Abstract
OBJECTIVE
Individuals with normal glucose tolerance (NGT), whose 1-h postload plasma glucose is ≥155 mg/dL (NGT 1h-high), have an increased risk of type 2 diabetes. The purpose of this study was to characterize their metabolic phenotype.
RESEARCH DESIGN AND METHODS
A total of 305 nondiabetic offspring of type 2 diabetic patients was consecutively recruited. Insulin secretion was assessed using both indexes derived from oral glucose tolerance test (OGTT) and intravenous glucose tolerance test (IVGTT). Insulin sensitivity was measured by hyperinsulinemic-euglycemic clamp.
RESULTS
Compared with individuals with a 1-h postload plasma glucose <155 mg/dL (NGT 1h-low), NGT 1h-high individuals exhibited lower insulin sensitivity after adjustment for age, sex, and BMI. Insulin secretion estimated from the OGTT did not differ between the two groups of individuals. By contrast, compared with NGT 1h-low individuals, the acute insulin response during an IVGTT and the disposition index were significantly reduced in NGT 1h-high individuals after adjustment for age, sex, and BMI. Incretin effect, estimated as the ratio between total insulin responses during OGTT and IVGTT, was higher in NGT 1h-high individuals compared with NGT 1h-low individuals.
CONCLUSIONS
NGT 1h-high individuals may represent an intermediate state of glucose intolerance between NGT and type 2 diabetes characterized by insulin resistance and reduced β-cell function, the two main pathophysiological defects responsible for the development of type 2 diabetes. Postload hyperglycemia is the result of an intrinsic β-cell defect rather than impaired incretin effect.
Impaired glucose tolerance (IGT) identifies individuals with a dysglycemic condition intermediate between normal glucose tolerance (NGT) and type 2 diabetes (1). Individuals with IGT are at high risk for the future development of type 2 diabetes, and several clinical trials have shown that both lifestyle changes and pharmacological intervention prevent/halt the progression from IGT to overt type 2 diabetes (2–5). The results of these intervention studies highlight the importance of identifying individuals at high risk for type 2 diabetes in order to offer them an intervention program to reduce the incidence of the disease, and recently new guidelines for the screening of individuals for type 2 diabetes risks and diabetes prevention have been developed by a European multidisciplinary consortium (the Development and Implementation of a European Guideline and Training Standards for Diabetes Prevention [IMAGE] project) to provide evidence-based recommendations for health care practitioners, organizations, and funders on the prevention of type 2 diabetes in European health care settings (6,7). It is important to note that all clinical trials that have evaluated the impact of intervention strategies for preventing type 2 diabetes have recruited subjects with IGT (2–5). However, longitudinal studies have demonstrated that over one-third of individuals who develop type 2 diabetes have NGT at baseline (8), indicating that the use of IGT or impaired fasting glucose (IFG) categories as the sole means of identifying individuals at high risk for type 2 diabetes may overlook a considerable proportion of individuals who will develop type 2 diabetes over time. Recently, it has been reported that a cutoff of 155 mg/dL for 1-h postload plasma glucose during an oral glucose tolerance test (OGTT) can identify individuals at high risk for development of type 2 diabetes among those who have NGT (NGT 1h-high) (9–11). Addition of HbA1c levels to 1-h postload plasma glucose levels significantly increased their respective power in predicting development of type 2 diabetes risk, indicating that additional information about type 2 diabetes risk is embedded in HbA1c (12). Importantly, NGT 1h-high individuals exhibit early signs of subclinical organ damage including vascular atherosclerosis (13), reduced estimated glomerular filtration rate (14), left ventricular hypertrophy (15), and left ventricular diastolic dysfunction (16). The metabolic abnormalities responsible for 1-h postload hyperglycemia remain to be elucidated. Impaired insulin sensitivity and failure of pancreatic β-cells to compensate for the enhanced insulin demand are the principal factors responsible for the development and progression of type 2 diabetes. It is possible that defects in β-cells function and/or in the incretin effect occur at an earlier stage than IGT, i.e., in individuals who are considered to have NGT according to current diagnostic criteria. To gain a more deep insight into the metabolic abnormalities characterizing NGT 1h-high individuals, we evaluated insulin sensitivity assessed by hyperinsulinemic-euglycemic clamp as well as insulin secretion and the incretin effect by using both OGTT and intravenous glucose tolerance test (IVGTT) in a cohort of nondiabetic offspring of type 2 diabetic patients.
