Abstract
Acute kidney injury (AKI) is a prevalent and devastating condition associated with significant morbidity and mortality. Despite marked improvements in clinical care, the outcomes for subjects with AKI have shown limited improvement in the past 50 years. A major factor inhibiting clinical progress in this field has been inability to accurately predict and diagnose early kidney dysfunction. The current gold-standard clinical and biochemical criteria for diagnosis of AKI, Risk Injury Failure Loss End-stage renal disease (RIFLE), and its modification, Acute Kidney Injury Network (AKIN) criteria, rely on urine output and serum creatinine (SCr) which are insensitive, non-specific, and late markers of disease. The recent development of a variety of analytical mass spectrometry (MS) based platforms have enabled separation, characterization, detection, and quantification of proteins (proteomics) and metabolites (metabolomics). These high-throughput platforms have raised hopes of identifying novel protein and metabolite markers, and recent efforts have led to several promising novel markers of AKI. However, substantial challenges remain including need to systematically evaluate incremental performance of these markers over and beyond current clinical and biochemical criteria for AKI. Here, we discuss the basic issues surrounding AKI biomarker development, highlight the most promising markers currently under development, and discuss the barriers towards wide-spread clinical implementation of these markers.
Keywords: Acute Kidney Injury, Biomarkers, Mass Spectrometry, Proteomics, Metabolomics
1. Acute Kidney Injury: A Diagnostic Dilemma
Acute kidney injury (AKI) is a prevalent and devastating condition associated with significant morbidity and mortality. 30–50% of critically ill patients suffer from AKI, and those requiring the initiation of renal replacement therapy carry a mortality that exceeds 50%.1 Currently, there are no effective therapies shown to improve outcomes in established AKI, and therefore, management has remained largely supportive in nature. One of the major limitations hindering clinical progress in this field has been the inability to accurately predict and identify early AKI. While ultrasound and other imaging techniques can often provide prognostic information in chronic kidney disease and identify cause in obstructive renal disease, currently there is limited imaging based diagnostic capability in detecting medical kidney injury in the acute setting.
Over the past decade, there has been a shift in nomenclature from “acute renal failure” to AKI, in recognition of the fact that injury may have significant clinical consequences without overt failure of kidney function. In spite of this conceptual change, current classification of AKI still relies on indirect measures of glomerular filtration rate (GFR). Both the Risk Injury Failure Loss End-stage renal disease (RIFLE) criteria,2 and its modification, the Acute Kidney Injury Network (AKIN) criteria,3 utilize serum creatinine (SCr) and urine output to define AKI leaving SCr the gold standard determiner of kidney injury for close to a century.4
The use of SCr as a marker of AKI has significant limitations (Table 1). Subjects with AKI (particularly those with baseline impairment in renal function) often have a significant decline in GFR prior to any observable change in SCr.5 Furthermore, SCr is a late marker of disease, and alterations are often not apparent until 48–72 hours post-injury leading to missed early therapeutic opportunities when treatments may be most effective.6 SCr is a non-specific marker both for the diagnosis of AKI and for specific disease etiology. Age, gender, muscle mass, and nutritional status all effect SCr levels. Additionally, as a non-discriminating marker of glomerular filtration, SCr can increase with volume depletion, non-steroidal anti-inflammatory drug use, or many other etiologies of decreased renal perfusion without kidney injury.7 Even in healthy stable subjects, SCr shows dramatic alterations with repeated measures bringing to further question its ability to accurately predict acute renal injury. It is for these well-recognized limitations of SCr and for the devastating clinical implications surrounding AKI that a search for new non-invasive markers of renal injury has been pushed to the forefront of active research.
Table 1.
Limitations of serum creatinine in diagnosing acute kidney injury
| Limitations of Serum Creatinine |
|---|
| Requires steady state GFR |
| Insensitive to small changes in GFR |
| Late marker of altered GFR |
| Non-specific to disease process |
| Altered by clinical characteristics (age, weight, gender, volume status, medication use) |
GFR = glomerular filtration rate
2. The Ideal AKI Super-Marker
While the perfect marker of AKI is unlikely to exist, there are several ideal characteristics to consider in renal biomarker development as outlined in Table 2.8, 9 First, a marker of AKI must be a sensitive and early predictor of disease. Ideally, basal levels of a marker would allow for risk stratification prior to insult, and following insult, would display a rapid and dramatic alteration spanning a large range proportional to degree of injury (e.g. cardiac troponin-I and T for acute myocardial infarction). Not only should levels increase rapidly with onset of injury, but they should remain elevated to allow a large diagnostic window.
Table 2.
Ideal characteristics of a biomarker for acute kidney injury.
| Ideal Biomarker Characteristics |
|---|
| Basal levels allow risk stratification |
| Sensitive and specific for kidney injury |
| Early marker of kidney injury |
| Wide spectrum of values correlating to degree of injury |
| Remains elevated for large diagnostic window |
| Levels can be monitored for therapeutic response |
| Unaffected by demographics and clinical factors |
| Samples readily available and non-invasive (urine, plasma) |
| Cost effective |
| Rapid results |
A useful AKI biomarker should be specific for renal injury and provide insight into etiology and location of injury, rather than just a non-specific marker of glomerular filtration. Unlike SCr, the marker should be relatively unaffected by patient characteristics or clinical status, and should play a direct mechanistic role in renal cell injury or recovery to allow prognostic utility and ability to monitor therapeutic response. Further, levels should predict hard clinical outcomes, such as need for renal replacement therapy and mortality, beyond capabilities of current clinical indicators like demographic data.
In addition to overall sensitivity and specificity in the prediction and diagnosis of AKI, a useful biomarker must also be appropriate for wide spread clinical use. Samples should be obtained non-invasively and be readily available making serum, plasma, or urine sampling ideal. Urinary analysis has particular benefits, in the absence of anuric renal failure, as it may provide a more direct marker of renal injury with less alteration by systemic processes than circulating plasma.8 Finally, level monitoring should be cost-effective with rapid results, utilizing clinically available enzyme-linked immunosorbent assays (ELISA) or similar techniques.
3. Challenges to Renal Biomarker Development
Despite these seemingly straight-forward criteria, AKI biomarker development faces numerous challenges. First, validation of novel biomarkers requires existence of a gold standard for diagnostic comparison. Highly accurate markers of GFR, such as inulin or radiolabeled markers, are too laborious for large scale clinical trials, thus SCr often serves this role. As previously discussed, the significant limitations of SCr in defining AKI are then extrapolated to new developing biomarkers. Specifically, studies often use correlation with SCr as a diagnostic necessity for further investigation. This leads to inclusion of markers with dependence on glomerular filtration and other similar flaws to SCr measurement, while excluding novel and perhaps more mechanism-based markers. Further, even with standardized AKI criteria,2, 3 a wide assortment of SCr based definitions are utilized in clinical research making cross-study comparisons difficult if not impossible. In addition to difficulties in defining AKI, the heterogeneous number of disease etiologies makes it difficult to distinguish kidney injury specific markers from those that arise from the underlying cause itself.
Identification of novel biomarkers has increasingly relied on high throughput technology. In the past two decades, a new field of science called systems biology has emerged. The goal of systems biology is to characterize an individual on a gene (genomics), transcript (transcriptomics), protein (proteomics), or metabolite (metabolomics) level. Initial large scale transcriptional based studies are more recently being followed by mass spectrometry (MS) based proteomic and metabolomic analyses as they have the advantage of utilizing readily available and non-invasively collected biofluids (plasma and urine). As such, the MS-based platforms are more amenable to biomarker discovery and translation into clinical practice. While these are powerful tools, they are limited by high cost, need for specialized equipment and expertise, and additional technique specific difficulties which restrict their wide scale application to general clinical medicine.10 In urinary proteomic analysis, albumin and other highly abundant proteins predominate in the sample.11 Because of the prevalence of such proteins, low abundant proteins, perhaps those most important to early disease pathogenesis, are often left undetected.12
The majority of current AKI biomarker investigation is further limited by sample availability. Clinical studies are for the most part small, single center, phase 1 or phase 2 trials, with very homogenous populations. Control groups often consist of healthy volunteers making it difficult to determine if altered biomarker levels are due to renal injury or are secondary markers of systemic disease. Despite these obstacles, significant advances have been made in AKI biomarker development. Here, we will briefly review several of the most promising markers and discuss future development strategies and needs.
