Abstract
OBJECTIVE
—Ecological data suggest an inverse correlation between fish consumption and diabetes prevalence. However, epidemiological data on fish intake and diabetes incidence are controversial and inconclusive. Therefore, we aimed to assess the literature and determine the association between fish consumption and diabetes risk quantitatively.
RESEARCH DESIGN AND METHODS
—Prospective cohort studies published through August 2011 in peer-reviewed journals indexed in PubMed were selected. Additional information was retrieved through Google or a hand search of the references from relevant articles. The weighted relative risk (RR) and corresponding 95% CI for incident diabetes was estimated using random-effects models.
RESULTS
—A database was derived from nine eligible studies (12 independent cohorts), including 438,214 individuals with an average 11.4-year follow-up. Compared with those who never consumed fish or ate fish less than once per month, the pooled RR of incident diabetes was 0.99 (95% CI 0.85–1.16) for individuals who ate fish five or more times per week (P trend = 0.80). Similar results were found for long-chain n-3 polyunsaturated fatty acid intake. Study location was an effect modifier. An inverse association between fish intake and diabetes incidence was found by combining studies conducted in Eastern but not Western countries.
CONCLUSIONS
—Accumulated evidence generated from this meta-analysis does not support an overall inverse association of fish or fish oil intake with incidence of diabetes. The null association was modified by study location (Eastern vs. Western countries), which may reflect the possible difference between Eastern and Western dietary patterns. Further studies are warranted.
Although cardioprotective effects of fish or long-chain n-3 polyunsaturated fatty acid (LCn3PUFA) intake have been documented (1), the role of these dietary factors in the development of diabetes remains uncertain. Since ecological data indicate low prevalence of diabetes in populations with a large amount of fish consumption (2) and laboratory studies suggest that insulin sensitivity is directly associated with the content of LCn3PUFA in cell membranes (3), it has been hypothesized that fish or LCn3PUFA intake may provide beneficial effects on diabetes risk. In the past decade, a number of longitudinal studies have examined the association between fish intake and incidence of diabetes, but the findings are conflicting. Although some studies reported a significant inverse association (4–6), other cohort studies (7–9) found a positive relation between fish consumption and incidence of diabetes after adjustment for various potential confounders. To provide a reliable quantitative assessment of the association of fish and LCn3PUFA intake with diabetes risk and to explore major sources of heterogeneity among studies, we conducted a meta-analysis of all eligible prospective cohort studies with the existing data.
RESEARCH DESIGN AND METHODS
We undertook this meta-analysis according to MOOSE (Meta-analysis of Observational Studies in Epidemiology) guidelines during all stages of design, implementation, and reporting (10).
Study selection
All prospective cohort studies published in English-language journals from 1966 to August 2011 that reported the association between fish consumption and incidence of diabetes were identified by searching PubMed using the terms “fish,” “seafood,” “animal products,” “omega-3 fatty acids,” “n-3 fatty acids,” “diabetes,” “glucose tolerance,” and “insulin resistance.” Additional information was retrieved through Google and a hand search of the references from relevant articles. Both authors independently reviewed all relevant papers and identified eligible studies. Discrepancies were resolved by discussion. An article would be included if the study was a prospective cohort design and the relative risks (RRs) and their corresponding 95% CIs of diabetes relating to fish and/or LCn3PUFA intake were presented or such information could be recalculated.
