Abstract
Healthcare quality improvement has the potential to reduce the striking disparities in health outcomes among patients with systemic lupus erythematosus (SLE). Donabedian’s framework for assessment of healthcare quality, which divides factors impacting quality into structures, processes and outcomes, provides a theoretical framework for research and interventions in quality improvement. This review applies Donabedian’s model to current research describing quality of care in SLE, highlighting structures and processes that may lead to improved outcomes. Work remains to be done to develop meaningful metrics to assess quality and to understand the structures and processes that improve outcomes. Quality indicators have emerged as an important tool to measure quality, but further validation is required to define their validity and feasibility in clinical practice, as well as their association with improved outcomes. Implementation science also shows promise as a means to create meaningful systematic improvements in healthcare quality for patients with SLE.
Keywords: access to care, healthcare quality, health disparities, health outcomes, systemic lupus erythematosus
Systemic lupus erythematosus (SLE) continues to cause significant morbidity and mortality, and has among the most striking disparities in outcomes among rheumatic diseases. Understanding factors in the healthcare system that are associated with improved outcomes in SLE is important since these factors are potentially modifiable. For patients with SLE, the need for quality healthcare, including adequate preventive care, monitoring for disease-specific morbidity, and effective patient self-care, is universal across the spectrum of disease. Healthcare for patients with SLE is often fragmented, with patients receiving care from multiple sources and needing to travel significant geographic distances to access routine specialty care. Therefore, there is an urgent need to better understand the components of quality healthcare in this condition.
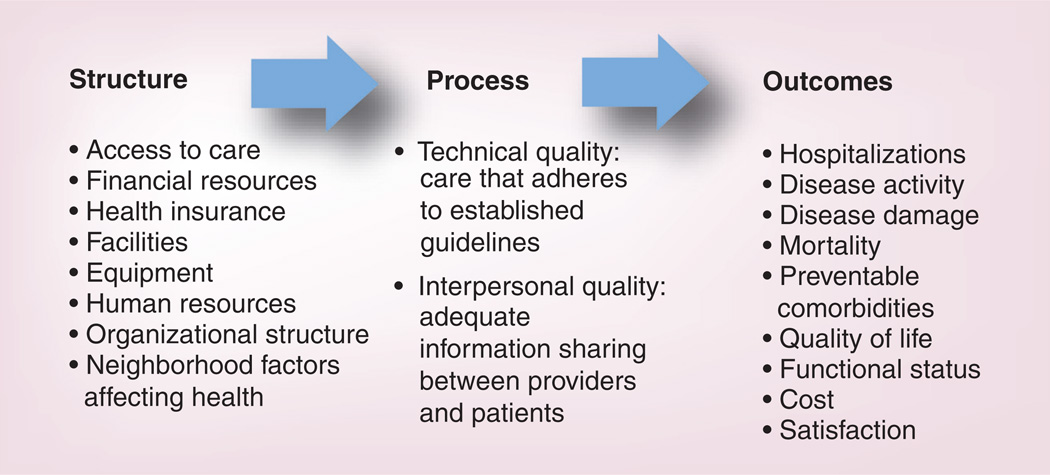
To systematically assess quality of care in SLE, a model is needed that comprehensively examines the components of healthcare quality. Such a model will outline potential mechanisms of variation in quality, and create a theoretical framework for healthcare quality research and design of quality improvement interventions. Donabedian’s framework for assessment of healthcare quality is perhaps the most widely used in the healthcare quality field and has been applied across a spectrum of medical specialties and illness diagnoses [1]. According to this framework, inferences about the quality of healthcare can be classified under three categories: structure, process and outcome (Figure 1). The structure category includes the attributes of the settings in which care occurs. These include financial resources, facilities, equipment, human resources and organizational structure. In the case of SLE, structures that affect healthcare quality include system factors that facilitate access to rheumatology care, adequacy of insurance coverage and neighborhood factors affecting health. The process category describes the actions performed in giving and receiving healthcare. These include the provision of effective SLE care that adheres to recommended guidelines (‘technical quality’), and adequate information sharing between providers and patients (‘interpersonal quality’). Finally, the outcome category describes the effects of care on the health status of patients and populations, with patient adherence to the plan of care as an intermediary outcome that directly impacts health status. In SLE, outcomes of interest include healthcare utilization such as hospitalizations, disease activity, disease damage, mortality and quality of life.
Figure 1.
The Donabedian model of healthcare quality applied to systemic lupus erythematosus.
This is the first review to propose a conceptual model that critically analyzes gaps in healthcare quality in SLE, with the aim of systematically understanding the discrete components of quality gaps and their potential mechanisms. Identifying specific areas of healthcare quality problems will provide actionable targets for future research and quality improvement in SLE.
Structure of care
Structural components of SLE care include characteristics of the healthcare system that facilitate the provision of health services. Examples include the setting in which healthcare is provided (both the physical structures and the community), as well as other factors such as financial and organizational arrangements. Increasingly, technological infrastructure is also an important consideration. In the USA, structural components of care are increasingly complex with significant variation in aspects such as the practice setting and type (solo vs group practices, size of group and its composition and staffing), healthcare financing (with public and private systems, and managed care and fee-for-service financial arrangements within each system), and variable use of information technology. Within this complex system, it is important to identify the financial incentives and organizational policies that facilitate the provision of high-quality care and encourage coordinated, evidence-based care. It is likely that a variety of structural components of care can positively impact quality for a complex chronic condition such as SLE. To date, several studies have demonstrated that insurance status and healthcare access are linked to health outcomes in SLE. However, other structural components of the healthcare system that may influence processes of care and outcomes remain to be explored.
In our current fragmented healthcare system, poor access to care has been shown to negatively impact health outcomes across a spectrum of diseases. Coordinated care has shown potential to reduce hospitalizations, decrease cost and improve quality of care in chronic illnesses [2]. For patients with SLE, the level of experience of the treating physician in managing SLE has been shown to influence mortality for hospitalized patients, suggesting that access to specialty care ultimately impacts outcomes [3]. Poor access to care in SLE has been associated with age, insurance, socioeconomic status, distance to healthcare providers, and neighborhood or geographic factors [4–6].
