Abstract
OBJECTIVE
To determine associations of gestational diabetes mellitus (GDM) and obesity with adverse pregnancy outcomes in the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study.
RESEARCH DESIGN AND METHODS
Participants underwent a 75-g oral glucose tolerance test (OGTT) between 24 and 32 weeks. GDM was diagnosed post hoc using International Association of Diabetes and Pregnancy Study Groups criteria. Neonatal anthropometrics and cord serum C-peptide were measured. Adverse pregnancy outcomes included birth weight, newborn percent body fat, and cord C-peptide >90th percentiles, primary cesarean delivery, preeclampsia, and shoulder dystocia/birth injury. BMI was determined at the OGTT. Multiple logistic regression was used to examine associations of GDM and obesity with outcomes.
RESULTS
Mean maternal BMI was 27.7, 13.7% were obese (BMI ≥33.0 kg/m2), and GDM was diagnosed in 16.1%. Relative to non-GDM and nonobese women, odds ratio for birth weight >90th percentile for GDM alone was 2.19 (1.93–2.47), for obesity alone 1.73 (1.50–2.00), and for both GDM and obesity 3.62 (3.04–4.32). Results for primary cesarean delivery and preeclampsia and for cord C-peptide and newborn percent body fat >90th percentiles were similar. Odds for birth weight >90th percentile were progressively greater with both higher OGTT glucose and higher maternal BMI. There was a 339-g difference in birth weight for babies of obese GDM women, compared with babies of normal/underweight women (64.2% of all women) with normal glucose based on a composite OGTT measure of fasting plasma glucose and 1- and 2-h plasma glucose values (61.8% of all women).
CONCLUSIONS
Both maternal GDM and obesity are independently associated with adverse pregnancy outcomes. Their combination has a greater impact than either one alone.
Gestational diabetes mellitus (GDM) and maternal obesity are independently associated with adverse maternal and neonatal outcomes (1,2). Both share common metabolic characteristics such as increased insulin resistance, hyperglycemia, and hyperinsulinemia, and GDM may impart distinct effects on clinical outcomes independent of obesity alone. The same is true for maternal obesity, although differences in metabolism may also exist among certain ethnic groups (3). Therefore, examination of the combined association of these common metabolic problems with pregnancy outcomes is an important question.
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study offers a unique opportunity to examine the independent associations of GDM and obesity alone and in combination with adverse pregnancy outcomes (4,5). This is particularly true because the HAPO Study was a purely observational study in which participants and caregivers were blinded to maternal glucose values and did not include any recommendations, diet or otherwise, for treatment of either glucose intolerance or obesity. The primary HAPO results showed a continuous positive relationship between glucose concentrations during a 2-h 75-g oral glucose tolerance test (OGTT) and the primary outcomes of birth weight >90th percentile, primary cesarean section, neonatal hypoglycemia, and cord serum C-peptide >90th percentile (4). Similarly, high maternal BMI (kg/m2) was independently associated with an increasing frequency of birth weight >90th percentile, percentage body fat >90th percentile, primary cesarean section, and cord C-peptide >90th percentile (5). Higher maternal glucose and BMI were also associated with secondary outcomes including preeclampsia (6). These perinatal problems have short- and long-term metabolic implications both for the mother and for her offspring (7–9). Hence, the purpose of this analysis is to examine the relative contributions of GDM based on the new International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria (10) and obesity defined as BMI ≥33.0 kg/m2 (5) at 24–32 weeks' gestation to adverse maternal and neonatal outcomes. We hypothesize that both maternal hyperglycemia and obesity alone are independently associated with adverse obstetrical outcomes, and the combination of the two factors is associated with greater risk than either GDM or obesity alone.
RESEARCH DESIGN AND METHODS
The protocol was approved by the Institutional Review Board in all 15 field centers. All participants provided written informed consent. An external data monitoring committee provided oversight. Study methods have been published previously (4), and only a brief overview is provided here. All pregnant women at each field center were eligible to participate unless they had one or more exclusion criteria as published previously.