4. Protein Biomarkers of AKI
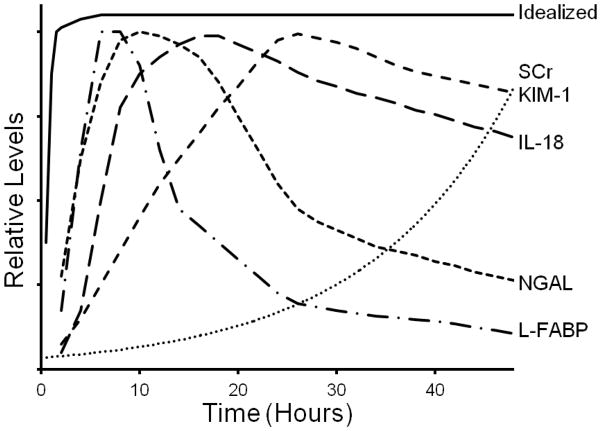
There is intense interest in several plasma and urinary proteins as markers of AKI, as summarized in Table 3. The schematized temporal rise of these markers following renal injury compared to an idealized biomarker and to SCr is depicted in Fig 1.
Table 3.
Advantages and disadvantages of novel clinical biomarkers of acute kidney injury
| Biomarker | Source | Characteristics | Advantages | Disadvantages |
|---|---|---|---|---|
| NGAL | Urine Plasma | Ubiquitous epithelial protein Freely filtered by glomerulus, reabsorption at proximal tubule |
Increased levels 1–2 hours post-insult 25, 26 Widely investigated in diverse populations 22, 23 Rapid point of care testing available |
Decreased specificity in critically ill populations 48 |
| IL-18 | Urine | Pro-inflammatory cytokine Injury induced production in proximal tubular epithelial cells |
Increased levels 4–6 hours post-insult 60 Widely investigated in diverse populations 41, 46, 58, 59, 61 |
Increased in general inflammatory conditions 62, 63 Research based testing only |
| KIM-1 | Urine | Membrane glycoprotein expressed in injured proximal tubular epithelial cells Directs phagocytosis of apoptotic cells |
Increased specificity for ischemic renal injury 74 Rapid point of care testing reported 72 |
Delayed increase up to 12 hours post-insult in certain populations 75 Lacking data in the majority of etiologies of acute kidney injury |
| L-FABP | Urine | Hepatically synthesized cystosolic protein in proximal tubule epithelial cells Limits cell toxicity via sequestration of lipid peroxidation products |
Increased levels 4 hours post-insult 6 Baseline levels may prognosticate acute kidney injury 90 |
May be a non-specific marker of ischemia 91 May be altered in sepsis and liver dysfunction 92 Research based testing only |
| Cys-C | Urine Plasma | Ubiquitously produced cysteine proteinase inhibitor Freely filtered at glomerulus without secretion or reabsorption Fully catabolized by prox tubule cells, undetectable in normal urine |
Increased specificity for proximal tubule dysfunction compared to SCr Rapid clinical testing available |
Non-specific marker of GFR suffering similar limitations to SCr Delayed increase up to 8–12 hours post-insult, but more rapid than SCr 100–102 Levels altered by thyroid disease and systemic inflammation 105 |
Figure 1. Schematic diagram of biomarker temporal rise following acute kidney injury.
Values are extrapolated from available clinical trials and show the variability in biomarker elevations compared to an idealized biomarker. Neutrophil gelatinase-associated lipocalin (NGAL), Interleukin-18 (IL-18), Kidney injury molecule-1 (KIM-1), Liver-type fatty acid binding protein-1 (L-FABP), Serum creatinine (SCr).
4.1 Neutrophil Gelatinase-Associated Lipocalin (NGAL)
Neutrophil gelatinase-associated lipocalin (NGAL) is a 25kDa protein originally isolated from neutrophil secondary granules,13 and is now known as a prevalent epithelial cell protein with wide distribution throughout the body. Siderophores, small iron-binding molecules, are the major ligands of NGAL, and chelation of bacterial siderophores leads to iron deficiency and bacteriostatic effects.14, 15 Through transcriptional regulation by NF-κβ, NGAL can also mediate controlled eukaryotic iron delivery and stimulate cell proliferation and preservation.16 These characteristics underscore NGAL’s role in both innate immunity and cellular recovery.17 NGAL is freely filtered at the glomerulus and almost completely reabsorbed in the proximal tubule. Thus, increased urinary excretion suggests proximal tubular damage with impaired reabsorption or increased primary synthesis and excretion by distal nephron segments.
4.1.1 Preclinical Investigation
A role for NGAL as a marker of AKI was first suggested by high through-put transcriptomic and proteomic studies in animal models. Supavekin and colleagues were the first to show increased NGAL gene expression in a mouse model of renal ischemia reperfusion injury.18 Mishra and colleagues not only demonstrated NGAL as one of seven highly up-regulated genes following ischemic AKI in mice, but also showed an early increase in detectable urinary NGAL excretion marking it as a potential biomarker of renal injury.16 These findings were later validated in both ischemic and nephrotoxic animal models of AKI prompting further clinical evaluation in humans.19–21
4.1.2 Clinical Investigation
Clinical studies examining the utility of NGAL as a biomarker of AKI are numerous and have been extensively reviewed.22, 23 The predictive and diagnostic utility of NGAL in AKI has been most widely examined in the post- cardiac surgery population as there is an easily identifiable time of insult and a high incidence of renal injury.24 Mishra and colleagues, in follow-up to their animal work, first demonstrated the predictive value of NGAL in human AKI through examination of 71 pediatric patients undergoing cardiopulmonary bypass (CPB).25 20 children developed AKI, defined as a 50% increase in SCr from baseline, and urinary NGAL (uNGAL), 2 hours post-bypass, was the strongest independent predictor of AKI in multivariate analysis. The area under the curve of the receiver operating characteristic (AUC-ROC) curve was 0.99 with a 2 hour uNGAL cut-off of 50ug/L.
In adult populations, multiple studies have confirmed increases in NGAL post cardiac surgery, but the role in predicting AKI has been less conclusive. In as study examining 81 adult patients before and after cardiac surgery, 16 patients developed AKI defined as a 50% increase in SCr. 26 UNGAL concentrations increased in all patients post-operatively, but the 16 patients with AKI had significantly higher 1 hour post-operative values compared to the patients without AKI (4,195 +/− 6,520 vs. 1,068 +/− 2,129 ng/ml; P < 0.01). Further, in the AKI group, levels remained significantly elevated at 18 hours post-operatively, while NGAL levels in the non-AKI group returned to baseline. Similar studies have also documented the ability of uNGAL to predict AKI in the immediate post-operative setting. 27–29 However, in a larger study by Wagener and colleagues,30 the AUC-ROC for uNGAL was only 0.60, and uNGAL levels directly correlated with bypass and aortic cross-clamp time suggesting a limited accuracy of NGAL in predicting AKI. Most recently, Parikh and colleagues have investigated the ability of NGAL to predict AKI in post-cardiac surgery patients utilizing two large parallel adult and pediatric cohorts.31, 32 Overall, plasma NGAL (pNGAL) had an AUC-ROC of 0.70 and 0.56 while uNGAL had an AUC-ROC of 0.67 and 0.71 for adult and pediatric cohorts respectively. After adjustment for demographics and intra-operative risk factors, adults with the highest quintile of pNGAL levels had a 5-fold increased risk of developing AKI, with no increased risk associated with uNGAL levels.32 Pediatric patients with the highest uNGAL levels had a 4-fold risk of AKI, with no increased risk associated with plasma levels.31 This suggests differences in adult and pediatric patients perhaps related to age and comorbidities.
To further examine the effects of medical comorbidities on NGAL levels, the ability of NGAL to predict AKI has been examined in various critically ill populations. Zappitelli and colleagues first reported that uNGAL had an AUC-ROC of 0.78 for predicting AKI in a heterogeneous population of critically ill children.33 Wheeler and colleagues showed pNGAL to be a sensitive but non-specific marker of AKI in critically ill children, with levels the highest in patients with co-existent septic shock.34 In a population of adult multi-trauma patients, NGAL levels on admission to the intensive care unit (ICU) were strongly predictive of AKI development (AUC-ROC = 0.98),35 however in more heterogeneous populations of adult critically ill patients, AUC-ROCs range from 0.64 to 0.78 suggesting a more moderate ability to predict subsequent AKI.36, 37 Constantin and colleagues showed that pNGAL on ICU admission was a strong predictor for the development of AKI using a cut-off of 155nmol/L, and NGAL increased 48 hours prior to diagnosis of AKI by RIFLE criteria.38 However, recent investigation continues to demonstrate a significant difference in NGAL levels in septic versus non-septic AKI further supporting the influence of comorbidities in the prognostic and diagnostic ability of NGAL.39
NGAL has also been found to predict delayed graft function (DGF) following renal transplantation, 40–42 and to predict contrast induced nephropathy (CIN) in both children and adult populations.43–46 Additionally, Nickolas and colleagues have shown that a single uNGAL level on admission strongly predicted AKI with an AUC-ROC of 0.95, and levels correlated with important clinical outcomes including nephrology consultation, dialysis, and admission to the ICU. 47 Finally, a recent meta-analysis by Haase and colleagues examined 2,538 patients in 19 studies.48 The overall standardized AUC-ROC of NGAL to predict AKI was 0.83, with improved predictive ability in children versus adults (AUC-ROC 0.93 vs. 0.78). Predictive value was the best for contrast induced nephropathy (AUC-ROC=0.89), followed by post-cardiac surgery patients (AUC-ROC=0.78), and only modestly predictive in critically ill patients (AUC-ROC=0.73).