In SLE, decreased access to care may correlate with increased renal damage. Using data from the United States Renal Data System (USRDS), Ward found that individuals with SLE had onset of end-stage renal disease (ESRD) at a younger age if they had Medicaid or lacked health insurance completely, as compared with individuals with private insurance [7]. Since type of insurance is unrelated to age at onset of lupus nephritis, these findings suggest that the rate of progression to ESRD among patients with lupus nephritis differs according to insurance status. Another population-based study utilizing the USRDS found that in California, incidence of ESRD due to SLE varied by ZIP code, with more ESRD seen in areas with higher rates of hospitalizations for ambulatory-care sensitive conditions and a greater proportion of Medicaid or uninsured hospitalizations, independent of mean socioeconomic status of individuals living in the ZIP code [8].
The Lupus Outcomes Study (LOS), a large, longitudinal cohort study of subjects with physician-confirmed SLE, has been used in several analyses to address questions regarding access to rheumatology care in SLE. Predictors of utilization of subspecialty care were identified among 982 subjects from 2002–2004 [5]. Older subjects and those with lower income were least likely to identify a rheumatologist as being primarily responsible for their SLE care. Decreased use of subspecialty care for the elderly existed in spite of insurance coverage through Medicare, suggesting that barriers other than insurance status exist in preventing access to a rheumatologist. Tonner et al. also found that subjects in the LOS cared for by a generalist had significantly fewer physician visits for SLE as compared with those cared for by a rheumatologist, even after controlling for a variety of characteristics including disease status [4]. Whether differences in utilization in this study are a proxy for barriers to access and are associated with differential processes of care or outcomes requires further exploration.
Geographic distance from medical providers may constitute a barrier to healthcare access. The 2002–2004 waves of the LOS were used to measure the distance from 982 subjects’ homes to their primary SLE provider, and to identify differences in healthcare utilization patterns based on insurance type [6]. Medicaid patients on average traveled further to their primary SLE provider as compared with subjects with private insurance, a finding that was most pronounced among subjects cared for by a rheumatologist. Subjects with Medicaid were also more likely to obtain care for their SLE from general practitioners and emergency departments as compared with subjects with private insurance. These findings suggests that public insurance negatively impacts patients’ ability to access coordinated SLE care as compared with patients with private insurance.
A longitudinal analysis of LOS data was used to define the impact of health maintenance organizations (HMOs) on healthcare utilization [9]. Subjects enrolled in HMOs utilized less healthcare overall as compared with subjects with fee-for-service insurance, both with and without adjustment for sociodemographic and health characteristics. The difference was most pronounced among subjects with government-based insurance; the impacts of these utilization differences require further research. In particular, it will be important in future studies to investigate whether differential access explains any part of the utilization differences for Medicare and Medicaid beneficiaries in an HMO system.
Individual and community-level socioeconomic status may also play an important role in access to healthcare for patients with SLE. Among 755 subjects from the 2004–2007 waves of the LOS, living in a community of poverty was significantly associated with fewer physician visits in subjects with SLE, independent of individual-level sociodemographic and health characteristics [4]. It is unclear whether communities with high concentrations of poverty lack a sufficient quantity of physicians to provide care, or whether other factors, such as neighborhood violence, make traveling to medical appointments more difficult.
Hospitalizations for conditions that can be treated with timely and appropriate outpatient management, such as pneumonia and cellulitis, are an indicator of poor access to healthcare or underutilization of available care [10,11]. Population-based data on 16,751 hospitalizations among patients with SLE in New York State found that 12.7% were classified as avoidable [12]. Risk of avoidable hospitalization was higher among elderly subjects and those with lower socioeconomic status, as well as patients with Medicare, suggesting that poor and elderly patients may have more difficulty accessing care. Hospitals that admitted larger numbers of patients with SLE had lower rates of avoidable hospitalizations as compared with hospitals that admitted fewer patients with SLE, possibly due to better outpatient care at those facilities.
Children with SLE and ESRD are also susceptible to disparities in healthcare access mediated by geography, race and insurance status. A study utilizing the USRDS sought to determine predictors of listing for kidney transplantation, receipt of kidney transplantation and mortality among 583 children aged 5–18 years with new-onset lupus-associated ESRD across the USA [13]. Children in the northeast and west were more than twice as likely to be listed for transplant and over 50% more likely to receive a transplant as compared with children living in the south. Older (OR: 0.59, p = 0.009), African–American (OR: 0.48, p < 0.001), and Hispanic (OR: 0.63, p = 0.03) children were less likely to receive a transplant as compared with younger, Caucasian and non-Hispanic children, respectively. Children with Medicaid were also less likely to receive a transplant (OR: 0.7, p = 0.03).
Although much work remains to be done to better define the relationship between structures of care in SLE and higher quality processes or improved heath outcomes, the above studies suggest that across at least two domains (access to care and healthcare financing/insurance), the structure of the healthcare plays an important role in quality. However, the evidence base in this area remains underdeveloped for SLE as with most chronic diseases.
Future research would benefit from studying key structural elements of the healthcare system that may facilitate higher quality care in SLE. In particular, studies should examine the role of structural characteristics that can be implemented across healthcare settings. For example, can organizational processes, such as the establishment of standardized evidence-based protocols improve care and outcomes? Can information technology infrastructure, including the use of electronic medical records for clinical decision-support and care coordination improve quality? The most compelling studies will be those that empirically explore the relationship between structure and outcome.
Process of care
The processes of healthcare include the actions performed in giving and receiving care. In the rheumatic diseases, including in SLE, most attempts to evaluate quality have included measures of process. This partly reflects the fact that process measures provide more actionable targets for quality improvement and require less complicated methodology (e.g., risk adjustment) than outcome measures to apply. Early studies in SLE suggested that processes of care potentially played an important role in explaining differential health outcomes. These included studies that evaluated the association between hospital and physician factors and health outcomes. More recent research has applied specific quality measures to different healthcare settings to investigate processes of care in SLE.
Hospital & physician factors
Hospital experience in caring for patients with SLE has been found to have a significant impact on in-hospital mortality, which is most pronounced for patients admitted for SLE flares or on an emergent basis [14]. An analysis of the California Hospital Discharge Database was used to identify 9989 patients with SLE hospitalized from 1991–1994; outcomes were compared between hospitals with less than 50 urgent or emergent SLE admissions per year, and those with 50 or more admissions. Patients admitted to hospitals with more SLE experience were found to have a lower risk of mortality as compared with those admitted to hospitals with less experience (mortality 3.8 vs 5.3%; adjusted OR: 0.72, 95% CI: 0.50–1.04). Among subjects admitted on an emergent basis (n = 2372), there was a 66% decreased risk of in-hospital mortality for those admitted to a hospital with more SLE experience (mortality 4.2 vs 11.3%; adjusted OR: 0.34, 95% CI: 0.19–0.58). Subjects admitted for SLE on an emergent basis (n = 405) had a 95% decrease in mortality risk if admitted to a hospital with more SLE experience (mortality 1.7 vs 10.0%; adjusted OR: 0.05, 95% CI: 0.006–0.34).