Maternal BMI
The measures of maternal height and weight, used to calculate BMI, were obtained at the time of the OGTT with outer garments and shoes removed. BMI was defined as weight/height squared (kg/m2). Height was measured twice to the nearest 0.5 cm with a stadiometer or wall-mounted measuring tape and the participant’s head facing forward in the horizontal plane. If the results differed by more than 1.0 cm, the measurement was repeated. Weight was measured twice to the nearest 0.1 kg on a scale calibrated each day. A third weight was taken if the results of the first two measurements differed by more than 0.5 kg. If a third measurement was taken, the average of the two nearest measures was used. Recalled maternal prepregnancy weight was recorded but was not the primary focus of the study because of its inherent subjectivity and the absence of data for 1,966 participants (8.4%). No center provided specific interventions to participants based on weight or BMI.
To take into account weight gain during pregnancy, category limits for BMI at the OGTT that could be considered comparable with nonpregnant World Health Organization (WHO) BMI categories were obtained from a regression of OGTT BMI on prepregnancy BMI and gestational age at the OGTT. This yielded a definition of obesity at 28 weeks as a BMI ≥33.0 kg/m2, of overweight at 28 weeks as a BMI of 28.5–32.9, and of normal weight or underweight as a BMI ≤28.4. As outlined previously (5), these cut points from regression are equivalent to the WHO categories of (nonpregnant) class 1 obesity, BMI ≥30.0 kg/m2, overweight 25.0–29.9, and normal or underweight <25.0, respectively (11).
OGTT
Participants underwent a 2-h 75-g OGTT between 24 and 32 weeks' gestation (as close to 28 weeks as possible). Data concerning smoking and alcohol use, first degree family history of diabetes and hypertension, and demographics were collected using standardized questionnaires. Race/ethnicity was self-identified. A sample for random plasma glucose (RPG) was collected at 34–37 weeks' gestation as a safety measure to identify case subjects with hyperglycemia above a predefined threshold.
Glucose analysis and unblinding
Aliquots of fasting and 2-h OGTT plasma glucose and RPG samples were analyzed at field center laboratories. Values were unblinded if fasting glucose exceeded 5.8 mmol/L, if the 2-h glucose was >11.1 mmol/L or if RPG was ≥8.9 mmol/L or if any plasma glucose value was <2.5 mmol/L. Otherwise, women, caregivers, and HAPO Study staff remained blinded to glucose values. To avoid confounding effects of center-to-center analytical variation, aliquots of all OGTT specimens were analyzed at the HAPO central laboratory, and these results were used for data analysis. Only women whose results remained blinded, with no additional glucose testing outside the HAPO protocol, are included in the analysis.
Diagnosis of GDM
The diagnosis of GDM reported in this article was made post hoc and did not affect pregnancy care during the HAPO Study. We have applied the new IADPSG recommendations (10). With this definition, the diagnosis of GDM is made when any of the following values from the 75-g OGTT is equaled or exceeded: fasting plasma glucose 5.1 mmol/L (92 mg/dL), 1-h plasma glucose 10.0 mmol/L (180 mg/dL), or 2-h plasma glucose 8.5 mmol/L (153 mg/dL).
Prenatal care and delivery
Prenatal care and timing of delivery were determined by standard field center practice. No field center arbitrarily delivered patients before full term or routinely performed cesarean delivery at a specified maternal or gestational age.
Neonatal care and anthropometrics
After delivery, infants received routine care. Medical records were abstracted to obtain data regarding prenatal problems, labor, and delivery including need for primary cesarean section, postpartum, and newborn course.
Neonatal anthropometrics were obtained within 72 h of delivery. To ensure accuracy and reliability of anthropometric data and consistency across all field centers, a training and certification procedure was established for HAPO Study staff as described previously (12). Anthropometrics included weight, length, head circumference and flank, subscapular, and triceps skinfold thickness.
Birth weight was obtained without a diaper using a calibrated electronic scale. Length was measured using a standardized plastic length board. Head circumference was measured across the occipital fontanel. Skinfold thickness was measured with skinfold calipers (Harpenden, Baty, U.K.). Flank skinfold was measured on the left side as described previously (12). The mean coefficients of variation for the anthropometric measurements were birth weight 0.04%, length 0.17%, and flank skinfold 2.91%.
Weight at delivery was used to determine birth weight >90th percentile. Weight at the anthropometric assessment was used to determine total and percent body fat.
Outcomes
Birth weight >90th percentile.
The 90th percentiles were determined using eight newborn sex-ethnic groups (Caucasian or other, Black, Hispanic, or Asian) with adjustment for gestational age, field center, and parity (0,1,2+). Birth weight >90th percentile was considered to be present if the birth weight was greater than the 90th percentile for the baby’s sex, gestational age, ethnicity, field center, and maternal parity with gestational ages of 30–44 weeks included.
