Abstract
OBJECTIVE
Metformin produced weight loss and delayed or prevented diabetes in the Diabetes Prevention Program (DPP). We examined its long-term safety and tolerability along with weight loss, and change in waist circumference during the DPP and its long-term follow-up.
RESEARCH DESIGN AND METHODS
The randomized double-blind clinical trial of metformin or placebo followed by a 7–8-year open-label extension and analysis of adverse events, tolerability, and the effect of adherence on change in weight and waist circumference.
RESULTS
No significant safety issues were identified. Gastrointestinal symptoms were more common in metformin than placebo participants and declined over time. During the DPP, average hemoglobin and hematocrit levels were slightly lower in the metformin group than in the placebo group. Decreases in hemoglobin and hematocrit in the metformin group occurred during the first year following randomization, with no further changes observed over time. During the DPP, metformin participants had reduced body weight and waist circumference compared with placebo (weight by 2.06 ± 5.65% vs. 0.02 ± 5.52%, P < 0.001, and waist circumference by 2.13 ± 7.06 cm vs. 0.79 ± 6.54 cm, P < 0.001 in metformin vs. placebo, respectively). The magnitude of weight loss during the 2-year double-blind period was directly related to adherence (P < 0.001). Throughout the unblinded follow-up, weight loss remained significantly greater in the metformin group than in the placebo group (2.0 vs. 0.2%, P < 0.001), and this was related to the degree of continuing metformin adherence (P < 0.001).
CONCLUSIONS
Metformin used for diabetes prevention is safe and well tolerated. Weight loss is related to adherence to metformin and is durable for at least 10 years of treatment.
Metformin is an established treatment for diabetes with a good safety profile (1). Its most common side effects are gastrointestinal (1). These symptoms are generally transient, resolve spontaneously, and can often be avoided by gradual escalation of dosage. Metformin treatment has not been associated with hypoglycemia unless used in conjunction with other glucose-lowering medicines (sulfonylureas or insulin). In U.S. clinical trials, about 4% of participants were unable to continue metformin due to adverse effects. Serious adverse events are infrequent and generally limited to lactic acidosis, which occurs only in persons with renal or hepatic insufficiency or other contraindications. Metformin is associated with weight loss when used to treat diabetes and thus differs from a number of other antidiabetic medications that are associated with weight stability or gain (2,3). To date, metformin is indicated only for diabetes management and not for weight loss in individuals with or without diabetes.
In the Diabetes Prevention Program (DPP), metformin reduced the development of diabetes by 31% over an average of 2.8 years of follow-up (4,5). The long-term follow-up of the DPP, the DPP Outcomes Study (DPPOS), included an open-label extension of metformin treatment in those randomly assigned to metformin in the DPP. After a median of 10 years of follow-up since DPP randomization, both the lifestyle and metformin intervention groups had significantly less diabetes than the placebo group (6). During the DPP, participants randomized to metformin experienced an average 2.1-kg weight loss (4). Weight loss was a strong predictor of diabetes prevention in both the metformin and placebo groups with weight loss accounting for 64% of the metformin versus placebo effect on diabetes prevention (7). Weight loss in the metformin group was maintained throughout the combined DPP and DPPOS period with metformin participants having an average 2.5-kg weight loss over time (6). This report updates these findings by documenting the long-term safety and tolerability of metformin, and in a post hoc analysis, it tests the hypothesis that greater adherence to metformin is associated with greater weight loss and reduction in waist circumference in participants randomly assigned to metformin compared with those randomly assigned to placebo.
RESEARCH DESIGN AND METHODS
Between 1996 and 1999, 3,234 participants from 27 clinics in the U.S. were enrolled in the DPP; the 2,155 randomly assigned to the metformin (1,073) or placebo (1,082) arms are included in this analysis (8). Participants were ≥25 years of age, had a BMI ≥24 kg/m2 (≥22 kg/m2 in Asian Americans), elevated fasting glucose (95–125 mg/dL), and impaired glucose tolerance (140–199 mg/dL) 2 h after a 75-g oral glucose load. Participants were excluded for a prior diagnosis of diabetes or conditions or medications that would impair their ability to participate or affect weight loss. All participants gave written informed consent as approved by each institutional review board.
Metformin or matching placebo was initiated at 850-mg once per day and increased by 1 month to 850-mg twice daily unless gastrointestinal symptoms warranted a longer titration period. Standard lifestyle recommendations and written information on healthy eating, healthy weight, and physical activity were provided annually (4). Case managers promoted adherence to the DPP study medications using a brief structured interview and a standard problem-solving approach.