Physician experience caring for patients with SLE has also been inversely associated with mortality [3]. Population-based data on 15,509 patients obtained from state health planning agencies in New York and Pennsylvania was used to assess risk of in-hospital mortality relative to the average annual number of patients with SLE hospitalized by the admitting physician. Physicians were divided into those who treated <1 hospitalized patient with SLE per year, 1–3 patients per year or >3 patients per year. As compared with physicians who treated <1 SLE patient in the hospital each year, the adjusted mortality risk was 20% lower among patients cared for by physicians who treated 1–3 hospitalized SLE patients per year, and 42% lower among patients of physicians who treated >3 hospitalized SLE patients per year. Among patients with nephritis, the effect of physician experience on mortality was even more substantial, with a 60% lower risk of mortality among patients cared for by physicians in the highest volume category.
Low patient socioeconomic status may be associated with a lower rate of diagnosis of SLE [15]. Mortality rates due to SLE were calculated using US Multiple Causes of Death data, accounting for age, gender and education. Among Caucasians, risk of death due to SLE increased with decreasing levels of education, similar to all-cause mortality risk. However, among African–American women and men, and Asian/Pacific Islander women, risk of death due to SLE was lower in individuals with less education, which contrasted with the association between education and all-cause mortality. It is suspected that there is underascertainment of deaths due to SLE in these groups, likely due to underreporting of SLE on death certificates versus failure of physicians to diagnose SLE in minorities with low levels of education.
This group of studies strongly suggests that hospital and physician factors influence outcomes in SLE, and that processes of care leading to improved outcomes likely exist and should be identified. Process–outcome links can be complex to unravel, particularly if the processes of care leading to improved outcomes are either difficult to identify or challenging to measure and quantify. To begin to address these issues, recent studies have attempted to use standardized measures to assess processes of care, as described below.
Development of quality indicators
Recent efforts to reach consensus on the processes that constitute high-quality care in SLE has led to the creation of healthcare quality indicator sets. Quality indicators (QIs) have been defined as “retrospectively measurable elements of practice performance for which there is evidence or consensus that can be used to assess the quality of care provided and hence change it” [16]. These measures are intended to represent a minimal standard of care that can be universally applied to patients with a particular condition. While the rarity of SLE may make universal application of quality measures more challenging, the complexity of SLE management and the prevalence of healthcare disparities in SLE make the implementation of QIs particularly important. Two recent studies sought to define quality indicators in SLE.
Our group developed QIs for the diagnosis and management of SLE using a combination of existing guidelines, scientific evidence and expert consensus [17]. Twenty candidate indicators were derived from a review of the literature, which were then revised by an expert panel and increased to 25 QIs. A second systematic literature review was then completed to evaluate the evidence for each QI. Finally, a second expert panel was convened, and a modified Research and Development/University of California (CA, USA) appropriateness method was used to review the scientific evidence and rate each process of care [18]. Twenty QIs were ultimately rated as both valid and feasible. These QIs describe minimum standards of care for diagnosis, preventive strategies, osteoporosis prevention, drug toxicity monitoring, management of renal disease, cardiovascular disease prevention and reproductive healthcare (Table 1).
Table 1.
A comparison of quality indicator sets.
| QI category | Yazdany et al. (2009) [17] | Mosca et al. (2011) [19] |
|---|---|---|
| Diagnosis | If a patient has a suspected diagnosis of SLE, then an initial work-up should include the following: ANA, CBC with differential, platelet count, serum creatinine and urinalysis If a patient is newly diagnosed with SLE, then the following laboratory tests should be ordered within 6 months of diagnosis: anti-dsDNA, complement levels and antiphospholipid antibodies |
If a patient is diagnosed with SLE, then the following autoantibodies should be evaluated at the first evaluation: ANA, anti-dsDNA, anti-Ro, anti-La, anti-RNP, anti-Sm and anti-phospholipid |
| Disease monitoring | If a patient has had evidence of SLE renal disease (increasing proteinuria, active urinary sediment, a rising creatinine level, or disease activity on renal biopsy) in the past 2 years, then the following should be obtained at 3-month intervals: CBC, serum creatinine, urinalysis with microscopic evaluation and measurement of urine protein using a quantitative assay | If a patient is diagnosed with SLE, then at least every 6 months the rheumatologist should request the following laboratory assessment: CBC, erythrocyte sedimentation rate, albumin, serum creatinine or estimated glomerular filtration rate, urinalysis and protein:creatinine ratio (or 24 h proteinuria), C3 and C4 If a patient is diagnosed with SLE, then the treating physician should assess and record disease activity using a validated index at each visit If a patient is diagnosed with SLE, then the treating physician should assess and record disease damage by the SLICC/ACR damage index annually If a patient is diagnosed with SLE, then he/she should provide an evaluation of quality of life at each visit If a patient is diagnosed with SLE, then the treating physician or a specialized nurse should record the presence of comorbid conditions at each visit |
| General preventive strategies | If a patient with SLE is on immunosuppressive therapy, then a pneumococcal vaccine should be administered, unless patient refusal or contraindications are noted If a patient with SLE is on immunosuppressive therapy, then an inactivated influenza vaccination should be administered annually, unless patient refusal or contraindications are noted If a patient has SLE, then education about sun avoidance should be documented at least once in the medical record (e.g., wearing protective clothing, applying sunscreens whenever outdoors and avoiding sunbathing) |
If a patient is diagnosed with SLE, then the patient’s history of vaccinations should be documented. Patients should be vaccinated against influenza and pneumococcus (preferably without adjuvant), if there are no contraindications to immunization |
| Osteoporosis | If a patient with SLE has received prednisone (or other glucocorticoid equivalent) ≥7.5 mg/day for ≥3 months, then the patient should have BMD testing documented in the medical record, unless the patient is currently receiving antiresorptive or anabolic therapy If a patient with SLE has received prednisone (or other glucocorticoid equivalent) ≥7.5 mg/ day for ≥3 months, then supplemental calcium and vitamin D should be prescribed or recommended and documented If a patient with SLE has received prednisone (or other glucocorticoid equivalent) ≥7.5 mg/day for ≥1 month, and has a central T score less than or equal to −2.5 or a history of fragility fracture, then the patient should be treated with an antiresorptive or anabolic agent, unless patient refusal or contraindications are noted |