Cord C-peptide >90th percentile.
Cord blood was collected at delivery for the measurement of serum C-peptide. The specimens were analyzed at the HAPO central laboratory by an immunoassay (AutoDELPHIA; PerkinElmer). Because hemolysis (affecting ∼15% of cord blood samples) is known to increase insulin degradation, but not to affect C-peptide levels (13), and because C-peptide and insulin are secreted in equimolar amounts, we used cord serum C-peptide concentrations rather than insulin as our index of fetal β-cell function. The 90th percentile for C-peptide for the total HAPO cohort (1.7 μg/L) was used to determine the presence of hyperinsulinemia.
Primary cesarean.
Primary cesarean section was defined as the need for the first cesarean delivery at the discretion of the subject’s primary obstetrical care provider. We elected not to use total cesarean deliveries as an outcome because of the various policies of repeat cesarean deliveries and trial of labor after a previous cesarean delivery at the various HAPO Study sites.
Preeclampsia.
Hypertension that was present at <20 weeks' gestation and did not progress to preeclampsia was classified as chronic hypertension. After 20 weeks, blood pressure measurements were used to classify hypertensive disorders in pregnancy according to the International Society for the Study of Hypertension in Pregnancy guidelines (14). Systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg on ≥2 occasions ≥6 h apart and proteinuria of ≥1+ dipstick or ≥300 mg per 24 h indicated preeclampsia. Meeting the criteria for elevated blood pressure, but not proteinuria, was classified as gestational hypertension.
Percent body fat >90th percentile.
Fat mass was calculated from birth weight, length, and flank skinfold according to the equation given by Catalano et al. as reported previously (15). Percent body fat was then calculated as 100 × fat mass/birth weight. Percent body fat >90th percentile was defined using the same methods as for birth weight >90th percentile, with gestational ages of 36–44 weeks included.
Shoulder dystocia/birth injury.
Additional data were abstracted at centers when either was suspected. Data were reviewed by two members of an outcome review committee who were blinded to the mother’s glycemic status, to confirm whether either was present.
Statistical analyses.
Descriptive statistics include means and SDs for continuous variables and numbers and percentages for categorical variables. To examine the associations of GDM and obesity, singly and in combination, HAPO participants were divided into four mutually exclusive groups: 1) no GDM, no obesity; 2) GDM, no obesity; 3) no GDM, obesity; and 4) GDM, obesity. Two logistic regression models were then fit for each outcome, with no GDM and no obesity used as the referent group. Model I included adjustment for field center or the variables used in estimating the 90th percentiles for birth weight and percent body fat for gestational age (sex, ethnicity, center, and parity). Model II included adjustment for multiple potential confounders, including maternal age and height at the OGTT, smoking, alcohol use, family history of diabetes, gestational age at the OGTT, baby’s sex, parity (0, 1, 2+) (except primary cesarean delivery), mean arterial pressure and hospitalization before delivery (except preeclampsia), family history of hypertension and maternal urinary tract infection (preeclampsia only), and cord glucose (cord serum C-peptide >90th percentile only).
In addition, to provide an example of the associations of BMI and glucose across the full range of BMI and OGTT glucose singly and in combination, we created a composite OGTT measure that used all three glucose values. This variable was created by calculating z scores for fasting plasma glucose and 1- and 2-h plasma glucose by subtracting the appropriate HAPO mean from each woman’s glucose measurements, dividing by the corresponding SD, and then summing the three resulting z scores for each woman. We then divided BMI and the sum of the three z scores into three categories each. The categories for BMI were normal or underweight (≤28.4), overweight (28.5–32.9), and obese (≥33.0). We divided the OGTT z score sum into normal (≤0.539, no GDM), intermediate (>0.539, no GDM), and GDM. The cut point for the intermediate glucose group was selected to have approximately the same proportion as in the overweight group. We then examined the associations of BMI and OGTT glucose with birth weight >90th percentile in a logistic regression analysis with Model II adjustment, and with birth weight in a multiple linear regression analysis with Model II adjustment, including adjustment for gestational age at delivery. These models included dummy (0–1) variables for the intermediate glucose and GDM categories and for the BMI categories of overweight and obese. Odds ratios (ORs) for birth weight >90th percentile relative to normal glucose and normal or underweight BMI were then obtained for all combinations of BMI and glucose categories by multiplying the OR corresponding to the appropriate glucose and BMI categories. Mean differences in birth weight relative to normal glucose and normal or underweight were obtained for all combinations of BMI and glucose categories by adding the mean differences in birth weight corresponding to the appropriate glucose and BMI categories.
