 months after delivery, the incidences of DM and prediabetes, i.e., IGT, IFG, and IFG-IGT, as single events or a composite outcome, were evaluated in the current analysis. In the non-PCOS controls with GDM, we demonstrated a sharp improvement in the glycemic pattern and metabolic condition after delivery, with an incidence of DM and prediabetes of 1.2 and 12.0%, respectively, at the 18-month follow-up. Alternatively, a persistence of a glucose alteration was observed in 45.2% patients with PCOS at the same follow-up visit. Specifically, DM, IFG-IGT, IFG, and IGT were found in 4.8, 9.5, 16.7, and 14.3% of PCOS women, respectively, which reached statistical significance compared with the controls for all end points except DM, which was likely due to the small sample studied.
Our findings demonstrated that women with PCOS and GDM had a 3.5-fold higher risk for impaired glucose metabolism after delivery than the non-PCOS controls. This increased risk reached statistical significance for IGT and the composite outcome. In addition, although the cases and controls had a comparable adherence to the treatment and were similarly treated with MNT and SMBG at diagnosis of GDM, the subjects affected by PCOS had a lower glycemic control and more frequently were in need of insulin therapy than controls.
At present, no specific data that could explain our findings are available in the literature. The multivariate logistic regression analysis, which was performed for this purpose, showed that the composite outcome was influenced by several factors occurring before pregnancy (such as BMI, hyperandrogenism, oligo-anovulation, glucose abnormalities, and insulin resistance), during pregnancy (such as the need for insulin therapy), and after pregnancy (such as the breast-feeding), suggesting that, probably, various factors interact, increasing the risk of glucose metabolism impairment after GDM in PCOS women.
PCOS patients have a higher risk of conversion from normal glycemia to IGT or DM, irrespective of pregnant state (14–16). This higher risk for glucose metabolism impairment seemed strongly related to insulin resistance and influenced by BMI (15). In fact, PCOS is a condition that is characterized by a chronic insulin-resistant state present before pregnancy, as was observed in the current study, and is exacerbated by the physiological changes that lead to insulin resistance during pregnancy (4). In this regard, adverse maternal/perinatal/neonatal outcomes, which are related to the insulin resistance state (such as pregnancy-induced hypertension, preeclampsia, the type of delivery, and the gestational age at delivery) (17), occurred more frequently in PCOS patients than in the controls.
After delivery, the insulin resistance remained worse in PCOS women than in non-PCOS controls. This abnormality, already described for PCOS, resulted from a combination of increased insulin resistance with defective pancreatic β-cell function (18). It has recently been demonstrated that in women with IGT during pregnancy, hepatic insulin resistance is an early determinant of declining β-cell function after gestational dysglycemia in the first postpartum year (13). The current analysis confirmed the impaired insulin resistance state in PCOS women during the early postpartum follow-ups. Moreover, at the same time, a β-cell dysfunction, assessed by means-appropriate test, was also observed more frequently in PCOS women.
It would be interesting to identify PCOS-related factors that influence the insulin resistance state and the β-cell function, with subsequent persistence of glucose alterations. In this regard, a subanalysis of our results based on the PCOS phenotypes would be clinically useful for evaluating major factors associated with abnormal glucose tolerance in PCOS. Unfortunately, this analysis was not done because our sample was too small for that aim. In addition, the roles of the different phenotypes of PCOS on the metabolic pattern are still debated (19–23).
Our data confirmed (24) that women with PCOS have a reduced breast-feeding rate and duration, losing the beneficial effect of lactation on glucose metabolism and insulin sensitivity that may reduce DM risk after GDM pregnancy (25). Thus, the reduced breast-feeding could be a further factor that interacts with others playing a role in the persistent impaired glucose metabolism after GDM in high-risk patients, such as PCOS subjects.
On the basis of these considerations, PCOS should be considered a high-risk condition for glucose impairment after GDM. Thus, two main points of debate should be discussed: 1) the need for an accurate follow-up in order to recognize early glucose impairments and 2) strategies to prevent the decline in glucose metabolism.
With regard to the first point, the American Diabetes Association has included PCOS among the criteria for DM testing of asymptomatic adult individuals at the first prenatal visit (evidence level B) (26). Moreover, a recent prospective study (27) provided insights into the relative accuracy of the various clinical methods used to detect prediabetes and DM in women with PCOS and demonstrated the accuracy of both OGTT and A1C for DM screening. Alternatively, a lower level of evidence has been demonstrated regarding postpartum surveillance, which suggests screening women with GDM for persistent DM 6–12 weeks postpartum and lifelong screening for the development of DM or prediabetes at least every 3 years (26). At present, no data on specific indications for postpartum screening in PCOS women are available, but screening these women more frequently has been advised because most will have additional risk factors for developing DM, including obesity, glucose intolerance, and insulin resistance (27). The same authors (27) suggested that PCOS women with prediabetes or DM should be considered as having insulin resistance and the remaining women should undergo HOMA-IR and insulin level assays to identify insulin resistance. With regard to preventative strategies, all interventions that are able to affect the risk factors for GDM and the persistence of glucose alterations, i.e., obesity, hyperandrogenism, oligo-anovulation, glucose abnormalities, and insulin resistance, should be recommended for PCOS women seeking pregnancy.
