Abstract
OBJECTIVE
This study evaluated the nerve conduction study (NCS) parameters of the most distal sensory nerves of the lower extremities—namely, the medial dorsal cutaneous (MDC), dorsal sural (DS), and medial plantar (MP) nerves—in diabetic (DM) and impaired glucose tolerance (IGT) patients who displayed normal findings on their routine NCSs.
RESEARCH DESIGN AND METHODS
Standard NCSs were performed on healthy control (HC), DM, and IGT groups (N = 147). The bilateral NCS parameters of the MDC, DS, and MP nerves were investigated. The Toronto Clinical Scoring System (TCSS) was assessed for the DM and IGT groups.
RESULTS
The mean TCSS scores of the IGT and DM groups were 2.5 ± 2.3 and 2.8 ± 2.2, respectively. No significant differences between the two groups were observed. After adjustment of age and BMI, the DM group showed significant NCS differences in DS and MDC nerves compared with the HC group (P < 0.05). These differences were also exhibited in the left DS of the IGT group (P = 0.0003). More advanced NCS findings were observed in the DM group. Bilateral abnormal NCS responses in these distal sensory nerves were found in 40 and 16% of DM and IGT patients, respectively.
CONCLUSIONS
These results showed that the simultaneous assessment of the most distal sensory nerves allowed the detection of early NCS changes in the IGT and DM groups, even when the routine NCS showed normal findings.
Diabetic peripheral neuropathy (DPN) is one of the most common long-term complications of diabetes mellitus (DM) and is the main initiation factor for foot ulceration and lower extremity amputation (1). Proper detection is important because, once these changes begin, there is no known treatment that can arrest, prevent, or slow the development of neuropathy (1). Current guidelines suggest that a combination of symptoms, signs, and electrophysiologic measurements provide the most accurate diagnosis (2). However, DPN may first develop well before the symptoms are manifested. Therefore, objective tests that can identify DPN during its early stages are important.
Nerve conduction studies (NCSs) are considered by many to be the gold standard or a consistent indicator of neuropathy (3). The typical DPN is length-dependent (1), and changes begin at the distal nerves, affecting the sensory nerves of the lower extremities first. The standard NCS protocol involves sensory NCSs of the lower extremity sensory nerves, mainly the sural and superficial peroneal nerves, along with motor NCSs of the peroneal and tibial nerves. However, even with normal NCS findings from conventional studies, NCS changes in the most distal portions of these nerves—the dorsal sural (DS), medial dorsal cutaneous (MDC), and medial plantar (MP) nerves—can be observed (4–9). These nerves are the distal nerve endings of the superficial peroneal, sural, and tibial nerves, respectively. Because these most distal nerves are not assessed by routine NCSs, some studies have advocated the assessment of these nerves in those with normal NCS findings to increase the diagnostic sensitivity of NCSs.
Impaired glucose tolerance (IGT) is a transitional stage to DM, and many previous research studies have suggested that IGT plays a role in the development of neuropathy (10,11). In fact, there is evidence that neuropathy occurs earlier in the course of hyperglycemia than other microvascular complications (12). Although there are still controversies about the relationship between IGT and DPN, studies that include epidermal fiber count (13) and NCSs (10,11,14) have confirmed that neuropathic changes occur in IGT well before the progression to DM. A previous study reported changes that are detectable by DS NCSs (15) in IGT patients, but little is known about whether NCSs of the other sensory nerves reflect early DPN changes in IGT patients.
We hypothesized that NCS changes of the most distal nerves (MDC, DS, and MP nerves) would be present in DM and possibly in IGT patients, even in those with normal NCS findings obtained from routine NCSs. We therefore sought to record the MDC, DS, and MP, nerves in (1) DM patients, (2) IGT patients, and (3) healthy control (HC) patients. We also sought to determine whether significant NCS changes are present in the most distal sensory nerves.
RESEARCH DESIGN AND METHODS
The institutional review board at the Catholic University of Korea, Bucheon St. Mary’s Hospital approved the protocol for this study (approval number HC09EISI0059).
DM and IGT recruitment
For the DM and IGT groups, men or women who were aged between 20 and 70 years were recruited. All of the IGT and DM (type 2) subjects had received a formal diagnosis according to the World Health Organization guidelines at the Department of Endocrinology. The endocrinologist referred potential candidates who agreed to undergo a prescreening NCS evaluation of the right sural and superficial peroneal nerves. Only those with normal NCS values (sensory nerve action potential [SNAP] values >10 µV) were enrolled. Clinical characteristics were recorded at the time of enrollment.