Because prepregnancy BMI is independently related to adverse pregnancy outcomes, we also examined the relationship of prepregnancy BMI with the outcome measures in Supplementary Data. Supplementary Tables A and B provide analyses comparable with those in Tables 2 and 3 based on self-reported prepregnancy weight using the WHO criteria above for normal or underweight, overweight, and obese.
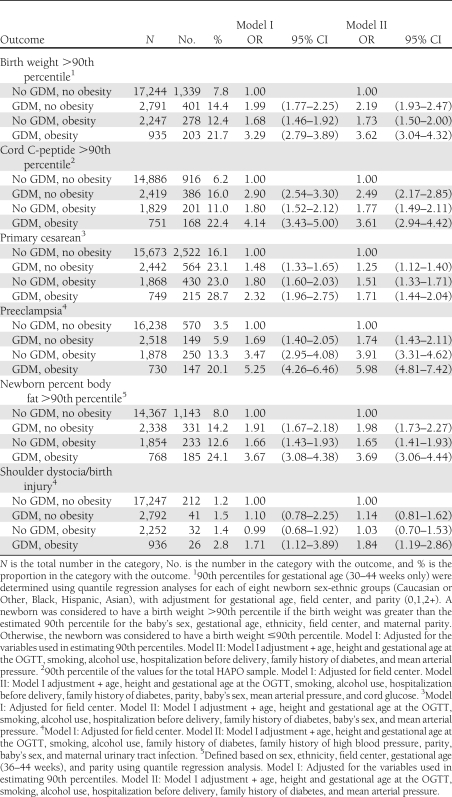
Table 2.
Relationship between maternal GDM, obesity, and outcomes
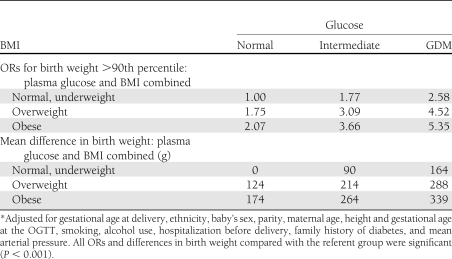
Table 3.
ORs for birth weight >90th percentile and mean differences in birth weight for combinations of plasma glucose and BMI with Model II adjustment*
All analyses were conducted in Stata 11.2.
RESULTS
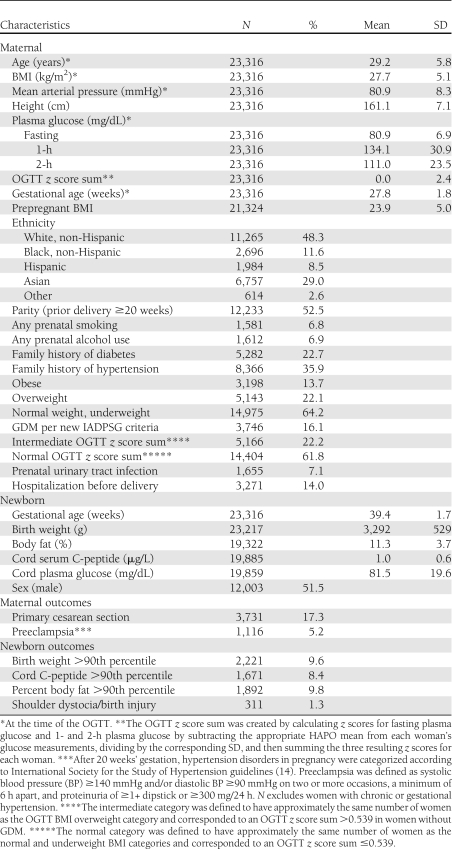
Among 53,295 eligible women from 15 centers in nine countries, 28,562 (53.6%) agreed to participate between July 2000 and April 2006. A total of 25,505 women completed the OGTT: 746 (2.9%) were excluded because of glucose unblinding, 1,412 (5.5%) were excluded primarily because they had undergone glucose testing or delivery outside the context of the HAPO Study, and 31 (0.1%) were excluded owing to missing key data or improbable results. The data from 23,316 women were available for analysis. Table 1 shows the characteristics and frequency of outcomes relative to the specific aims of this study. The mean maternal BMI at the time of the OGTT was 27.7 kg/m2. Obesity was present in 13.7% of participants, and 16.1% met the new IADPSG criteria for GDM; 25% of those diagnosed with GDM were obese.
