Abstract
Background: Intercellular communication between epithelial and mesenchymal cells is central to mammalian craniofacial development. β-catenin is the gateway of canonical Wnt signaling, one of the major evolutionarily conserved cell–cell communication pathways in metazoa. In this study, we report an unexpected stage- and tissue-specific function of β-catenin during mammalian jaw development. Results: Using a unique mouse genetic tool, we have discovered that epithelial β-catenin is essential for lower jaw formation, while attenuation of β-catenin is required for proper upper jaw development. Changes in β-catenin in vivo alter major epithelial Fgf8, Bmp4, Shh, and Edn1 signals, resulting in partial transcriptional reprogramming of the neural crest-derived mesenchyme, the primary source of jawbones. Conclusions: The Wnt/β-catenin signal coordinates expression of multiple epithelial signals and has stage-specific asymmetric functions during mammalian upper and lower jaw development. In addition, these findings suggest that evolutionary changes of the canonical Wnt/β-catenin signaling pathway may lead to innovation of jaws. Developmental Dynamics 241:663–674, 2012. © 2012 Wiley Periodicals, Inc.
Key Findings
Mammalian epithelial Wnt/β-catenin signaling has asymetric functions in the upper and lower jaw development.
The canonical Wnt/β-catenin pathway coordinates expression of multiple epithelial signals including Fgf8, Bmp4, Shh, and Edn1.
Activation of epithelial Wnt/β-catenin signaling induces molecular transformation of the upper jaw to the lower jaw mesenchymal phenotype.
Evolutionary changes of the canonical Wnt/β-catenin signaling pathway may lead to innovation of jaws.
Keywords: craniofacial, pharyngeal arch, jaw; Wnt, ß-catenin, Fgf8, Bmp4, Shh, endothelin, Dlx5, Hand2, Cre
INTRODUCTION
Exquisite vertebrate facial structures are results of gradual transformations of relatively simple embryonic mesenchymal swellings or prominences, including βthe first pharyngeal arch (PA1; Helms and Schneider,2003; Santagati and Rijli,2003; Kuratani,2005; Depew and Simpson,2006). The dorsal maxillary (mx) and ventral mandibular (md) components of the PA1 are the principal sources of upper jaw and lower jaw structures, respectively. Although mesenchymal cells of the PA1 prominences have intrinsic jaw identity (Schneider and Helms,2003), growth and patterning of pharyngeal mesenchyme depends on molecular cues from the surrounding epithelial cells. For instance, the foregut endoderm and the frontonasal ectoderm play instructive roles in specification of lower and upper beak, respectively (Couly et al.,2002; Hu et al.,2003). The molecular basis underlying epithelial and mesenchymal cell interactions during jaw specification has yet to be fully elucidated.
The Wnt/β-catenin signaling pathway is one of the most ancient intercellular communication pathways, in which intracellular β-catenin acts as a central node to transduce canonical Wnt signals (Richards and Degnan,2009; Pang et al.,2010). Canonical Wnt signaling stabilizes and enhances β-catenin function as a transcription coactivator to regulate downstream target gene expression. The Wnt/β-catenin pathway has multiple roles including establishing primary body axis, formation of individual organs, and maintenance of tissue homeostasis. One of the conserved functions of this pathway is to convey positional information to promote posterior or caudal identity (Martin and Kimelman,2009; Petersen and Reddien,2009). This function can be traced to prebilaterians, the basal metazoans that diverged from bilaterians over 500 million years ago (Hobmayer et al.,2000; Wikramanayake et al.,2003; Kusserow et al.,2005; Pang et al.,2010).
Genetic analyses of mouse β-catenin have uncovered diverse functions such that the level and timing of β-catenin activity triggers different and sometimes opposing biological outcomes (Grigoryan et al.,2008). Studies in chick and mouse have shown that levels of the Wnt/β-catenin signaling correlate with region-specific growth and fusion of craniofacial prominences, suggesting that changes in Wnt/β-catenin activity may underlie evolutionary adaptations and variations of species-specific facial appearances, such as chick beak and mouse muzzle (Brugmann et al.,2007). Wnt/β-catenin signaling induces neural crest cell formation (Garcia-Castro et al.,2002), and is required for survival of PA1 mesenchymes during craniofacial morphogenesis (Brault et al.,2001; Reid et al.,2011; Wang et al.,2011b). In this study, we examined surface epithelial-specific functions of β-catenin in the PA1 development. Our findings demonstrate that β-catenin is a key regulator of multiple epithelial signals and has distinct functions in the patterning upper and lower jaws.