Due to the strong clinical evidence supporting NGAL as a marker of AKI, it is now being utilized as a clinical endpoint of renal injury in many clinical trials.49, 50 This use is likely to further increase with the recent development of rapid point of care testing methods for both plasma (Biosite Incorporated, San Diego, CA, USA) and urinary levels (Abbott Diagnostics, Illinois, USA). Still, some concern remains as NGAL appears to most accurately predict AKI in patients with normal baseline renal function and limited comorbidities, perhaps explaining the increased predictive values in pediatric populations.51 As with the other biomarkers currently in development, further clinical trials in heterogeneous populations are required to confirm the validity and clinical utility of NGAL as a biomarker of AKI.
4.2 Interleuken-18 (IL-18)
Interleukin-18 (IL-18) is a pro-inflammatory cytokine that is induced in proximal tubular epithelial cells in response to injury. Following renal injury, IL-18 is secreted in the urine prior to a significant decrease in renal function. Therefore, urinary IL-18 (uIL-18) has been identified as a potential early marker of AKI.
4.2.1 Preclinical Investigation
The rationale for examining the role of IL-18 in AKI arose from the observation that caspases play a key role in mediating renal damage. 52 Caspase-1, previously known as IL-1β converting enzyme, cleaves the inactive pro-form of IL-18 into the mature active form with high specificity. Early studies of caspase-1 deficient mice found that IL-18 production was defective in response to lipopolysaccharide, and that the mice were resistant to the effects of endotoxemia. 53 A follow-up study found that these mice also experienced less severe renal failure, defined by rise in BUN or Cr and histologic changes, than wild type mice in response to ischemic renal insults. Furthermore, the administration of IL-18-neutralizing rabbit anti-sera to wild type mice was protective of ischemic AKI.54 Later, a study of IL-18 binding protein transgenic mice, where IL-18 is neutralized, found that these mice were also protected against ischemic renal injury.55 Together, these studies have established that IL-18 is not only a marker of AKI, but also plays a pathogenic role in ischemic renal injury.56
Early animal studies measured uIL-18 with an electrochemiluminescence assay that detected both the pro and active forms of IL-18.57 U-IL-18 levels are also expressed as a ratio to urinary Cr levels in order to account for differences in urinary concentration. Using this method in a mouse model, uIL-18 was significantly elevated in animals exposed to ischemic AKI compared to virtually undetectable levels in controls.54 Thus a potential diagnostic role for uIL-18 was suggested.
4.2.2 Clinical Investigation
One of the first clinical studies of the diagnostic potential of uIL-18 examined 72 patients with a variety of renal disorders.58 In patients with a clinical diagnosis of acute tubular necrosis (ATN), uIL-18 was significantly elevated compared to normal controls (mean uIL-18 814+151 vs. 23+9 pg/mg Cr, p<.0001). Importantly, uIL-18 levels also distinguished patients with ATN from patients with pre-renal azotemia, chronic kidney disease (CKD), urinary tract infection, and nephrotic syndrome. In a subgroup of transplant patients, uIL-18 levels measured in the first 24 hours post-transplant were significantly higher in patients receiving a deceased donor transplant who subsequently met criteria for delayed graft function (DGF, defined as requiring dialysis within the first week post-transplant) compared to transplant patients with prompt kidney function. In this study, the AUC-ROC for the diagnosis of ATN and DGF was 0.95.
Parikh and colleagues evaluated the diagnostic role of uIL-18 in a cohort of 138 critically ill patients enrolled in the Acute Respiratory Distress Syndrome Network trial and specifically examined the timing of uIL-18 levels relative to standard AKI definitions. Urinary IL-18 levels were significantly greater in AKI compared to non-AKI patients at 24 and 48hrs before Cr-based criteria for AKI were met. The AUC-ROC of uIL-18 for predicting AKI in the next 24hrs was 0.73. An additional important finding was that uIL-18 level at the time of study enrollment was an independent predictor of subsequent mortality.59 A subsequent study further characterized the time-course of uIL-18 by demonstrating that levels begin rising as early as 4 to 6 hours following an ischemic insult, peak at 12 hours, and remain elevated for up to 48 hours.60
Several additional studies have demonstrated the potential utility of uIL-18 as an early biomarker for AKI in a variety of clinical settings, including CIN,46 critically ill pediatric patients,61 and renal transplant patients.41 Recently, Siew and colleagues measured uIL-18 in a general cohort of 451 ICU patients, 86 of whom developed AKI. Interestingly, in this study uIL-18 was not predictive of AKI development within 24 hours (AUC-ROC 0.62), although levels were associated with 28 day mortality.62 A possible explanation is that IL-18 is also produced in lymphocytes, macrophages, fibroblasts and intestinal epithelial cells, raising the possibility that levels may be influenced by generalized inflammatory states.63
While this initial clinical data supports uIL-18 as a useful biomarker of human AKI, several limitations remain. In contrast to early testing in animal models, measurement of uIL-18 in humans is done by ELISA. While testing kits are commercially available, they are labor intensive, have long turnaround times, and thus are useful for research purposes only. Further, while studies of discrete patient populations have suggested AUC values of greater than 0.90 for the diagnosis of AKI, recent studies suggest that the diagnostic characteristics of uIL-18 may be more modest in heterogeneous populations. In particular, future studies are needed to clarify the potential confounding impact of generalized inflammation on uIL-18 levels. In addition, no consensus thresholds have been identified for risk stratification, with suggested cutoffs ranging from 100 to 500 pg/mg Cr.59
4.3 Kidney Injury Molecule-1 (KIM-1)
Kidney injury molecule 1 (KIM-1) is a membrane glycoprotein that is expressed in proximal renal tubular epithelial cells in response to cellular injury. Interest in KIM-1 first arose from genomic screening approaches, in which KIM-1 was observed to be the most up-regulated gene in a rat model of ischemic AKI.64 KIM-1 directs phagocytosis of apoptotic cells in the tubular lumen by epithelial cells,65 and does not appear to be expressed in normal kidneys.66
4.3.1 Preclinical Investigation
Since the identification of KIM-1 up-regulation in the rat model of renal ischemia, additional studies have confirmed this finding and examined the diagnostic role of KIM-1 in other models of AKI. An important observation was that the extracellular domain of KIM-1 is cleaved and excreted, 67 spurring investigation of urinary KIM-1 (uKIM-1) as a biomarker of AKI.
Ichimura and colleagues examined tissue and uKIM-1 expression in a rat model of toxic AKI. Following cisplatin administration, uKIM-1 was qualitatively detectable by immunoblot assay on day 1 while serum Cr levels did not rise until day 3.68 A further advance was the development of a sensitive ELISA test to quantitatively measure uKIM-1 in rodents.69 Subsequent studies demonstrated a similar pattern of KIM-1 elevation in response to a variety of other nephrotoxic insults.70, 71 As a result, the Food and Drug Administration has recognized uKIM-1 as an appropriate biomarker for renal injury in preclinical studies of pharmacologic agents.72, 73
4.3.2 Clinical Investigation
Han and colleagues conducted the first biomarker evaluations of uKIM-1 in humans.74 In 6 patients with biopsy-proven ischemic ATN, uKIM-1 levels, normalized to urinary creatinine, were significantly higher than in controls without renal failure, where uKIM-1 was nearly undetectable. Patients with a variety of other renal conditions, including CIN, other AKI, and CKD, were also found to have elevated uKIM-1 levels compared to controls. Interestingly, these conditions had significantly lower KIM-1 levels compared to ischemic ATN, suggesting that KIM-1 may be useful in differentiating between various etiologies of AKI.