RESEARCH DESIGN AND METHODS
The study group comprised 305 nondiabetic offspring participating in the EUGENE2 (European Network on Functional Genomics of Type 2 Diabetes) project (17) who had one parent with type 2 diabetes and one parent without a history of type 2 diabetes and a normal response to an OGTT. All subjects were Caucasian and were consecutively recruited at the Department of Internal Medicine of the University of Rome “Tor Vergata” and at the Department of Medical and Surgical Sciences of the University “Magna Graecia” of Catanzaro. All subjects were characterized according to a previously described protocol (17,18). Briefly, after 12-h fasting, all individuals underwent anthropometrical evaluation including body composition evaluated by bioelectrical impedance, and a 75-g OGTT was performed with 0-, 30-, 60-, 90-, and 120-min sampling for plasma glucose and insulin. On the second visit, the subjects underwent an IVGTT and a hyperinsulinemic-euglycemic clamp. At 8 a.m., after a 12-h overnight fast, an intravenous catheter was placed in the antecubital vein for the infusion of glucose. Another cannula for blood sampling was inserted into a wrist vein surrounded by a heated box. After baseline blood collection, a bolus of glucose (300 mg/kg in a 50% solution) was given (within 30 s) into the antecubital vein to acutely increase the blood glucose level. Samples for the measurement of blood glucose and plasma insulin were drawn at 2, 4, 6, 8, 10, 20, 30, 40, 50, and 60 min. At 60 min after the glucose bolus, a continuous insulin infusion was initiated at the rate of 40 mU/m2 body surface area per min, after a priming dose, in order to reach and maintain a steady-state plasma insulin of 85 ± 9 μU/mL. Plasma glucose was assessed at 5-min intervals during the 2-h clamp study by a glucose analyzer. In the study subjects, mean plasma glucose concentration during the last hour of the clamp was 92 ± 4 mg/dL. The study was approved by institutional ethics committees, and informed consent was obtained from each subject in accordance with the principles of the Declaration of Helsinki.
Calculation
Subjects were classified as NGT (fasting plasma glucose [FPG] <126 mg/dL and 2-h postload <140 mg/dL) or IGT (FPG <126 mg/dL and 2-h postload 140–199 mg/dL). Acute insulin response (AIR) during the IVGTT was calculated as the incremental area under the curve (AUC) for insulin during the first 10 min of the IVGTT using the trapezoidal rule. Two indexes of insulin secretion were calculated from the OGTT data using the Stumvoll index (first-phase secretion = 1,283 + 1.829 × Ins30 − 138.7 × Gluc30 + 3.772 × Ins0) (19), where Ins is insulin and Gluc is glucose, and the ratio of total insulin AUC to total glucose AUC during 0–30 min of the OGTT (InsAUC30/GluAUC30). Glucose disposal (M) was calculated as the mean rate of glucose infusion measured during the last 60 min of the clamp examination (steady state) and is expressed as milligrams per minute per kilogram fat-free mass (MFFM) measured with the use of electrical bioimpedance. To evaluate β-cell function, the so-called disposition index was calculated as AIR × MFFM (20). The incretin effect was estimated as the ratio between total insulin responses during OGTT and IVGTT and expressed as percentage [100% × (AUCins OGTT – AUCins IVGTT)/AUCins OGTT] (21). Three indexes of insulin sensitivity, which have been shown to strongly correlate with insulin sensitivity measured by hyperinsulinemic-euglycemic clamp (22), have been calculated from the OGTT data: the Matsuda index [10,000/square root of (fasting glucose in mmol/L) × fasting insulin in mU/L] × [mean glucose × mean insulin during OGTT] (23); the simple index assessing insulin sensitivity using OGTT (SIISOGTT), 1/[log(G0 + G30 + G90 + G120) + log(I0 + I30 + I90 + I120)] (24); and the Avignon SiM index, ([0.137 × insulin sensitivity index derived from insulin and glucose concentrations in the basal state {Sib}] + Si2h)/2, where Sib = 108/[fasting insulin × fasting glucose × glucose distribution volume (VD)], Si2h = 108/(2-h insulin × 2-h glucose × VD), and VD = 150 mL/kg body weight (25).
