Abstract
OBJECTIVE
To determine exposure to hyper- and hypoglycemia using blinded continuous glucose monitoring (CGM) profiles in youth with type 1 diabetes (T1D) with residual β-cell function during the first year of insulin treatment.
RESEARCH DESIGN AND METHODS
Blinded, 3–7 day CGM profiles were obtained in 16 short-term T1D patients (age 8–18 years, T1D duration 6–52 weeks) who had peak C-peptide levels ranging from 0.46 to 1.96 nmol/L during a mixed-meal tolerance test. Results in this short-term group were compared with those in 34 patients with well-controlled, longer-term T1D (duration ≥5 years), matched for age and A1C with the short-term T1D group, and with those in 26 age-matched nondiabetic individuals.
RESULTS
Despite matching for A1C, and therefore similar mean sensor glucose levels in the two T1D groups, short-term T1D participants had a lower frequency of hypoglycemia (0.3 vs. 7.6%, P < 0.001), a trend toward less hyperglycemia (17 vs. 32%, P = 0.15), and a greater percentage in the target range (median 77 vs. 60%, P = 0.02). Indeed, the percentage of sensor glucose levels ≤70 mg/dL in the short-term T1D group (0.3%) did not differ from those in the nondiabetic group (1.7%, P = 0.73). The coefficient of variation of sensor glucose levels (an index of glucose variability) was lower in short-term vs. longer-term T1D participants (27 vs. 42%, respectively, P < 0.001).
CONCLUSIONS
In youth with short-term T1D who retain residual β-cell function, there is negligible exposure to hypoglycemia and lower glucose variability than in youth with well-controlled T1D of longer duration.
The impact of residual β-cell function on the safety and efficacy of intensive insulin treatment of type 1 diabetes (T1D) was first demonstrated in the Diabetes Control and Complications Trial (DCCT). In that study, intensively treated adolescent and adult patients with duration of diabetes between 1 and 5 years and a C-peptide response to a standard mixed-meal stimulus between 0.2 and 0.5 pmol/mL had significantly lower A1C levels, daily insulin doses, and rates of severe hypoglycemia than intensively treated participants who were C-peptide nonresponders (1). Nevertheless, the influence of residual β-cell function on exposure to biochemical hyper- and hypoglycemia early in the treatment of youth with T1D has not been established.
The Diabetes Research in Children Network (DirecNet) has undertaken a study to examine the relationship between residual β-cell function (assessed by a mixed-meal tolerance test [MMTT]) and preservation of the plasma glucagon response to hypoglycemia (assessed by the hypoglycemic clamp technique) in children and adolescents during the first 12 months of treatment of T1D. As part of the study protocol, each participant wore a blinded continuous glucose monitor (CGM) for 3–7 days before the hypoglycemic clamp. In this report, we present analyses of the blinded CGM profiles in the group of C-peptide responders in the DirecNet study. Blinded CGM results in this group of participants with short-term T1D were compared with results of blinded CGM profiles obtained by the Juvenile Diabetes Research Foundation (JDRF) CGM Randomized Clinical Trial study group. The comparison groups included age-matched nondiabetic control subjects (2) and age-matched youth with longer-term (≥5 years duration) T1D who were also matched for A1C levels to the DirecNet cohort of recent-onset individuals (3,4).
RESEARCH DESIGN AND METHODS
The DirecNet study protocol involving the short-term T1D participants is available on the DirecNet website, http://direcnet.jaeb.org/viewpage.aspx?pagename=home, and is briefly summarized here. The study was approved by the DirecNet Data and Safety Monitoring Board and the institutional review boards at each of the five DirecNet centers. A parent or guardian gave written consent, and each subject gave written assent. Major eligibility criteria included the following: 1) clinical diagnosis of T1D between 6 and 52 weeks, 2) positive antibody (GAD65 and islet cell autoantigen 512) determination, and 3) age 8 to <19 years. Participants were asked to wear a Guardian Clinical (Medtronic MiniMed, Northridge, CA) for 3–7 days prior to a hypoglycemic clamp test. The Guardian Clinical is the same as a Guardian Real-Time CGM, but it is a “blinded” device, meaning the glucose values are recorded in the receiver but the values are not visible to the patient. The C-peptide concentration during MMTT was measured at Northwest Lipid Metabolism and Diabetes Research Laboratories (Seattle, WA) using Tosoh AIA 1800 (Tosoh, Montgomeryville, PA). Among the 20 participants who completed both tests, 1 had a C-peptide response <0.2 nmol/L during MMTT and 3 did not wear the CGM device. This analysis included the other 16 participants who had a mean ± SD peak C-peptide level of 0.96 ± 0.42 nmol/L, ranging from 0.46 to 1.96 nmol/L, and a median duration of glucose data of 77 h (25th–75th percentile 62–117), ranging from 34 to 154 h. A1C was measured using the DCA Vantage analyzer (Siemens Healthcare Diagnostics).