RESULTS
Epithelial β-catenin Is Essential for Craniofacial Morphogenesis
A Cre transgenic line driven by a Pitx1 enhancer, Pitx1/Cre, is expressed in the oral epithelium but not the pharyngeal mesenchymes (Olson et al.,2006) Using a R26RlacZ reporter line (Soriano,1999), we demonstrated its epithelial-specific Cre activity in the PA1 (Fig. 1). Whole-mount X-gal staining of embryonic day (E) 10.5 embryos demonstrated strong Cre activity in the surface epithelium of the PA1, including both ventral mandibular (mdPA1) and dorsal maxillary (mxPA1) components, and the oral epithelium (Fig. 1). The X-gal-positive cells extended dorsally and rostrally to the optic region and to the ventral third of the nasal placode, respectively. The caudal limits reached to the second PA. Sagittal sections of E12.5 embryos demonstrated that the entire oral epithelium was stained with X-gal (Fig. 1D). Sharp caudal boundaries of the oral epithelium were found near pituitary gland and tongue primordium (Fig. 1D), demonstrating that Cre activity was restricted to ectoderm lineages. At E15.5, strong X-gal staining was found in surface epithelial cells covering the mandible, maxilla, and to a lesser extent, the optic and external ear regions (Fig. 1C,E).
Fig. 1.
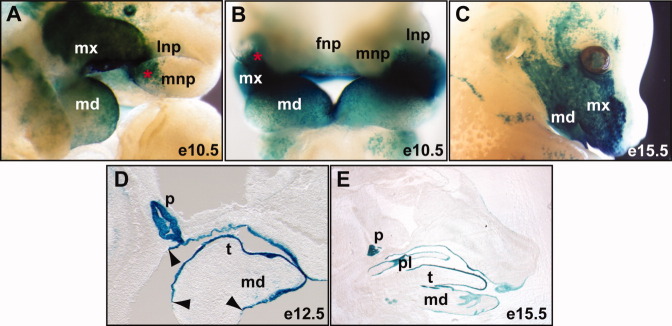
Epithelial-specific Pitx1-Cre transgenic mice. X-gal staining (blue) of Cre activities of the Pitx1/Cre and R26RlacZ double heterozygous embryos. A–C: Whole-mount staining. D,E: Middle sagittal sections. Arrowheads point to the caudal limits of Cre activity; asterisk, ventral nasal placode; fnp, frontal nasal prominence; lnp, lateral nasal prominence; md, mandible prominence; mnp, medial nasal prominence; mx, madillary prominence; p, pituitary; pl, palate; t, tongue.
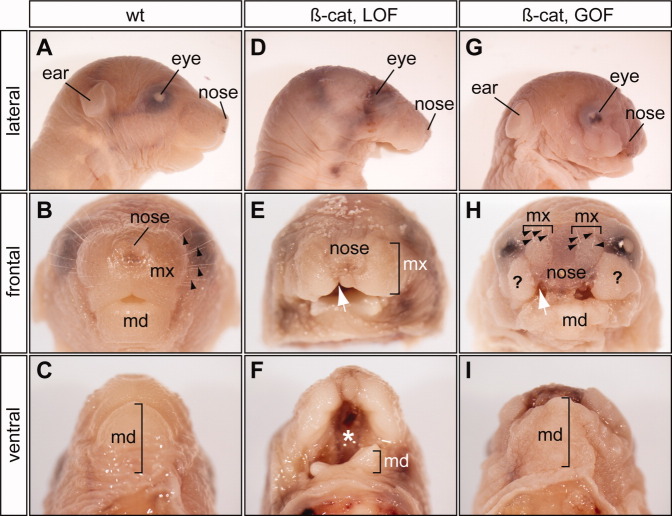
To determine potential roles of epithelial Wnt/β-catenin signaling during craniofacial development, we combined Pitx1/Cre with two different β-catenin alleles that permit conditional deletion of the β-catenin gene (β-catex2-6; Brault et al.,2001) or stabilization of β-catenin protein (β-catex3; Harada et al.,1999). We first confirmed changes of β-catenin levels in Pitx1/Cre;β-Catex2-6/ex2-6 and Pitx1/Cre;β-Catex3/+ embryos, the PA1 epithelial-specific β-catenin LOF (β-cateLOF) and β-catenin GOF (β-cateGOF) mutants respectively (Fig. 2). Immunohistochemical staining using β-catenin-specific antibody indicated high levels of β-catenin in the PA1 epithelium and Rathke's pouch in wild-type controls at E10.5. As expected, β-catenin was undetectable in β-cateLOF mutants, while it was markly increased in the β-cateGOF mutants. β-Catenin expression was unaffected in regions where Cre was not expressed. We next examined gross defects of β-cateLOF and β-cateGOF mutants (Fig. 3). All β-cateLOF and β-cateGOF mutants survived to birth but exhibited severe craniofacial defects (Fig. 3). The mandibles of β-cateLOF mutants were absent (Fig. 3D–F), but were present in β-cateGOF mutants (Fig. 3G–I). Conversely, the maxillas of β-cateGOF mutants were severely deformed (Fig. 3G–I). The area of maxillary soft tissue and whisker pads was much smaller in β-cateGOF mutants than in wild-type controls and was displaced dorsally by an unidentified soft tissue mass (Fig. 3H). The frontal nasal structures in both mutants were hypoplastic (Fig. 3), but nasal capsule cartilage was not affected (Fig. 4). Consistent with the essential role of epithelial β-catenin function during the second palate formation (He et al.,2011), both mutants had cleft lip and palate defects (Fig. 3F and data not shown). Together, these gross phenotypes demonstrated the important role of epithelial β-catenin during craniofacial development.
Fig. 2.
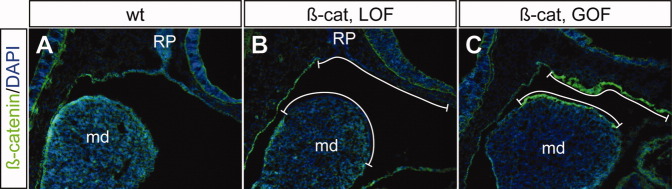
Conditional manipulation of epithelial β-catenin. A–C: Immunohistochemical analyses of sagittal cryostat sections from E10.5 wild-type (A, wt) and β-catenin loss-of-function (B, LOF) and gain-of-function (C, GOF) mutants using β-catenin-specific antibody. bracket, epithelial subdomain where epithelial β-catenin is conditionally altered; md, mandibular; RP, Rathke's pouch.
Fig. 3.
Asymetric requirements for epithelial β-catenin in upper and lower jaw formation. D–F: Newborn lower jaw defects of epithelial-specific β-catenin loss-of-function mutants (β-cat, LOF), and upper jaw defects of the gain-of-function mutants (β-cat, GOF, G-I). A–C: Wild-type controls. White arrow, cleft lips; black arrow, whiskers; asterisk, cleft palate; md, mandible; mx, maxillary; question mark, ectopic soft tissue.
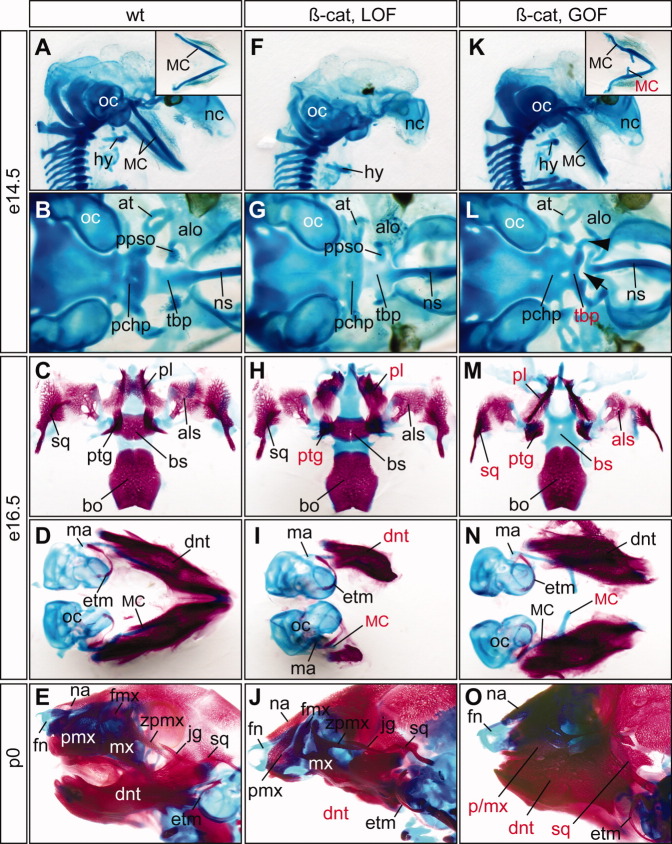
Fig. 4.