Statistical analysis
Continuous variables are expressed as means ± SD. Categorical variables were compared by χ2 test. A general linear model with post hoc Bonferroni correction for multiple comparisons was used to compare differences of continuous variables between groups. For all analyses, a P value ≤0.05 was considered statistically significant. All analyses were performed using SPSS software, version 16.0, for Windows.
RESULTS
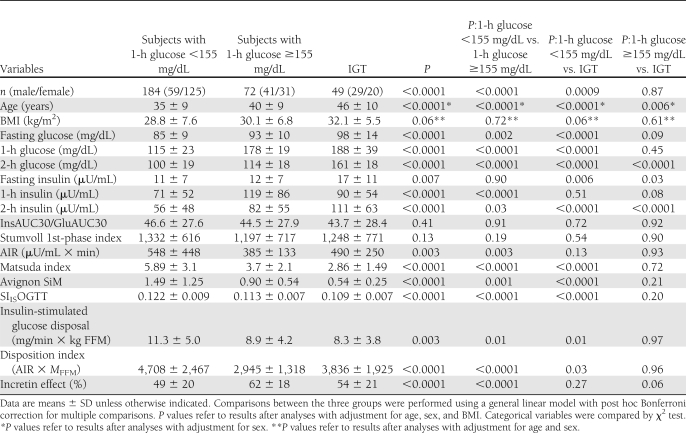
Of 305 nondiabetic individuals examined, 49 had IGT. We divided the subjects with NGT into two groups: 184 individuals with 1-h postload plasma glucose <155 mg/dL (NGT 1h-low) and 72 individuals with 1-h postload plasma glucose ≥155 mg/dL (NGT 1h-high). Table 1 shows the clinical characteristics and laboratory findings of the three study groups. Significant differences between the three groups were observed with respect to sex (higher prevalence of men among NGT 1h-high and IGT compared with NGT 1h-low subjects), age (NGT 1h-high and IGT subjects were older than NGT 1h-low subjects), and BMI (IGT subjects were heavier than NGT 1h-high and NGT 1h-low subjects). Therefore, all analyses were adjusted for age, sex, and BMI.
Table 1.
Anthropometric and clinical characteristics of the study subjects stratified according to glucose tolerance
By design, NGT 1h-high and IGT individuals had significantly higher 1-h and 2-h postload plasma glucose levels in addition to higher fasting plasma glucose compared with NGT 1h-low individuals. A significant reduction in peripheral insulin sensitivity, evaluated by the hyperinsulinemic-euglycemic clamp, was observed in NGT 1h-high and IGT individuals compared with NGT 1h-low individuals, but no differences were observed between the two former groups. Similarly, a significant reduction in insulin sensitivity was observed in NGT 1h-high and IGT individuals compared with NGT 1h-low individuals by using three validated OGTT-derived surrogate indexes such as the Matsuda, SIISOGTT, and Avignon SiM indexes (Table 1).