These 16 short-term T1D participants were matched for age and A1C to 34 participants with ≥5 years T1D duration (“longer-term”) from the JDRF CGM Randomized Clinical Trial cohort (3,4). CGM data were taken from an approximately 7-day period of blinded sensor use at baseline prior to randomization. The median duration of glucose data was 150 h (25th–75th percentile 131–181), ranging from 97 to 241 h in the 26 (76%) participants using the Guardian Clinical device and 8 (24%) using the FreeStyle Navigator (Abbott Diabetes Care, Alameda, CA). A1C was measured using the DCA 2000 analyzer (Bayer, Tarrytown, NY) in 32 (94%) participants and Tosoh G7 (Tosoh) in 2 (6%) participants. The correlation between the DCA 2000 assay and the Tosoh G7 assay is r = 0.94 (5).
In addition, the 16 short-term T1D participants were matched for age to 26 nondiabetic participants who were diabetes-antibody negative from a separate JDRF-funded study (2), including 12 (46%) participants who used the Guardian Clinical device for 3 days and 14 (54%) who used a blinded Navigator for 5 days. The median duration of the glucose data was 71 h (25th–75th percentile 67–103), ranging from 51 to 141 h. A previous study showed that results were similar comparing the Navigator and Guardian Clinical CGM devices (mean glucose 98 ± 11 and 98 ± 9 mg/dL, respectively (2). A1C was measured using the DCA 2000 for 23 (88%) participants and Tosoh G7 for 3 (12%) participants.
CGM glucose indices were calculated giving equal weight to each of the 24 h of a calendar day. As the long-duration group had a greater amount of data than the short-term T1D and nondiabetic groups, group comparisons were made using both 3 and 6 days of data. Since the results were similar regardless of the length of data used (Supplementary Table 1), the data from ∼6 days of sensor wear are presented here. Coefficient of variation (CV) as a measure of glucose variability was defined as SD divided by mean glucose.
Means ± SD or median (25th–75th percentiles) was summarized as appropriate to the distribution for demographic and clinical characteristics and various measures of glucose separately for each of three groups. The comparisons between short-term T1D participants and nondiabetic participants were performed using regression models based on van der Waerden rank normal scores adjusted for age, sex, and race/ethnicity (white vs. nonwhite). Comparisons of the two T1D groups were also adjusted for A1C level. In addition, comparisons of SD between groups were adjusted for mean glucose because of the high correlation between SD and the mean glucose. The models were not adjusted for CGM device type because the short-term T1D group only used the Guardian Clinical. Previous studies have shown that the accuracy of the Real-Time system and FreeStyle Navigator are similar (6,7). Moreover, the results were similar when the analysis was restricted to the Guardian Clinical data (data not shown). Analyses were performed with SAS version 9.2 (SAS Institute, Cary, NC). Only P values <0.01 were considered statistically significant because multiple statistical comparisons.
