Abstract
OBJECTIVE
The metabolic syndrome (MetS) is highly prevalent and confers an increased risk for diabetes and cardiovascular disease (CVD). While MetS is a proinflammatory state, there is a paucity of data on cellular inflammation in MetS. Toll-like receptors (TLRs) are classical pattern recognition receptors of the innate immune response.
RESEARCH DESIGN AND METHODS
The aim of this study was to examine monocyte TLR2 and TLR4 in MetS patients without diabetes or CVD and control subjects since both of the receptors have been implicated in atherosclerosis and insulin resistance. Fasting blood was obtained for TLR expression and activity.
RESULTS
Circulating levels of high-sensitivity C-reactive protein, interleukin (IL)-1β, IL-6, IL-8, and soluble tumor necrosis factor receptor 1 (sTNFR1) were significantly increased in MetS versus control subjects following adjustment for waist circumference. There was a significant increase in both TLR2 and TLR4 surface expression and mRNA on monocytes after adjustment for waist circumference. In addition to increased nuclear factor-κB nuclear binding, there was significantly increased release of IL-1β, IL-6, and IL-8 in MetS versus control subjects following priming of the monocytes with lipopolysaccharides. While both plasma free fatty acids and endotoxin were increased in MetS, they correlated significantly with TLR4 only.
CONCLUSIONS
In conclusion, we make the novel observation that both TLR2 and TLR4 expression and activity are increased in the monocytes of patients with MetS and could contribute to increased risk for diabetes and CVD.
Metabolic syndrome (MetS) affects one in three U.S. adults and confers an increased risk for both diabetes and cardiovascular disease (CVD) (1,2). It is clearly becoming apparent that, in addition to the diagnostic criteria used for MetS by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III), both insulin resistance and low-grade inflammation, as documented by circulating biomarkers, are also evident (3–5). However, with regards to the low-grade inflammation, there is a paucity of data with regards to the cellular basis for the proinflammatory phenotype of MetS uncomplicated by diabetes and/or CVD (6). Recently, we showed that subcutaneous adipose tissue of nascent MetS patients without diabetes or CVD produced increased levels of adipokines compared with matched control subjects, and they correlated with both the proinflammatory state and the insulin resistance of these patients (6). However, the sentinel cell of inflammation and innate immunity is the monocyte/macrophage. There is scanty data on monocyte function in patients with MetS. Natal et al. (7) showed increased monocyte CD40L/CD40 expression in MetS but did not report on downstream signaling or biomediators of this dyad. In this study, we provide further evidence for the proinflammatory state of MetS by studying the classical pathogen recognition receptors of the innate immune response, Toll-like receptors (TLRs). The TLRs that we studied included TLR2 and TLR4 since both of the receptors have been incriminated in both atherosclerosis and insulin resistance (8–13).
RESEARCH DESIGN AND METHODS
Patients and methods
All subjects were recruited from Sacramento County, California, through fliers and advertisements in the newspaper. The subjects (aged 21–70 years) with MetS (n = 49) and healthy control subjects (n = 41) were studied. MetS was defined using the criteria of the NCEP ATP III (1,2). Briefly, subjects classified as MetS must have at least three risk factors to sustain the diagnosis, including central obesity, hypertension, dyslipidemia (low HDL, high triglycerides), and/or hypertension on antihypertensive medications. Control subjects needed to have ≤2 features of MetS and not be on blood pressure medications. Other exclusion criteria for control subjects were fasting plasma glucose (>100 mg/dL) and triglycerides (TGs) (>200 mg/dL) (14).