The prevention of DM, and translationally of GDM, was defined as a long and winding road without a shortcut, and a persistent and prolonged intensive lifestyle intervention seems to be the most effective treatment (28). In PCOS women, lifestyle modification improves body composition, hyperandrogenism, and insulin resistance (29). In addition, for women with GDM, a lifestyle intervention that starts during pregnancy and continues postpartum is feasible and may prevent pregnancy weight retention and also may help overweight women lose weight (30).
However, the main challenge of “real life” behavioral changes is the adherence to the program; therefore, pharmacological therapies alone or in combination with lifestyle programs have been proposed to reduce development of DM in high-risk subjects (31). In PCOS women, insulin-sensitizing drugs, such as metformin, have been proven to be effective on hormonal and metabolic features of the syndrome (32). However, the effect of metformin on GDM prevention in pregnant PCOS subjects is still controversial (33–37). Additional well-designed studies on an adequate sample population to evaluate the effect of metformin administration before and during pregnancy on GDM development in PCOS are needed.
In conclusion, patients with PCOS are at an increased risk for persistent impaired glucose metabolism after GDM. In this population, accurate follow-ups and preventative strategies should be adopted to avoid long-term consequences.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
S.P. contributed to study design and data analysis and wrote the manuscript. A.F. performed statistical analysis and wrote the manuscript. T.R., M.O., and A.G.C. researched data and reviewed the manuscript. L.R. researched data. R.V. researched data and contributed to data analysis. C.C. researched data. A.T. and A.C. researched data and contributed to discussion. F.Z. contributed to data analysis and discussion. F.O. contributed to study design and researched data. S.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1971/-/DC1.
References
- 1.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19–25 [DOI] [PubMed] [Google Scholar]
- 2.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol 2011;7:219–231 [DOI] [PubMed] [Google Scholar]
- 3.Wild RA, Carmina E, Diamanti-Kandarakis E, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab 2010;95:2038–2049 [DOI] [PubMed] [Google Scholar]
- 4.Ryan EA. Hormones and insulin resistance during pregnancy. Lancet 2003;362:1777–1778 [DOI] [PubMed] [Google Scholar]
- 5.Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update 2006;12:673–683 [DOI] [PubMed] [Google Scholar]
- 6.Toulis KA, Goulis DG, Kolibianakis EM, Venetis CA, Tarlatzis BC, Papadimas I. Risk of gestational diabetes mellitus in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Fertil Steril 2009;92:667–677 [DOI] [PubMed] [Google Scholar]
- 7.Kjerulff LE, Sanchez-Ramos L, Duffy D. Pregnancy outcomes in women with polycystic ovary syndrome: a metaanalysis. Am J Obstet Gynecol 2011;204:558.e1–558.e6 [DOI] [PubMed] [Google Scholar]
- 8.Kitzmiller JL, Dang-Kilduff L, Taslimi MM. Gestational diabetes after delivery. Short-term management and long-term risks. Diabetes Care 2007;30(Suppl. 2):S225–S235 [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2008;31(Suppl. 1):S55–S60 [DOI] [PubMed] [Google Scholar]
- 10.Getahun D, Fassett MJ, Jacobsen SJ. Gestational diabetes: risk of recurrence in subsequent pregnancies. Am J Obstet Gynecol 2010;203:467.e1–467.e6 [DOI] [PubMed] [Google Scholar]
- 11.Lapolla A, Dalfrà MG, Fedele D. Diabetes related autoimmunity in gestational diabetes mellitus: is it important? Nutr Metab Cardiovasc Dis 2009;19:674–682 [DOI] [PubMed] [Google Scholar]
- 12.Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet 2011;378:169–181 [DOI] [PubMed] [Google Scholar]
- 13.Retnakaran R, Qi Y, Ye C, et al. Hepatic insulin resistance is an early determinant of declining β-cell function in the first year postpartum after glucose intolerance in pregnancy. Diabetes Care 2011;34:2431–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;25:1862–1868 [DOI] [PubMed] [Google Scholar]
- 15.Norman RJ, Masters L, Milner CR, Wang JX, Davies MJ. Relative risk of conversion from normoglycaemia to impaired glucose tolerance or non-insulin dependent diabetes mellitus in polycystic ovarian syndrome. Hum Reprod 2001;16:1995–1998 [DOI] [PubMed] [Google Scholar]
- 16.Boudreaux MY, Talbott EO, Kip KE, Brooks MM, Witchel SF. Risk of T2DM and impaired fasting glucose among PCOS subjects: results of an 8-year follow-up. Curr Diab Rep 2006;6:77–83 [DOI] [PubMed] [Google Scholar]