RESULTS
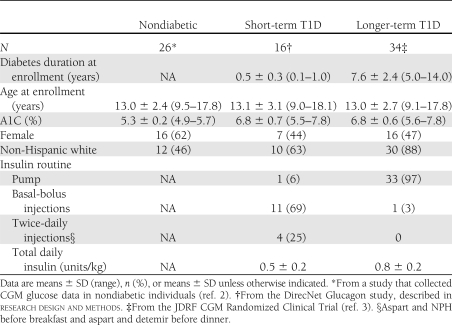
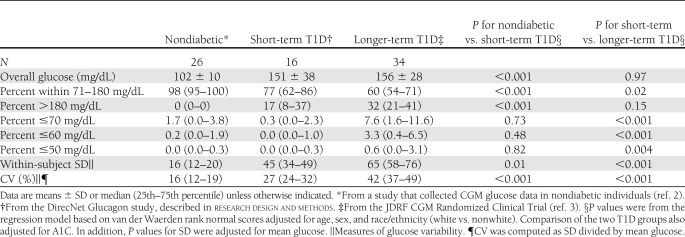
Table 1 provides the characteristics of the 16 short-term T1D participants, the 34 longer-term T1D participants, and the 26 nondiabetic participants. As shown in Table 2, the short-term T1D participants had a mean glucose level similar to that of the longer-term T1D participants (151 ± 38 vs. 156 ± 28 mg/dL, respectively, P = 0.97), but they had a significantly lower percentage of glucose values <70 mg/dL (0.3 vs. 7.6%, P < 0.001). Similar results were observed for the percentage of glucose <60 and 50 mg/dL. There were trends toward greater percentage of glucose values in the 71–180 mg/dL target range (median 77 vs. 60%, P = 0.02) and fewer glucose values >180 mg/dL in the short-term T1D participants (17 vs. 32%, P = 0.15). Glucose variability measured by CV was significantly lower in the short-term T1D group (median 27 vs. 42%, P < 0.001).
Table 1.
Clinical characteristics
Table 2.
Summary of glycemic indices
Compared with the nondiabetic participants, the short-term T1D participants had a higher mean glucose (151 ± 38 vs. 102 ± 10 mg/dL, P < 0.001), fewer values in the target range (median 77 vs. 98%, P < 0.001), higher percentage in the hyperglycemic range (17 vs. 0%, P < 0.001), and increased glucose variability (CV 27 vs. 16%, P < 0.001). Overall, there were few sensor glucose values in the hypoglycemic range in both the short-term diabetic and nondiabetic participants (Table 2).
CONCLUSIONS
The availability of blinded CGM profiles in nondiabetic children and adolescents and in youth with longer-term diabetes who were successfully achieving target A1C goals with intensive treatment provided us with a unique opportunity to examine how glucose profiles in these two groups of participants compared with those in youth with well-controlled short-term T1D who retained residual insulin secretion. Since both groups of T1D participants were matched for A1C levels, it was not surprising that overall mean CGM glucose values were also similar. Nevertheless, youth with short-term T1D had a lower frequency of hypoglycemia and a trend toward less hyperglycemia than youth with longer-term T1D. In addition, glucose variability as assessed by the CV of sensor glucose levels was significantly reduced in the short-term versus longer-term T1D participants; short-term patients also had reduced daily insulin requirements (Table 1). In the DCCT, subjects with residual β-cell function had lower total daily insulin doses than subjects who were C-peptide nonresponders (1).
As expected, A1C levels, mean sensor glucose values, and glucose variability in the short-term T1D participants were higher than in nondiabetic youth of similar age. In addition to insulin deficiency, recent studies indicate that patients with short-term T1D have inappropriate increases in plasma glucagon levels during mixed-meal feedings, which may also contribute to postprandial hyperglycemia (8). On the other hand, there was no significant difference between the nondiabetic and the short-term T1D groups in the frequency of sensor values in the hypoglycemic range, which is particularly striking. In contrast, participants in the longer-term T1D group achieved A1C levels near or within the <7.5% pediatric target range (9) at the expense of a sharp increase in exposure to biochemical hypoglycemia. Using a one-step hyperinsulinemic, hypoglycemic clamp, we have demonstrated that 56% of the subjects in the short-term group maintained a glucagon response to hypoglycemia, which may have contributed to their low incidence of biochemical hypoglycemia (10). Since recurrent episodes of mild biochemical hypoglycemia have been implicated in the development of hypoglycemia-associated autonomic failure (11) and hypoglycemia unawareness, the absence of such exposure in our short-term participants may also help explain why the risk of severe hypoglycemic events was reduced in C-peptide responders vs. C-peptide nonresponders in the DCCT (1).