Cartilage and bone defects of epithelial-specific β-catenin mutants. A–O: Alcian blue cartilage staing of of embryonic day (E) 14.5 (A,B,F,G,K,L), E16.5 embryos (C,D,H,I,M,N), and newborn pups (E,J,O) and alizarin red bone staining of E16.5 embryos and newborn pups. A–E: Wild-type controls. F–J: β-cateLOF mutants display severe truncation of Meckel's cartilage (MC) and dentary bone, deformation of palatine (pl) and pterygoid (ptg). K–O: β-cateGOF mutants have ectopic MC and upper jaw defects. Red labels indicate defective structures. All pictures are ventral views except A, E, F, J, K and O, which are sagittal views. Inserts in A and K show ventral view of dissected MC. Note that the dentary bones of the β-cateGOF (N) are connected distally but dissociated during staining and dissection process. Arrow, gap; arrowhead, unidentified cartilage rod; at, ala temporalis; alo, ala orbitalis; als, alisphenoid; bo, basioccipital; bs, basisphenoid; dnt, dentary; etm, ectotympanic; fmx, frontal process of maxilla; fn, frontal nasal cartilage; hy, hyoid; jg, jugal; ma, malleus; mx, maxilla; mc, Meckel's cartilage; na, nasal bone; nc, nasal capsule; ns, nasal septum; oc, otic capsule; pchp, parachordal plate; pl, palatine; ppso, pila postoptica; ptg, pterygoid; sq, squamosal; tbp, trabecular basal plate; zpmx, zygomatic process of maxilla.
Asymetric Requirements for Epithelial β-catenin in Upper and Lower Jaw Formation
The phenotypic differences of β-cateLOF and β-cateGOF mutants is intriguing because it suggests that epithelial β-catenin has a differential impact on upper and lower jaw formation (Fig. 3). To further examine whether epithelial β-catenin regulates formation of the neural crest-derived skeletal structures, we analyzed craniofacial cartilages and bones of β-cateLOF and β-cateGOF mutants (Fig. 4). Overall, the frontal nasal cartilages from both LOF (Fig. 4F,J) and GOF (Fig. 4K,O) mutants were intact. Formation of the upper and lower jaw skeletal structures, however, exhibited distinct sensitivities to altered levels of epithelial β-catenin (Fig. 4).
The β-cateLOF mutants exhibited severe distal truncation of the mdPA1-derived lower jaw Meckel's cartilage at E14.5 (Fig. 4F). The most ventral mdPA1-derived structures, including the rostral process, lower incisor, and distal dentary bones, were all missing from the E16.5 embryos and the newborn pups (Fig. 4H–J). The dorsal mdPA1-derived bones, including the ectotympanic ring, gonial, and malleus, were not affected (Fig. 4I,J). Consistent with the cleft palate phenotype, the palatine and pterygoid bones were deformed in β-cateLOF mutants (Fig. 4H). However, other mxPA1-derived skeletal elements, including maxilla, jugal, lamina obturans, squamosal, incus, and the associated skeletal structures including parachordal and trabecular cartilage plates, and basioccipital and basisphenoid bones, were normal (Fig. 4J). These findings demonstrate that formation of the lower jaw, but not the upper jaw, is particularly sensitive to reduced levels of epithelial β-catenin.