|
| Drug monitoring | If a patient is prescribed a new medication for SLE (e.g., NSAIDs, DMARDs or glucocorticoids), then a discussion with the patient about the risks versus benefits of the chosen therapy should be documented If a patient with SLE is newly prescribed an NSAID, DMARD or glucocorticoid, then baseline studies should be documented within an appropriate period of time from the original prescription If a patient with SLE has established treatment with an NSAID, DMARD or glucocorticoid, then monitoring for drug toxicity should be performed If a patient with SLE is taking prednisone (or other steroid equivalent) ≥10 mg for ≥3 months, then an attempt should be made to taper the prednisone, add a steroid-sparing agent or escalate the dose of an existing steroid-sparing agent, unless patient refusal or contraindications are noted |
If a patient is diagnosed with SLE and is prescribed high-dose corticosteroids and/or immunosuppressive drugs, then, based on patient’s history, the rheumatologist should consider the evaluation of HCV, HBV and tuberculosis screening and record the results into the clinical chart before starting therapy If a patient is diagnosed with SLE, then the treating physician should assess the presence of drug toxicity at each visit and record the data in the clinical chart. Alternatively, the physician should record the absence of drug toxicity If a patient is diagnosed with SLE and treated with hydroxycloroquine/chloroquine, then he/she should undergo an ophthalmologic assessment according with the existing guidelines and this should be documented in the clinical chart If a patient is diagnosed with SLE and treated with corticosteroids, then he/she should undergo an ophthalmologic assessment for the presence of cataracts and/or glaucoma according with the existing guidelines. This should be documented in the clinical chart |
| Management of renal disease | If a patient is diagnosed with proliferative SLE nephritis (WHO or ISN/RPS class III or IV), then therapy with corticosteroids combined with another immunosuppressant agent should be provided and documented within 1 month of this diagnosis, unless patient refusal or contraindications are noted If a patient with SLE has renal disease (proteinuria ≥300 mg/day or estimated glomerular filtration rate <60 ml/min) and ≥2 BP readings, including the last reading, with systolic BP >130 mmHg or diastolic BP >80 mmHg over 3 months, then pharmacologic therapy for hypertension should be initiated or the current regimen should be changed or escalated, unless patient refusal or contraindications are noted If a patient with SLE has proteinuria ≥300 mg/day, then the patient should be treated with an ACE inhibitor or ARB, unless patient refusal or contraindications are noted |
|
| Cardiovascular disease | If a patient has SLE, then risk factors for cardiovascular disease, including smoking status, BP, BMI, diabetes and serum lipids (including total cholesterol, HDL, LDL and triglycerides), should be evaluated annually | |
| Reproductive health | If a patient with SLE is pregnant, then anti-SSA, anti-SSB and antiphospholipid antibodies should be documented in the medical record If a patient has had pregnancy complications as a result of APS, then the patient should be offered aspirin and heparin (i.e., heparin or low-molecular-weight heparin) during subsequent pregnancies If a woman between 18 and 45 years of age is started on any of the following medications for SLE: chloroquine, quinacrine, methotrexate, azathioprine, leflunomide, mycophenolate mofetil, cyclosporine, cyclophosphamide or thalidomide, then a discussion with the patient about the potential teratogenic risks of therapy and about contraception should be documented prior to drug initiation, unless the patient is unable to conceive (e.g., has had a hysterectomy, oophorectomy, tubal ligation or is postmenopausal) |
ACE: Angiotensin-converting enzyme; ACR: American College of Rheumatology; ANA: Antinuclear antibody; anti-RNP: Anti-ribonucleoprotein; anti-Sm: Anti-Smith; APS: Antiphospholipid syndrome; ARB: Angiotensin receptor blocker; BMD: Bone mineral density; BP: Blood pressure; CBC: Complete blood count; DMARD: Disease-modifying antirheumatic drug; HBV: Hepatitis B virus; HCV: Hepatitis C virus; ISN: International Society of Nephrology; QI: Quality indicator; RPS: Renal Pathology Society; SLE: Systemic lupus erythematosus; SLICC: Systemic Lupus International Collaborating Clinics; SSA: Anti-ssA antibody; SSB: Anti-ssB antibody.
More recently, Mosca et al. also developed a set of QIs for use in SLE care based on European League Against Rheumatism (EULAR) recommendations [19]. Nominal group technique, Delphi surveys, small group discussion, systematic literature review and two rounds of Delphi technique for agreement were used to develop a preliminary list of QIs. An additional Delphi survey was then administered to assess priority, definitions and feasibility of QIs. These results then defined the final set of 11 QIs for use in the diagnosis and management of SLE. Measures focus on routine monitoring for disease activity, disease damage, drug toxicity, comorbidities and quality of life. Minimum necessary laboratory evaluation at diagnosis is also defined. Finally, preventive measures such as vaccination and infectious disease screening prior to immunosuppression are included. Techniques for the application of QIs in clinical practice are also described, including designation of responsibility for measuring each QI, frequency of QI assessment and the data sources used to assess QIs.
Assessment of QIs
Since the first set of QIs for the diagnosis and management of SLE were defined in 2009, several studies have sought to evaluate adherence to QIs in clinical practice.
The 2008–2009 wave of the LOS was used to assess pregnancy intentions, contraceptive use and self-reported receipt of contraceptive counseling among 206 premenopausal women aged <45 years who were sexually active with men [20]. Many women were using contraceptives inconsistently (22%), and 53% were relying on barrier methods alone. Less than half reported having received contraceptive counseling in the past year (41%). Women using potentially teratogenic medications were no more likely to have received contraceptive counseling, use contraception consistently or use more effective contraceptive methods. In addition, two out of 11 women with a history of thrombosis and two of 24 women with antiphospholipid antibodies were inappropriately taking estrogen-containing contraceptives. These findings suggest significant deficiencies in reproductive healthcare for women with SLE, especially in light of the frequent use of teratogenic medications and the risk of thrombosis in this population.
Rates of cancer screening and immunizations among 685 patients with SLE were assessed by comparing data from the 2004–2005 wave of the LOS with two samples derived from a statewide health interview survey, a general population sample (n = 18,013) and a sample with nonrheumatic chronic conditions (n = 4515) [21]. Rates of preventive care were similar in both the SLE sample and the two comparison samples. Among subjects with SLE, 70% of eligible respondents reported receipt of cervical cancer screening and mammography and 62% reported receiving colon cancer screening. Influenza vaccine had been received by 59% of eligible respondents and 60% had received pneumococcal vaccination. In multivariate analysis, subjects of younger age and lower educational attainment were less likely to receive preventive services.