The InsAUC30/GluAUC30 and the Stumvoll first-phase indexes of insulin secretion, estimated from the OGTT, did not differ between the three groups of individuals. By contrast, compared with NGT 1h-low individuals, the AIR during an IVGTT, a direct measure of insulin secretion from β-cells, was significantly reduced in NGT 1h-high individuals but not in IGT individuals. As insulin secretion is dependent on actual insulin sensitivity, we compared the disposition index, calculated as the product of the insulin-stimulated glucose disposal and AIR indexes in the three groups of individuals. The disposition index was significantly lower in NGT 1h-high and IGT individuals compared with NGT 1h-low individuals, but no differences were observed between the two former groups.
The divergent results between estimates of glucose-stimulated insulin secretion during oral versus intravenous glucose administration in NGT 1h-high individuals compared with NGT 1h-low individuals raise the possibility that an increased incretin effect may account for these disparities. Since the glucose AUC during OGTT (219 ± 31, 264 ± 15, and 292 ± 29 for NGT 1h-low, NGT 1h-high, and IGT groups, respectively) was similar to the glucose AUC during IVGTT (220 ± 39, 250 ± 27, and 280 ± 30 for NGT 1h-low, NGT 1h-high, and IGT groups, respectively) in each of the three groups, we compared the incretin effect estimated as the ratio between total insulin responses during OGTT and IVGTT. The incretin effect was higher in NGT 1h-high individuals compared with NGT 1h-low individuals.
CONCLUSIONS
There is evidence that a significant proportion of individuals with NGT are at risk for type 2 diabetes (8), and recent studies have shown that a cutoff value of 155 mg/dL for 1-h postload glucose during an OGTT can identify NGT individuals at high risk for development of type 2 diabetes (9–11). In this study, we provide evidence that subjects with NGT, whose 1-h postload plasma glucose is ≥155 mg/dL, have a reduction in both insulin sensitivity and β-cell dysfunction, the two main pathophysiological defects responsible for the development of type 2 diabetes. We found that, compared with NGT 1h-low individuals, NGT 1h-high and IGT individuals had a similar impairment of insulin sensitivity, but only NGT 1h-high individuals had a defect in first-phase insulin secretion assessed by IVGTT. Because the amount of insulin secreted by the β-cell is strongly dependent on the prevailing degree of insulin sensitivity, accounting for differences in insulin sensitivity is a critical point when evaluating β-cell function. Thus, adjusting insulin secretion for the level of insulin sensitivity using the disposition index (insulin sensitivity × β-cell function) may be a better measure of β-cell function (20). Using this approach, we found that both NGT 1h-high and IGT individuals had a lower disposition index compared with NGT 1h-low individuals after adjusting for age, sex, and BMI. Consistent with the present data, a recent report from the Relationship between Insulin Sensitivity and Cardiovascular Risk (RISC) study group showed that NGT individuals, whose 1-h postload plasma glucose was >161 mg/dL, exhibited lower insulin sensitivity and β-cell dysfunction compared with NGT individuals with 1-h postload plasma glucose ≤161 mg/dL (26).
Conceptually, in the presence of increased insulin resistance, 1-h postload hyperglycemia may arise either from an intrinsic β-cell defect or from an impairment of incretin’s ability to potentiate insulin secretion. We found that NGT 1h-high individuals have a normal insulin response to oral glucose ingestion as estimated by two OGTT-derived indexes of insulin secretion, but they exhibit a significant impairment in insulin response to intravenous glucose administration. Intriguingly, NGT 1h-high individuals showed a higher incretin effect estimated as the ratio between total insulin responses during OGTT and IVGTT. These results suggest that postload hyperglycemia may be the result of an intrinsic β-cell defect rather than of an impaired incretin effect. The enhanced incretin effect could be due to a compensatory increase in either secretion or action of incretin hormones or to both. Another possibility is that similar insulin responses to oral glucose load between the NGT 1h-low, NGT 1h-high, and IGT individuals in the face of higher glucose levels during the OGTT in the latter group may indicate a lower sensitivity of the β-cells to glucose in NGT 1h-high individuals. Accordingly, it has indeed been shown that NGT individuals, whose 1-h postload plasma glucose was >161 mg/dL, have a reduction in β-cell glucose sensitivity (26).