- 17.Hauth JC, Clifton RG, Roberts JM, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network Maternal insulin resistance and preeclampsia. Am J Obstet Gynecol 2011;204:327.e1–327.e621458622 [Google Scholar]
- 18.Salley KE, Wickham EP, Cheang KI, Essah PA, Karjane NW, Nestler JE. Glucose intolerance in polycystic ovary syndrome—a position statement of the Androgen Excess Society. J Clin Endocrinol Metab 2007;92:4546–4556 [DOI] [PubMed] [Google Scholar]
- 19.Amato MC, Verghi M, Galluzzo A, Giordano C. The oligomenorrhoic phenotypes of polycystic ovary syndrome are characterized by a high visceral adiposity index: a likely condition of cardiometabolic risk. Hum Reprod 2011;26:1486–1494 [DOI] [PubMed] [Google Scholar]
- 20.Guo M, Chen ZJ, Macklon NS, et al. Cardiovascular and metabolic characteristics of infertile Chinese women with PCOS diagnosed according to the Rotterdam consensus criteria. Reprod Biomed Online 2010;21:572–580 [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Qu J, Wu X, Hou L, Erkkola R, Wang Y. Different phenotypes of polycystic ovary syndrome by Rotterdam criteria are differently steroidogenic but similarly insulin resistant. Fertil Steril 2010;93:1362–1365 [DOI] [PubMed] [Google Scholar]
- 22.Svendsen PF, Madsbad S, Nilas L. The insulin-resistant phenotype of polycystic ovary syndrome. Fertil Steril 2010;94:1052–1058 [DOI] [PubMed] [Google Scholar]
- 23.Moran LJ, Strauss BJ, Teede HJ. Diabetes risk score in the diagnostic categories of polycystic ovary syndrome. Fertil Steril 2011;95:1742–1748 [DOI] [PubMed] [Google Scholar]
- 24.Vanky E, Isaksen H, Moen MH, Carlsen SM. Breastfeeding in polycystic ovary syndrome. Acta Obstet Gynecol Scand 2008;87:531–535 [DOI] [PubMed] [Google Scholar]
- 25.Gunderson EP, Hedderson MM, Chiang V, et al. Lactation intensity and postpartum maternal glucose tolerance and insulin resistance in women with recent GDM: the SWIFT cohort. Diabetes Care 2012;35:50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Diabetes Association Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hurd WW, Abdel-Rahman MY, Ismail SA, Abdellah MA, Schmotzer CL, Sood A. Comparison of diabetes mellitus and insulin resistance screening methods for women with polycystic ovary syndrome. Fertil Steril 2011;96:1043–1047 [DOI] [PubMed] [Google Scholar]
- 28.Misra A. Prevention of type 2 diabetes: the long and winding road. Lancet 2009;374:1655–1656 [DOI] [PubMed] [Google Scholar]
- 29.Moran LJ, Hutchison SK, Norman RJ, Teede HJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev 2011;7:CD007506. [DOI] [PubMed] [Google Scholar]
- 30.Ferrara A, Hedderson MM, Albright CL, et al. A pregnancy and postpartum lifestyle intervention in women with gestational diabetes mellitus reduces diabetes risk factors: a feasibility randomized control trial. Diabetes Care 2011;34:1519–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garber AJ. Combined pharmacologic/nonpharmacologic intervention in individuals at high risk of developing type 2 diabetes: pro pharmacologic therapy. Diabetes Care 2009;32(Suppl. 2):S184–S188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palomba S, Falbo A, Zullo F, Orio F., Jr Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: a comprehensive review. Endocr Rev 2009;30:1–50 [DOI] [PubMed] [Google Scholar]
- 33.Vanky E, Stridsklev S, Heimstad R, et al. Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: a randomized, controlled multicenter study. J Clin Endocrinol Metab 2010;95:E448–E455 [DOI] [PubMed] [Google Scholar]
- 34.Glueck CJ, Goldenberg N, Sieve L, Wang P. An observational study of reduction of insulin resistance and prevention of development of type 2 diabetes mellitus in women with polycystic ovary syndrome treated with metformin and diet. Metabolism 2008;57:954–960 [DOI] [PubMed] [Google Scholar]
- 35.Glueck CJ, Pranikoff J, Aregawi D, Wang P. Prevention of gestational diabetes by metformin plus diet in patients with polycystic ovary syndrome. Fertil Steril 2008;89:625–634 [DOI] [PubMed] [Google Scholar]
- 36.Glueck CJ, Goldenberg N, Wang P, Loftspring M, Sherman A. Metformin during pregnancy reduces insulin, insulin resistance, insulin secretion, weight, testosterone and development of gestational diabetes: prospective longitudinal assessment of women with polycystic ovary syndrome from preconception throughout pregnancy. Hum Reprod 2004;19:510–521 [DOI] [PubMed] [Google Scholar]
- 37.Glueck CJ, Wang P, Kobayashi S, Phillips H, Sieve-Smith L. Metformin therapy throughout pregnancy reduces the development of gestational diabetes in women with polycystic ovary syndrome. Fertil Steril 2002;77:520–525 [DOI] [PubMed] [Google Scholar]