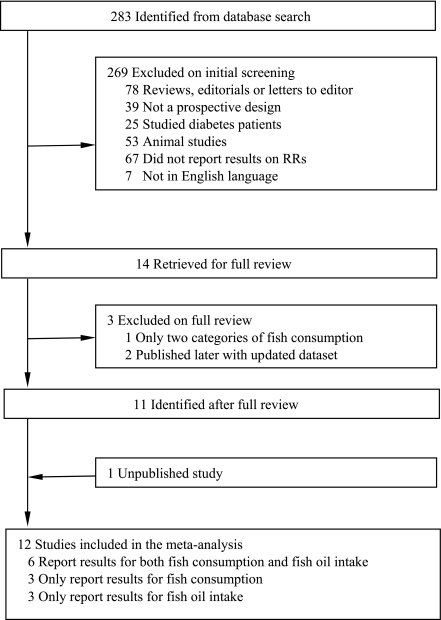
Of 283 identified abstracts from the PubMed, we excluded 269 by screening because they were reviews, editorials, or letters to the editor; were not prospective cohort studies; studied diabetes patients; were animal studies; did not report RR of fish or fish oil and incident diabetes; or were not in English (Fig. 1). Two investigators independently examined the full text of the remaining 14 studies (4–9,11–18) to confirm eligibility for inclusion. Of the 14 studies, 1 study (11) was excluded because it only categorized fish consumption to two groups (yes vs. no). Two studies (14,15) were excluded because more detailed results were published (7) later with the same dataset. In addition, 1 study (7) reports findings from 3 independent cohorts and another study (5) presents results from 2 independent cohorts so that these 2 studies were counted as 5 separate cohorts in the meta-analysis. Moreover, we generated unpublished de novo results for this meta-analysis by analyzing data from the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Thus, 12 studies (15 independent cohorts) (4–9,12,13,16–18) were included for the meta-analysis. Of them, 9 studies (12 cohorts) (4–9,12,13) reported results on fish consumption and 9 studies (11 cohorts) (5,7–9,13,16–18) reported results on LCn3PUFA intake.
Figure 1.
Process of study selection.
Quality assessment and data extraction
To evaluate the quality of the studies included, we designed a scoring system with several a priori selected important characteristics that may affect study quality based on literature (19). The system was created to account for study eligibility (1 point if appropriate inclusion and exclusion criteria was clearly stated), exposure (1 point if fish intake was assessed by a validated tool; and 1 point if fish intake was standardized [1]), outcome (1 point if diabetes cases were diagnosed at clinical examination or self-reported cases verified by clinicians on the basis of established clinical criteria [20]), and statistical analysis (1 point if adjustment included age, BMI, energy intake, and family history of diabetes). The scoring system was designed with reference to MOOSE (10) and allowed a total score from 0 to 5 points, with 5 reflecting the highest quality. Two authors independently assessed the quality for all identified studies.
The data included the first author’s name, year of publication, study population, country of origin, sample size, proportion of men, duration of follow-up, range or mean of participants’ age, number of events, case identification methods, categories of fish or LCn3PUFA intake, the amount of fish or LCn3PUFA intake for each category, methods for diet measurement, adjusted covariates, as well as RRs and 95% CIs of incident diabetes in the corresponding categories. RRs transformed to their natural logarithms (ln) and the 95% CIs were used to compute the corresponding SEs.
To standardize fish intake, we first converted frequency into grams per day. The amount of fish consumption (g/day) was estimated by multiplying the frequency of consumption (serving/day) by the corresponding portion size (g/serving). For example, the derived average portion size in Kaushik et al. (7) was 105 g/serving. The range of fish consumption for one to three times per month (shown as, for example, 1–3/month hereafter) was converted to 3.5 g/day (105 × 1/30) to 10.5 g/day (105 × 3/30). The median or mean value between two adjacent categories was used as the cut-off point. When the portion size of fish intake in an individual study was not available from the published paper, the information was acquired from authors of the primary study or the value was determined based on data from Kaushik et al. because this study includes three large cohort studies (the Nurses’ Health Study [NHS], the Nurses’ Health Study 2 [NHS2] and the Health Professionals Follow-up Study [HPFS]) and taking into consideration that the food frequency questionnaire (FFQ) used in these three studies has been validated (21). If the upper-bound of the highest fish intake category was uncertain, for instance, fish intake ≥5/week, we assigned 1 serving/day of fish as the upper limit. If the information from the primary study was insufficient for standardizing fish consumption, especially if the reference level of fish intake was substantially higher than the standardized level, de novo results based on the standardized fish consumption cut-off points were requested from authors of the primary study (4–6).