Laboratory assessment
To rule out the presence of other possible causes of peripheral polyneuropathy, IGT and DM participants underwent laboratory tests of thyroid hormones, lipid profile, and vitamin levels, in addition to routine glucose tests, on the same day that the NCS was performed.
Toronto Clinical Scoring System
Participants in the IGT and DM groups were assessed using the Toronto Clinical Scoring System (TCSS) (16). Reflex and sensory tests, including pinprick, temperature, light touch, vibration, and position sensation, were performed as part of the TCSS.
HC recruitment
Healthy volunteers with no known risk factors of neuropathy or abnormality upon neurologic examination were enrolled as the HC group. A prescreening NCS was performed to confirm normal SNAP values in the sural and superficial peroneal nerves. All subjects underwent an additional neurologic examination that included the TCSS. Anyone with positive findings from the TCSS was excluded.
Subjects who met the following criteria were excluded: 1) skin lesion or edema of the lower extremities that would interfere with NCS procedures; 2) previous diagnosis or clinical symptoms that would indicate the presence of mononeuropathy or entrapment neuropathy of the lower extremities; a history of 3) alcohol drinking >170 g/week 4) substance abuse, 5) use of medications such as chemotherapeutic or antituberculosis drugs; and 6) a former diagnosis of other systemic conditions related to peripheral polyneuropathy, other than that caused by diabetes in the two hyperglycemic groups.
Electrophysiologic evaluation
NCS of conventional nerves.
All NCSs were performed with Viking Select electromyography (Viasys Healthcare, Nicolet Biomedical Inc., Madison, WI) at the setting of 20 Hz for the lower filter, 3 kHz for the higher filter for sensory studies, and 5 Hz–10 kHz for motor studies. A sweep speed of 2 ms/division and sensitivity of 20 µV/division were used for the distal sensory nerves. The stimulus was 0.1 ms in pulse duration. All NCSs were performed with 10-mm-diameter silver chloride cup electrodes (Viasys Healthcare). NCSs of the right sural, superficial peroneal, median sensory, and tibial motor nerves were performed. Delayed responses were obtained with minimum F-response latencies from the right tibial and median nerve, along with H-reflex latencies from tibial nerve stimulation.
NCS of the most distal sensory nerves.
Bilateral distal sensory NCSs were performed on all subjects. All NCS of the distal sensory nerves were performed according to previous protocols. The MDC nerve was assessed with antidromic electrical stimulation performed 10 cm proximal to the recording electrodes (6); the MP nerve was assessed with orthodromic stimulation at 14 cm (5), and the DS nerve conduction was performed with electrical stimulation applied 10 cm from the recording electrodes (4). Signal averaging of 5–10 responses was used when SNAPs were too small to be obtained with a single stimulation. A follow-up of NCS evaluation of the right leg in the IGT (n = 5) and DM (n = 5) groups was performed to document any longitudinal changes of the distal sensory nerves.
Statistical analysis
We compared the clinical, laboratory parameters, blood pressure, and TCSS between the IGT and DM group using the unpaired t test. Side-to-side differences in the NCS values were tested using the paired t test. To compare the demographic features and NCS parameters of the three groups, we performed one-way ANOVA, followed by post hoc Duncan analysis. We performed ANCOVA with adjustments for the covariates of age, BMI, and folate. All NCS values were subject to logarithmic transformation. Abnormal responses were defined as those beyond the limit of the normal range values of mean ± 2 SDs from the logarithmic transformed values (17) and were calculated for each distal sensory nerve. We performed the Cochran-Armitage trend test to compare the number of subjects with abnormal responses among the three groups. We considered a two-sided P < 0.05 statistically significant. All statistical analyses were performed using SAS software (SAS Institute, Inc., Cary, NC).