Other exclusion criteria for both groups were diabetes, clinical atherosclerosis (coronary artery disease, peripheral arterial disease, CVD, etc.), smoking, hypo- or hyperthyroidism, malabsorption, anticoagulant therapy, steroid therapy, anti-inflammatory drugs, statin and other hypolipidemic therapy, hypoglycemic agents, angiotensin receptor blockers, TG >400 mg/dL (for MetS subjects), oral contraceptives, use of antioxidant supplements in the past 6 months, pregnancy, abnormal complete blood count, alcohol consumption >1 oz/day, consumption of n-3 polyunsaturated fatty acid, postmenopausal women on estrogen replacement therapy, active wounds, recent surgery, inflammatory or malignant disease, C-reactive protein (CRP) >10 mg/L, and chronic high-intensity exercisers (exercise >100 min/week). Diabetes was excluded by two fasting glucose levels (screening and day of monocyte isolation) <126 mg/dL and an HbA1c <6.5%.
Informed consent was obtained from participants in the study, which was approved by the institutional review board at the University of California Davis. All human investigation was conducted according to the principles expressed in the Declaration of Helsinki. After history and physical examination, fasting blood was obtained. A complete blood count, plasma lipid and lipoprotein profile, urea nitrogen, creatinine, aspartate aminotransferase, alanine aminotransferase, glucose, and thyrotropin were assayed by standard laboratory techniques in the Clinical Pathology Laboratory. Insulin levels were assayed by enzyme-linked immunosorbent assay (Linco Biosystems), and homeostasis model assessment of insulin resistance (HOMA-IR) was calculated from glucose and insulin levels as previously described (14–16).
After subjects were screened, if they met the selection criteria, they were requested to come to the University of California Davis Clinical and Translational Science Center Clinical Research Center for a fasting blood draw. Plasma free fatty acid (FFA) levels were assayed as previously described (16), and endotoxin levels were quantitated using reagents from Lonza (Limulus Amebocyte Lysate, QCL 1000; Walkersville, MD).
Levels of soluble tumor necrosis factor receptor 1 (sTNFR1) and 2 (sTNFR2) were assayed using a quantitative sandwich enzyme immunoassay technique using monoclonal antibodies to sTNFR1 and 2, respectively (R&D Biosystems, MN). All the above assays were performed in duplicate.
Monocyte isolation
Mononuclear cells were isolated from fasting heparinized blood by Ficoll Hypaque centrifugation followed by magnetic separation using the depletion technique (Miltenyi Biotech, Auburn, CA) as previously described (15). Using this technique, more than 92% of cells were identified as monocytes by CD14 staining. Isolated monocytes were studied before and after activation with lipopolysaccharides (LPS) (from Escherichia coli 026:B6, 100 ng/mL; Sigma Chemicals, St. Louis, MO). Supernatants were collected after an overnight incubation for assessment of cytokines/chemokines by multiplex immunoassays as previously described (15,16).
Surface expression of TLR2 and TLR4
Monocytes from control and MetS subjects were incubated with anti-human TLR2 and TLR4 antibodies (InvivoGen) or isotype controls, and surface expression of TLR2 and TLR4 were analyzed using BD FACSArray (Franklin Lakes, NJ) after gating for CD14 as previously described (15,16). Results were expressed as mean fluorescence intensity of 10,000 cells. Intra-assay and interassay coefficient of variation for TLR2 and TLR4 expression was <5% and <15%, respectively.
TLR2 and TLR4 mRNA expression
RNA was isolated from the monocytes using TRIzol reagent (Invitrogen, Carlsbad, CA). RT-PCR was performed using TLR2, TLR4, and 18s RNA primers (Invivogen, San Diego, CA) following the manufacturer's cycling parameters. Band intensities were determined using Image Quant Software (GE Healthcare Biosciences, Piscataway, NJ) as previously described (15) and expressed as ratio of TLR mRNA/18s RNA.
Cell signaling studies
Monocyte nuclear extracts were prepared as previously described (15,16), and nuclear factor (NF)-κB p65 activity in the nuclear extracts were assessed using reagents from Bio-Rad using the Bioplex multiplex assays following the manufacturer's instructions. The intra-assay coefficients of variations of the assays were <14%.