Statistical analysis
Fish consumption was standardized into five categories: never or <1/month, 1–3/month, 1/week, 2–4/week, and ≥5/week. We created a dataset by assigning each RR extracted from each primary study into its corresponding standardized category according to the range or median amount of fish intake in the category. If the median amount of fish consumption from more than one group in a single study fell into the same standardized category of fish intake in our meta-analysis, we pooled these RRs and used the combined estimate for that category. Also, if the range of fish intake covered more than one standardized category, we allocated RR based on the median fish intake. We estimated the pooled RRs and 95% CIs of diabetes for each standardized category of fish consumption compared with the lowest category using random-effects models. In addition, the Cochran test was used to test for heterogeneity among studies, and I 2 was computed to determine the degree of inconsistency across studies. A variance-weighted least squares regression was used to model the ln RR for diabetes as a linear function of fish intake. The median intake of fish for each category was derived. Publication bias was assessed by visualizing the funnel plot and determined by the Egger asymmetry test (22).
We further carried out subgroup analysis to explore potential sources of heterogeneity based on sex, study location (Eastern vs. Western countries), duration of follow-up (<11.4 vs. ≥11.4 years), and outcome identification method (clinical examination vs. self-report) by using random-effects models.
Two sensitivity analyses were conducted to test the robustness of our findings: 1) we evaluated whether the results could have been affected markedly by a single study; and 2) we restricted our meta-analysis to studies that specified type 2 diabetes in the outcome.
P ≤ 0.05 was considered statistically significant for all tests. All analyses were performed using STATA statistical software (Version 11.0. STATA Corp., College Station, TX).
RESULTS
Characteristics of included studies
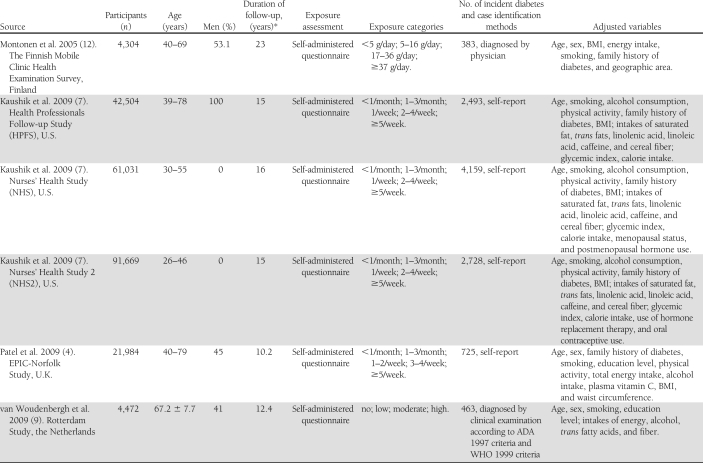
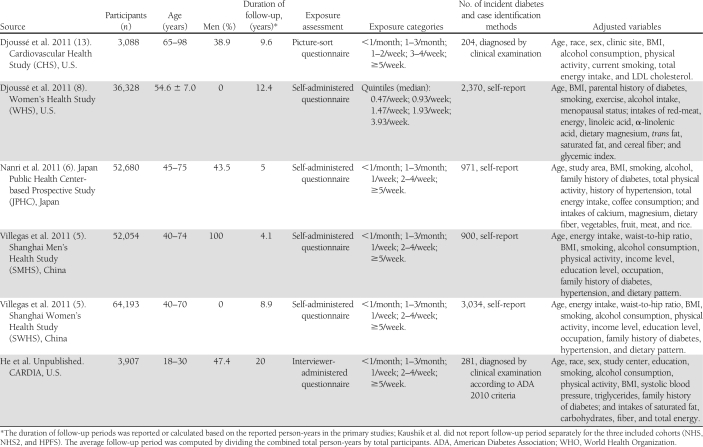
The final dataset for our meta-analysis of fish consumption and incident diabetes included 12 independent cohorts from nine studies comprising 438,214 individuals (18,711 incident diabetes cases) aged between 18 and 98 years. Of the 12 cohorts, 9 were from Western countries and 3 from Asia. The sample sizes varied across studies from 3,088 (the Cardiovascular Health Study) (13) to 91,669 (NHS2) (7). The average duration of follow-up was 11.4 years (range 4–23 years), calculated based on the person-times of primary studies. Diet including fish consumption was collected by either self-administered or interview-based FFQ. Fish intake in primary studies was classified into four to five categories. Incident diabetes cases were self-reported (8 cohorts) or diagnosed at clinical examinations (5 cohorts). Two studies did not specify type of diabetes in the outcome. The quality scores for all included studies are ≥3 points based on our 5-point scoring system. All reported RRs (95% CI) of diabetes in each study were adjusted for multiple covariates (Table 1).