In a study of pediatric patients undergoing CPB surgery, urinary KIM-1 levels were prospectively monitored following surgery. Patients that developed AKI, defined as a 50% increase in serum creatinine, had a significant rise in normalized KIM-1 by 12 hours (but not at 2 or 6 hours) post-op when compared to patients not developing AKI, and KIM-1 levels remained elevated for 48 hours. In this study, the 12 hour KIM-1 level yielded an AUC-ROC of 0.83 for the diagnosis of AKI.75 In contrast, a study of 103 adult patients undergoing CPB found that normalized KIM-1 levels were significantly elevated by 2 hours in AKI versus non-AKI patients, with an AUC-ROC of 0.78. Based on test characteristics, an optimal diagnostic threshold value of KIM-1 was determined to be 0.42 ng/mg Cr.76 Another study of adult cardiac surgery patients confirmed that normalized uKIM-1 levels were significantly elevated in the early, immediately and at 3 hours, post-operative period in patients who developed AKI within the first 24 hours post-op compared to non-AKI patients.77
A recent study of 201 patients with established AKI found that higher normalized uKIM-1 levels were independently associated with a greater risk for the composite outcome of death or need for dialysis.78 Therefore, KIM-1 may hold important prognostic value in addition to diagnostic utility.
Recently, a rapid testing method for KIM-1 has been described, yielding semi-quantitative results in just 15 minutes.72 This assay provides the potential for rapid diagnosis of AKI in advance of traditional markers such as SCr. Commercial kits using this assay have become available. However, as with uIL-18, all tests remain labeled for research purposes only and further clinical evaluation is required.
While initial studies suggested that KIM-1 elevations may be delayed relative to other novel biomarkers of AKI, more recent studies suggest that KIM-1 elevations do occur within hours of renal injury. Potential advantages of this biomarker include the apparent specificity for ischemic renal injury and the availability of a rapid assay. However, there have also been inconsistent results between studies79 and further research is needed to clarify the value of KIM-1 as a diagnostic or prognostic biomarker in AKI.
4.4 Liver FABP-1 (L-FABP)
Fatty acid binding proteins (FABPs) are a family of small cytosolic proteins which facilitate β-oxidation through binding and transportation of long chain fatty acids.80 Additionally, selective binding to lipid peroxidation products limits subsequent cellular toxicity,81 and this protective role has triggered interest in FABPs as potential markers of cellular injury. There are currently nine tissue-specific FABPs identified. Liver-type (L)-FABP (or FABP-1) is a 14-kDa hepatically synthesized protein localized to the liver, the intestinal tract, and the renal proximal tubule epithelium, a cell type reliant on primary fatty acid energy metabolism.
4.4.1 Pre-clinical Investigation
An antioxidant role of L-FABP was displayed by Wang and colleagues by subjecting liver cells to oxidative stress in vitro.82 Transfected cells, with increased L-FABP expression, displayed a significant decrease in the generation of reactive oxidant species. To allow examination of L-FABP in vivo, a humanized transgenic mouse model was generated, Tg-hL-FABP, which has significant L-FABP expression compared to minimal expression in wild type mice.83 Expression of L-FABP was found to be protective against renal tubulointerstitial damage and prevented accumulation of lipid peroxidation products following ureteral obstruction.84 Later, Yamamoto and colleagues demonstrated L-FABP transitions from the cytoplasm to the proximal tubular lumen in Tg-hL-FABP mice following ischemia-reperfusion injury, further supporting the acute response of L-FABP to oxidative stress and promoting urinary L-FABP (uL-FABP) as an early marker of kidney injury.85
Negishi and colleagues showed that cisplatin-mediated nephrotoxicity was associated with increased uL-FABP excretion in the Tg-hL-FABP mice 1 day post exposure while elevations in BUN were not detected until 3 days later.86, 87 In a follow-up study, the same group showed that uL-FABP could be used as a marker of differing degrees of renal histological injury following cisplatin or ischemia-reperfusion induced AKI.88 Here, uL-FABP levels showed a better correlation with renal histological injury scores than either BUN or N-acetyl-d-glucosaminidase, and urinary levels of L-FABP increased as early as 1–2 hours post injury. The correlation of uL-FABP excretion with degree of histologic renal injury has been confirmed in subsequent studies.89 Collectively, this evidence suggests a protective role for L-FABP against oxidant stress and cellular toxicity, with urinary excretion acting as a rapid mechanistic marker of AKI.
4.4.2 Clinical Investigation
The role of L-FABP in human AKI has been investigated in several recent studies. Nakamura and colleagues measured uL-FABP in 66 patients, with baseline SCr of 1.2–2.5mg/dL, before and after elective coronary catheterization.90 CIN, defined as an increase in SCr of greater than 0.5 mg/dL or a relative increase of more than 25% at 2 to 5 days post procedure, occurred in 13 patients. Baseline values of uL-FABP were significantly elevated in the 13 CIN patients compared to the remaining 53 patients (18.5 ± 12.8 vs. 7.4 ± 4.4μg/gCr; p<0.01). Levels significantly increased post procedure only in the CIN group (1 day post procedure 46.8 ± 30.5 μg/gCr; p<0.01) and remained elevated at 14 days despite return to baseline of SCr, supporting both a predictive and diagnostic role of uL-FABP in CIN. More recently, Yamamoto and colleagues measured uL-FABP in 12 patients receiving living-related kidney transplants immediately after organ reperfusion was achieved.85 They found a significant inverse correlation of uL-FABP levels with peritubular capillary flow, measured with intravital video analysis, and a significant direct correlation with transplant ischemia time (both p<0.0001). This supported the role of uL-FABP as a marker of ischemic renal injury.
Portilla and colleagues measured uL-FABP before and after CPB in 40 pediatric patients.6 AKI, defined as a 50% increase in SCr, occurred in 21 patients with increases in uL-FABP, 20 ± 4 ng/mgCr to 1885 ± 500ng/mgCr at 4hrs to 905 ± 320ng/mgCr at 12hrs, while increases in SCr were not seen until 24–72hrs post-surgery. Both bypass time and 4hr uL-FABP were independent risk factors for AKI, and 4hr L-FABP had an AUC-ROC of 0.810 for predicting AKI. Additionally, in patients with AKI, there were no detectable changes in serum L-FABP until 12 hours post bypass suggesting that uL-FABP is a specific marker for AKI, and not merely a marker of high plasma levels from a non-renal source. Interestingly, in a study evaluating patients undergoing cardiac catheterization, Fukuda and colleagues concluded that uL-FABP was a marker of acute coronary syndrome, sensitivity 0.80 and specificity 0.96, and not a marker of AKI, although none of the studied patients developed CIN.91
Most recently, the diagnostic ability of uL-FABP has been examined in septic shock. Nakamura and colleagues retrospectively examined L-FABP levels in 40 patients with septic shock, 20 patients with sepsis without shock, and 20 hospitalized patients with AKI with mean SCr 2.8mg/dL and baseline SCr <1.2mg/dL.92 The 40 patients with septic shock had the highest uL-FABP levels even compared to those with AKI (1860 ± 1260 vs. 120 ± 84 μg/gCr; p<0.0001). uL-FABP did not correlate with SCr or with need for dialysis. Ferguson and colleagues performed a cross-sectional study of 92 hospitalized patients with AKI compared to 68 primarily ambulatory controls.93 UL-FABP was a significant independent predictor of need for renal replacement therapy. When comparing AKI versus hospitalized controls, the AUC-ROC of uL-FABP for identification of AKI was 0.93 using a cutoff of 47.1ng/mgCr. Even comparing AKI to intensive care unit controls without AKI, the AUC-ROC remained at 0.82 for the diagnosis of AKI. No adjustment for severity of illness or liver dysfunction was made in this analysis.
Collectively, these studies demonstrate the utility of uL-FABP as an early indicator of AKI, however, further investigation is needed. The clinical trials have been small and largely cross-sectional; large prospective trials including multiple etiologies of renal disease are needed to truly assess the diagnostic and prognostic ability of L-FABP levels. Further investigation is also needed to clarify systemic illness, particularly liver injury, on the role of increased uL-FABP excretion. Finally, while ELISA testing is possible, no standardized assays for L-FABP exist to allow widespread clinical use.
4.5 Cystatin C (CysC)
Cystatin C (CysC) is a cysteine proteinase inhibitor that is produced at a constant rate by all nucleated cells.94 This middle molecular weight molecule, 13-kDa, which does not exhibit significant protein binding, is freely filtered by the glomerulus but not secreted or reabsorbed into plasma, and therefore serum cystatin C (sCysC) levels have been extensively evaluated as a measure of GFR in CKD patients.95–98 Following filtration, CysC is fully catabolized by proximal tubular epithelial cells99 and is usually undetectable in the urine of patients with normal renal function. However, tubular injury can result in measurable urinary levels, suggesting CysC as a potential biomarker for AKI.