The first phase of the DPP was completed in 2001 after an average of 3.2 years of follow-up on the advice of the Data and Safety Monitoring Board due to the effectiveness of the lifestyle and metformin interventions in preventing diabetes (4). After the DPP results were announced, participants underwent a 1- to 2-week study/drug washout period followed by a repeat glucose tolerance test (9). Subsequently, they were unblinded and offered a 6-month, 16-session, group-implemented program with content identical to the original DPP lifestyle intervention (10). All DPP participants, regardless of whether diabetes had developed, were encouraged to join the DPPOS, and 88% did (6). Participants followed their original treatment assignments, and all were offered quarterly group lifestyle classes. Placebo treatment was terminated. Participants initially assigned to metformin continued taking study-provided open-label metformin unless there were contraindications, or fasting plasma glucose was ≥140 mg/dL in the DPP, or HbA1c was ≥7% during the DPPOS, which required management outside of the protocol.
A summary of data collection during the DPP and the DPPOS can be found in Supplementary Appendix Table 1. Adverse events were ascertained as follows: 1) During the DPP but not the DPPOS, all study participants were queried every quarter by asking “During the interval since the last visit, has the participant had any new symptoms, injuries, illness or side effects, or worsening of pre-existing conditions?” Responses were coded using the U.S. Food and Drug Administration’s (FDA) COSTART (Coding Symbols for a Thesaurus of Adverse Reaction Terms) coding system. Possible hypoglycemic events were identified by searching for “HYPOGLY,” “CONSCIOUS,” and “COMA,” and for possible anemia as “ANEMIA” or “B12.” Serious adverse events (SAEs) were handled as defined by the FDA. 2) Gastrointestinal symptoms were identified annually throughout the DPP and the DPPOS by asking participants about any stomach pain, bloating, nausea, diarrhea, or loss of appetite. 3) Gastrointestinal symptoms attributed to study medication were ascertained during the DPP and for those participants actively taking metformin during the DPPOS.
Weight was measured twice yearly and waist circumference annually (4,8). Hemoglobin and hematocrit were measured annually in each clinic’s laboratory on all participants randomized to medication during the DPP and for participants actively taking metformin during the DPPOS. Low hematocrit was defined as <40% in men and <36% in women and low hemoglobin as <13 mg/dL in men and <12 mg/dL in women.
Statistical analysis
Analyses are presented in two parts: 1) the first 2 years of the double-blind randomized placebo-controlled trial and 2) the complete follow-up period since randomization, including the open-label phase, lasting an additional 7–8 years (until 27 August 2008, the closing date for this analysis). Two years was selected because all participants completed two full years in the double-blind period in the DPP and for comparability with many other drug trials for weight loss. We also show the results at 9 years, the minimum combined DPP + DPPOS follow-up time. We examine the weight and waist circumference changes stratified by level of adherence to placebo and metformin during the 2-year blinded phase and to metformin throughout both phases.
Adherence to metformin was assessed at all regularly scheduled clinic visits and recorded as ≥80% (adherent) or <80% (nonadherent) of assigned pills taken, based on pill counts. Four categories of long-term adherence were defined (Table 1). Participants were censored from the metformin adherence grouping when study-supplied metformin was discontinued due to uncontrolled hyperglycemia, and diabetes drug treatment was managed outside the protocol. All participants, other than those censored, were included in the adherence measures regardless of reasons for low adherence (e.g., safety or standard contraindications) to explore fully the exposure to metformin and weight loss.
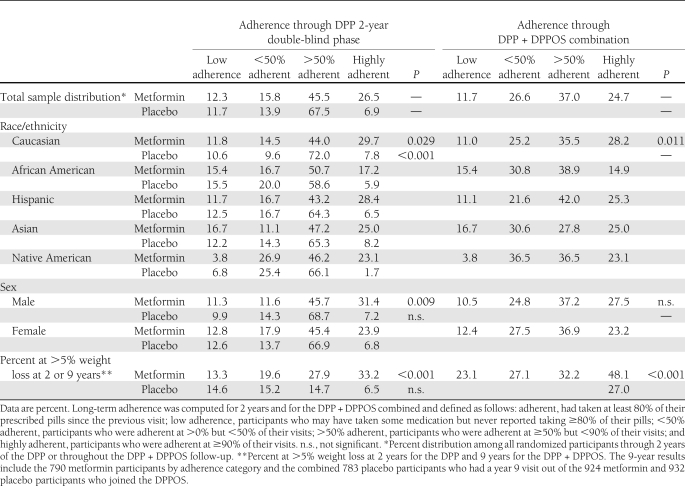
Table 1.