Statistical analysis
Data are expressed as mean ± SD or, for skewed variables, as median and interquartile range. Log transformations were applied to skewed data prior to parametric analyses. Comparisons between the control and MetS groups were made with two-sample t tests. Spearman rank correlation coefficients were computed to assess the association between metabolic risk factors and monocyte activity. Multiple regression models were constructed to evaluate predictors of monocyte-dependent variables. Independent variables assessed included age, BMI, waist circumference, blood pressure, plasma glucose, TGs, HOMA-IR, LDL cholesterol, HDL cholesterol, and CRP, FFA, and endotoxin. Data were analyzed using SAS version 9.1.3 (SAS Institute, Cary, NC).
RESULTS
Table 1 shows salient characteristics of the studied population. In addition to the features of MetS, which are significantly abnormal in the patients with MetS, it is important to appreciate that both high-sensitivity CRP (hs-CRP) levels and HOMA-IR are also increased. Also circulating levels of IL-1β, IL-6, and IL-8 and sTNFR1 levels were significantly increased in MetS versus control subjects even following adjustment for waist circumference. However, both TNF and monocyte chemoattractant protein (MCP)-1 levels were similar, and there was a trend to significance in sTNFR2 (P = 0.08, following waist circumference adjustment).
Table 1.
Baseline characteristics
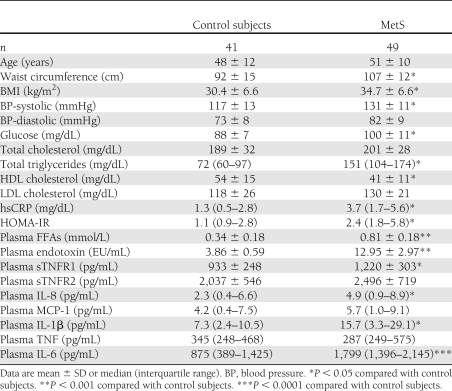
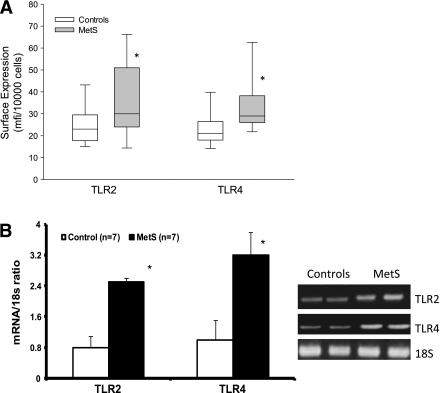
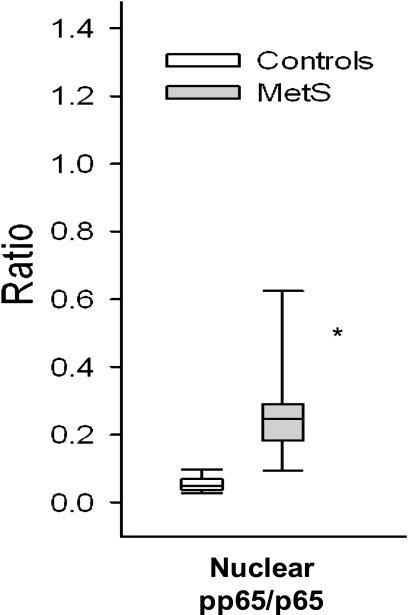
With regard to monocyte biology, Fig. 1A depicts TLR2 and TLR4 levels quantitated by flow cytometry. There was a significant increase in both TLR2 and TLR4 in MetS that persisted after adjustment for waist circumference (Fig. 1) and also BMI (data not shown). In a subgroup we showed increased TLR2 and TLR4 mRNA expression on monocytes in MetS compared with control subjects (Fig. 1B). A pivotal downstream readout of the activated TLR2 and TLR4 pathways is NF-κB, cytokines, and chemokines. As shown in Fig. 2, there is increased nuclear NF-κB binding in MetS (P < 0.001). Figure 3 shows significant increases in IL-1β, IL-6, and IL-8 in MetS versus control subjects following priming of the monocytes with the classical TLR4 agonist, LPS (P < 0.05). However with regard to MCP-1 and TNF-α, the differences between the two groups were not significant (MCP-1: controls 156 [129–998] vs. MetS 432 [171–895] pg/mL; TNF: controls 345 [248–468] vs. MetS 287 [250–583] pg/mL; data presented as median and interquartile range, P > 0.05).