RESULTS
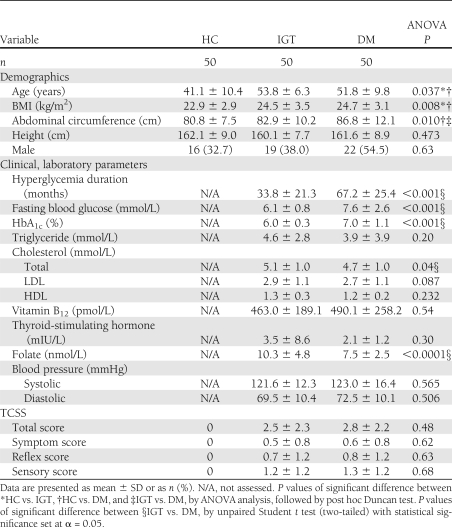
Each group comprised 50 participants (N = 150). The characteristics and laboratory parameters of the hyperglycemic subjects are reported in Table 1. The eldest participant was 65 years old. One patient from the IGT group displayed abnormal levels of thyroid hormones. Two patients from the DM group displayed abnormal thyroid and folate levels. Their NCS findings were excluded from the statistical analysis (Supplementary Fig. 1).
Table 1.
Baseline demographic and clinical characteristics of the HC, IGT, and DM groups
TCSS
TCSS scores from 75% of the IGT participants and 83% of the DM participants displayed positive symptoms or signs. All participants in the HC group were preselected after confirmation that their TCSS scores were 0; thus, comparisons of total scores and subscores were performed only between the two hyperglycemic groups (Table 1). No significant differences in TCSS scores was observed between the two groups.
NCS results
NCS results for conventional nerves.
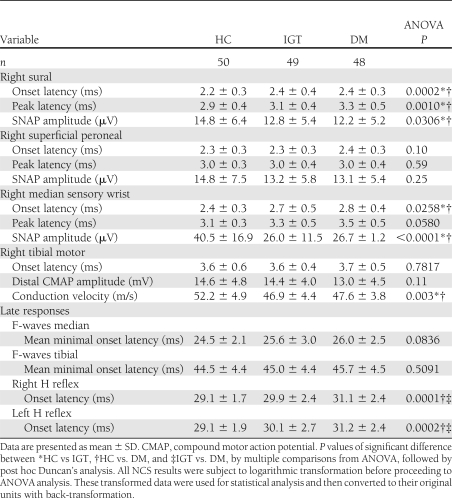
We obtained SNAP responses in all of the tested nerves. The statistical analysis showed no side-to-side differences in NCS values for all tested nerves in any of the test groups (results not shown). Although all of the NCS values were within the normal range, the two hyperglycemic groups showed significant differences in their NCS values obtained for the sural and median sensory nerves compared with the HC group (Table 2).
Table 2.
Comparison of the nerve conduction results of sensory, motor nerves, and late responses in the HC, IGT, and DM groups
NCS results of distal sensory nerves.
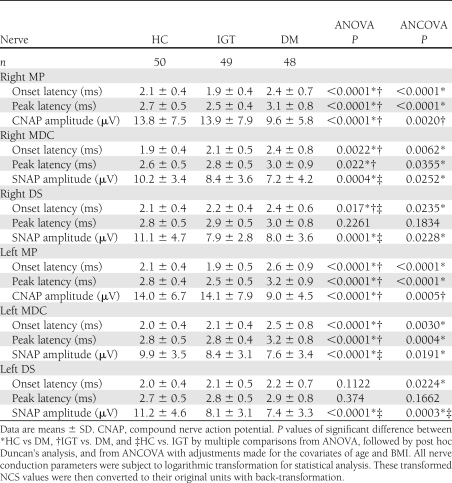
The SNAP amplitudes of the three distal sensory nerves were significantly smaller in the DM group than in the HC group. Even after adjustment of the covariates of age and BMI, the DM group still showed these differences (Table 3) in the MDC and DS. In the IGT group, the SNAP amplitudes of the MDC and DS nerves, but not of the MP nerve, were significantly smaller than those of the HC group (Table 3). After adjustment of age and BMI, these differences in SNAP amplitudes were only observed in the left DS nerve. After adjustment of folate, age, and BMI, no statistical difference was observed between the IGT and DM group in the MDC and DS SNAP amplitudes (Supplementary Table 1); this was similar to findings before adjustment.
Table 3.