Distribution of adherence to metformin and placebo overall and by race/ethnicity and sex during the DPP (2 years) and for the DPP + DPPOS combined (9 years), and percent of the DPP/DPPOS participants achieving greater than 5% weight loss during each time period
For the assessment of long-term metformin safety and tolerability, all visits after the diagnosis of diabetes were excluded in order to avoid confounding by diabetes treatment (e.g., by use of nonstudy drugs).
Fixed-effects models with the assumption of normally distributed errors were used to compute repeated-measures adjusted means in body weight and waist circumference among the adherence categories and treatment groups. Models were adjusted for baseline weight and waist circumference (11). Generalized estimating equations were used to assess symptoms and adverse events over time by treatment group (11).
RESULTS
DPP—results from the double-blind phase
Characteristics of the DPP participants have been reported (4). The proportion of participants taking ≥80% of the prescribed dose over time during the DPP were lower in the metformin (71%) than in the placebo group (77%) (P < 0.001).
Medication adherence varied by race/ethnicity with African American participants having the lowest adherence during both the DPP (metformin and placebo) and the full follow-up period (metformin only) (Table 1). Men were more adherent to metformin during the DPP but not over the total follow-up period. No differences were observed among placebo participants. At the end of year 1, weight loss in the metformin group was 2.7 ± 4.7% (mean ± SD) compared with a loss of 0.43 ± 4.7% in the placebo group (P < 0.001). After 2 years, weight loss was 2.1 ± 5.7% in the metformin group compared with 0.02 ± 5.5% (P < 0.001) in the placebo group. Waist circumference was reduced at year 1 in the metformin group by 2.2 ± 6.2 cm vs. 0.71 ± 5.6 cm in the placebo group (P < 0.001) and at 2 years by 2.1 ± 7.1 cm in the metformin group vs. 0.79 ± 6.5 cm in the placebo group (P < 0.001 for both time periods). At year 1, 29% in the metformin group had lost ≥5% of their initial body weight, compared with 13% of the placebo group, and at 2 years, 26% of the metformin and 14% of the placebo group had lost ≥5% of baseline body weight (P < 0.001 for both time periods). The percentage losing ≥10% of their body weight at 1 year was 8% for the metformin versus 4% for the placebo group and at 2 years was 10% for the metformin and 5% for the placebo group (P < 0.001 for both time periods).
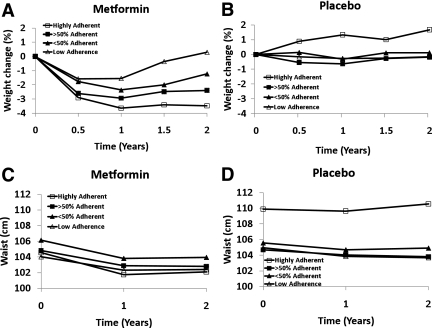
Figure 1 shows changes in body weight and waist circumference in each treatment group according to pill-taking adherence during the 2-year double-blind phase. Seventy-two percent of the metformin group and 74% of the placebo group were in the ≥50% adherent or highly adherent categories (Table 1), although fewer placebo participants were highly adherent. Adherence was strongly associated with weight loss in the metformin-treated group. The durability of weight loss was also affected by adherence. Average weight of highly adherent participants was 3.5 ± 0.35% below baseline at 2 years, very close to their 1-year weight loss. Those with low adherence had returned to baseline weight by year 2 (Fig. 1A) (P < 0.001). Placebo participants in all adherence subgroups remained within 1% of their baseline weight, on average, over the 2 years (Table 1), except for the 7% who were highly adherent with placebo, who had a small weight gain of 1.2% (P < 0.05 for the highly adherent compared with the >50% adherent group; all other P values >0.05). The associations of adherence to medication with changes in waist circumference were not statistically significant for either the metformin or placebo groups. (Fig. 1C and D).
Figure 1.
Effect of adherence to metformin or placebo on percent weight change (A and B) and change in waist circumference (C and D) during 2 years of treatment during the double-blind phase of the DPP.