Complementary to the agenesis of the Meckel's cartilage of the LOF mutants, β-cateGOF mutants formed ectopic Meckel's cartilage at E14.5 and E16.5 (Fig. 4K,N). These ectopic cartilage were formed at the proximal region of the bent Meckel's cartilage rods at E14.5, and the dentary bones were deformed at E16.5 (Fig. 4K,N). The resulting lower jawbones were enlarged and fused with the putative upper jawbones of the newborn β-cateGOF mutants (Fig. 4O). The mxPA1-derived upper jaw structures, including alisphenoid and squamosal bones, were underdeveloped (Fig. 4M), and were completely fused with dentary bones and severely deformed in the newborn pups (Fig. 4O). The premaxilla and maxilla were also fused with the dentary bones. The jugal bone was missing. In addition, the trabecular plate failed to connect to the nasal septum (Fig. 4L). A conspicuous rod shaped cartilage structure, most likely the deformed cartilage of the postoptic pillar of optic capsule, extended bilaterally from rostral end of the trabecular plate toward the ala orbitalis cartilage (Fig. 4L, arrowhead). The basisphenoid bone was missing in β-cateGOF mutants (Fig. 4M). The palatine bones failed to meet at the midline and the pterygoid bones were severely deformed (Fig. 4M). Thus, in contrast to the LOF mutants, stabilization of epithelial β-catenin protein in the GOF mutants resulted in severe defects of the mxPA1-derived skeletal structures.
Collectively, facial skeletal analyses of both β-catenin LOF and GOF mutants suggested that epithelial β-catenin is required for formation of the lower jawbones while attenuation of epithelial β-catenin is necessary for proper upper jawbone development.
β-catenin Is Genetically Upstream of Multiple Epithelial Signals
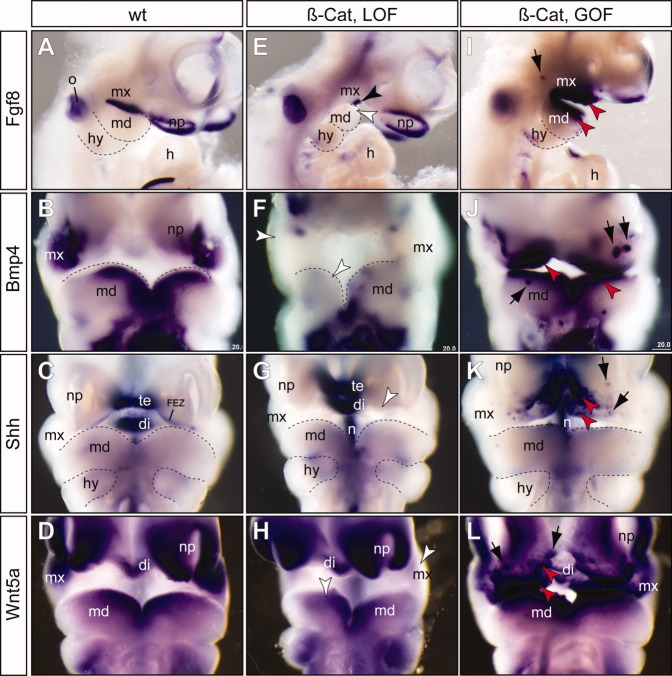
To determine whether changes in epithelial β-catenin caused alterations in epithelial signals with consequent changes of mesenchymal cell patterning, we analyzed expression of Fgf8 and Bmp4 (Fig. 5). Fgf8 was expressed in PA epithelium of wild-type control embryos at E10.5 (Fig. 5A). Previous studies demonstrated that conditional inactivation of Fgf8 at E9.0 resulted in truncation of the proximal and intermediate regions of both mxPA1 and mdPA1 (Trumpp et al.,1999). In all β-cateLOF mutants analyzed (n = 5), Fgf8 expression in the PA1 was reduced to a small patch of cells at the cleft region between mxPA1 and mdPA1 (Fig. 5E). A downstream transcriptional target of Fgf8, Lhx7 (Trumpp et al.,1999), was concordantly reduced (Fig. 5C), confirming that epithelial Fgf8 signaling activity was reduced. Complementary to the observations of β-cateLOF mutants, we found that Fgf8 and Lhx7 expression levels were increased and their expression domains were expanded in the β-cateGOF mutants (Figs. 5I, 6E).
Fig. 5.
β-catenin functions upstream of other epithelial signals. E–L: RNA in situ hybridization (purple color) of embryonic day (E) 10.5 embryos using gene-specific probes indicates decreased expression of Fgf8, Bmp4, Shh, and Wnt5a in the β-catenin loss-of-function (β-cat, LOF) mutants (E–H), while expression of these genes were enhanced in the β-catenin gain-of-function (β-cat, GOF) mutants (I–L). A–D: Wild-type controls. I–L: Patchy ectopic staining (black arrows) of Fgf8 (I), Bmp4 (J), Shh (K), and Wnt5a (L) were observed in the β-catenin GOF mutants. All pictures are frontal views except A, E and I, which are lateral views. di, diencephalon; FEZ, frontonasal ectoderm zone; h, heart; hy, hyoid arch; md, mandibular prominence; mx, maxillary prominence; n, notochord/floor plate; np, nasal placode; o, otic vesicle; te, telencephalon; black arrowhead, residual expression of Fgf8 at the cleft of between mx and md; white arrowhead, reduced expression; red arrowhead, enhanced expression.