Results of the MP, DS, and MDC nerves in the HC, IGT, and DM groups
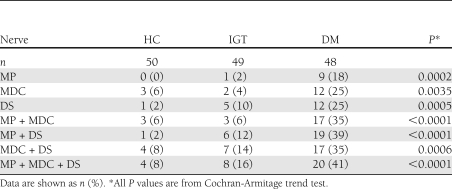
The findings from the three distal sensory nerves showed abnormal NCS responses in 20 (41%) and 8 (16%) of the DM and IGT participants, respectively. Statistical analysis showed a significant increase in the number of subjects with abnormal SNAP responses for the distal sensory nerves with disease progression from HC to IGT to DM (Table 4).
Table 4.
HC, IGT, and DM group participants who showed bilateral NCS values that fell below the normal range for the distal sensory nerves
Follow-up NCS studies
At the 2-year follow-up assessment, SNAP amplitudes of the MDC and DS nerves in the IGT group were significantly smaller compared with those assessed 2 years earlier (Supplementary Table 2). A similar trend was observed in the DM group, although it did not reach statistical significance.
CONCLUSIONS
The objectives of this study were to investigate the NCS results of the most distal sensory nerves (the MP, DS and MDC nerves) in DM and IGT subjects who otherwise displayed normal values from their routine NCS evaluations. These values were then compared with those of the HC group. Even after adjustment of age and BMI, the DM group exhibited significant NCS differences compared with the HC group in the DS and MDC nerves. These differences were also exhibited in the left DS for the IGT group. In addition, NCS assessments of these three distal nerves together showed that 16 and 40% of IGT and DM subjects displayed abnormal SNAP responses.
We used the TCSS, a valid instrument that reflects the clinical symptoms and signs of diabetic polyneuropathy (16). The TCSS is designed to emphasize subtle sensory symptoms and deficits rather than motor symptoms. Although participants in the two hyperglycemic groups displayed mild neuropathic symptoms and signs, we were able to detect significant NCS changes in the most distal sensory nerves in the IGT and DM groups. Notably, although a statistical analysis revealed no significant differences in the TCSS scores between these two groups, the DM group displayed more advanced NCS findings in the distal sensory nerves than the IGT group.
An analysis of the clinical characteristics of these two hyperglycemic group showed that the DM group had more prolonged exposure to hyperglycemia, as evidenced in their higher fasting blood glucose level, higher HbA1c, and longer duration of hyperglycemia. These factors are positively related to DPN (18). Folate levels in the DM group, although within the normal range, were significantly reduced compared with those recorded for the IGT group. A recent report has suggested the role of folate in type 1 diabetes (19); however, further studies will be necessary to determine whether the reduced levels of folate observed in DM play a role in DPN. Values within normal reference ranges were found for other factors known to be related to the incidence of DPN, including blood pressure, elevated triglyceride level, BMI (20), and lipid profiles (21). Other anthropometric factors that may affect NCS results, such as age, height, and sex, were similar in both groups. In consideration of these factors, we suggest that the advanced NCS findings in the distal sensory nerves of the DM group may be attributable to the more prolonged glycemic exposure.
Electrodiagnostic tests are objective and validated tests of peripheral polyneuropathy (2). Among the various NCS measures available, the sural SNAP amplitude is reported to show the earliest alteration in DPN (22). The DS nerve, which is the distal portion of the sural nerve, also manifested these early changes in the IGT and DM groups, confirming previous findings that DPN is a length-dependent process, with the earliest abnormalities appearing at the ends of the longest nerve fibers, which extend to the toes. Because of their superficial position, distal sensory nerves of the feet can be prone to injury or entrapment, but these injuries usually manifest as unilateral abnormalities. Because DPN is a symmetric disease, abnormal SNAPs can be observed on both sides; therefore, we think that the bilateral abnormal responses observed in these distal sensory nerves are more closely related to occult DPN changes than to focal trauma or entrapment.
IGT and impaired fasting glucose are prediabetic stages. Recent studies have suggested that the prevalence of polyneuropathy is increased in IGT (10,11,14) and even in impaired fasting glucose (23) compared with normal glucose tolerance. Results of the current study were in accordance to these previous studies and showed that there were significantly more patients with abnormal NCS responses of the most distal sensory nerves in the IGT group than in the HC group. In addition, supplementary NCS studies of IGT patients (Supplementary Table 2) at 2 years of follow-up indicated that these NCS parameters show gradual deterioration with smaller values. Although we did not include individuals with impaired fasting glucose, the issue whether the most distal sensory nerves can reflect the early DPN changes in this group is a topic that needs to be addressed in future studies.