Results including DPPOS—open-label treatment
During the total follow-up period, 62% of the metformin participants were in the ≥50% or highly adherent categories compared with 72% in the 2-year double-blind phase (Table 1). Placebo was discontinued when the open-label phase began and adherence could not be assigned.
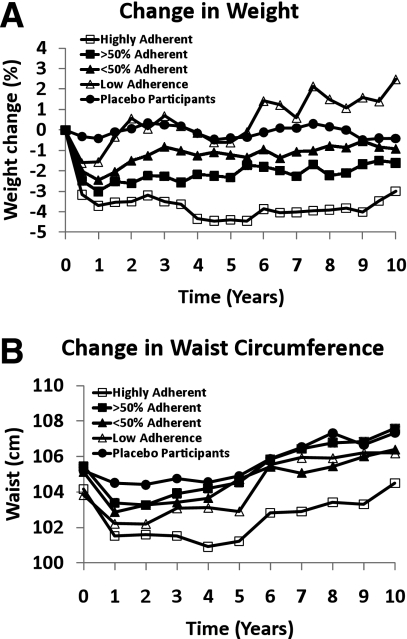
Among placebo participants, body weight was relatively stable but waist circumference increased after the fourth year, continuing throughout the entire open-label follow-up (Fig. 2). Over the total follow-up, average weight loss from baseline in the metformin treatment group, independent of adherence, was 2.0% (1.9 kg). Among those highly adherent to metformin, weight loss from baseline was 3.5% (3.1 kg). Among those with partial adherence to metformin, weight loss was intermediate between the highly adherent group and those on placebo. In the low adherence group, weight initially fell, followed by weight change similar to the placebo participants until 5 years, followed by weight increase. The percent of metformin participants who lost at least 5% was positively associated with metformin adherence (Table 1). Waist circumference remained significantly lower than at baseline in the highly adherent group (P < 0.05) through the 7-year visit, whereas in the partially adherent groups it was lower up to the 4-year visit, and in the low-adherence group only through 2 years. Although weight and waist changes over time varied by race/ethnicity and sex, there was no interaction between level of adherence and either race/ethnicity or sex indicating that the effect of metformin adherence on weight loss was consistent across race/ethnic groups and in men and women.
Figure 2.
Change in weight (A) and change in waist circumference (B) throughout the DPP and the DPPOS by placebo and adherence to metformin.
Safety and tolerability
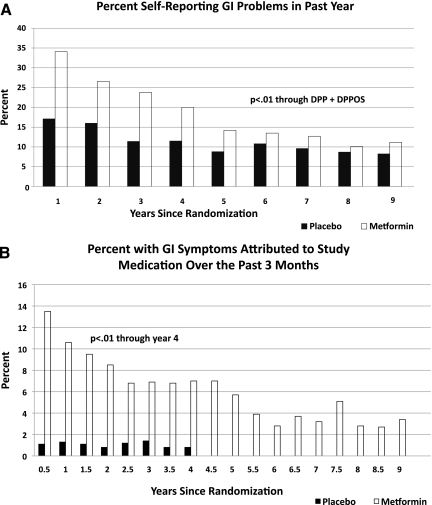
During the DPP (through year 4), reports of gastrointestinal symptoms were more common among metformin compared with placebo participants (average 28% vs. 16%, P = 0.01) (Fig. 3A). Metformin participants self-reported “study medication-related” gastrointestinal symptoms more frequently than placebo participants (9.5% vs. 1.1%, P < 0.001) (Fig. 3A). Both types of gastrointestinal symptom reports declined throughout the DPP. Rates of gastrointestinal symptoms were similar between groups by years 6 through 9 (P > 0.10). These results persisted after excluding all visits for participants who had low adherence to the study medication. Although reported symptoms varied by race/ethnicity and sex, changes in symptoms over time were similar by demographic group (data not shown).
Figure 3.
Self-reported gastrointestinal (GI) problems (A) and gastrointestinal symptoms attributed to study medication (B) through the DPP and the DPPOS.
No unexpected adverse events or treatment group differences in adverse events were identified, either by the study’s Data and Safety Monitoring Board or in this analysis. Adverse events during the DPP were previously reported (4). The rate of gastrointestinal symptoms was higher in the metformin group. Nonserious adverse events for hypoglycemia and anemia during the DPP were also uncommon and similar in metformin and placebo participants, with seven metformin participants and eight placebo participants ever reporting hypoglycemia, and fifty metformin participants and thirty-eight placebo participants ever reporting anemia. SAEs potentially related to study medication were rare. There were three SAE reports for anemia (two metformin and one placebo participant), and there were none for lactic acidosis or hypoglycemia during nearly 18,000 patient-years of follow-up.