Cardiovascular screening and osteoporosis QIs were assessed in a population of 200 patients seen in at least two visits for SLE at the Brigham and Women’s Hospital Arthritis Center between June 2007 and July 2008 [22]. Among eligible subjects, 59% met the QI for bone mineral density testing, 62% for calcium and vitamin D supplementation, and 86% for receipt of anti-absorptive or anabolic osteoporosis medications. However, rates for cardiovascular screening QIs were significantly lower. Only 3% of subjects had five cardiac risk factors assessed within the last year; 26% had four risk factors assessed. Having a primary care physician within the same hospital network improved the likelihood of receiving care recommended in QIs for osteoporosis and cardiovascular disease screening.
Application of 2009 SLE QIs related to bone health were assessed using the 2007–2008 wave of the LOS [23]. One hundred and twenty seven patients met criteria for the recommendation of osteoporosis screening and preventive treatment with calcium and vitamin D (taking at least 7.5 mg of prednisone per day for at least 3 months); 91 patients met the criteria for the recommendation of antiresorptive or anabolic osteoporosis medications (taking at least 7.5 mg of prednisone per day for at least 1 month, and having either a central T score of less than or equal to −2.5 or a history of fragility fracture). Among subjects for whom it was recommended, 74% were receiving osteoporosis screening, 58% were receiving calcium and vitamin D supplementation, and 56% were receiving antiresorptive treatment. In adjusted analysis, female sex, older age, Caucasian race and longer disease duration were associated with higher-quality bone care. Overall, rates of screening, prevention and treatment for osteoporosis were suboptimal. Furthermore, the percentage of patients meeting QIs were similar in this study of the LOS and the previous study performed at the Brigham and Women’s Hospital Arthritis Center, suggesting that these rates may be similar across different healthcare systems.
Hydroxychloroquine has been shown to reduce disease activity in SLE in two double-blind, randomized, placebo-controlled trials [24,25], and has been shown to reduce mortality in multiple observational trials [26–28]. The LOS was used to examine hydroxychloroquine use in 881 patients from 2004 to 2007 [29]. Prevalence of hydroxychloroquine use was 55 per 100 person-years, and did not change over the course of the study. Patients receiving care from a rheumatologist for their SLE were nearly twice as likely to be taking hydroxychloroquine as compared with those receiving care from a generalist or a nephrologist. Patients with shorter disease duration were also more likely to be taking hydroxychloroquine regardless of age. This study suggests that hydroxychloroquine use could be improved by targeting interventions towards patients with long disease duration and those cared for by nonrheumatologists.
Patients with renal failure who begin renal replacement therapy on an emergent basis have higher complication rates as compared with those who begin treatment on an elective basis. Emergency renal replacement therapy is also associated with higher serum creatinine and more severe anemia. Therefore, laboratory markers at the start of renal replacement therapy may serve as an indicator of quality of care for patients with renal failure. The USRDS was used to evaluate for disparities in treatment of ESRD based on race and ethnicity [30]. Among 6018 subjects with lupus-related ESRD, serum creatinine levels were lowest in Caucasian patients and highest in African–American patients. African–American patients also had the lowest hematocrit among all racial and ethnic groups. Subjects without medical insurance had higher creatinine levels and lower hematocrits as compared with insured subjects. There was no independent association between laboratory values and socioeconomic status. Given the known higher incidence of renal failure in non-Caucasian patients with lupus nephritis, this data suggest that African–American race, independent of socioeconomic status, may have a negative impact on quality of care in lupus nephritis-related ESRD.
Together, these studies begin to identify potential gaps in healthcare processes for patients with SLE in the areas of preventive care, reproductive health and osteoporosis screening and management. Processes such as hydroxychloroquine use and prompt initiation of renal replacement therapy also show room for improvement. However, it is important to acknowledge that although many of the SLE QIs have a strong theoretical process–outcome link, it remains to be proven whether improving these processes of care in routine clinical practice will indeed improve important patient outcomes. In other more prevalent chronic conditions, even significant improvements in care processes have sometimes yielded disappointing results in terms of improving outcomes [31]. Therefore, future studies that can quantify, to the extent possible, the effect of improving care processes on key SLE health outcomes are important. Ultimately, resources are best invested in healthcare processes that are the most likely to improve outcomes. In addition, missing from the existing literature are studies measuring the patient’s experience and satisfaction with healthcare in SLE.
The scientific evidence underlying the SLE QIs will evolve over time, and it is likely that some of the QIs will become outdated. QIs should therefore not be viewed as static – they should be continually updated as new evidence and testing of their validity and feasibility becomes available. In addition, as the field of quality measurement and improvement evolves, there is a need to avoid proliferation of multiple, conflicting measures on the same topic. Organizations such as the National Quality Forum now require ‘measure harmonization’ if there are multiple measures on the same topic, the ‘best of set’ – the most valid, reliable and broadly applicable – will be selected. As an example, it is anticipated that the SLE QIs pertaining to glucocorticoid-induced osteoporosis will eventually be supplanted with measures applicable to all patients with glucocorticoid-induced osteoporosis based in a recent American College of Rheumatology guideline (not just SLE) [32]. However, the preliminary work performed in SLE will likely help inform these new measures by demonstrating substantial gaps in care.
Outcomes
Donabedian’s model has directionality in that the ultimate goal of healthcare delivery and quality assessment is to improve patient outcomes. Establishing definitive links between structure or process and outcome is difficult. However, many of the studies above suggest an association between structures, processes of care and outcomes. For example, healthcare structures such as insurance status, geographic region and systems that facilitate access to care have been shown to impact health outcomes such as rate of progression to renal failure and avoidable hospitalizations, with some evidence of increased disparities among racial minorities. Other processes, aimed at prevention of conditions to which lupus patients may be particularly susceptible, have been shown to improve outcomes in the general population. These include fracture prevention via osteoporosis screening and management, and prevention of cardiovascular morbidity and mortality via risk factor screening. Finally, certain characteristics of healthcare providers or systems that are associated with health outcomes may serve as proxies for processes of care, such as physician and hospital experience in the management of SLE.
Intermediary outcomes: patient factors
Patients play a critical role in their own healthcare outcomes. While hospitals and physicians may determine diagnostic evaluations and treatment plans, patients managing disease as outpatients determine the quality of their own disease self-management. When patients do not accept or adhere to the plan of care set in motion by their providers, healthcare quality can be compromised. Adherence to medications and regular medical visits have been shown to impact lupus outcomes [33]. Therefore, adherence to a medical regimen may be seen as an intermediary outcome in the structure–process–outcomes model, with processes of care such as screening and treatment for depression or adequate provider–patient communication able to improve adherence and, ultimately, health outcomes.