4.5.1 Preclinical Investigation
Because of the extensive background research establishing the role of CysC as a measure of GFR, there has been a relative paucity of pre-clinical studies of its use in AKI models. Instead, most studies have been focused on humans in various clinical settings as reviewed below.
4.5.2 Clinical Investigation
In contrast to some other novel biomarkers, a rise in sCysC levels reflects a decrement in GFR and therefore there is an inherent delay between renal injury and a detectable increase in levels. However, studies have shown that sCysC levels rise prior to creatinine levels and therefore may provide an earlier diagnosis of AKI. Herget-Rosenthal and colleagues found that a 50% rise in sCysC preceded a similar rise in serum creatinine (i.e. RIFLE stage R AKI) by 2 days. In their study of 85 ICU patients at risk for developing AKI, sCysC performed well as a diagnostic test for AKI with an AUC-ROC of 0.82. Cystatin C measurements were also predictive of the need for renal replacement therapy.100 A more recent study by Royakkers and colleagues also found that sCysC levels of ICU patients developing AKI were elevated 2 days before meeting RIFLE criteria, providing an AUC of 0.72. Interestingly, however, sCysC levels no longer differed 24 hours preceding AKI diagnosis.101 In a study of patients receiving radiocontrast, Rickli and colleagues reported that sCysC levels were not elevated at 5 hours after contrast administration but subsequently peaked at 24 hours, while serum creatinine was still rising at 48 hours.102 Other studies have provided more mixed results regarding the utility of sCysC rise compared to serum creatinine.27, 103, 104 In addition, there have been reports of confounding clinical conditions that can lead to sCysC elevations, including thyroid disease and/or generalized inflammation.105
Because uCysC should only be detectable when proximal renal tubular function is impaired, this marker may provide earlier diagnosis of renal injury and be more specific than sCysC. Koyner and colleagues evaluated a series of biomarkers following cardiac surgery and found that uCysC levels measured 6 hours post-operatively were predictive of AKI with an AUC of 0.72, outperforming sCysC and NGAL measured at the same time point.27 More recent studies have reported less favorable results using uCysC, and a recent meta-analysis (which included the above study) concluded that uCysC has only modest diagnostic value with a pooled AUC-ROC of 0.64.106
Unlike other novel biomarkers for AKI, CysC measurement is readily available for clinical measurement using automated and standardized assays that provide rapid turnaround. However, there is wide variation in the reporting of both serum and urinary CysC levels, and no consensus has emerged regarding threshold levels of CysC by which to define AKI diagnosis or risk.106 Additionally, as a marker of GFR, sCysC shares many of the same limitations as serum creatinine for the diagnosis of AKI, including lack of specificity in differentiating acute versus chronic kidney disease and a delay of approximately 24 hours before rising after renal injury. As such, the primary use of sCysC measurements will likely remain in the evaluation of CKD. Conversely, uCysC theoretically holds more promise for AKI diagnosis. However, clinical studies to date have reported largely mixed results, and several studies suggest fairly poor diagnostic test characteristics compared to other novel biomarkers. Additional large studies will be needed to clarify any potential role of CysC in the diagnosis of AKI.
4.6 Other Protein Biomarkers of AKI
In addition to these widely investigated potential biomarkers, multiple studies have now used various MS platforms to examine the wide spectrum of urinary proteins in AKI. These studies have identified several novel urinary proteins with biomarker potential and have been recently reviewed by Bennett.11 Nguyen and colleagues examined the urine proteome of 60 pediatric CPB patients. Aprotinin, alpha-1 microglobulin, alpha-1 acid glycoprotein, and albumin were significantly increased in patients that subsequently developed AKI, defined as a 50% increase in SCr.107–109 Similar studies in adult CPB populations have been performed. Ho and colleagues originally identified hepcidin-25 to be significantly decreased in patients with AKI following CPB.110 This finding has now been validated in a recent nested case control analysis using ELISA based measurement techniques, suggesting a role of iron homeostasis in the pathogenesis of AKI.111 Aregger and collegues examined the urinary proteome of 36 adults undergoing CPB.112 In the 6 patients that developed AKI, defined by RIFLE criteria, urinary albumin was increased, and zinc-alpha-2-glycoprotein and a fragment of adrenomedullin-binding protein were decreased. Most recently, Heller and colleagues have shown that urinary calprotectin, an activator of the innate immune system, reliably differentiates pre-renal azotemia from intrinsic AKI in a group of hospitalized patients, supporting the predictive role of the urinary proteome even in heterogeneous patient populations.113 While these initial exploratory urinary proteome studies are intriguing and will undoubtedly lead to improved mechanistic understanding and diagnostic capabilities surrounding AKI, larger clinical trials are needed for validation.
5. Metabolite Biomarkers of AKI
In addition to proteomics, recent biomarker development has utilized the growing field of metabolomics. Metabolomics attempts to identify and quantitate small molecules from biological samples and includes the analysis of amino acids, lipids, nucleic acids, and organic acids to understand the overall disease phenotype. Beger and colleagues examined the urine of 40 pediatric patients following cardiac surgery and found a significant elevation of homovanillic acid sulfate in those that developed AKI.114 The level drawn at 4 hours post CPB had an AUC-ROC of 0.78 in predicting AKI. Metabolomic techniques have also been utilized in kidney transplant recipients, with the hopes of identifying an early biomarker of rejection and avoidance of high risk allograft biopsy. Foxall and others have consistently found elevations in urinary trimethylamine-N-oxide, an organic amine, in patients with early allograft dysfunction.115, 116 Metabolic profiles have also been widely utilized in animal models of drug induced kidney injury, which has recently been reviewed elsewhere.117 Continued advances in bioinformatics and analytical strategies will likely increase the widespread investigation of metabolomics in human AKI.
6. Summary and Perspective
Despite the significant progress in AKI biomarker discovery, further investigation is needed to validate clinical utility. Currently, the majority of investigation has involved small single center trials. Larger trials, with more heterogeneous populations, will be required to better understand the applicability to routine clinical practice. Additionally, future studies are needed to compare various biomarkers to each other. As each individual marker has independent biological properties and kinetics, it is likely that a panel, combining several biomarkers, will have greater diagnostic and predictive capabilities (Figure 1). Several studies have in fact demonstrated the benefits of combination panels in clinical trials.75, 118 For example, Metzger and colleagues identified a panel of urinary proteins which predict AKI in a heterogeneous ICU population with an AUC-ROC of 0.91.119 Most recently, Hall and colleagues have shown that a panel combining novel biomarker levels with other clinical variables leads to a significant increase in ability to predict worsening renal failure and in-hospital mortality. 120 In the future, the biomarkers, either alone or as a panel, will require validation within interventional trials to determine their ability to predict important clinical outcomes, particularly related to therapeutic response. Finally, in order to fully validate a given biomarker, more accurate gold standard measures of AKI, without reliance on SCr, will have to be utilized. As of now, none of the novel biomarkers or panel has demonstrated a clear superiority to current diagnostic gold-standards. While a single super-marker is unlikely to exist, future investigation in this needed field will undoubtedly lead to greater mechanistic understanding of AKI. Until then careful clinical evaluation and measurement of SCr will continue to guide clinical decision making process.
Acknowledgments
This work is supported in part by grants from the National Institutes of Health (DK082841, DK094292, DK089503 and K12HD001438) and the Doris Duke Foundation Clinical Scientist Development Award (SP).