Table 1.
Characteristics of 12 included cohorts (from nine studies) on fish consumption and incidence of diabetes
Association of fish consumption and LCn3PUFA intake with incidence of diabetes
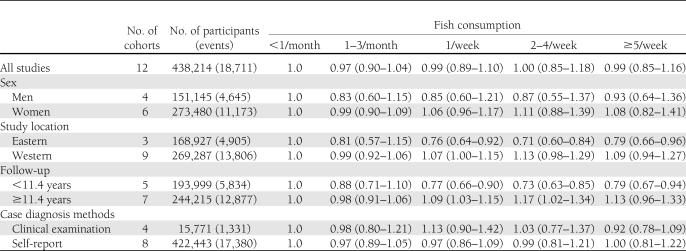
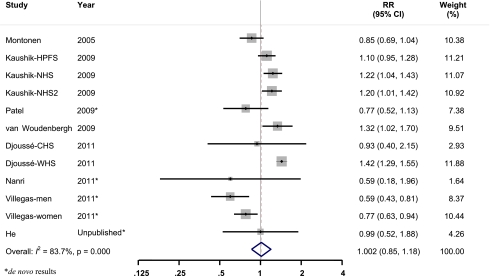
After pooling data from the identified studies, neither fish consumption nor LCn3PUFA intake was significantly associated with incidence of diabetes. Compared with those who never consumed fish or ate fish less than once per month, the pooled RR of incident diabetes was 0.99 (95% CI 0.85–1.16) for 5 or more times per week (P trend = 0.80) (Table 2). We tested the heterogeneity among the included cohorts by calculating I 2 for each categories of fish intake with the lowest group as the reference. The range of I 2 values was from 4.5 to 83.7% for fish consumption. As shown in Fig. 2, there was significant heterogeneity between studies (I 2 = 83.7%, P < 0.01) when comparing participants who consumed fish 2–4/week with those in the reference group. However, a sensitivity analysis revealed that none of the studies significantly influenced the pooled estimate, with the pooled RR ranging from 0.96 (0.82–1.12) after excluding Djoussé et al. (8) to 1.06 (0.91–1.23) after excluding the study on men by Villegas et al. (5).
Table 2.
Pooled RRs and 95% CI of incident diabetes according to fish consumption
Figure 2.
Multivariable adjusted RR and 95% CI of incident diabetes. The pooled estimates were obtained using a random-effects model. The dots indicate the adjusted RRs by comparing fish consumption 2–4 times per week to never or less than once per month. The size of the shaded square is proportional to the percent weight of each study. The horizontal lines represent 95% CIs. The diamond data markers indicate the pooled RRs. (A high-quality color representation of this figure is available in the online issue.)
Similar results were found for LCn3PUFA intake. The pooled RR (95% CI) of incident diabetes across quintiles of LCn3PUFA intake were 1.00, 1.00 (0.96–1.05), 1.00 (0.93–1.08), 1.04 (0.93–1.17), and 1.04 (0.92–1.18; P trend=0.38).
Stratified analysis
We conducted stratified analyses based on the available data (Table 2). The observed null associations were modified by study location (Eastern vs. Western countries) and follow-up period (<11.4 vs. ≥ 11.4 years). An inverse association between fish consumption and diabetes risk was documented by combining studies conducted in Asia and studies with a follow-up period <11.4 years. However, the associations of fish consumption with risk of diabetes were not appreciably modified by sex and outcome identification methods (clinical examination vs. self-report). In addition, two studies did not specify type of diabetes in the outcome. Nevertheless, the pooled results were consistent when excluding these two studies (data not shown).
Publication bias
Publication bias was assessed by Egger test, and no evidence was found (P = 0.14).
CONCLUSIONS
In this quantitative meta-analysis of prospective cohort studies, we did not find an overall pooled association of fish consumption or LCn3PUFA intake with diabetes incidence. However, evidence from studies conducted in Asia suggests an inverse association between fish consumption and diabetes risk. The follow-up period appeared to also modify the association, which may reflect the fact that all studies with a follow-up period longer than the average follow-up were conducted in Western countries and most studies with a follow-up period shorter than the average were conducted in Eastern countries. No other effect modifiers were identified.