The current study has several strengths. Data were collected by a trained staff, following a standardized protocol (17), and metabolic and hormonal analyses were performed in a centralized laboratory. Our results are also strengthened by the use of state-of-the-art techniques for assessments of insulin sensitivity and insulin secretion. Most previous studies in NGT 1h-high versus NGT 1h-low individuals have used only oral glucose tests, and in none of these studies were simultaneous measurements of insulin sensitivity by the gold standard hyperinsulinemic-euglycemic clamp, and insulin secretion by OGTT and IVGTT, performed (9–13,26). The present results point to the importance of discriminating between insulin secretion measurements derived from oral or from intravenous glucose challenge tests, the relevance of estimating the incretin effect when evaluating insulin secretion, and the impact of adjusting for the actual insulin sensitivity when evaluating β-cell function.
Nevertheless, the current study needs to be interpreted in the context of some potential limitations. First, the cross-sectional nature of our study does not provide insight into the time course of the development of alterations in insulin secretion and insulin sensitivity leading to deterioration in glucose homeostasis; therefore, no conclusions regarding cause-effect relationships can be made. A second limitation of the current study is that each test (OGTT, IVGTT, and euglycemic clamp study) assessing insulin sensitivity and glucose-stimulated insulin secretion was only performed once. Although such an approach reflects clinical practice, intraindividual variation in levels of these variables cannot be taken into account, and some individuals might have been misclassified. Moreover, the observed differences in insulin secretion and insulin sensitivity may be, in part, due to differences in sex, age, and BMI among the groups with different glucose tolerance; however, all measures of insulin secretion and insulin action were adjusted for these variables. Additionally, it can also be argued that the findings of the current study might have been influenced by the presence of a family history of diabetes. However, all of the subjects in this study came from families with only one parent affected by type 2 diabetes, and many subjects who develop the disease have a family history of diabetes. Finally, the current results are only based on Caucasian individuals, and generalizing them to other ethnic groups must be done with caution because differences between ethnic groups in insulin resistance and β-cell function have been reported. The present cross-sectional study should be considered hypothesis generating and requiring confirmation by both cross-sectional and prospective studies in other ethnic populations.
Acknowledgments
This work was supported, in part, by the Italian Ministry of Health (grant RF-FSR-2007-631176) to G.S.
No potential conflicts of interest relevant to this article were reported.
M.A.M., E.S., S.F., S.M., F.A., and A.S. acquired, analyzed, and interpreted data. R.L. contributed to discussion and reviewed the manuscript. M.L.H. reviewed and edited the manuscript. F.P. contributed to discussion and reviewed the manuscript. G.S. designed the study, analyzed and interpreted the data, and wrote the manuscript. G.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuomilehto J, Lindström J, Eriksson JG, et al. ; Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 3.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trial Research Group Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002;359:2072–2077 [DOI] [PubMed] [Google Scholar]
- 4.Gerstein HC, Yusuf S, Bosch J, et al. ; DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096–1105 [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Tripathy D, Schwenke DC, et al. ; ACT NOW Study Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011;364:1104–1115 [DOI] [PubMed] [Google Scholar]
- 6.Paulweber B, Valensi P, Lindström J, et al. A European evidence-based guideline for the prevention of type 2 diabetes. Horm Metab Res 2010;42(Suppl. 1):S3–S36 [DOI] [PubMed] [Google Scholar]