Abbreviations
- AKI
Acute Kidney Injury
- AKIN
Acute Kidney Injury Network
- AUC-ROC
area under the curve of the receiver operating characteristic curve
- ATN
acute tubular necrosis
- BUN
blood urea nitrogen
- CKD
chronic kidney disease
- CIN
contrast induced nephropathy
- CPB
cardiopulmonary bypass
- CysC
Cystatin C
- DGF
delayed graft function
- ELISA
enzyme-linked immunosorbent assays
- GFR
glomerular filtration rate
- ICU
intensive care unit
- IL-18
Interleukin-18
- KIM-1
Kidney injury molecule-1
- L-FABP
Liver-type fatty acid binding protein-1
- MS
mass spectrometry
- NGAL
neutrophil gelatinase-associated lipocalin
- RIFLE
Risk Injury Failure Loss End-stage renal disease
- SCr
serum creatinine
- UTI
urinary tract infection
Footnotes
Conflicts: None of the authors have potential conflicts of interest. All authors have read the journal’s policy on disclosure of potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Metnitz PG, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30(9):2051–8. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folin ODJ. On the creatinine and creatinine content of blood. Biol Chem. 1914;17:487–91. [Google Scholar]
- 5.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009;20(3):672–9. doi: 10.1681/ASN.2008070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Portilla D, Dent C, Sugaya T, Nagothu KK, Kundi I, Moore P, et al. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008;73 (4):465–72. doi: 10.1038/sj.ki.5002721. [DOI] [PubMed] [Google Scholar]
- 7.Doi K, Yuen PS, Eisner C, Hu X, Leelahavanichkul A, Schnermann J, et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol. 2009;20(6):1217–21. doi: 10.1681/ASN.2008060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parikh CR, Devarajan P. New biomarkers of acute kidney injury. Crit Care Med. 2008;36(4 Suppl):S159–65. doi: 10.1097/CCM.0b013e318168c652. [DOI] [PubMed] [Google Scholar]
- 9.Devarajan P. Proteomics for biomarker discovery in acute kidney injury. Semin Nephrol. 2007;27(6):637–51. doi: 10.1016/j.semnephrol.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang JH, Byun J, Pennathur S. Analytical approaches to metabolomics and applications to systems biology. Semin Nephrol. 2010;30(5):500–11. doi: 10.1016/j.semnephrol.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett MR, Devarajan P. Proteomic analysis of acute kidney injury: biomarkers to mechanisms. Proteomics Clin Appl. 2011;5(1–2):67–77. doi: 10.1002/prca.201000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urine Glycoprotein Profile Reveals Novel Markers for Chronic Kidney Disease. International Journal of Proteomics. 2011 doi: 10.1155/2011/214715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu SY, Carlson M, Engstrom A, Garcia R, Peterson CG, Venge P. Purification and characterization of a human neutrophil lipocalin (HNL) from the secondary granules of human neutrophils. Scand J Clin Lab Invest. 1994;54(5):365–76. doi: 10.3109/00365519409088436. [DOI] [PubMed] [Google Scholar]
- 14.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10(5):1033–43. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 15.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432(7019):917–21. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 16.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14(10):2534–43. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18(2):407–13. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- 18.Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003;63(5):1714–24. doi: 10.1046/j.1523-1755.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- 19.Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24(3):307–15. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- 20.Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115(3):610–21. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuen PS, Jo SK, Holly MK, Hu X, Star RA. Ischemic and nephrotoxic acute renal failure are distinguished by their broad transcriptomic responses. Physiol Genomics. 2006;25(3):375–86. doi: 10.1152/physiolgenomics.00223.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devarajan P. Neutrophil gelatinase-associated lipocalin: a promising biomarker for human acute kidney injury. Biomark Med. 2010;4(2):265–80. doi: 10.2217/bmm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soni SS, Cruz D, Bobek I, Chionh CY, Nalesso F, Lentini P, et al. NGAL: a biomarker of acute kidney injury and other systemic conditions. Int Urol Nephrol. 2010;42(1):141–50. doi: 10.1007/s11255-009-9608-z. [DOI] [PubMed] [Google Scholar]
- 24.Cruz DN, Ronco C, Katz N. Neutrophil gelatinase-associated lipocalin: a promising biomarker for detecting cardiac surgery-associated acute kidney injury. J Thorac Cardiovasc Surg. 2010;139(5):1101–6. doi: 10.1016/j.jtcvs.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 26.Wagener G, Jan M, Kim M, Mori K, Barasch JM, Sladen RN, et al. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006;105(3):485–91. doi: 10.1097/00000542-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Koyner JL, Bennett MR, Worcester EM, Ma Q, Raman J, Jeevanandam V, et al. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 2008;74(8):1059–69. doi: 10.1038/ki.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xin C, Yulong X, Yu C, Changchun C, Feng Z, Xinwei M. Urine neutrophil gelatinase-associated lipocalin and interleukin-18 predict acute kidney injury after cardiac surgery. Ren Fail. 2008;30(9):904–13. doi: 10.1080/08860220802359089. [DOI] [PubMed] [Google Scholar]
- 29.Tuladhar SM, Puntmann VO, Soni M, Punjabi PP, Bogle RG. Rapid detection of acute kidney injury by plasma and urinary neutrophil gelatinase-associated lipocalin after cardiopulmonary bypass. J Cardiovasc Pharmacol. 2009;53(3):261–6. doi: 10.1097/FJC.0b013e31819d6139. [DOI] [PubMed] [Google Scholar]
- 30.Wagener G, Gubitosa G, Wang S, Borregaard N, Kim M, Lee HT. Urinary neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery. Am J Kidney Dis. 2008;52(3):425–33. doi: 10.1053/j.ajkd.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Parikh CR, Devarajan P, Zappitelli M, Sint K, Thiessen-Philbrook H, Li S, et al. Postoperative Biomarkers Predict Acute Kidney Injury and Poor Outcomes after Pediatric Cardiac Surgery. J Am Soc Nephrol. 2011;22(9):1737–47. doi: 10.1681/ASN.2010111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, et al. Postoperative Biomarkers Predict Acute Kidney Injury and Poor Outcomes after Adult Cardiac Surgery. J Am Soc Nephrol. 2011;22(9):1748–57. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, et al. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care. 2007;11(4):R84. doi: 10.1186/cc6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wheeler DS, Devarajan P, Ma Q, Harmon K, Monaco M, Cvijanovich N, et al. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med. 2008;36(4):1297–303. doi: 10.1097/CCM.0b013e318169245a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makris K, Markou N, Evodia E, Dimopoulou E, Drakopoulos I, Ntetsika K, et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) as an early marker of acute kidney injury in critically ill multiple trauma patients. Clin Chem Lab Med. 2009;47(1):79–82. doi: 10.1515/CCLM.2009.004. [DOI] [PubMed] [Google Scholar]
- 36.Siew ED, Ware LB, Gebretsadik T, Shintani A, Moons KG, Wickersham N, et al. Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol. 2009;20(8):1823–32. doi: 10.1681/ASN.2008070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cruz DN, de Cal M, Garzotto F, Perazella MA, Lentini P, Corradi V, et al. Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med. 2010;36(3):444–51. doi: 10.1007/s00134-009-1711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Constantin JM, Futier E, Perbet S, Roszyk L, Lautrette A, Gillart T, et al. Plasma neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in adult critically ill patients: a prospective study. J Crit Care. 2010;25(1):176 e1–6. doi: 10.1016/j.jcrc.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Bagshaw SM, Bennett M, Haase M, Haase-Fielitz A, Egi M, Morimatsu H, et al. Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive Care Med. 2010;36(3):452–61. doi: 10.1007/s00134-009-1724-9. [DOI] [PubMed] [Google Scholar]
- 40.Mishra J, Ma Q, Kelly C, Mitsnefes M, Mori K, Barasch J, et al. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol. 2006;21(6):856–63. doi: 10.1007/s00467-006-0055-0. [DOI] [PubMed] [Google Scholar]
- 41.Parikh CR, Jani A, Mishra J, Ma Q, Kelly C, Barasch J, et al. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant. 2006;6(7):1639–45. doi: 10.1111/j.1600-6143.2006.01352.x. [DOI] [PubMed] [Google Scholar]