This meta-analysis comprises more than 435,000 male and female adults with a wide age range. Most cohorts or studies included in this meta-analysis had a large sample size and long-term follow-up periods that increased the statistical power to examine the overall associations of fish intake and incidence of diabetes. The prospective cohort study design also reduced the likelihood of selection bias and recall bias. Although randomized placebo controlled trials are the best approach to establish causal inference, no randomized data on fish consumption and diabetes incidence are available yet, presumably, in part because of the feasibility considerations such as participant’s long-term adherence. Thus, evidence generated from meta-analysis of cohort studies is very valuable, especially for reliably estimating the long-term association between fish consumption and diabetes risk. Another major strength to be highlighted is that we standardized fish consumption in the meta-analysis. Specifically, for those primary studies (4–6) with insufficient information for fish consumption standardization, de novo results were obtained from the investigators of the primary studies, which substantially reduced the misclassification of fish intake.
Because this meta-analysis is based on observational studies, the inherent limitations of primary studies may have affected our findings. For example, the individual RR estimate included in the meta-analysis was adjusted for different covariates in the different studies. Thus, the possibility of residual confounding due to inclusion of different factors or bias due to measurement errors or unmeasured factors cannot be ruled out. Alternatively, a meta-analysis based on primary data with subsequent adjustment for identified covariates may provide more solid evidence. In addition, differences in method of case identification (i.e., clinical examination vs. self-report) might lead to difficulties in estimating true effects of fish intake on diabetes risk. For instance, individuals with high fish intake are generally more health conscious, and therefore they may be less likely to have undiagnosed cases of diabetes. By contrast, those in the low fish intake groups may be more likely to have undiagnosed diabetes, thus underestimation of diabetes cases could occur particularly in the studies with self-reported cases. This limitation may bias the results toward the null. Nevertheless, our stratified analyses did not support the presence of substantial effect modifications by case identification methods.
In this meta-analysis, we found that study location modified the association between fish consumption and diabetes risk. A combined inverse association was observed in 3 studies conducted in Eastern countries (Japan and China). The reasons for this modification are unclear. Presumably, fish consumption may be a marker of healthy dietary pattern in the observational studies. Thus, the effect modification of study location we observed may reflect the differences in dietary pattern in relation to diabetes risk between Eastern and Western population. For example, fast food and sweetened beverages—possible risk factors of diabetes—are more popular in Western countries (23). Also, food preparation methods may partially explain this effect modification. Raw, boiled, and steamed fish are major preparation methods in Eastern cuisine. In contrast, fried fish may be more popular in Western food. Studies suggest that frying may modify the lipid profile through a decrease in LCn3PUFA content (24). Deep frying (e.g., fried fast food) may also cause the presence of trans-fatty acids and lipid oxidation products that may consequently increase the risk of diabetes (18) by promoting systemic inflammation (25) and oxidant stress (26). In addition, we noted that the amounts of fish consumption in studies conducted in Asia were much higher than the amounts of fish intake in Western countries. The possibility cannot be completely ruled out that the potential benefit of fish consumption on diabetes development would only be seen at a high LCn3PUFA levels. In other words, a relatively low LCn3PUFA intake may not be able to outweigh the risk of contaminants in fish. Further studies are clearly warranted.
In addition, we observed effect modification by study follow-up period in this meta-analysis. Of note, this modification may simply be a surrogate of effect modification of study location because all studies with a follow-up period (mean ∼16 years) above the average study duration (∼11 years) were conducted in Western countries, whereas most studies with a follow-up period (mean ∼8 years) below the average study duration were conducted in Asia. Another possible explanation of this effect modification may be that the incident diabetes cases were delayed rather than completely prevented by fish consumption so that an inverse association was more likely to be observed in studies with a relatively short follow-up period.
Our results are robust in a couple of sensitivity analyses. First, none of the primary studies significantly influenced the pooled estimate when we excluded one study at a time in the meta-analysis. Second, the results remained when we excluded two studies without specified type 2 diabetes.