A survey of 68 African–American and 54 Caucasian women with SLE recruited from two urban, tertiary-care medical centers assessed the effect of several processes of care on adherence behaviors [34]. Among Caucasian patients, perception of poor treatment efficacy was associated with decreased medication adherence. African–American subjects with concerns about side effects of medication and a need for child or elder care were less likely to take medications consistently. A study of 106 patients with SLE in New Zealand also showed that concerns about medication use was a strong predictor of non-adherence [35]. These results suggest that support for patients in need of child or elder care, routine assessment and treatment of depression, and attention to interpersonal processes of care when starting medications may be areas worth exploring as we work to define processes of care that could improve adherence and, ultimately, health outcomes.
Final outcomes
Focusing on outcomes creates a shared goal among all stakeholders in the healthcare system. What is the significance of assessing structures, such as access to care, or processes, such as QIs, when health outcomes are what matter most to patients, providers, insurers, governments and other stakeholders? Porter describes healthcare quality as value of care to the individual patient, further defined as health outcomes achieved per dollar spent [36]. The assessment of outcome measures, as compared with process measures, allows us to learn about the ultimate impact of healthcare processes and facilitates innovations in healthcare, as opposed to merely comparing provider behavior. Outcome measures must be condition-specific and multidimensional, and must encompass the entire cycle of care for a patient’s medical condition in order to be meaningful. However, the current organization of our health system around physician, department, hospital and billing measures makes patient-centered measures difficult to assess.
Although measuring outcomes (and their associated value) in SLE is the ultimate goal of quality measurement, there are inherent challenges to using health outcomes as the sole marker of healthcare quality in lupus. Lupus is an extremely heterogeneous disease, in which health outcomes are strongly influenced by genetics and environment, making the course of disease difficult to predict. Some patients require minimal intervention to remain healthy, while others may develop organ-threatening diseases in spite of optimal medical management. In addition, ethnic minorities and individuals of low socioeconomic status often have more severe disease, which may bias quality assessment against providers who care for underserved populations. All of these factors bring up issues of how to best risk-adjust outcomes in developing metrics, and much basic methodological work is needed before outcome measures in key domains such as disease activity, disease damage or how health-related quality of life can be applied.
Conclusion
The field of quality assessment and improvement in healthcare continues to evolve rapidly, as does the financing and infrastructure of health systems in the USA. For most chronic diseases, including SLE, much work remains to be done to develop meaningful metrics to assess quality and to understand the structures and processes that improve outcomes within complex systems. Applying Donabedian’s conceptual framework for healthcare quality to SLE allows us to highlight potential gaps in care as well as structures or processes that can potentially improve quality.
Moving forward, future research studies should have two primary goals. The first is to continue to develop the scientific evidence base to further establish the relationships between structure, process and outcome within Donabedian’s conceptual framework for SLE. The second, discussed below, is the completion of systematic investigations in the field of implementation science or applying research findings to effect change in the healthcare system. The former goal will be greatly facilitated by the development and application of meaningful metrics to understand quality in SLE. The two available QI sets provide a useful starting place for these activities, and will likely evolve over time. Validating these measures, both in terms of studying the feasibility of their application to clinical practice and in terms of continuing to develop the evidence that links structures or processes to outcomes will be critical. In addition, preliminary identification and application of risk-adjusted outcome measures would help move towards the ultimate goal of aligning stakeholders towards a common goal of improving outcomes in SLE.
Donabedian’s conceptual model provides an important framework for systematic investigations of quality, but additional guidance is needed to address the second primary goal in this field: implementing change to bring about quality improvement. In SLE, no studies have yet addressed this vital area. However, future research can draw on the rapidly expanding field of implementation science. A variety of validated frameworks have been developed and are helpful in framing quality improvement interventions, such as the plan–do–study–act cycle [37] or total quality management [38]. These frameworks can be applied to effect change at the individual practice, group or larger organizational level. The basic premise is that small, systematic tests of change that involve the input of multiple stakeholders can often effectively improve care. These frameworks can be used both to start a quality improvement project de novo and for continuous quality improvement.
Rapid, iterative change with frequent performance measurement and feedback, as exemplified by the plan–do–study–act cycle, has proved to be effective in creating quality improvement within a hospital system. Quality improvement efforts utilizing this approach include performance measurement with regular disclosure of results to providers and systems, and electronic systems to facilitate communication, monitoring and outcome measurement. For example, in San Francisco’s public safety-net health system, an electronic consultation request process allows subspecialists to screen consultation requests from primary care physicians to evaluate urgency, choice of specialties, whether sufficient work-up information is provided, and whether a specialist needs to see the patient or can guide the primary care clinician through the electronic system [39]. This system successfully decreased waiting times for nonurgent visits by up to 90% in the first 6 months of use. For patients with a potentially severe condition such as SLE, this system has greatly improved access to timely subspecialty care.
There are unique challenges to measuring and executing quality improvement for the care of rare or chronic illnesses such as SLE. First, there is little understanding of how to best organize care for these patients. Few data exist to determine the structure of optimal care for complex chronic illnesses, and optimal structure will likely vary among geographic regions and healthcare systems. Second, many quality improvement efforts involving small, local tests of change are not reported in the medical literature. Third, research addressing these issues in the USA has been hampered by the relative lack of data streams that reliably capture the SLE population. Available national datasets (e.g., Medicare and Veterans Affairs) do not have generalizable representation of SLE patients (who are largely younger and female). National Medicaid data will perhaps provide the first glimpse at this important issue, at least among those with low socioeconomic status, and work using this dataset is underway.
Future perspective
A pragmatic approach to quality improvement in SLE will likely entail leveraging work done in other rheumatic diseases or other chronic conditions to improve quality when possible [40]. For some aspects of SLE, however, novel quality improvement interventions are likely needed. We must continue to refine quality measures to assure that they are meaningful, and to implement them in a way that will effectively promote positive change in healthcare practices. Collaboration among health systems will be essential to assure that quality improvement recommendations are generalizable among settings, and to overcome the challenge of small numbers of patients with this rare disease. Importantly, in the next few years, we expect that dramatic advances in health information technology, including the widespread use of electronic medical records that can exchange information (health information exchanges), will greatly improve our ability to conduct health services research in less common diseases like SLE.