Figure 1.
A: Monocyte TLR expression. Monocytes were isolated by negative magnetic separation, and surface expression of TLR2 and TLR4 were assessed by flow cytometry as described in Research Design and Methods. *P < 0.01 compared with control subjects. B: TLR2 and TLR4 mRNA expression. Representative RT-PCR gel showing significantly increased TLR2 and TLR4 mRNA expression in monocytes of MetS compared with control subjects. Densitometric values are normalized to 18 sRNA and expressed as mean ratio ± SD (n = 7) as described in Research Design and Methods. *P < 0.05 compared with control subjects.
Figure 2.
Monocyte signaling. Monocytes were isolated by negative magnetic separation and nuclear extracts for p65 of NF-κB assay by multiplex immunoassays as described in Research Design and Methods. *P < 0.001 compared with control subjects.
Figure 3.
Monocyte cytokines in MetS. Monocytes were isolated by negative magnetic separation and incubated overnight with LPS (100 ng/mL). Cell supernatants were used for multiplex immunoassay analyses of chemokines/cytokines and expressed per milligram cell protein as described in Research Design and Methods. *P < 0.05 compared with control subjects.
We correlated features of MetS with TLR2 and TLR4. TLR2 correlated significantly with waist circumference (r = 0.22, P < 0.05) and blood pressure (r = 0.32, P = 0.002). TLR4 correlated significantly with plasma glucose (r = 0.21, P < 0.05) and TGs (r = 0.28, P = 0.01). TLR4 correlated significantly with the following: IL-Iβ (r = 0.22, P = 0.045), MCP-1 (r = 0.23, P = 0.04), and NF-κB activity (r = 0.31, P = 0.006). TLR2 correlated significantly with IL-6 (r = 0.28, P = 0.02) and NF-κB activity (r = 0.32, P = 0.006). While there was a trend to a positive correlation between HOMA and TLR2 (r = 0.19, P = 0.08), there was a significant correlation between TLR4 and HOMA (r = 0.26, P = 0.020).
To gain some mechanistic insights, we assayed both FFA and endotoxin levels in this clinical study. Compared with control subjects, patients with MetS had significantly elevated levels of both FFA and endotoxin (P < 0.001) (Table 1). Hence, we undertook correlations between TLR2 and TLR4 and endotoxin and FFA. Not surprisingly, we found a significant correlation between TLR4 and endotoxin (r = 0.61, P < 0.0001), but not with TLR2 (r = 0.15, P = 0.37). Also, we found a significant correlation between FFA and TLR4, (r = 0.53, P = 0.0006), but not with TLR2 (r = 0.12, P = 0.47).
We have previously shown a decreased number of progenitor cells (CD34 positivity) and endothelial progenitor cells (dual CD34 and kinase insert domain-containing receptor [KDR] positivity) in patients with MetS (14). As recently reviewed, both these cells predict cardiovascular events and are emerging as a biomarker of vascular health (17). Both TLR2 and TLR4 correlated significantly with progenitor cell numbers (r = −0.38, P = 0.002 and r = −0.25, P = 0.048, respectively), while only TLR4 correlated significantly with endothelial progenitor cells (r = −0.25, P = 0.049) with a trend for TLR2 (r = −0.23, P = 0.07).