The possibility of publication bias is always a concern in a meta-analysis. By visualizing the funnel plot and conducting statistical tests, we found no strong evidence of publication bias in our results. Nevertheless, a potential bias resulting from excluding studies published in other languages (if any) is possible.
Because the prevalence of diabetes is very rare in populations with high fish consumption (27) and animal studies indicate a beneficial effect of fish oil intake on glucose metabolism (28), it was hypothesized that dietary enrichment with LCn3PUFAs may reduce risk of diabetes (2). However, the present meta-analysis does not generate strong evidence supporting this hypothesis. In addition to the heterogeneity of individual studies, some other important issues that may explain the overall null association between fish intake and diabetes incidence merit consideration. Although LCn3PUFAs are the key nutrients in fish responsible for the potential cardioprotective effect of fish consumption, fish is not only LCn3PUFAs but a package of nutrients and contaminants (29). Notably, fish is a major source of exposure to methylmercury and polychlorinated biphenyls (PCBs) (30,31). Studies suggest that PCBs may be a risk factor of type 2 diabetes (32). Also, laboratory studies indicate that even low dose mercury-induced oxidative stress and phosphoinositide 3-kinase activation can cause pancreatic β-cell dysfunction and increase risk of diabetes (33). Hence, assuming LCn3PUFAs are beneficial to risk of diabetes development, contaminants in fish—particularly PCBs and methylmercury—may substantially attenuate or even cancel the benefit of LCn3PUFAs. In addition, cooking methods may alter the nutrient content in fish and generate unexpected chemicals (24), which may confound the effects of fish intake. In this meta-analysis, we also combined studies that reported results on LCn3PUFA intake and incidence of diabetes. We found no association between LCn3PUFAs and diabetes, which was generally in concordance with what we observed in fish. In fact, consistent associations of fish and LCn3PUFA intake with diabetes were found in all studies with data available on both fish and LCn3PUFAs, which may reflect the fact that LCn3PUFA intake assessed by diet measurement instrument is more likely to be a surrogate marker of fish consumption because total LCn3PUFA intake is derived from the nutrient table based on the frequency of fish intake. The effects of dietary LCn3PUFA intake cannot be isolated from fish intake. Data on objective biomarkers of LCn3PUFAs and diabetes are sparse. Three studies found no significant correlations between serum or plasma LCn3PUFAs and diabetes (34–36), and one study reported a borderline significant inverse association (13). Because the amount of endogenous LCn3PUFA is small (37), the biomarker levels of LCn3PUFAs should be parallel with the total amount of fish consumption as well as the content of other constituents in fish. Thus, findings from studies using the objective biomarkers of LCn3PUFA are still subject to potential confounding from other components in fish such as methylmercury and PCBs.
In future studies, randomized placebo-controlled trials on fish oil supplements and glucose metabolism or diabetes risk are needed to elucidate the potential mechanism of action and to help us better understand the key nutrients—LCn3PUFAs—in fish in relation to incidence of diabetes. In cohort studies, investigators should pay attention to collecting information on types of fish, cooking methods, and other important constituents in fish. Besides methylmercury and PCBs, fish is rich in selenium and vitamin D (38). Recent studies suggest that high levels of selenium may be associated with elevated risk of diabetes (39). Also, studies indicate that vitamin D may be a protective factor of diabetes development (40). Unless we clearly understand these both the nutritious and the possible harmful factors as well as their interactions in relation to diabetes risk, it may be too early for us to make any definitive recommendation on fish consumption with respect to primary prevention of diabetes.
In summary, this meta-analysis of prospective cohort studies does not find an overall association between fish consumption and diabetes risk. However, results from this meta-analysis suggest that fish consumption may be beneficial for diabetes development among Asians who had a relatively large amount of fish intake and different dietary pattern compared with Western populations. Until more studies are available, findings from this meta-analysis should not alter current advisories on fish intake or diminish the fact that fish is overall a healthy food, as a large body of evidence supports the cardioprotective effects of fish consumption.
Acknowledgments
P.X. and K.H. were partially supported by a grant (R21DK073812) from the National Institutes of Health.
No potential conflicts of interest relevant to this article were reported.