By merging the classical concepts in Donabedian’s model of quality and modern implementation science, there will be an important opportunity to make the healthcare system more effective for patients with SLE. Moreover, it is hoped that quality improvement will also serve as one tool in a broader approach to decrease disparities in SLE. Such an approach recognizes the critical role played by high-quality clinical care in affecting disease outcomes, but also addresses the psychosocial and environmental factors that play a role in driving disease outcomes.
Executive summary.
Quality of care & the Donabedian framework
-
▪
Striking disparities in outcomes exist among patients with systemic lupus erythematosus (SLE).
-
▪
Donabedian’s classic framework describes factors impacting healthcare quality as a continuum of structures, processes and outcomes.
-
▪
This framework can help us to understand the implications of current research in quality of care in SLE.
Structure of care
-
▪
Access to care and healthcare financing may have a significant impact on the quality of SLE care received.
-
▪
Further research should focus on key structural elements of the healthcare system and their association with improved outcomes in SLE, such as standardized evidence-based protocols and technology infrastructure.
Process of care
-
▪
Hospital and physician factors
-
–
Hospital and physician experience caring for SLE may have a significant impact on in-hospital mortality.
-
–
Low patient socioeconomic status may be associated with underdiagnosis of SLE and with subsequent underascertainment of morbidity and mortality.
-
–
-
▪
Development of quality indicators (QIs)
-
–
QIs are designed to capture retrospectively measurable elements of practice performance which can be used to assess and change the quality of care.
-
–
Two sets of QIs for the diagnosis and management of SLE have been developed.
-
–
-
▪
Assessment of QIs
-
–
Gaps in SLE healthcare processes may exist for preventive care, reproductive health, osteoporosis care and hydroxychloroquine use.
-
–
Improvement in care processes must be strongly linked to health outcomes to assure maximum validity of QIs.
-
–
Outcomes
-
▪
Intermediary outcomes: patient factors
-
–
Adherence to a plan of medical care has been shown to impact outcomes in SLE.
-
–
Inadequate child or elder care, depression and poor communication with providers may negatively impact adherence.
-
–
-
▪
Final outcomes
-
–
Focusing on health outcomes such as mortality, disease damage and health-related quality of life creates shared goals among all stakeholders in the healthcare system.
-
–
The use of outcome measures to assess healthcare quality in lupus is limited by heterogeneity of disease, requiring the development of risk-adjusted assessments.
-
–
Conclusion
-
▪
As a rare and chronic disease, the measurement and improvement of SLE healthcare quality is associated with unique challenges.
-
▪
Future research should focus on the development and application of meaningful metrics to assess and improve quality using QIs, risk-adjusted outcome measures and implementation science.
Acknowledgments
Grant Support: Arthritis Foundation, K23AR060259 and NIH T32.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪▪ of considerable interest
- 1. Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260(12):1743–1748. doi: 10.1001/jama.260.12.1743. ▪▪ Describes Donabedian’s framework for the assessment of healthcare quality.
- 2.Peikes D, Chen A, Schore J, Brown R. Effects of care coordination on hospitalization, quality of care, and health care expenditures among medicare beneficiaries: 15 randomized trials. JAMA. 2009;301(6):603–618. doi: 10.1001/jama.2009.126. [DOI] [PubMed] [Google Scholar]
- 3.Ward MM. Association between physician volume and in-hospital mortality in patients with systemic lupus erythematosus. Arthrit. Rheum. 2005;52(6):1646–1654. doi: 10.1002/art.21053. [DOI] [PubMed] [Google Scholar]
- 4.Tonner C, Trupin L, Yazdany J, Criswell L, Katz P, Yelin E. Role of community and individual characteristics in physician visits for persons with systemic lupus erythematosus. Arthrit. Care Res. (Hoboken) 2010;62(6):888–895. doi: 10.1002/acr.20125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yazdany J, Gillis JZ, Trupin L, et al. Association of socioeconomic and demographic factors with utilization of rheumatology subspecialty care in systemic lupus erythematosus. Arthrit. Rheum. 2007;57(4):593–600. doi: 10.1002/art.22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillis JZ, Yazdany J, Trupin L, et al. Medicaid and access to care among persons with systemic lupus erythematosus. Arthrit. Rheum. 2007;57(4):601–607. doi: 10.1002/art.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward MM. Medical insurance, socioeconomic status, and age of onset of endstage renal disease in patients with lupus nephritis. J. Rheumatol. 2007;34(10):2024–2027. [PubMed] [Google Scholar]
- 8.Ward MM. Access to care and the incidence of endstage renal disease due to systemic lupus erythematosus. J. Rheumatol. 2010;37(6):1158–1163. doi: 10.3899/jrheum.091199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yelin E, Trupin L, Katz P, et al. Impact of health maintenance organizations and fee-for-service on health care utilization among people with systemic lupus erythematosus. Arthrit. Rheum. 2007;57(3):508–515. doi: 10.1002/art.22625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Billings J, Zeitel L, Lukomnik J, Carey TS, Blank AE, Newman L. Impact of socioeconomic status on hospital use in New York city. Health Aff. (Millwood) 1993;12(1):162–173. doi: 10.1377/hlthaff.12.1.162. [DOI] [PubMed] [Google Scholar]
- 11.Weissman JS, Gatsonis C, Epstein AM. Rates of avoidable hospitalization by insurance status in Massachusetts and Maryland. JAMA. 1992;268(17):2388–2394. [PubMed] [Google Scholar]
- 12.Ward MM. Avoidable hospitalizations in patients with systemic lupus erythematosus. Arthrit. Rheum. 2008;59(2):162–168. doi: 10.1002/art.23346. [DOI] [PubMed] [Google Scholar]
- 13.Hiraki LT, Lu B, Alexander SR, et al. End-stage renal disease due to lupus nephritis among children in the US, 1995–2006. Arthrit. Rheum. 2011;63(7):1988–1997. doi: 10.1002/art.30350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward MM. Hospital experience and mortality in patients with systemic lupus erythematosus. Arthrit. Rheum. 1999;42(5):891–898. doi: 10.1002/1529-0131(199905)42:5<891::AID-ANR7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 15.Ward MM. Education level and mortality in systemic lupus erythematosus (SLE): evidence of underascertainment of deaths due to SLE in ethnic minorities with low education levels. Arthrit. Rheum. 2004;51(4):616–624. doi: 10.1002/art.20526. [DOI] [PubMed] [Google Scholar]
- 16.Campbell SM, Braspenning J, Hutchinson A, Marshall MN. Research methods used in developing and applying quality indicators in primary care. BMJ. 2003;326(7393):816–819. doi: 10.1136/bmj.326.7393.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yazdany J, Panopalis P, Gillis JZ, et al. A quality indicator set for systemic lupus erythematosus. Arthrit. Rheum. 2009;61(3):370–377. doi: 10.1002/art.24356. ▪▪ Describes the development of the first quality indicator set in systemic lupus erythematosus.