During the DPP, average hemoglobin and hematocrit levels were the same at baseline and over time were slightly lower in the metformin group than in the placebo group (hemoglobin: 13.6 vs. 13.8 mg/dL; hematocrit: 40.6 vs. 41.1%; P < 0.001 for both). Hemoglobin and hematocrit levels varied by race/ethnicity and sex; however, changes over time were similar by demographic group (data not shown). The percent of participants with low hemoglobin was not significantly different between metformin and placebo participants (11.2 vs. 7.6%, P = 0.17) whereas the percent of participants with low hematocrit was higher in metformin than placebo participants (12.6 vs. 8.4%, P = 0.035). Among metformin participants, changes in hemoglobin and hematocrit occurred during the first year following randomization with no further changes observed over time.
CONCLUSIONS
We report the longest follow-up to date of metformin on body weight changes and on safety and tolerability. Metformin used in overweight or obese individuals with elevated fasting glucose and impaired glucose tolerance was associated with modest but durable weight loss and was safe and well tolerated over many years.
On an intent-to-treat basis, metformin produced a significant weight loss that persisted during the 2-year double-blind treatment period and for the entire duration of follow-up. Long-term follow-up was excellent at 92%, in contrast with other weight loss drug trials (12). Effects of metformin on weight have been reported in several trials in diabetes (2,3,13,14,15), one in obese adolescents (16), and in a recent meta-analysis (17). One report included changes in waist-to-hip circumference ratio, and only three provided data for more than 1 year of follow-up. In a meta-analysis of metformin and weight loss, weight change at 1 year was −1.52 kg (95% CI −2.82 to −0.21) (17). The mean weight change at 1 year in our study was −2.7 kg in the metformin group and −0.4 kg in the placebo group.
Adherence to metformin improved the magnitude of weight loss—but not waist circumference—during the first 2 years. Adherence to placebo did not affect weight loss, suggesting that metformin, rather than nonspecific adherence to positive health behaviors, was the relevant factor.
Waist circumference increased in all groups after year 2, except for the highly adherent participants where the increase began after 5 years and waist circumference remained lower than baseline. Since body weight did not increase, this suggests that central adiposity increased by redistribution of body fat.
Metformin participants in the low adherence group lost weight but placebo participants with low adherence did not. Many of those in the low adherence metformin group took some metformin but not enough to be labeled “adherent.” In addition, those with low adherence in the placebo group may have personality traits related to accepting advice, ability to change habits, etc., whereas low adherence in the metformin group may also be related to gastro-intestinal side effects. Therefore, one would not expect those with low adherence in the two groups to have the same characteristics.
Metformin lowers glucose and reduces risk for diabetes in part through weight loss (2,7,16,17). Although basal energy metabolism is highly correlated with body mass, early studies showed that despite appreciable reductions in body weight with metformin treatment, basal energy expenditure remains unchanged (18). This is because metformin-induced weight loss is almost exclusively confined to reductions in adipose mass (2,16,18) with little change in lean tissue. This pattern is different from that seen with caloric restriction, which tends to induce loss of lean tissue as well as adipose tissue. Metformin has several effects on energy metabolism that parallel physical exercise. Both exercise and metformin stimulate phosphorylation of AMP-activated protein kinase (AMPK) (19). AMPK is an important regulator of mitochondrial biogenesis (20), hepatic and muscle fatty acid oxidation, glucose transport, insulin secretion, and lipogenesis (21). Whether metformin directly affects energy expenditure from physical activity is unknown. Metformin might also influence weight loss through reduced food intake owing to irritation of the gastrointestinal tract, which may motivate a reduction in food intake or change in nutritional preference.
Adherence to metformin was high. In the double-blind phase of the trial, 72% were highly adherent, and throughout the entire trial, 62% of the metformin group was highly adherent.
No significant safety issues were identified. Both hemoglobin and hematocrit declined slightly in the metformin group over the first year after randomization and stabilized after that. Metformin participants reported more gastrointestinal symptoms than placebo participants, however these abated over time and both types of gastrointestinal symptom reports were similar between groups by the latter years of the DPPOS.