Certain limiting factors of this study need to be considered: First, the HC participants were significantly younger than those in the two hyperglycemic groups. Although age is known to have a significant effect on NCS parameters, NCS changes are known to be more prominent in those older than 60 years (24). However, most subjects in all of the three groups were aged 40–60 years, and reference values are usually provided for this age group as a whole. Also, even after adjustment of age and BMI, significant differences in the NCS values of the distal sensory nerves were detected in the DM group compared with the HC group.
Second, the early stages of DPN are postulated to involve small fibers first; in IGT patients, this may, but not always, manifest as painful neuropathy (10). The methods used to measure nerve conduction in this study primarily assess the large fibers; therefore, the results do not provide information on the extent of small-fiber involvement. Further studies that assess the dysfunction of small, unmyelinated fibers are warranted.
It is important to diagnose DPN at the earliest stage possible, because early intervention for prediabetic patients is reported to slow or even halt the progression of DPN (25) and to prevent the occurrence of more debilitating consequences such as foot ulceration and amputation. Current guidelines (2) mandate that no further NCSs are necessary when unilateral NCSs of the lower extremities, which include the sural nerve, reveal normal findings. However, according to the results of our study, hyperglycemic subjects, including IGT patients, already display changes in the most distal sensory nerves, despite mild symptoms or equivocal clinical findings and normal results on conventional NCS studies.
The results of this study confirm previous reports on the clinical utility of assessing these nerves in hyperglycemic patients (4–9,15). Also, these results are clinically significant in that this is the first study to date that has performed NCSs of all three distal sensory nerves simultaneously and compared the results among IGT, DM, and HC groups. Our results indicate that assessment of the distal sensory nerves can help to detect occult abnormalities in DM and IGT patients, possibly even before conventional studies and clinical scales show positive findings.
Acknowledgments
The Catholic Medical Center Research Foundation provided financial support in 2009. The statistical analysis was supported by the Catholic Research Coordinating Center of the Korean Health 21 R&D Project (A070001).
No potential conflicts of interest relevant to this article were reported.
S.I. wrote the manuscript and analyzed the data. S.-R.K. helped with the recruitment of subjects. J.H.P. revised the manuscript. Y.S.K. researched the data. G.-Y.P. contributed to the overall design of the study protocol.
This work was presented as part of the poster presentation at the 57th American Association of Neuromuscular and Electrodiagnostic Medicine Annual Meeting, Québec City, Québec, Canada, 6–9 October 2010.
The authors thank Hyeon-Woo Yim, MD, PhD; Seung-Hee Jeong, MPH; and MiSun Park at the Catholic Medical Center Clinical Research Coordinating Center, for their advice on statistics.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1001/-/DC1.
Clinical trial reg. no. NCT01094418, clinicaltrials.gov.
References
- 1.Boulton AJ, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care 2004;27:1458–1486 [DOI] [PubMed] [Google Scholar]
- 2.England JD, Gronseth GS, Franklin G, et al. ; American Academy of Neurology; American Association of Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2005;64:199–207 [DOI] [PubMed] [Google Scholar]
- 3.Dyck PJ, O’Brien PC, Litchy WJ, Harper CM, Klein CJ, Dyck PJ. Monotonicity of nerve tests in diabetes: subclinical nerve dysfunction precedes diagnosis of polyneuropathy. Diabetes Care 2005;28:2192–2200 [DOI] [PubMed] [Google Scholar]
- 4.Balci K, Karacayir S, Varol G, Utku U. Utility of dorsal sural nerve in early determination of diabetic polyneuropathy. J Peripher Nerv Syst 2005;10:342–343 [DOI] [PubMed] [Google Scholar]