- 42.Hall IE, Yarlagadda SG, Coca SG, Wang Z, Doshi M, Devarajan P, et al. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol. 2010;21(1):189–97. doi: 10.1681/ASN.2009030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirsch R, Dent C, Pfriem H, Allen J, Beekman RH, 3rd, Ma Q, et al. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol. 2007;22(12):2089–95. doi: 10.1007/s00467-007-0601-4. [DOI] [PubMed] [Google Scholar]
- 44.Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Dobrzycki S. Neutrophil-gelatinase-associated lipocalin and renal function after percutaneous coronary interventions. Am J Nephrol. 2006;26(3):287–92. doi: 10.1159/000093961. [DOI] [PubMed] [Google Scholar]
- 45.Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Dobrzycki S. Neutrophil gelatinase-associated lipocalin (NGAL) correlations with cystatin C, serum creatinine and eGFR in patients with normal serum creatinine undergoing coronary angiography. Nephrol Dial Transplant. 2007;22(1):295–6. doi: 10.1093/ndt/gfl408. [DOI] [PubMed] [Google Scholar]
- 46.Ling W, Zhaohui N, Ben H, Leyi G, Jianping L, Huili D, et al. Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clin Pract. 2008;108(3):c176–81. doi: 10.1159/000117814. [DOI] [PubMed] [Google Scholar]
- 47.Nickolas TL, O’Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148(11):810–9. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54(6):1012–24. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 49.Boldt J, Suttner S, Brosch C, Lehmann A, Rohm K, Mengistu A. Cardiopulmonary bypass priming using a high dose of a balanced hydroxyethyl starch versus an albumin-based priming strategy. Anesth Analg. 2009;109(6):1752–62. doi: 10.1213/ANE.0b013e3181b5a24b. [DOI] [PubMed] [Google Scholar]
- 50.Wagener G, Gubitosa G, Wang S, Borregaard N, Kim M, Lee HT. Increased incidence of acute kidney injury with aprotinin use during cardiac surgery detected with urinary NGAL. Am J Nephrol. 2008;28(4):576–82. doi: 10.1159/000115973. [DOI] [PubMed] [Google Scholar]
- 51.McIlroy DR, Wagener G, Lee HT. Neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery: the effect of baseline renal function on diagnostic performance. Clin J Am Soc Nephrol. 2010;5(2):211–9. doi: 10.2215/CJN.04240609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edelstein CL, Shi Y, Schrier RW. Role of caspases in hypoxia-induced necrosis of rat renal proximal tubules. J Am Soc Nephrol. 1999;10(9):1940–9. doi: 10.1681/ASN.V1091940. [DOI] [PubMed] [Google Scholar]
- 53.Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80(3):401–11. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 54.Melnikov VY, Ecder T, Fantuzzi G, Siegmund B, Lucia MS, Dinarello CA, et al. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest. 2001;107(9):1145–52. doi: 10.1172/JCI12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He Z, Lu L, Altmann C, Hoke TS, Ljubanovic D, Jani A, et al. Interleukin-18 binding protein transgenic mice are protected against ischemic acute kidney injury. Am J Physiol Renal Physiol. 2008;295(5):F1414–21. doi: 10.1152/ajprenal.90288.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu H, Craft ML, Wang P, Wyburn KR, Chen G, Ma J, et al. IL-18 contributes to renal damage after ischemia-reperfusion. J Am Soc Nephrol. 2008;19(12):2331–41. doi: 10.1681/ASN.2008020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fantuzzi G, Reed DA, Dinarello CA. IL-12-induced IFN-gamma is dependent on caspase-1 processing of the IL-18 precursor. J Clin Invest. 1999;104(6):761–7. doi: 10.1172/JCI7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis. 2004;43(3):405–14. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 59.Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005;16(10):3046–52. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- 60.Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70(1):199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 61.Washburn KK, Zappitelli M, Arikan AA, Loftis L, Yalavarthy R, Parikh CR, et al. Urinary interleukin-18 is an acute kidney injury biomarker in critically ill children. Nephrol Dial Transplant. 2008;23(2):566–72. doi: 10.1093/ndt/gfm638. [DOI] [PubMed] [Google Scholar]
- 62.Siew ED, Ikizler TA, Gebretsadik T, Shintani A, Wickersham N, Bossert F, et al. Elevated urinary IL-18 levels at the time of ICU admission predict adverse clinical outcomes. Clin J Am Soc Nephrol. 2010;5(8):1497–505. doi: 10.2215/CJN.09061209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol. 2003;73(2):213–24. doi: 10.1189/jlb.0602313. [DOI] [PubMed] [Google Scholar]
- 64.Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273(7):4135–42. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 65.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118(5):1657–68. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonventre JV. Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant. 2009;24(11):3265–8. doi: 10.1093/ndt/gfp010. [DOI] [PubMed] [Google Scholar]
- 67.Bailly V, Zhang Z, Meier W, Cate R, Sanicola M, Bonventre JV. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem. 2002;277(42):39739–48. doi: 10.1074/jbc.M200562200. [DOI] [PubMed] [Google Scholar]
- 68.Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286(3):F552–63. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 69.Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290(2):F517–29. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 70.Prozialeck WC, Vaidya VS, Liu J, Waalkes MP, Edwards JR, Lamar PC, et al. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int. 2007;72(8):985–93. doi: 10.1038/sj.ki.5002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou Y, Vaidya VS, Brown RP, Zhang J, Rosenzweig BA, Thompson KL, et al. Comparison of kidney injury molecule-1 and other nephrotoxicity biomarkers in urine and kidney following acute exposure to gentamicin, mercury, and chromium. Toxicol Sci. 2008;101(1):159–70. doi: 10.1093/toxsci/kfm260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vaidya VS, Ford GM, Waikar SS, Wang Y, Clement MB, Ramirez V, et al. A rapid urine test for early detection of kidney injury. Kidney Int. 2009;76(1):108–14. doi: 10.1038/ki.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28(5):478–85. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–44. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 75.Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, Devarajan P, et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73(7):863–9. doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liangos O, Tighiouart H, Perianayagam MC, Kolyada A, Han WK, Wald R, et al. Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers. 2009;14(6):423–31. doi: 10.1080/13547500903067744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2009;4(5):873–82. doi: 10.2215/CJN.04810908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liangos O, Perianayagam MC, Vaidya VS, Han WK, Wald R, Tighiouart H, et al. Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007;18(3):904–12. doi: 10.1681/ASN.2006030221. [DOI] [PubMed] [Google Scholar]
- 79.Huang Y, Don-Wauchope AC. The clinical utility of kidney injury molecule 1 in the prediction, diagnosis and prognosis of acute kidney injury: a systematic review. Inflamm Allergy Drug Targets. 2011;10(4):260–71. doi: 10.2174/187152811796117735. [DOI] [PubMed] [Google Scholar]
- 80.Chmurzynska A. The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet. 2006;47(1):39–48. doi: 10.1007/BF03194597. [DOI] [PubMed] [Google Scholar]
- 81.Ek-Von Mentzer BA, Zhang F, Hamilton JA. Binding of 13-HODE and 15-HETE to phospholipid bilayers, albumin, and intracellular fatty acid binding proteins. implications for transmembrane and intracellular transport and for protection from lipid peroxidation. J Biol Chem. 2001;276(19):15575–80. doi: 10.1074/jbc.M011623200. [DOI] [PubMed] [Google Scholar]
- 82.Wang G, Gong Y, Anderson J, Sun D, Minuk G, Roberts MS, et al. Antioxidative function of L-FABP in L-FABP stably transfected Chang liver cells. Hepatology. 2005;42(4):871–9. doi: 10.1002/hep.20857. [DOI] [PubMed] [Google Scholar]
- 83.Kamijo A, Sugaya T, Hikawa A, Okada M, Okumura F, Yamanouchi M, et al. Urinary excretion of fatty acid-binding protein reflects stress overload on the proximal tubules. Am J Pathol. 2004;165(4):1243–55. doi: 10.1016/S0002-9440(10)63384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kamijo-Ikemori A, Sugaya T, Obama A, Hiroi J, Miura H, Watanabe M, et al. Liver-type fatty acid-binding protein attenuates renal injury induced by unilateral ureteral obstruction. Am J Pathol. 2006;169(4):1107–17. doi: 10.2353/ajpath.2006.060131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamamoto T, Noiri E, Ono Y, Doi K, Negishi K, Kamijo A, et al. Renal L-type fatty acid--binding protein in acute ischemic injury. J Am Soc Nephrol. 2007;18(11):2894–902. doi: 10.1681/ASN.2007010097. [DOI] [PubMed] [Google Scholar]
