Abstract
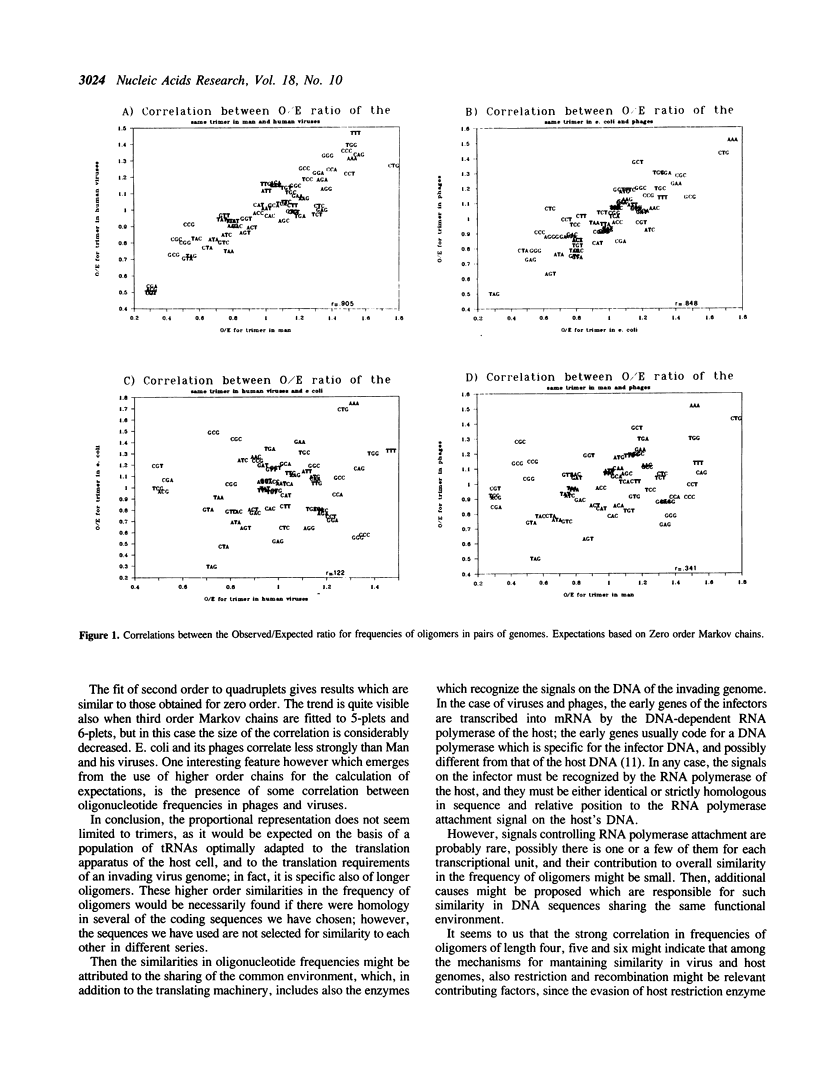
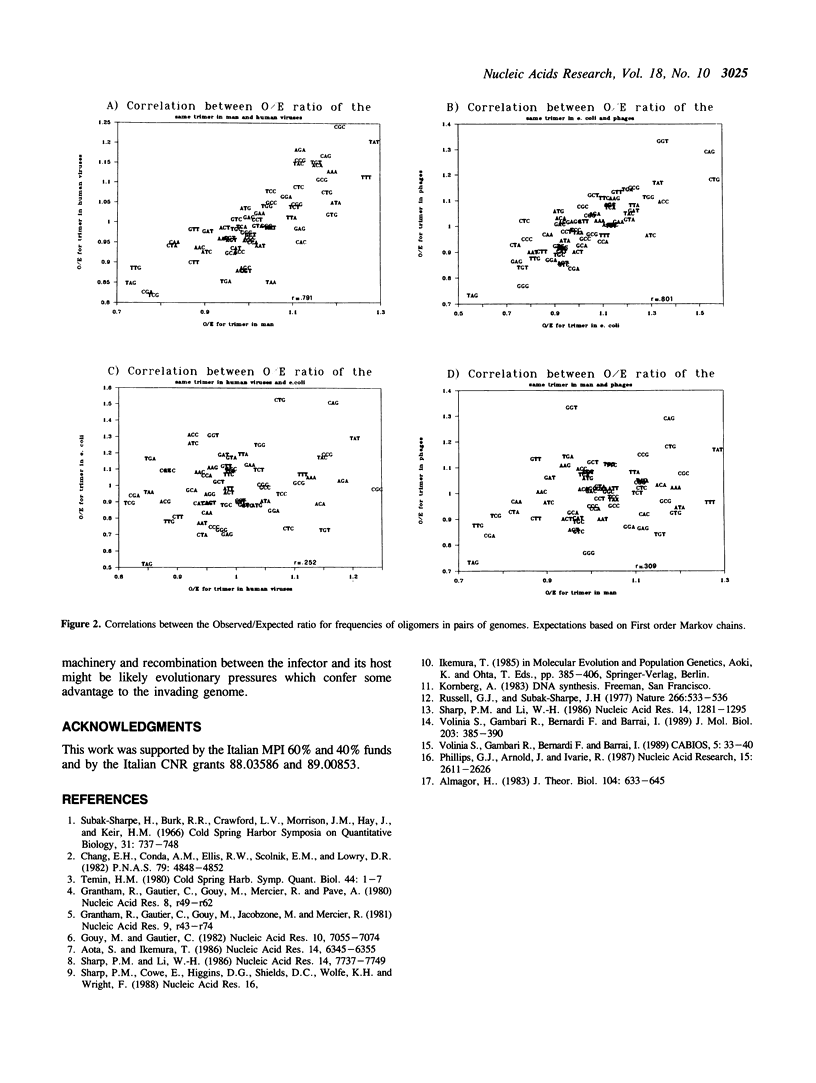
The frequencies of oligonucleotides of length 3-6 were studied in 211 sequences of human DNA (659 kilobases), 22 sequences of DNA of human viruses (120 kbs), in 181 sequences of E. coli (442 kbs), and in 42 sequences of phages of E. coli (137 kbs). The sequences were obtained from Genbank(R) 48. The observed frequencies (O) were compared to the expected frequencies (E) obtained in two ways: 1) according to nucleotide composition for each series, and 2) according to first order Markow chains for triplets, second order for quadruplets, and third order for quintuplets and sextuplets. The ratio O/E was obtained for each oligonucleotide. Then, the correlation between the ratio O/E in a pair of series was calculated. Strong correlations were observed for sequences of man and human viruses, and for E. coli and its phages. Other correlations were small. For higher order Markov chains, there is indication of some correlation also between viruses and phages. It was concluded that through analysis of parallel oligonucleotide series it may be possible to infer some of the complex evolutionary relationships existing between cells and their infectors beyond the level of codon usage.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almagor H. A Markov analysis of DNA sequences. J Theor Biol. 1983 Oct 21;104(4):633–645. doi: 10.1016/0022-5193(83)90251-5. [DOI] [PubMed] [Google Scholar]
- Aota S., Ikemura T. Diversity in G + C content at the third position of codons in vertebrate genes and its cause. Nucleic Acids Res. 1986 Aug 26;14(16):6345–6355. doi: 10.1093/nar/14.16.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. H., Gonda M. A., Ellis R. W., Scolnick E. M., Lowy D. R. Human genome contains four genes homologous to transforming genes of Harvey and Kirsten murine sarcoma viruses. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4848–4852. doi: 10.1073/pnas.79.16.