Table 1.
Characteristics of participants and frequency of outcomes
Table 2 shows the associations of maternal GDM and obesity with outcomes. There were significantly greater odds of birth weight, newborn percent body fat and cord C-peptide >90th percentile, primary cesarean delivery, and preeclampsia for GDM or obesity alone compared with the reference group in both Models I and II. The combination of GDM and obesity showed substantially higher ORs compared with those for either GDM or obesity alone. Shoulder dystocia/birth injury was uncommon (1.3% overall), and odds for these outcomes were significantly greater than in the reference group only when GDM and obesity were both present.
Supplementary Table A based on prepregnancy BMI shows a similar pattern with significantly greater odds of birth weight, newborn percent body fat and cord C-peptide >90th percentile, primary cesarean delivery, and preeclampsia for GDM or obesity alone compared with the reference group in both Models I and II and substantially higher ORs compared with those for either GDM or obesity alone. However, the ORs for prepregnancy obesity are smaller than those for OGTT obesity, particularly for birth weight, cord C-peptide, and newborn percent body fat >90th percentile. Although there was a strong correlation between prepregnancy BMI and BMI at the time of the OGTT (0.918), these data need to be interpreted with caution because prepregnancy weight was obtained by recall, 8.4% of the data were missing, and the frequency of missing data was not evenly distributed among the centers.
Table 3 shows the relative odds of birth weight >90th percentile for combinations of maternal BMI and OGTT glucose. When compared with women who are normal/underweight with normal glucose, the relative odds of birth weight >90th percentile is progressively greater with both higher glucose and maternal BMI, 2.58 for GDM vs. normal glucose, and 2.07 for obese vs. normal or underweight. The difference in birth weight in grams (bottom half of Table 3) shows a similar pattern. There is a progressively larger difference in birth weight with both higher OGTT glucose and maternal BMI compared with the referent group with the largest difference of 339 g being associated with GDM and obesity in combination. Supplementary Table B based on prepregnancy weight shows a similar pattern. However, ORs and differences for overweight and obesity based on prepregnancy BMI are smaller than those for OGTT BMI.
CONCLUSIONS
The results of previous reports from the HAPO Study have shown significant independent associations of higher maternal glucose concentrations (4,6) and maternal obesity (5) with adverse pregnancy outcomes. This study adds to the previous reports by examining the impact of GDM and obesity alone as well as their combined impact on adverse pregnancy outcomes. The combination of these factors shows a greater risk of adverse pregnancy outcomes than either GDM or obesity alone.
In the U.S., ∼7% or 200,000 pregnant women are currently diagnosed with GDM (16). Using the IADPSG criteria will increase the number of women diagnosed with GDM (10). Much of this potential increase in the frequency of GDM in the U.S. and other developed countries can be attributed to the increase in obesity in women of reproductive age (17). Approximately 60% of women of reproductive age are overweight or obese in the U.S. and other developed countries (17). Obesity is an increasing problem in other areas of the world, where many of the HAPO field centers were located. Although we defined obesity in pregnancy corresponding to WHO criteria (11), lower BMI in many developing populations, particularly in Asia, may correlate better with the BMI of ≥30 in Western countries because of the relative increase in visceral adiposity (18). However, a WHO consultation concluded that available data do not necessarily indicate a clear BMI cutoff point for all Asians for overweight or obesity and that the WHO BMI cutoff points should be retained as international classifications (19).
GDM and maternal obesity alone and in combination are independently associated with adverse pregnancy outcomes, and it is tempting to compare the Model II associations among subgroups. We have a number of concerns about using this approach. First, among the several outcomes summarized in Table 2, there is no consistent pattern regarding Model II OR for GDM without obesity compared with obesity without GDM. Furthermore, when BMI of the participants was classified from estimated prepregnancy weight the ORs were more variable and associations with obesity without GDM tended to be weaker. What is most striking is that the combination of GDM and obesity is most strongly associated with each outcome. In addition, Table 3 clearly illustrates the strong association of a combination of overweight and intermediate levels of maternal glucose with outcomes. Finally, GDM and obesity seem to influence a number of the outcomes through similar mechanisms.