Fig. 6.
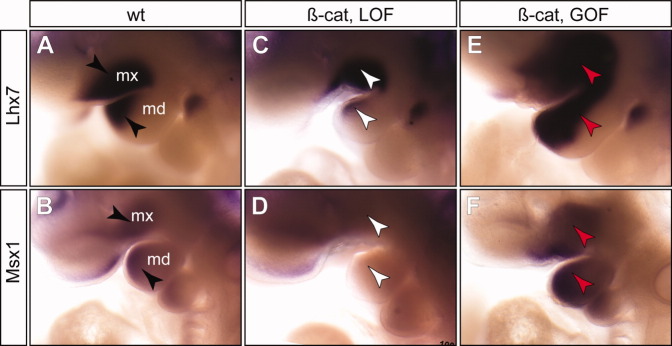
Epithelial β-catenin indirectly regulates expression of genes in the mesenchymal cells. Whole-mount RNA in situ hybridization using Lhx7 and Msx1 probes. A,B: Wild-type controls. C,D: Epithelial-specific β-catenin loss-of-function (β-cat, LOF) mutants. E,F: β-catenin gain-of-function (β-cat, GOF) mutants. black arrowhead, normal expression pattern; white arrowhead, reduced expression; red arrowhead, increased expression.
Conditional deletion of Bmp4 results in reduction of Msx1/Msx2 expression and severe defects of the jawbones (Liu et al.,2005). While expression of Bmp4 is independent of Fgf8 (Trumpp et al.,1999), Bmp4 acts an activator of Fgf8 in the proximal mdPA1, and a repressor of Fgf8 in the distal mdPA1 (Liu et al.,2005). In wild-type arches, we detected Bmp4 at the distal ends of maxillary and mandibular epithelia of the PA1 (Fig. 5B). In β-cateLOF mutants, Bmp4 was largely undetectable, and only small patches of cells at the most distal ends exhibited weak expression (Fig. 5F). Expression of a Bmp4 downstream target gene, Msx1, was not detected in β-cateLOF mutants, consistent with the deficiency of Bmp4 signaling in the mutants (Fig. 6D). In β-cateGOF mutants, we observed enhanced and expanded expression of Bmp4 in epithelial cells and Msx1 in mesechymal cells (Figs. 5J, 6F). Thus, we observed concerted shifts of epithelial Bmp4 signal and its downstream target in mesenchymal cells, Msx1. Together, these results demonstrate that β-catenin is genetically upstream of critical epithelia signals including Fgf8 and Bmp4 during mammalian jaw development.
We also examined expression patterns of Shh and Wnt5a (Fig. 5). The Shh-positive frontal ectodermal zone and foregut endoderm are critical for craniofacial development (Couly et al.,2002; Hu et al.,2003; Brito et al.,2006; Hu and Marcucio,2009). In addition, Shh signaling controls reciprical epithelial-mesenchymal interactions in the outgrowth of the facial primordia (Jeong et al.,2004; Young et al.,2010), and the secondary palate development (Lan and Jiang,2009). In β-cateLOF mutants, Shh expression in the frontal ectodermal zone was undetectable, while expression in internal control regions including telencephalon, diencephalon and pharyngeal endoderm, were not affected (Fig. 5G). Previous studies in the chick have shown that Shh-expressing cells can induce ectopic supernumerary jaws (Brito et al.,2008). We found that in β-cateGOF mutants, Shh expression was enhanced at the frontal ectodermal zone and ectopically expressed at the distal mxPA1 and frontonasal regions, and to a lesser extent, the mdPA1 (Fig. 5K). Similar to Bmp4 (Fig. 5J), ectopic Shh expression was often patchy in the frontal nasal region (Fig. 5K). Wnt5a is critical for outgrowth of the craniofacial prominences including the mdPA1 and mxPA1 (Yamaguchi et al.,1999). We found that Wnt5a was down regulated in β-cateLOF mutants (Fig. 5H), and increased and ectopically expressed in β-cateGOF mutants (Fig. 5L). Collectively, these findings demonstrated that epithelial β-catenin is an upstream regulator of multiple critical epithelial signals including Fgf8, Bmp4, Shh, and Wnt5a.