- 86.Negishi K, Noiri E, Sugaya T, Li S, Megyesi J, Nagothu K, et al. A role of liver fatty acid-binding protein in cisplatin-induced acute renal failure. Kidney Int. 2007;72(3):348–58. doi: 10.1038/sj.ki.5002304. [DOI] [PubMed] [Google Scholar]
- 87.Negishi K, Noiri E, Maeda R, Portilla D, Sugaya T, Fujita T. Renal L-type fatty acid-binding protein mediates the bezafibrate reduction of cisplatin-induced acute kidney injury. Kidney Int. 2008;73(12):1374–84. doi: 10.1038/ki.2008.106. [DOI] [PubMed] [Google Scholar]
- 88.Negishi K, Noiri E, Doi K, Maeda-Mamiya R, Sugaya T, Portilla D, et al. Monitoring of urinary L-type fatty acid-binding protein predicts histological severity of acute kidney injury. Am J Pathol. 2009;174(4):1154–9. doi: 10.2353/ajpath.2009.080644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yokoyama T, Kamijo-Ikemori A, Sugaya T, Hoshino S, Yasuda T, Kimura K. Urinary excretion of liver type fatty acid binding protein accurately reflects the degree of tubulointerstitial damage. Am J Pathol. 2009;174(6):2096–106. doi: 10.2353/ajpath.2009.080780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nakamura T, Sugaya T, Node K, Ueda Y, Koide H. Urinary excretion of liver-type fatty acid-binding protein in contrast medium-induced nephropathy. Am J Kidney Dis. 2006;47(3):439–44. doi: 10.1053/j.ajkd.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 91.Fukuda Y, Miura S, Zhang B, Iwata A, Kawamura A, Nishikawa H, et al. Significance of urinary liver-fatty acid-binding protein in cardiac catheterization in patients with coronary artery disease. Intern Med. 2009;48(19):1731–7. doi: 10.2169/internalmedicine.48.2410. [DOI] [PubMed] [Google Scholar]
- 92.Nakamura T, Sugaya T, Koide H. Urinary liver-type fatty acid-binding protein in septic shock: effect of polymyxin B-immobilized fiber hemoperfusion. Shock. 2009;31(5):454–9. doi: 10.1097/SHK.0b013e3181891131. [DOI] [PubMed] [Google Scholar]
- 93.Ferguson MA, Vaidya VS, Waikar SS, Collings FB, Sunderland KE, Gioules CJ, et al. Urinary liver-type fatty acid-binding protein predicts adverse outcomes in acute kidney injury. Kidney Int. 2010;77(8):708–14. doi: 10.1038/ki.2009.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brzin J, Popovic T, Turk V, Borchart U, Machleidt W. Human cystatin, a new protein inhibitor of cysteine proteinases. Biochem Biophys Res Commun. 1984;118(1):103–9. doi: 10.1016/0006-291x(84)91073-8. [DOI] [PubMed] [Google Scholar]
- 95.Grubb AO. Cystatin C--properties and use as diagnostic marker. Adv Clin Chem. 2000;35:63–99. doi: 10.1016/S0065-2423(01)35015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coll E, Botey A, Alvarez L, Poch E, Quinto L, Saurina A, et al. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36(1):29–34. doi: 10.1053/ajkd.2000.8237. [DOI] [PubMed] [Google Scholar]
- 97.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40(2):221–6. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 98.Roos JF, Doust J, Tett SE, Kirkpatrick CM. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children--a meta-analysis. Clin Biochem. 2007;40(5–6):383–91. doi: 10.1016/j.clinbiochem.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 99.Kaseda R, Iino N, Hosojima M, Takeda T, Hosaka K, Kobayashi A, et al. Megalin-mediated endocytosis of cystatin C in proximal tubule cells. Biochem Biophys Res Commun. 2007;357(4):1130–4. doi: 10.1016/j.bbrc.2007.04.072. [DOI] [PubMed] [Google Scholar]
- 100.Herget-Rosenthal S, Marggraf G, Husing J, Goring F, Pietruck F, Janssen O, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66(3):1115–22. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 101.Royakkers AA, Korevaar JC, van Suijlen JD, Hofstra LS, Kuiper MA, Spronk PE, et al. Serum and urine cystatin C are poor biomarkers for acute kidney injury and renal replacement therapy. Intensive Care Med. 2011;37(3):493–501. doi: 10.1007/s00134-010-2087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rickli H, Benou K, Ammann P, Fehr T, Brunner-La Rocca HP, Petridis H, et al. Time course of serial cystatin C levels in comparison with serum creatinine after application of radiocontrast media. Clin Nephrol. 2004;61(2):98–102. doi: 10.5414/cnp61098. [DOI] [PubMed] [Google Scholar]
- 103.Ahlstrom A, Tallgren M, Peltonen S, Pettila V. Evolution and predictive power of serum cystatin C in acute renal failure. Clin Nephrol. 2004;62(5):344–50. doi: 10.5414/cnp62344. [DOI] [PubMed] [Google Scholar]
- 104.Soto K, Coelho S, Rodrigues B, Martins H, Frade F, Lopes S, et al. Cystatin C as a marker of acute kidney injury in the emergency department. Clin J Am Soc Nephrol. 2010;5(10):1745–54. doi: 10.2215/CJN.00690110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cruz DN, de Geus HR, Bagshaw SM. Biomarker strategies to predict need for renal replacement therapy in acute kidney injury. Semin Dial. 2011;24(2):124–31. doi: 10.1111/j.1525-139X.2011.00830.x. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Z, Lu B, Sheng X, Jin N. Cystatin C in prediction of acute kidney injury: a systemic review and meta-analysis. Am J Kidney Dis. 2011;58(3):356–65. doi: 10.1053/j.ajkd.2011.02.389. [DOI] [PubMed] [Google Scholar]
- 107.Nguyen MT, Ross GF, Dent CL, Devarajan P. Early prediction of acute renal injury using urinary proteomics. Am J Nephrol. 2005;25(4):318–26. doi: 10.1159/000086476. [DOI] [PubMed] [Google Scholar]
- 108.Nguyen MT, Dent CL, Ross GF, Harris N, Manning PB, Mitsnefes MM, et al. Urinary aprotinin as a predictor of acute kidney injury after cardiac surgery in children receiving aprotinin therapy. Pediatr Nephrol. 2008;23(8):1317–26. doi: 10.1007/s00467-008-0827-9. [DOI] [PubMed] [Google Scholar]
- 109.Devarajan P, Krawczeski CD, Nguyen MT, Kathman T, Wang Z, Parikh CR. Proteomic identification of early biomarkers of acute kidney injury after cardiac surgery in children. Am J Kidney Dis. 2010;56(4):632–42. doi: 10.1053/j.ajkd.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ho J, Lucy M, Krokhin O, Hayglass K, Pascoe E, Darroch G, et al. Mass spectrometry-based proteomic analysis of urine in acute kidney injury following cardiopulmonary bypass: a nested case-control study. Am J Kidney Dis. 2009;53(4):584–95. doi: 10.1053/j.ajkd.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 111.Ho J, Reslerova M, Gali B, Gao A, Bestland J, Rush DN, et al. Urinary hepcidin-25 and risk of acute kidney injury following cardiopulmonary bypass. Clin J Am Soc Nephrol. 2011;6(10):2340–6. doi: 10.2215/CJN.01000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aregger F, Pilop C, Uehlinger DE, Brunisholz R, Carrel TP, Frey FJ, et al. Urinary proteomics before and after extracorporeal circulation in patients with and without acute kidney injury. J Thorac Cardiovasc Surg. 2010;139(3):692–700. doi: 10.1016/j.jtcvs.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 113.Heller F, Frischmann S, Grunbaum M, Zidek W, Westhoff TH. Urinary Calprotectin and the Distinction between Prerenal and Intrinsic Acute Kidney Injury. Clin J Am Soc Nephrol. 2011;6(10):2347–55. doi: 10.2215/CJN.02490311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Beger RD, Holland RD, Sun J, Schnackenberg LK, Moore PC, Dent CL, et al. Metabonomics of acute kidney injury in children after cardiac surgery. Pediatr Nephrol. 2008;23(6):977–84. doi: 10.1007/s00467-008-0756-7. [DOI] [PubMed] [Google Scholar]
- 115.Foxall PJ, Mellotte GJ, Bending MR, Lindon JC, Nicholson JK. NMR spectroscopy as a novel approach to the monitoring of renal transplant function. Kidney Int. 1993;43(1):234–45. doi: 10.1038/ki.1993.37. [DOI] [PubMed] [Google Scholar]
- 116.Wishart DS. Metabolomics: the principles and potential applications to transplantation. Am J Transplant. 2005;5(12):2814–20. doi: 10.1111/j.1600-6143.2005.01119.x. [DOI] [PubMed] [Google Scholar]
- 117.Boudonck KJ, Rose DJ, Karoly ED, Lee DP, Lawton KA, Lapinskas PJ. Metabolomics for early detection of drug-induced kidney injury: review of the current status. Bioanalysis. 2009;1(9):1645–63. doi: 10.4155/bio.09.142. [DOI] [PubMed] [Google Scholar]
- 118.Vaidya VS, Waikar SS, Ferguson MA, Collings FB, Sunderland K, Gioules C, et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci. 2008;1(3):200–8. doi: 10.1111/j.1752-8062.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Metzger J, Kirsch T, Schiffer E, Ulger P, Mentes E, Brand K, et al. Urinary excretion of twenty peptides forms an early and accurate diagnostic pattern of acute kidney injury. Kidney Int. 2010;78(12):1252–62. doi: 10.1038/ki.2010.322. [DOI] [PubMed] [Google Scholar]
- 120.Hall IE, Coca SG, Perazella MA, Eko UU, Luciano RL, Peter PR, et al. Risk of Poor Outcomes with Novel and Traditional Biomarkers at Clinical AKI Diagnosis. Clin J Am Soc Nephrol. 2011;6(12):2740–9. doi: 10.2215/CJN.04960511. [DOI] [PMC free article] [PubMed] [Google Scholar]