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouy M., Gautier C. Codon usage in bacteria: correlation with gene expressivity. Nucleic Acids Res. 1982 Nov 25;10(22):7055–7074. doi: 10.1093/nar/10.22.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M., Jacobzone M., Mercier R. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 1981 Jan 10;9(1):r43–r74. doi: 10.1093/nar/9.1.213-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M., Mercier R., Pavé A. Codon catalog usage and the genome hypothesis. Nucleic Acids Res. 1980 Jan 11;8(1):r49–r62. doi: 10.1093/nar/8.1.197-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G. J., Arnold J., Ivarie R. Mono- through hexanucleotide composition of the Escherichia coli genome: a Markov chain analysis. Nucleic Acids Res. 1987 Mar 25;15(6):2611–2626. doi: 10.1093/nar/15.6.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell G. J., Subak-Sharpe J. H. Similarity of the general designs of protochordates and invertebrates. Nature. 1977 Apr 7;266(5602):533–536. doi: 10.1038/266533a0. [DOI] [PubMed] [Google Scholar]
- Sharp P. M., Li W. H. Codon usage in regulatory genes in Escherichia coli does not reflect selection for 'rare' codons. Nucleic Acids Res. 1986 Oct 10;14(19):7737–7749. doi: 10.1093/nar/14.19.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subak-Sharpe H., Bürk R. R., Crawford L. V., Morrison J. M., Hay J., Keir H. M. An approach to evolutionary relationships of mammalian DNA viruses through analysis of the pattern of nearest neighbor base sequences. Cold Spring Harb Symp Quant Biol. 1966;31:737–748. doi: 10.1101/sqb.1966.031.01.094. [DOI] [PubMed] [Google Scholar]
- Volinia S., Bernardi F., Gambari R., Barrai I. Co-localization of rare oligonucleotides and regulatory elements in mammalian upstream gene regions. J Mol Biol. 1988 Sep 20;203(2):385–390. doi: 10.1016/0022-2836(88)90006-x. [DOI] [PubMed] [Google Scholar]
- Volinia S., Gambari R., Bernardi F., Barrai I. The frequency of oligonucleotides in mammalian genic regions. Comput Appl Biosci. 1989 Feb;5(1):33–40. doi: 10.1093/bioinformatics/5.1.33. [DOI] [PubMed] [Google Scholar]