P.X. analyzed data, drafted the manuscript, and contributed to the critical revision of the manuscript. K.H. conceived the study concept and design, drafted the manuscript, and contributed to the critical revision of the manuscript. K.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Drs. Nita Forouhi and Pinal Patel (Medical Research Council Epidemiology Unit, Institute of Metabolic Science, Cambridge, U.K.) and the investigators and participants of the European Prospective Investigation of Cancer (EPIC)-Norfolk Study; Dr. Akiko Nanri (Department of Epidemiology and Prevention, International Clinical Research Center, National Center for Global Health and Medicine, Tokyo, Japan) and the investigators and participants of the Japan Public Health Center–based Prospective (JPHC) Study; and Dr. Raquel Villegas (Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee) and the investigators and participants of the Shanghai Women’s Health Study (SWHS) and Shanghai Men’s Health Study (SMHS) for kindly providing de novo results for this meta-analysis.
Footnotes
References
- 1. He K, Song Y, Daviglus ML, et al. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation 2004;109:2705–2711 [DOI] [PubMed] [Google Scholar]
- 2. Lardinois CK. The role of omega 3 fatty acids on insulin secretion and insulin sensitivity. Med Hypotheses 1987;24:243–248 [DOI] [PubMed] [Google Scholar]
- 3. Borkman M, Storlien LH, Pan DA, Jenkins AB, Chisholm DJ, Campbell LV. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med 1993;328:238–244 [DOI] [PubMed] [Google Scholar]
- 4. Patel PS, Sharp SJ, Luben RN, et al. Association between type of dietary fish and seafood intake and the risk of incident type 2 diabetes: the European Prospective Investigation of Cancer (EPIC)-Norfolk cohort study. Diabetes Care 2009;32:1857–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villegas R, Xiang YB, Elasy T, et al. Fish, shellfish, and long-chain n-3 fatty acid consumption and risk of incident type 2 diabetes in middle-aged Chinese men and women. Am J Clin Nutr 2011;94:543–551 [DOI] [PMC free article] [PubMed]
- 6.Nanri A, Mizoue T, Noda M, et al. Fish intake and type 2 diabetes in Japanese men and women: the Japan Public Health Center-based Prospective Study. Am J Clin Nutr 2011;94:884–891 [DOI] [PubMed]
- 7. Kaushik M, Mozaffarian D, Spiegelman D, Manson JE, Willett WC, Hu FB. Long-chain omega-3 fatty acids, fish intake, and the risk of type 2 diabetes mellitus. Am J Clin Nutr 2009;90:613–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Djoussé L, Gaziano JM, Buring JE, Lee IM. Dietary omega-3 fatty acids and fish consumption and risk of type 2 diabetes. Am J Clin Nutr 2011;93:143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Woudenbergh GJ, van Ballegooijen AJ, Kuijsten A, et al. Eating fish and risk of type 2 diabetes: A population-based, prospective follow-up study. Diabetes Care 2009;32:2021–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–2012 [DOI] [PubMed] [Google Scholar]
- 11. Feskens EJ, Bowles CH, Kromhout D. Inverse association between fish intake and risk of glucose intolerance in normoglycemic elderly men and women. Diabetes Care 1991;14:935–941 [DOI] [PubMed] [Google Scholar]
- 12. Montonen J, Järvinen R, Heliövaara M, Reunanen A, Aromaa A, Knekt P. Food consumption and the incidence of type II diabetes mellitus. Eur J Clin Nutr 2005;59:441–448 [DOI] [PubMed] [Google Scholar]
- 13.Djoussé L, Biggs ML, Lemaitre RN, et al. Plasma omega-3 fatty acids and incident diabetes in older adults. Am J Clin Nutr 2011;94:527–533 [DOI] [PMC free article] [PubMed]
- 14. Schulze MB, Manson JE, Willett WC, Hu FB. Processed meat intake and incidence of type 2 diabetes in younger and middle-aged women. Diabetologia 2003;46:1465–1473 [DOI] [PubMed] [Google Scholar]
- 15.van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 2002;25:417–424 [DOI] [PubMed]