- 7.Lindström J, Neumann A, Sheppard KE, et al. Take action to prevent diabetes—the IMAGE toolkit for the prevention of type 2 diabetes in Europe. Horm Metab Res 2010;42(Suppl. 1):S37–S55 [DOI] [PubMed] [Google Scholar]
- 8.Unwin N, Shaw J, Zimmet P, Alberti KGMM. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med 2002;19:708–723 [DOI] [PubMed] [Google Scholar]
- 9.Abdul-Ghani MA, Williams K, DeFronzo RA, Stern M. What is the best predictor of future type 2 diabetes? Diabetes Care 2007;30:1544–1548 [DOI] [PubMed] [Google Scholar]
- 10.Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care 2008;31:1650–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdul-Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L. Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes: results from the Botnia Study. Diabetes Care 2009;32:281–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdul-Ghani MA, Abdul-Ghani T, Müller G, et al. Role of glycated hemoglobin in the prediction of future risk of T2DM. J Clin Endocrinol Metab 2011;96:2596–2600 [DOI] [PubMed] [Google Scholar]
- 13.Succurro E, Marini MA, Arturi F, et al. Elevated one-hour post-load plasma glucose levels identifies subjects with normal glucose tolerance but early carotid atherosclerosis. Atherosclerosis 2009;207:245–249 [DOI] [PubMed] [Google Scholar]
- 14.Succurro E, Arturi F, Lugarà M, et al. One-hour postload plasma glucose levels are associated with kidney dysfunction. Clin J Am Soc Nephrol 2010;5:1922–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sciacqua A, Miceli S, Carullo G, et al. One-hour postload plasma glucose levels and left ventricular mass in hypertensive patients. Diabetes Care 2011;34:1406–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sciacqua A, Miceli S, Greco L, et al. One-hour postload plasma glucose levels and diastolic function in hypertensive patients. Diabetes Care 2011;34:2291–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laakso M, Zilinskaite J, Hansen T, et al. ; EUGENE2 Consortium Insulin sensitivity, insulin release and glucagon-like peptide-1 levels in persons with impaired fasting glucose and/or impaired glucose tolerance in the EUGENE2 study. Diabetologia 2008;51:502–511 [DOI] [PubMed] [Google Scholar]
- 18.Succurro E, Andreozzi F, Marini MA, et al. Low plasma insulin-like growth factor-1 levels are associated with reduced insulin sensitivity and increased insulin secretion in nondiabetic subjects. Nutr Metab Cardiovasc Dis 2009;19:713–719 [DOI] [PubMed] [Google Scholar]
- 19.Stumvoll M, Mitrakou A, Pimenta W, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 2000;23:295–301 [DOI] [PubMed] [Google Scholar]
- 20.Kahn SE. Clinical review 135: The importance of β-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab 2001;86:4047–4058 [DOI] [PubMed] [Google Scholar]
- 21.Lyssenko V, Lupi R, Marchetti P, et al. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest 2007;117:2155–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorenzo C, Haffner SM, Stancáková A, Laakso M. Relation of direct and surrogate measures of insulin resistance to cardiovascular risk factors in nondiabetic finnish offspring of type 2 diabetic individuals. J Clin Endocrinol Metab 2010;95:5082–5090 [DOI] [PubMed] [Google Scholar]
- 23.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 24.Bastard JP, Vandernotte JM, Faraj M, et al. Relationship between the hyperinsulinemic-euglycaemic clamp and a new simple index assessing insulin sensitivity in overweight and obese postmenopausal women. Diabetes Metab 2007;33:261–268 [DOI] [PubMed] [Google Scholar]
- 25.Avignon A, Boegner C, Mariano-Goulart D, Colette C, Monnier L. Assessment of insulin sensitivity from plasma insulin and glucose in the fasting or post oral glucose-load state. Int J Obes Relat Metab Disord 1999;23:512–517 [DOI] [PubMed] [Google Scholar]
- 26.Manco M, Panunzi S, Macfarlane DP, et al. ; Relationship between Insulin Sensitivity and Cardiovascular Risk (RISC) Consortium One-hour plasma glucose identifies insulin resistance and beta-cell dysfunction in individuals with normal glucose tolerance: cross-sectional data from the Relationship between Insulin Sensitivity and Cardiovascular Risk (RISC) study. Diabetes Care 2010;33:2090–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]