The HAPO Study supports the Pedersen hypothesis that increased maternal glucose concentration shows a strong continuous relationship with fetal growth as does the strong association between cord C-peptide and fetal adiposity (20). As indicated in Table 2 and published reports (5,21), obesity is also independently associated with fetal hyperinsulinemia, birth weight, and newborn adiposity. In a recent study that used continuous glucose monitoring, Harmon et al. (22) found that obese women with normal glucose tolerance have higher daytime and nocturnal glucose profiles compared with normal weight women. There is also evidence that circulating levels of other nutrients such as lipids and amino acids, which are influenced by insulin and insulin resistance, are increased in both GDM (23) and obesity (24) and may contribute to hyperinsulinemia, fetal growth, and adiposity. The pathways for fatty acid esterification in fetal adipose tissue are not well described. However, emerging data on the characterization of fatty acid binding proteins, lipid transporters, and enzymes for fatty acid esterification in the human placenta have now improved our understanding of how maternal lipids may contribute to increased fetal fat accretion (25).
Maternal obesity has a strong independent relationship with adverse perinatal outcomes (8), and the pathophysiology of some of the associations may have similarities with GDM as described above. Other associations may have different mechanisms. For example, we found a higher risk of preeclampsia in obese non-GDM women (OR 3.91, Model II; Table 2) than in nonobese GDM (OR 1.74, Model II). Obese women are more insulin resistant as compared with normal weight women (8); hence increased insulin resistance may be relevant to the development of preeclampsia in obese women and women developing GDM. However, obesity in addition to GDM was associated with a greater risk of preeclampsia than either factor alone (OR 5.98, Model II; Table 2), thereby implicating other potential mechanisms such as inflammation in the development of preeclampsia in this high-risk group.
The utility of the HAPO Study is that it provides objective evidence upon which to base future strategies to improve perinatal health. The randomized controlled trials of Crowther et al. (26) and Landon et al. (27) for the treatment of mild GDM, using current management protocols, in which only 8–20% of mild GDMs required insulin therapy, reported improved outcomes including decreased risks of birth weight >90th percentile and preeclampsia. Maternal weight gain was decreased in the treated GDM as compared with the control group in both studies. Avoidance of excessive gestational weight gain in obese women may improve perinatal outcomes such as birth weight >90th percentile. Because 50–60% of overweight and obese women gain more weight during pregnancy than that recommended in the 2009 Institute of Medicine guidelines (28), avoidance of excessive gestational weight gain should result in decreased postpartum weight retention for future pregnancies, thereby decreasing the vicious cycle of obesity affecting obese pregnant women and their offspring. However, further research is needed to determine which lifestyle treatment options best improve perinatal outcomes in obese women.
In summary, both maternal GDM and obesity are independently associated with adverse pregnancy outcomes. The combination of the two, however, has a greater impact than either one alone. Although management of GDM requires strict glucose control, it results in lower frequencies of adverse outcomes. Optimal management of maternal obesity per se has yet to be defined. Until the results of ongoing research studies are available, avoidance of excessive gestational weight gain, moderate exercise, and a prudent diet are reasonable recommendations for obese pregnant women.
Acknowledgments
The study was funded by grants R01-HD34242 and R01-HD34243 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Diabetes and Digestive and Kidney Diseases; by the National Center for Research Resources (M01-RR00048, M01-RR00080); and by the American Diabetes Association. Support has also been provided to local field centers by Diabetes UK (RD04/0002756), Kaiser Permanente Medical Center, KK Women’s and Children’s Hospital, Mater Mother’s Hospital, Novo Nordisk, and the Howard and Carol Bernick Family Foundation.
No potential conflicts of interest relevant to this article were reported.
P.M.C. researched the data, contributed to the discussion, wrote the manuscript, and reviewed and edited the manuscript. H.D.M. and J.K.C. researched the data, contributed to the discussion, and reviewed and edited the manuscript. D.R.M., A.R.D., B.E.M., and L.P.L. researched the data, contributed to the discussion, wrote the manuscript, and reviewed and edited the manuscript. E.R.T. and D.R.C. researched the data, contributed to the discussion, and reviewed and edited the manuscript. D.R.H. researched the data, contributed to the discussion, wrote the manuscript, and reviewed and edited the manuscript. B.P. researched the data and reviewed and edited the manuscript. M.H. and J.J.N.O. researched the data, contributed to the discussion, and reviewed and edited the manuscript. B.E.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1790/-/DC1.