- 16. Brostow DP, Odegaard AO, Koh WP, et al. Omega-3 fatty acids and incident type 2 diabetes: the Singapore Chinese Health Study. Am J Clin Nutr 2011;94:520–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meyer VF, Redente EF, Barbarick KA, Brobst R. Biosolids applications affect runoff water quality following forest fire. J Environ Qual 2001;30:1528–1532 [DOI] [PubMed] [Google Scholar]
- 18. Salmerón J, Hu FB, Manson JE, et al. Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr 2001;73:1019–1026 [DOI] [PubMed] [Google Scholar]
- 19. Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. BMJ 2010;341:c4229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 22. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou BF, Stamler J, Dennis B, et al. ; INTERMAP Research Group Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: the INTERMAP study. J Hum Hypertens 2003;17:623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Echarte M, Zulet MA, Astiasaran I. Oxidation process affecting fatty acids and cholesterol in fried and roasted salmon. J Agric Food Chem 2001;49:5662–5667 [DOI] [PubMed] [Google Scholar]
- 25. Lopez-Garcia E, Schulze MB, Meigs JB, et al. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr 2005;135:562–566 [DOI] [PubMed] [Google Scholar]
- 26. Tomey KM, Sowers MR, Li X, et al. Dietary fat subgroups, zinc, and vegetable components are related to urine F2a-isoprostane concentration, a measure of oxidative stress, in midlife women. J Nutr 2007;137:2412–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mouratoff GJ, Carroll NV, Scott EM. Diabetes mellitus in Eskimos. JAMA 1967;199:107–112 [DOI] [PubMed] [Google Scholar]
- 28. Storlien LH, Kraegen EW, Chisholm DJ, Ford GL, Bruce DG, Pascoe WS. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science 1987;237:885–888 [DOI] [PubMed] [Google Scholar]
- 29. He K. Fish, long-chain omega-3 polyunsaturated fatty acids and prevention of cardiovascular disease—eat fish or take fish oil supplement? Prog Cardiovasc Dis 2009;52:95–114 [DOI] [PubMed] [Google Scholar]
- 30. Groth E., 3rd Ranking the contributions of commercial fish and shellfish varieties to mercury exposure in the United States: implications for risk communication. Environ Res 2010;110:226–236 [DOI] [PubMed] [Google Scholar]
- 31. Humphrey HE, Gardiner JC, Pandya JR, et al. PCB congener profile in the serum of humans consuming Great Lakes fish. Environ Health Perspect 2000;108:167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee DH, Steffes MW, Sjödin A, Jones RS, Needham LL, Jacobs DR., Jr Low dose of some persistent organic pollutants predicts type 2 diabetes: a nested case-control study. Environ Health Perspect 2010;118:1235–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen YW, Yang CY, Huang CF, Hung DZ, Leung YM, Liu SH. Heavy metals, islet function and diabetes development. Islets 2009;1:169–176 [DOI] [PubMed] [Google Scholar]
- 34. Wang L, Folsom AR, Zheng ZJ, Pankow JS, Eckfeldt JH; ARIC Study Investigators Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2003;78:91–98 [DOI] [PubMed] [Google Scholar]
- 35. Vessby B, Aro A, Skarfors E, Berglund L, Salminen I, Lithell H. The risk to develop NIDDM is related to the fatty acid composition of the serum cholesterol esters. Diabetes 1994;43:1353–1357 [DOI] [PubMed] [Google Scholar]
- 36. Hodge AM, English DR, O’Dea K, et al. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr 2007;86:189–197 [DOI] [PubMed] [Google Scholar]
- 37. Wijendran V, Hayes KC. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu Rev Nutr 2004;24:597–615 [DOI] [PubMed] [Google Scholar]
- 38. Sheeshka J, Murkin E. Nutritional aspects of fish compared with other protein sources. Comments Toxicol 2002;8:375–397 [Google Scholar]
- 39. Stranges S, Marshall JR, Natarajan R, et al. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med 2007;147:217–223 [DOI] [PubMed] [Google Scholar]
- 40. Pittas AG, Sun Q, Manson JE, Dawson-Hughes B, Hu FB. Plasma 25-hydroxyvitamin D concentration and risk of incident type 2 diabetes in women. Diabetes Care 2010;33:2021–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]