CONCLUSIONS
MetS is a cluster of cardiometabolic risk factors that predisposes to both diabetes and CVDs. Although there is a large body of data on inflammation in obesity in both animal models and humans, not all individuals with obesity are at a high metabolic risk, and in the National Health and Nutrition Examination Survey data, 31.7% of obese persons appeared to have a low-risk metabolic phenotype (18). Hence in this study, we decided to probe the sentinel cell of inflammation in a high-risk obesity state, MetS (6). In accord with our previous findings for both type 1 and type 2 diabetes (15,16), in this article we show that patients with nascent MetS, without the confounders of diabetes or CVD, have increased TLR2 and TLR4 activity that cannot be simply explained by adiposity.
This was manifest by both increased cell surface receptor numbers, increased mRNA levels, increase in signaling through NF-κB activity, and increased levels of IL-1, IL-8, IL-6 and sTNFR1. We are unclear why MCP-1 levels are not increased in these patients’ serum- and LPS-primed monocytes in spite of previously showing increased release from subcutaneous adipose tissue (6). While serum TNF levels were not increased, there was a significant increase in sTNFR1 and a trend to an increase in sTNFR2. Interestingly, most of the biological functions of TNF are mediated via TNFR1 (19). Furthermore, the sTNFRs can bind TNF with high affinity and functions as TNF antagonists. Thus, the observed increase in sTNFRs could explain the failure to detect elevated levels of TNF since they bind to TNF.
Previous studies in animal models have shown that knockout of both these receptors, TLR2 and TLR4, attenuates the progression of atherosclerosis insulin resistance and diabetes (8–13). Hence the increased activity reported here in these patients could be advanced as a plausible mechanism for their increased risk for diabetes and CVD since cellular receptor abundance correlated with HOMA, a measure of insulin resistance, and biomediators such as IL-6, IL-1, and IL-8. However, given that this is a rapidly evolving field, we cannot conclude that either TLR2 or TLR4 is more important in the genesis of diabetes. With respect to diabetic vascular complications, Nymark et al. (20) showed that the classical ligand for TLR4, LPS, predicted the progression of diabetic nephropathy. Most recently, our group showed that compared with wild-type, streptozotocin-induced diabetes, TLR2 knockout mice attenuated the progression of diabetic nephropathy (21).
With respect to nascent MetS, in the only previous study that has looked at cellular inflammation, the investigators focused on the monocyte CD40L/CD40 dyad and showed that it was upregulated supporting increased cellular inflammation (7). In that study, however, the investigators did not examine the signaling pathway and the relevant biomediators. They also used BMI and not waist circumference as a criteria to define MetS. In patients with MetS with diabetes, Fortuño et al. (22) showed increased NADPH oxidase activity and increased plasma levels of both nitrotyrosine and oxidized LDL levels, clearly supporting increased oxidative stress. Also, Nakagomi et al. (23) showed increased tissue factor procoagulant activity in patients with MetS, which correlated with increased endotoxin levels, CRP, and HOMA. However, it is unclear from their reports if they excluded patients with diabetes and smokers because in their second report, which showed a correlation with carotid intima-media thickness (24), 67% of the patients were diabetic and 63% were smokers. Nonetheless, their studies at the cellular level support the posit that MetS is a procoagulant state. Since there is such sparse data on monocyte function in MetS and, in our view, it is a high-risk obesity state, it is important to point out that in patients with obesity, mononuclear cell, monocyte and adipose tissue TLRs, and other accepted biomarkers of inflammation have been reported. Ghanim et al. (5) made a novel observation that in 16 obese patients compared with control subjects, obesity was a proinflammatory state by showing increased NF-κB activity and increased levels of IL-6, TNF, migration inhibitory factor, and matrix metalloproteinase (MMP)-9. They also showed that the inflammatory mediators were related to BMI. Thus, we confirm this finding in a high-risk obesity state and argue that MetS is a proinflammatory state independent of adiposity since all our reported findings persisted following correction for both waist circumference and BMI.