One potential bias is that 12% of DPP participants chose not to continue into the DPPOS. It is likely that some of those who discontinued the study or who continued but chose not to take open-label metformin did so because of side effects, which may have influenced the safety and tolerability profile of metformin during the open-label DPPOS period.
In summary, metformin produces a highly significant reduction in body weight and waist circumference with minimal safety issues and limited issues of tolerability (22). The weight reduction persists for up to 10 years and is related to adherence to metformin. Waist circumference initially declines, then steadily increases after a nadir at 12–36 months in all groups except in the highly adherent group, in which this increase was delayed for 5 years. Metformin was well tolerated with few side effects.
Acknowledgments
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health provided funding to the clinical centers and the DPP Coordinating Center for the design and conduct of the study, and collection, management, analysis, and interpretation of the data. The Southwestern American Indian Centers were supported directly by the NIDDK, including its Intramural Research Program, and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, supported data collection at many of the clinical centers. Funding was also provided by the National Institute of Child Health and Human Development; the National Institute on Aging; the National Eye Institute; the National Heart, Lung, and Blood Institute; the Office of Research on Women’s Health; the National Institute on Minority Health and Health Disparities; the Centers for Disease Control and Prevention; and the American Diabetes Association.
Bristol-Myers Squibb and Parke-Davis provided additional funding and material support during the DPP. Lipha (Merck-Sante) provided medication, and LifeScan Inc. donated materials during the DPP and the DPPOS. P.W.F. was supported in part by grants from Novo Nordisk, the Swedish Heart-Lung Foundation, the Swedish Diabetes Association, and the Swedish Research Council. No other potential conflicts of interest relevant to this article were reported.
The full research group designed and undertook the study. G.A.B. researched data, participated in the reviewing and modifying of data analyses, drafted the first draft, wrote the manuscript, reviewed and edited the manuscript, and is the guarantor of the manuscript and, as such, had full access to all the data in the study and takes responsibitility for the integrity of the data and the accuracy of the data analysis. S.L.E. researched data, participated in the reviewing and modifying of data analyses, performed all statistical analyses, wrote the manuscript, and reviewed and edited the manuscript. J.P.C. researched data, participated in the reviewing and modifying of data analyses, wrote the manuscript, and reviewed and edited the manuscript. V.R.A. participated in the reviewing and modifying of data analyses and reviewed and edited the manuscript. P.W.F. participated in the reviewing and modifying of data analyses, wrote the manuscript, and reviewed and edited the manuscript. W.F. participated in the reviewing and modifying of data analyses and reviewed and edited the manuscript. E.H. researched data, participated in the reviewing and modifying of data analyses, and reviewed and edited the manuscript. S.J. participated in the reviewing and modifying of data analyses and reviewed and edited the manuscript. M.M. participated in the reviewing and modifying of data analyses and reviewed and edited the manuscript. S.M. participated in the reviewing and modifying of data analyses and reviewed and edited the manuscript. F.X.P.-S. researched data, participated in the reviewing and modifying of data analyses, and reviewed and edited the manuscript. N.H.W. researched data, participated in the reviewing and modifying of data analyses, wrote the manuscript, and reviewed and edited the manuscript. W.C.K. researched data, participated in the reviewing and modifying of data analyses, wrote the manuscript, and reviewed and edited the manuscript.
The research group gratefully acknowledges the commitment and dedication of the participants of the DPP and the DPPOS.
APPENDIX
Members of the writing group are George A. Bray, MD1; Sharon L. Edelstein, ScM2; Jill P. Crandall, MD3; Vanita R. Aroda, MD4; Paul W. Franks, MD5,6; Wilfred Fujimoto, MD7; Edward Horton, MD8; Susan Jeffries, BSN, MSN9; Maria Montez, RN, MSHP, CDE, CCRA10; Sunder Mudaliar, MD11; F. Xavier Pi-Sunyer, MD12; Neil H. White, MD13; and William C. Knowler, MD, DrPH14.
From the 1Pennington Biomedical Research Center, Baton Rouge, Louisiana; 2The George Washington University Biostatistics Center, Rockville, Maryland; the 3Albert Einstein College of Medicine, Bronx, New York; the 4Medstar Health Research Institute, Hyattsville, Maryland; the 5Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden; the 6Harvard School of Public Health, Boston, Massachusetts; the 7University of Washington, Seattle, Washington; the 8Joslin Diabetes Center, Boston, Massachusetts; the 9University of Pittsburgh, Pittsburgh, Pennsylvania; the 10University of Texas Health Sciences Center at San Antonio, San Antonio, Texas; the 11University of California, San Diego, San Diego, California; the 12St. Luke’s-Roosevelt Hospital Center, New York, New York; the 13Washington University School of Medicine, St. Louis, Missouri; and the14National Institute of Diabetes and Digestive and Kidney Diseases, Phoenix, Arizona.