Epithelial β-catenin Induces Molecular Reprogramming of Mesenchymal Cells
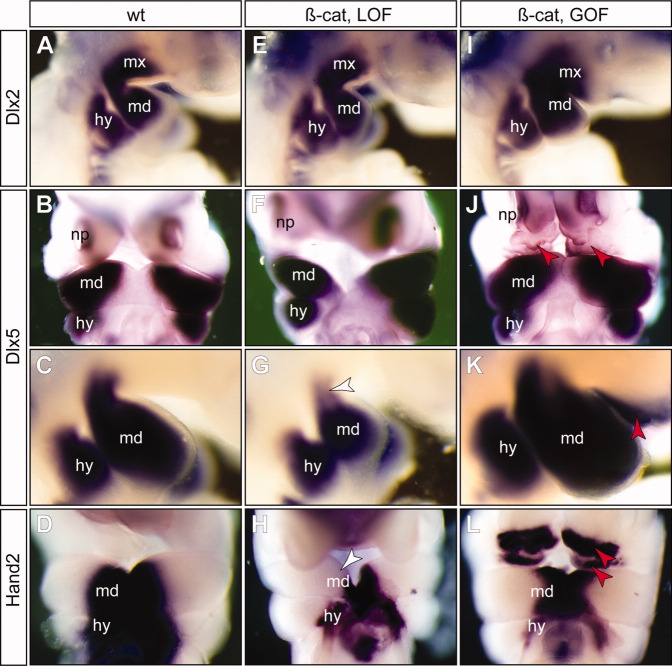
To determine whether altered epithelial β-catenin had the potential to alter mesenchymal cell differentiation programs, we examined expression patterns of transcription factors involved in the PA1 specification (Minoux and Rijli,2010; Fig. 7). Dlx-family homeodomain transcription factors are critical regulators of the PA1 (Depew et al.,2005; Jeong et al.,2008). Dlx1 and 2 are expressed in both mdPA1 and mxPA1, whereas Dlx5 and 6 are restricted to the mdPA1. Inactivation of Dlx5 and Dlx6 results in homeotic transformation of the lower jaw into the upper jaw structures (Beverdam et al.,2002; Depew et al.,2002). In our experiments, expression of Dlx2 appeared to be independent of epithelial β-catenin as both LOF and GOF mutants had similar levels of Dlx2 when compared with the wild-type littermate controls (Fig. 7A,D,G). However, Dlx5 expression was slightly reduced at the dorsal mdPA1 region of β-cateLOF mutants (Fig. 7G, white arrowhead, and Fig. 8F) and ectopically expressed in β-cateGOF mutants at the frontal nasal region (Fig. 7J, red arrowheads, and 8F) and the oral region of mxPA1 (Fig. 7K, red arrowhead, and Fig. 8F).
Fig. 7.
A–L: β-catenin induces partial reprogramming of maxillary mesenchyme. While expression of Dlx2 is maintained in both β-catenin loss-of-function (β-cat, LOF) mutants (E) and gain-of-function (β-cat, GOF) mutants (I), expression of the mdPA1 genes Dlx5 (B,C,F,G,J,K) and Hand2 (D,H,L) are reduced in the LOF mutants (white arrowheads in G and H), and ectopically induced in the GOF mutants (red arrowheads in K and L). A,E,I,C,G,K: Lateral views and the rest are frontal views. See Figure 4 for abbreviations.
Fig. 8.