Thus, a brief summary of the published studies support the notion that MetS at the cellular level is a proinflammatory state (adipose tissue and monocytes contributing), a procoagulant state, and associated with increased oxidative stress. A weakness of the current study is that we provide little in the way of mechanistic insight for the increased TLR activity. Potential culprits include FFA, endotoxin, and CRP (13,25,26). We found no significant correlation with CRP and TLRs. However since TLR4 receptor abundance correlated with both endotoxin and FFA levels, it appears that both of these factors could be accounting in part for the increase in TLR4 activity. Mechanisms that have been advanced to explain TLR2 and TLR4 ligand recognition and receptor activation with FFA include increased reactive oxygen species and fatty acid acylation in lipid (essential moiety of LPS) or bacterial lipoproteins (27). Importantly, neither endotoxin nor FFA correlated significantly with TLR2 receptor abundance suggesting that other factors could be at play. In patients with diabetes, we have shown (15,28) increased levels of endogenous ligands such as high-mobility group box 1 (HMGBI), heat shock protein 60 (HSP60), and HSP70. In future studies, we will determine the contribution of these ligands on both TLR2 and TLR4.
Since this study in its entirety examined endothelial progenitor cell biology (14), adipose tissue dysregulation (6), and monocyte TLR activity, we were limited by the amount of blood obtained to undertake all analyses.
In conclusion, we make the novel observation that both of the functional receptors TLR2 and TLR4 expression and activity are increased in the monocytes of patients with MetS and could contribute to their increased risk for both diabetes and CVD.
Acknowledgments
This study was supported by a grant from the National Institutes of Health (K24AT 00596) and an American Diabetes Association Clinical Research Grant (to I.J.).
No potential conflicts of interest relevant to this article were reported.
I.J. and S.D. wrote the manuscript. B.A.H. performed all the statistical analyses for the manuscript. H.K., A.C., and S.D. researched data. I.J. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Long Wang, PhD, Syracuse University, and Uma Singh, PhD, Seminole Community College, for help with subject recruitment while at the University of California Davis Medical Center. Gerred Smith, University of California Davis Medical Center, assisted in manuscript preparation.
References
- 1.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005;365:1415–1428 [DOI] [PubMed] [Google Scholar]
- 2.Alberti KGMM, Eckel RH, Grundy SM, et al. International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 3.Devaraj S, Rosenson RS, Jialal I. Metabolic syndrome: an appraisal of the pro-inflammatory and procoagulant status. Endocrinol Metab Clin North Am 2004;33:431–453 [DOI] [PubMed] [Google Scholar]
- 4.Devaraj S, Goyal R, Jialal I. Inflammation, oxidative stress, and the metabolic syndrome. Endocrinology 2008;4:32–37 [Google Scholar]
- 5.Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation 2004;110:1564–1571 [DOI] [PubMed] [Google Scholar]
- 6.Bremer A, Devaraj S, Afify A, Jialal I. Adipose tissue dysregulation I patients with metabolic syndrome. J Clin Endocrinol Metab 2011;96:E1782–E1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Natal C, Restituto P, Iñigo C, Colina I, Díez J, Varo N. The proinflammatory mediator CD40 ligand is increased in the metabolic syndrome and modulated by adiponectin. J Clin Endocrinol Metab 2008;93:2319–2327 [DOI] [PubMed] [Google Scholar]
- 8.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 2007;56:1986–1998 [DOI] [PubMed] [Google Scholar]
- 9.Ehses JA, Meier DT, Wueest S, et al. Toll-like receptor 2-deficient mice are protected from insulin resistance and beta cell dysfunction induced by a high-fat diet. Diabetologia 2010;53:1795–1806 [DOI] [PubMed] [Google Scholar]