- 18.Brook RH. The RAND/UCLA appropriateness method. In: McCormick KA, Moore SR, Siegel RA, editors. Methodology Perspectives. Rockville, MD, USA: US Department of Health and Human Services; 1994. pp. 59–70. [Google Scholar]
- 19. Mosca M, Tani C, Aringer M, et al. Development of quality indicators to evaluate the monitoring of SLE patients in routine clinical practice. Autoimmun. Rev. 2011;10(7):383–388. doi: 10.1016/j.autrev.2010.12.008. ▪▪ Describes the development of the European League Against Rheumatism quality indicator set for systemic lupus erythematosus.
- 20.Yazdany J, Trupin L, Kaiser R, et al. Contraceptive counseling and use among women with systemic lupus erythematosus: a gap in health care quality? Arthrit. Care Res. (Hoboken) 2010 doi: 10.1002/acr.20402. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yazdany J, Tonner C, Trupin L, et al. Provision of preventive health care in systemic lupus erythematosus: data from a large observational cohort study. Arthrit. Res. Ther. 2010;12(3):R84. doi: 10.1186/ar3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demas KL, Keenan BT, Solomon DH, Yazdany J, Costenbader KH. Osteoporosis and cardiovascular disease care in systemic lupus erythematosus according to new quality indicators. Semin. Arthrit. Rheum. 2010;40(3):193–200. doi: 10.1016/j.semarthrit.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmajuk G, Yelin E, Chakravarty E, Nelson LM, Panopolis P, Yazdany J. Osteoporosis screening, prevention, and treatment in systemic lupus erythematosus: application of the systemic lupus erythematosus quality indicators. Arthrit. Care Res. (Hoboken) 2010;62(7):993–1001. doi: 10.1002/acr.20150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsakonas E, Joseph L, Esdaile JM, et al. A long-term study of hydroxychloroquine withdrawal on exacerbations in systemic lupus erythematosus. The Canadian Hydroxychloroquine Study Group. Lupus. 1998;7(2):80–85. doi: 10.1191/096120398678919778. [DOI] [PubMed] [Google Scholar]
- 25.Levy RA, Vilela VS, Cataldo MJ, et al. Hydroxychloroquine (HCQ) in lupus pregnancy: double-blind and placebo-controlled study. Lupus. 2001;10(6):401–404. doi: 10.1191/096120301678646137. [DOI] [PubMed] [Google Scholar]
- 26.Alarcon GS, McGwin G, Bertoli AM, et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L) Ann. Rheum. Dis. 2007;66(9):1168–1172. doi: 10.1136/ard.2006.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fessler BJ, Alarcon GS, Mcgwin G, Jr, et al. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthrit. Rheum. 2005;52(5):1473–1480. doi: 10.1002/art.21039. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz-Irastorza G, Egurbide MV, Pijoan JI, et al. Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus. 2006;15(9):577–583. doi: 10.1177/0961203306071872. [DOI] [PubMed] [Google Scholar]
- 29.Schmajuk G, Yazdany J, Trupin L, Yelin E. Hydroxychloroquine treatment in a community-based cohort of patients with systemic lupus erythematosus. Arthrit. Care Res. (Hoboken) 2010;62(3):386–392. doi: 10.1002/acr.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward MM. Laboratory abnormalities at the onset of treatment of end-stage renal disease: are there racial or socioeconomic disparities in care? Arch. Intern. Med. 2007;167(10):1083–1091. doi: 10.1001/archinte.167.10.1083. [DOI] [PubMed] [Google Scholar]
- 31.Trivedi AN, Grebla RC, Wright SM, Washington DL. Despite improved quality of care in the Veterans Affairs health system, racial disparity persists for important clinical outcomes. Health Aff. (Millwood) 2011;30(4):707–715. doi: 10.1377/hlthaff.2011.0074. [DOI] [PubMed] [Google Scholar]
- 32.Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthrit. Care Res. (Hoboken) 2010;62(11):1515–1526. doi: 10.1002/acr.20295. [DOI] [PubMed] [Google Scholar]
- 33.Petri M, Perez-Gutthann S, Longenecker JC, Hochberg M. Morbidity of systemic lupus erythematosus: role of race and socioeconomic status. Am. J. Med. 1991;91(4):345–353. doi: 10.1016/0002-9343(91)90151-m. [DOI] [PubMed] [Google Scholar]
- 34.Mosley-Williams A, Lumley MA, Gillis M, Leisen J, Guice D. Barriers to treatment adherence among African American and white women with systemic lupus erythematosus. Arthrit. Rheum. 2002;47(6):630–638. doi: 10.1002/art.10790. [DOI] [PubMed] [Google Scholar]
- 35.Daleboudt GM, Broadbent E, McQueen F, Kaptein AA. Intentional and unintentional treatment non-adherence in patients with systemic lupus erythematosus. Arthrit. Care Res. (Hoboken) 2010 doi: 10.1002/acr.20411. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 36. Porter ME. What is value in health care? N. Engl. J. Med. 2010;363(26):2477–2481. doi: 10.1056/NEJMp1011024. ▪▪ Establishes a novel framework for the assessment of value in healthcare.
- 37.Langley GN, Moen R, Nolan KM, et al. The Improvement Guide: A Practical Approach To Enhancing Organizational Performance. San Francisco, CA, USA: Jossey-Bass Publishers; 1996. [Google Scholar]
- 38.Al-Hoqail R, Al-Shlash S, Helmi A, et al. Total quality management in plastic surgery. J. Craniofac. Surg. 2010;21(1):10–19. doi: 10.1097/SCS.0b013e3181c3463c. [DOI] [PubMed] [Google Scholar]
- 39.Chen AH, Kushel MB, Grumbach K, Yee HF., Jr Practice profile. A safety-net system gains efficiencies through ‘eReferrals’ to specialists. Health Aff. (Millwood) 2010;29(5):969–971. doi: 10.1377/hlthaff.2010.0027. [DOI] [PubMed] [Google Scholar]
- 40.Harrington JT, Lease J. System-based coordination of postfracture osteoporosis care compared with traditional approaches. Arthrit. Care Res. (Hoboken) 2010;62(11):1646–1649. doi: 10.1002/acr.20271. [DOI] [PubMed] [Google Scholar]