Interpretation of the findings of this study may be somewhat limited because the C-peptide response status of participants in the longer-term T1D group was not determined. Nevertheless, as the minimum diabetes duration in the longer-term T1D group was 5 years, we would expect the C-peptide response and insulin secretion to be minimal. In addition, if some of the longer-term T1D patients did retain C-peptide responses, it would have likely decreased our ability to demonstrate the differences in CGM profiles that were found between the two groups of T1D participants. The vast majority of long-term T1D subjects were using continuous subcutaneous insulin infusion compared with only 6% of the short-term subjects, 25% of whom were receiving only twice-daily insulin. Since continuous subcutaneous insulin infusion has been associated with a decreased frequency of hypoglycemia (12–14), the high frequency of pump use in the long-term subjects would also have been expected to limit our ability to demonstrate a difference with respect to exposure to hypoglycemia. It should also be noted that the short-term T1D group only used the Medtronic Guardian system for CGM monitoring, whereas both the Guardian and Abbott Navigator systems were used in the other two groups. However, previous studies from the JDRF CGM study group have demonstrated that glucose profiles and hypoglycemia detection using these two systems are very similar (2).
Our data demonstrate that during the honeymoon phase of T1D in youth, when residual β-cell function is retained, A1C levels can be lowered to <7.0% with negligible exposure to hypoglycemia and considerably lower glucose variability than is seen in youth with equally well-controlled T1D of ≥5 years’ duration. Immunologic and other approaches directed at preserving β-cell function in patients with newly diagnosed T1D are areas of intense study (15). Should such approaches be successful, our findings suggest that the therapeutic benefits of β-cell preservation may include considerably lower glucose variability and exposure to the risks of hypoglycemia, clinical outcomes that may be as important as A1C and total daily insulin dose metrics that are currently used to measure the success of such interventions.
Acknowledgments
This research was supported by the following National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development grants: HD41890-10, HD41906-10, HD41908-10, HD41915, HD41918, UL1RR024992, U10HD041906, UL1RR024139, UL1RR024979, and UL1RR025744.
Medtronic MiniMed (Northridge, CA) provided MiniLink transmitters at no cost and sensors at a discounted price. B.B. reports serving on the medical advisory boards for Medtronic MiniMed, UnoMedical, and Animas. C.K. has served as a paid consultant to Diabetes Technology Management, which was hired by Medtronic MiniMed to form a Veo advisory board to formulate a consensus statement on the design and analysis of a trial to evaluate the Veo LGS system. No other potential conflicts of interest relevant to this article were reported.
J.S. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. W.V.T. researched data, contributed to discussion, and reviewed and edited the manuscript. D.X. researched data and wrote the manuscript. E.T., N.M., B.B., N.H.W., and A.M.A. researched data, contributed to discussion, and reviewed and edited the manuscript. R.W.B. contributed to discussion and reviewed and edited the manuscript. C.K. and K.R. researched data, contributed to discussion, and reviewed and edited the manuscript. R.W.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
Appendix
The DirecNet Study Group: Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, Iowa: E.T. (principal investigator [PI]); Michael J. Tansey, MD (coinvestigator [I]); Julie Coffey, MSN (coordinator [C]); and Joanne Cabbage (C).
Nemours Children’s Clinic, Jacksonville, Florida: N.M. (PI); Larry A. Fox, MD (I); Kim Englert, RN (C); and Joe Permuy, ARNP (C).
Division of Pediatric Endocrinology and Diabetes, Stanford University, Stanford, California: B.B. (PI); Darrell M. Wilson, MD (I); Paula Clinton, RD, CDE (C); and Kimberly Caswell, APRN (C).
Department of Pediatrics, Yale University School of Medicine, New Haven, Connecticut: Stuart A. Weinzimer, MD (PI); W.V.T. (I); J.S. (I); Amy Steffen, BS (C); Kate Weyman, MSN (C); Melinda Zgorski, BSN (C); and Eileen Tichy, MMS (C).
Washington University in St. Louis, St. Louis, Missouri: N.H.W. (PI); A.M.A. (I); Lucy Levandoski, PA-C (C); and Angie Starnes, RN, BSN, CDE (C).