A slide set summarizing this article is available online.
References
- 1.Owens LA, O’Sullivan EP, Kirwan B, Avalos G, Gaffney G, Dunne F; ATLANTIC DIP Collaborators ATLANTIC DIP: the impact of obesity on pregnancy outcome in glucose-tolerant women. Diabetes Care 2010;33:577–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landon MB, Mele L, Spong CY, et al. ; Eunice Kennedy Shriver National Institute of Child Health, and Human Development (NICHD) Maternal–Fetal Medicine Units (MFMU) Network The relationship between maternal glycemia and perinatal outcome. Obstet Gynecol 2011;117:218–22421309194 [Google Scholar]
- 3.World Health Organization Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a WHO Consultation. Part 1: Diagnosis and classification of diabetes mellitus. Geneva, Switzerland, World Health Organization, 1999 [Google Scholar]
- 4.Metzger BE, Lowe LP, Dyer AR, et al. ; HAPO Study Cooperative Research Group Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 5.HAPO Study Cooperative Research Group Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index. BJOG 2010;117:575–584 [DOI] [PubMed] [Google Scholar]
- 6.The HAPO Study Cooperative Research Group Hyperglycemia and adverse Pregnancy Outcome (HAPO) study: preeclampsia. Am J Obstet Gynecol 2010;202:255.e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;115:e290–e296 [DOI] [PubMed] [Google Scholar]
- 8.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG 2006;113:1126–1133 [DOI] [PubMed] [Google Scholar]
- 9.Metzger BE. Long-term outcomes in mothers diagnosed with gestational diabetes mellitus and their offspring. Clin Obstet Gynecol 2007;50:972–979 [DOI] [PubMed] [Google Scholar]
- 10.Metzger BE, Gabbe SG, Persson B, et al. ; International Association of Diabetes and Pregnancy Study Groups Consensus Panel International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization Obesity: preventing and management of global epidemic. World Health Organ Tech Rep Ser 2000;984:1–4 [PubMed] [Google Scholar]
- 12.HAPO Study Cooperative Research Group Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes 2009;58:453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Rahilly S, Burnett MA, Smith RF, Darley JH, Turner RC. Haemolysis affects insulin but not C-peptide immunoassay. Diabetologia 1987;30:394–396 [DOI] [PubMed] [Google Scholar]
- 14.Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy 2001;20:IX–XIV [DOI] [PubMed] [Google Scholar]
- 15.Catalano PM, Thomas AJ, Avallone DA, Amini SB. Anthropometric estimation of neonatal body composition. Am J Obstet Gynecol 1995;173:1176–1181 [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association Gestational diabetes mellitus. Diabetes Care 2003;26(Suppl. 1):S103–S105 [DOI] [PubMed] [Google Scholar]
- 17.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010;303:235–241 [DOI] [PubMed] [Google Scholar]
- 18.Deurenberg P. Universal cut-off BMI points for obesity are not appropriate. Br J Nutr 2001;85:135–136 [DOI] [PubMed] [Google Scholar]
- 19.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–163 [DOI] [PubMed] [Google Scholar]
- 20.Pedersen J. Diabetes and pregnancy. Blood sugar of newborn infants. Copenhagen, Danish Science Press, 1952 [Google Scholar]
- 21.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 2009;32:1076–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harmon KA, Gerard L, Jensen DR, et al. Continuous glucose profiles in obese and normal-weight pregnant women on a controlled diet: metabolic determinants of fetal growth. Diabetes Care 2011;34:2198–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metzger BE, Phelps RL, Freinkel N, Navickas IA. Effects of gestational diabetes on diurnal profiles of plasma glucose, lipids, and individual amino acids. Diabetes Care 1980;3:402–409 [DOI] [PubMed] [Google Scholar]
- 24.Schaefer-Graf UM, Graf K, Kulbacka I, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 2008;31:1858–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catalano PM, Hauguel-De Mouzon S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol 2011;204:479–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS; Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477–2486 [DOI] [PubMed] [Google Scholar]
- 27.Landon MB, Spong CY, Thom E, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009;361:1339–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmussen KM, Yaktine AL, Eds. Weight gain during pregnancy: reexamining the recommendations. Washington, DC, The National Academies Press, 2009 [PubMed] [Google Scholar]