- 5.Herrmann DN, Ferguson ML, Pannoni V, Barbano RL, Stanton M, Logigian EL. Plantar nerve AP and skin biopsy in sensory neuropathies with normal routine conduction studies. Neurology 2004;63:879–885 [DOI] [PubMed] [Google Scholar]
- 6.Kushnir M, Klein C, Kimiagar Y, Pollak L, Rabey JM. Medial dorsal superficial peroneal nerve studies in patients with polyneuropathy and normal sural responses. Muscle Nerve 2005;31:386–389 [DOI] [PubMed] [Google Scholar]
- 7.Løseth S, Nebuchennykh M, Stålberg E, Mellgren SI. Medial plantar nerve conduction studies in healthy controls and diabetics. Clin Neurophysiol 2007;118:1155–1161 [DOI] [PubMed] [Google Scholar]
- 8.Turgut N, Karasalihoglu S, Kücükugurluoglu Y, Balci K, Ekuklu G, Tütüncüler F. Clinical utility of dorsal sural nerve conduction studies in healthy and diabetic children. Clin Neurophysiol 2004;115:1452–1456 [DOI] [PubMed] [Google Scholar]
- 9.Uluc K, Isak B, Borucu D, et al. Medial plantar and dorsal sural nerve conduction studies increase the sensitivity in the detection of neuropathy in diabetic patients. Clin Neurophysiol 2008;119:880–885 [DOI] [PubMed] [Google Scholar]
- 10.Singleton JR, Smith AG, Bromberg MB. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care 2001;24:1448–1453 [DOI] [PubMed] [Google Scholar]
- 11.Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 2003;60:108–111 [DOI] [PubMed] [Google Scholar]
- 12.Gordon Smith A, Robinson Singleton J. Idiopathic neuropathy, prediabetes and the metabolic syndrome. J Neurol Sci 2006;242:9–14 [DOI] [PubMed] [Google Scholar]
- 13.Smith AG, Ramachandran P, Tripp S, Singleton JR. Epidermal nerve innervation in impaired glucose tolerance and diabetes-associated neuropathy. Neurology 2001;57:1701–1704 [DOI] [PubMed] [Google Scholar]
- 14.Nebuchennykh M, Løseth S, Jorde R, Mellgren SI. Idiopathic polyneuropathy and impaired glucose metabolism in a Norwegian patient series. Eur J Neurol 2008;15:810–816 [DOI] [PubMed] [Google Scholar]
- 15.Koçer A, Domaç FM, Boylu E, Us O, Tanridağ T. A comparison of sural nerve conduction studies in patients with impaired oral glucose tolerance test. Acta Neurol Scand 2007;116:399–405 [DOI] [PubMed] [Google Scholar]
- 16.Bril V, Perkins BA. Validation of the Toronto Clinical Scoring System for diabetic polyneuropathy. Diabetes Care 2002;25:2048–2052 [DOI] [PubMed] [Google Scholar]
- 17.Campbell WW, Robinson LR. Deriving reference values in electrodiagnostic medicine. Muscle Nerve 1993;16:424–428 [DOI] [PubMed] [Google Scholar]
- 18.Jurado J, Ybarra J, Romeo JH, Pou JM. Clinical screening and diagnosis of diabetic polyneuropathy: the North Catalonia Diabetes Study. Eur J Clin Invest 2009;39:183–189 [DOI] [PubMed] [Google Scholar]
- 19.Giannattasio A, Calevo MG, Minniti G, et al. Folic acid, vitamin B12, and homocysteine levels during fasting and after methionine load in patients with Type 1 diabetes mellitus. J Endocrinol Invest 2010;33:297–299 [DOI] [PubMed] [Google Scholar]
- 20.Tesfaye S, Chaturvedi N, Eaton SE, et al. ; EURODIAB Prospective Complications Study Group Vascular risk factors and diabetic neuropathy. N Engl J Med 2005;352:341–350 [DOI] [PubMed] [Google Scholar]
- 21.Vinik AI, Erbas T, Stansberry KB, Pittenger GL. Small fiber neuropathy and neurovascular disturbances in diabetes mellitus. Exp Clin Endocrinol Diabetes 2001;109(Suppl. 2):S451–S473 [DOI] [PubMed] [Google Scholar]
- 22.Dyck PJ, Overland CJ, Low PA, et al. ; Cl vs. NPhys Trial Investigators Signs and symptoms versus nerve conduction studies to diagnose diabetic sensorimotor polyneuropathy: Cl vs. NPhys trial. Muscle Nerve 2010;42:157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A; KORA Study Group Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care 2008;31:464–469 [DOI] [PubMed] [Google Scholar]
- 24.Preston DC. Artifacts and technical factors. In Electromyography and Neuromuscular Disorders. 2nd ed. Preston DC, Shapiro BE, Eds. Philadelphia, Elsevier, 2005, p. 89–90 [Google Scholar]
- 25.Smith AG, Russell J, Feldman EL, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care 2006;29:1294–1299 [DOI] [PubMed] [Google Scholar]