Coordinating center: Jaeb Center for Health Research, Tampa, Florida:
R.W.B., K.R., C.K., D.X., and Beth Stevens.
National Institutes of Health, Bethesda, Maryland: Gilman D. Grave, MD; Karen K. Winer, MD; and Ellen Leschek, MD.
Data and Safety Monitoring Board: Mark Sperling, MD; Dorothy M. Becker, MBBCh; Patricia Cleary, MS; Carla Greenbaum, MD; and Antoinette Moran, MD.
University of Minnesota Central Laboratory: Michael W. Steffes, MD, PhD; Jean M. Bucksa, CLS; Maren L. Nowicki, CLS; and Vicky Makky, CLS.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-2190/-/DC1.
*A complete list of the members of the DirecNet Study Group can be found in the appendix.
References
- 1.The Diabetes Control and Complications Trial Research Group Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. Ann Intern Med 1998;128:517–523 [DOI] [PubMed] [Google Scholar]
- 2.Fox LA, Beck RW, Xing D; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Variation of interstitial glucose measurements assessed by continuous glucose monitors in healthy, nondiabetic individuals. Diabetes Care 2010;33:1297–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamborlane WV, Beck RW, Bode BW, et al. ; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 4.Beck RW, Hirsch IB, Laffel L, et al. ; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care 2009;32:1378–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamborlane WV, Kollman C, Steffes MW, et al. ; Diabetes Research in Children Network (DirecNet) Study Group Comparison of fingerstick hemoglobin A1C levels assayed by DCA 2000 with the DCCT/EDIC central laboratory assay: results of a Diabetes Research in Children Network (DirecNet) Study. Pediatr Diabetes 2005;6:13–16 [DOI] [PubMed] [Google Scholar]
- 6.Diabetes Research in Children Network (DirecNet) Study Group The accuracy of the Guardian RT continuous glucose monitor in children with type 1 diabetes. Diabetes Technol Ther 2008;10:266–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson DM, Beck RW, Tamborlane WV, et al. ; DirecNet Study Group The accuracy of the FreeStyle Navigator continuous glucose monitoring system in children with type 1 diabetes. Diabetes Care 2007;30:59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown RJ, Sinaii N, Rother KI. Too much glucagon, too little insulin: time course of pancreatic islet dysfunction in new-onset type 1 diabetes. Diabetes Care 2008;31:1403–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanas RJ, John G; International HbA Consensus Committee 2010 consensus statement on the worldwide standardization of the hemoglobin A1C measurement. Pediatr Diabetes 2010;11:209–211 [DOI] [PubMed] [Google Scholar]
- 10.Sherr J, Tamborlane WV, Tsalikian E, et al. Lack of association between residual insulin production and glucagon response to hypoglycemia in youth with short duration of type 1 diabetes (Abstract). Diabetes 2011;60(Suppl. 1):A136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cryer PE, Gerich JE. Glucose counterregulation, hypoglycemia, and intensive insulin therapy in diabetes mellitus. N Engl J Med 1985;313:232–241 [DOI] [PubMed] [Google Scholar]
- 12.Boland EA, Grey M, Oesterle A, Fredrickson L, Tamborlane WV. Continuous subcutaneous insulin infusion. A new way to lower risk of severe hypoglycemia, improve metabolic control, and enhance coping in adolescents with type 1 diabetes. Diabetes Care 1999;22:1779–1784 [DOI] [PubMed] [Google Scholar]
- 13.Weinzimer SA, Ahern JH, Doyle EA, et al. Persistence of benefits of continuous subcutaneous insulin infusion in very young children with type 1 diabetes: a follow-up report. Pediatrics 2004;114:1601–1605 [DOI] [PubMed] [Google Scholar]
- 14.Scrimgeour L, Cobry E, McFann K, et al. Improved glycemic control after long-term insulin pump use in pediatric patients with type 1 diabetes. Diabetes Technol Ther 2007;9:421–428 [DOI] [PubMed] [Google Scholar]
- 15.Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002;346:1692–1698 [DOI] [PubMed] [Google Scholar]