- 10.Caricilli AM, Nascimento PH, Pauli JR, et al. Inhibition of toll-like receptor 2 expression improves insulin sensitivity and signaling in muscle and white adipose tissue of mice fed a high-fat diet. J Endocrinol 2008;199:399–406 [DOI] [PubMed] [Google Scholar]
- 11.Hasu M, Thabet M, Tam N, Whitman SC. Specific loss of toll-like receptor 2 on bone marrow derived cells decreases atherosclerosis in LDL receptor null mice. Can J Physiol Pharmacol 2011;89:737–742 [DOI] [PubMed] [Google Scholar]
- 12.Mullick AE, Tobias PS, Curtiss LK. Toll-like receptors and atherosclerosis: key contributors in disease and health? Immunol Res 2006;34:193–209 [DOI] [PubMed] [Google Scholar]
- 13.Erridge C. The roles of Toll-like receptors in atherosclerosis. J Innate Immun 2009;1:340–349 [DOI] [PubMed] [Google Scholar]
- 14.Jialal I, Devaraj S, Singh U, Huet BA. Decreased number and impaired functionality of endothelial progenitor cells in subjects with metabolic syndrome: implications for increased cardiovascular risk. Atherosclerosis 2010;211:297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dasu MR, Devaraj S, Park S, Jialal I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 2010;33:861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab 2008;93:578–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devaraj S, Jialal I. Dysfunctional endothelial progenitor cells in metabolic syndrome. Exp Diabetes Res 2012;2012:585018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med 2008;168:1617–1624 [DOI] [PubMed] [Google Scholar]
- 19.Apostolaki M, Armaka M, Victoratos P, Kollias G. Cellular mechanisms of TNF function in models of inflammation and autoimmunity. Curr Dir Autoimmun 2010;11:1–26 [DOI] [PubMed] [Google Scholar]
- 20.Nymark M, Pussinen PJ, Tuomainen AM, Forsblom C, Groop PH, Lehto M. FinnDiane Study Group. Serum lipopolysaccharide activity is associated with the progression of kidney disease in Finnish patients with type 1 diabetes. Diabetes Care 2009;32:1689–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devaraj S, Tobias P, Kasinath BS, Ramsamooj R, Afify A, Jialal I. Knockout of toll-like receptor-2 attenuates both the proinflammatory state of diabetes and incipient diabetic nephropathy. Arterioscler Thromb Vasc Biol 2011;31:1796–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fortuño A, San José G, Moreno MU, Beloqui O, Díez J, Zalba G. Phagocytic NADPH oxidase overactivity underlies oxidative stress in metabolic syndrome. Diabetes 2006;55:209–215 [DOI] [PubMed] [Google Scholar]
- 23.Nakagomi A, Sasaki M, Ishikawa Y, et al. Upregulation of monocyte tissue factor activity is significantly associated with low-grade chronic inflammation and insulin resistance in patients with metabolic syndrome. Circ J 2010;74:572–577 [DOI] [PubMed] [Google Scholar]
- 24.Nakagomi A, Sasaki M, Ishikawa Y, et al. Upregulation of monocyte tissue factor activity is significantly associated with carotid intima-media thickness in patients with metabolic syndrome. J Atheroscler Thromb 2011;18:475–486 [DOI] [PubMed] [Google Scholar]
- 25.Dasu MR, Jialal I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am J Physiol Endocrinol Metab 2011;300:E145–E154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boden G. Obesity and free fatty acids. Endocrinol Metab Clin North Am 2008;37:635–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JY, Zhao L, Hwang DH. Modulation of pattern recognition receptor-mediated inflammation and risk of chronic diseases by dietary fatty acids. Nutr Rev 2010;68:38–61 [DOI] [PubMed] [Google Scholar]
- 28.Devaraj S, Dasu MR, Park SH, Jialal I. Increased levels of ligands of Toll-like receptors 2 and 4 in type 1 diabetes. Diabetologia 2009;52:1665–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]