Footnotes
Clinical trial reg. nos. NCT00004992 (DPP) and NCT00038727 (DPPOS), clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1299/-/DC1.
*A complete list of the members of the Diabetes Prevention Program Research Group, centers, and staff can be found in the Supplementary Data online, and members of the writing group are listed in the appendix.
The opinions expressed are those of the investigators and do not necessarily reflect the views of the funding agencies.
References
- 1. DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med 1999;131:281–303 [DOI] [PubMed] [Google Scholar]
- 2. UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 3. Bray GA, Greenway FL. Pharmacological treatment of the overweight patient. Pharmacol Rev 2007;59:151–184 [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crandall JP, Knowler WC, Kahn SE, et al. ; Diabetes Prevention Program Research Group The prevention of type 2 diabetes. Nat Clin Pract Endocrinol Metab 2008;4:382–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knowler WC, Fowler SE, Hamman RF, et al. ; Diabetes Prevention Program Research Group 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lachin JM, Christophi CA, Edelstein SL, et al. ; DDK Research Group Factors associated with diabetes onset during metformin versus placebo therapy in the Diabetes Prevention Program. Diabetes 2007;56:1153–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. The Diabetes Prevention Program Research Group The Diabetes Prevention Program: baseline characteristics of the randomized cohort. Diabetes Care 2000;23:1619–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diabetes Prevention Program Research Group Effects of withdrawal from metformin on the development of diabetes in the Diabetes Prevention Program. Diabetes Care 2003;26:977–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Venditti E, Bray GA, Carrion-Petersen ML, et al. ; the Diabetes Prevention Program Research Group. First versus repeat treatment with a lifestyle intervention program: attendance and weight loss outcomes. Int J Obes 2008;32:1537–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diggle PJ, Liang K-Y, Zeger SL. Analysis of longitudinal data. New York, Oxford University Press, 1994. [Google Scholar]
- 12. Simons-Morton DG, Obarzanek E, Cutler JA. Obesity research—limitations of methods, measurements, and medications. JAMA 2006;295:826–828 [DOI] [PubMed] [Google Scholar]
- 13. Fontbonne A, Charles MA, Juhan-Vague I, et al. ; BIGPRO Study Group The effect of metformin on the metabolic abnormalities associated with upper-body fat distribution. Diabetes Care 1996;19:920–926 [DOI] [PubMed] [Google Scholar]
- 14. Teupe B, Bergis K. Prospective randomized two-years clinical study comparing additional metformin treatment with reducing diet in type 2 diabetes. Diabete Metab 1991;17:213–217 [PubMed] [Google Scholar]
- 15. Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V; Indian Diabetes Prevention Programme (IDPP) The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–297 [DOI] [PubMed] [Google Scholar]
- 16. Yanovski JA, Krakoff J, Salaita CG, et al. Effects of metformin on body weight and body composition in obese insulin-resistant children: a randomized clinical trial. Diabetes 2011;60:477–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Avenell A, Broom J, Brown TJ, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess 2004;8:iii–iv, 1–182 [DOI] [PubMed] [Google Scholar]
- 18. Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med 1995;333:550–554 [DOI] [PubMed] [Google Scholar]
- 19. Schimmack G, DeFronzo RA, Musi N. AMP-activated protein kinase: role in metabolism and therapeutic implications. Diabetes Obes Metab 2006;8:591–602 [DOI] [PubMed] [Google Scholar]
- 20. Zong H, Ren JM, Young LH, et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci USA 2002;99:15983–15987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol 1996;270:E299–E304 [DOI] [PubMed] [Google Scholar]
- 22. Fujioka K, Brazg RL, Raz I, et al. Efficacy, dose-response relationship and safety of once-daily extended-release metformin (Glucophage XR) in type 2 diabetic patients with inadequate glycaemic control despite prior treatment with diet and exercise: results from two double-blind, placebo-controlled studies. Diabetes Obes Metab 2005;7:28–39 [DOI] [PubMed] [Google Scholar]