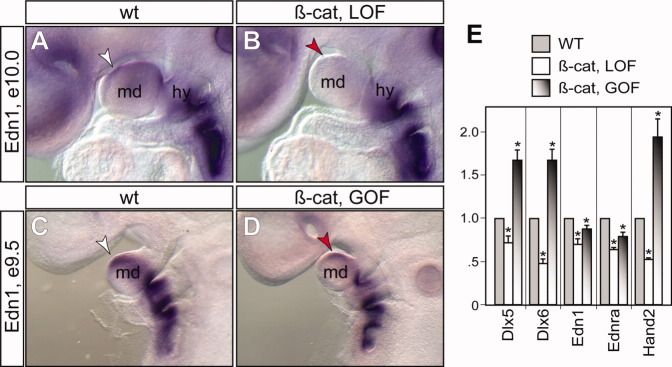
Expression of Edn1 depends on epithelial β-catenin. A–D: Whole-mount RNA in situ hybridization using Edn1 specific probe. white arrowhead, normal expression in mandibular prominence (md); red arrowhead, reduced expression in mutants. expression in hyoid arch (hy) and the rest of caudal arches are unaffected. E: Real-time quantitative polymerase chain reaction analyses of relative expression levels of genes (x-axis) in the microdissected first pharyngeal arch at embryonic day (E) 10.5 using β-actin as an internal control. Asterisk, P < 0.05 (y-axis), Student t-test, n = 4.
We also examined the expression pattern of Hand2, which is an effector of Dlx5 and Dlx6 transcription factors (Charite et al.,2001; Sato et al.,2008). Hand2 was reduced in the mdPA1 of β-cateLOF mutants (Figs. 7H, 8F). Furthermore, strong ectopic Hand2-positive cells were found at the frontal nasal and oral regions of the mxPA1 of β-cateGOF mutants (Fig. 7L, red arrowheads, and 8F). Conversion of the maxillary into a mandibular fate can occur through over expression of Hand2 (Sato et al.,2008). Local ectopic activation of Dlx5 and Hand2 in β-cateGOF mutants therefore suggests that high levels of epithelial β-catenin can induce molecular reprogramming of mesenchymal cells from a maxillary to mandibular fate. Because Dlx5/6 and Hand2 are downstream effectors of the Endothelin-1 (Edn1)/Endothelin receptor type-A (Ednra) signaling pathway, and constitutive activation of Edn1/Ednra is sufficient to transform maxillary to mandibular identity (Sato et al.,2008), we therefore examined whether expressions of Edn1 and Ednra were also affected by alteration of epithelial β-catenin levels (Fig. 8). Both Edn1 and Ednra were significantly reduced in the mandibular region of β-cateLOF mutants, while their expressions were unaffected in the caudal arch regions where β-catenin expression was not perturbed (Fig. 8 and data not shown). Surprisingly, their expressions were also down-regulated, but to a lesser extent in β-cateGOF mutants. These molecular changes are consistent with the gross phenotypic observations that loss of β-catenin results in a lower jaw agenesis phenotype while overexpression of β-catenin is not sufficient to fully transform the first arch identity.
DISCUSSION
Cell–cell communications are a quintessential feature of multicellular organisms and are, therefore, under tight evolutionary constraint. Key signaling pathways, including Wnt, TGF-β, Hedgehog, and Notch, were already present in the last common ancestors to all living bilaterians (Richards and Degnan,2009). Accordingly, variation and redeployment of these signaling pathways is likely to mediate the evolution of novel features such as evolution of beak shapes of Darwin's finches (Abzhanov et al.,2004) and acquisition of butterfly eyespots (Keys et al.,1999).
In this study, we demonstrated that epithelial β-catenin coordinates expression of several critical signaling molecules required for vertebrate jaw development. We found that high levels of epithelial β-catenin support lower jaw development while inducing molecular reprogramming of mesenchymal cells from a maxillary to mandibular-like fate. Deletion of epithelial β-catenin resulted in severe truncation or absence of the ventral mdPA1-derived skeletal structures, particularly the dentary bone, while stabilization of epithelial β-catenin enhanced dentary bone formation (Figs. 3, 4). These observations suggest that epithelial β-catenin activities have gradient effects controlling dorsoventral patterning of the PA1 and formation of jawbones. While epithelial β-catenin is essential for lower jaw formation, attenuation of its activities at later stages seems to be a prerequisite for normal development of the upper jaw. These surprising findings demonstrate that epithelial β-catenin has asymmetric functions during vertebrate jaw development: high levels promote lower jaw formation while low levels are required for upper jaw development. Changing levels of Wnt/β-catenin signaling have been implicated in the evolutionary adaptations and variations of species-specific facial appearances (Brugmann et al.,2007; Mallarino et al.,2011). Our studies support a hypothesis that changes of Wnt/β-catenin signaling were involved in vertebrate jaw evolution.
Different aspects of epithelial β-catenin functions in craniofacial development at earlier developmental stages were previously reported using a similar genetic strategy but with different Cre-expressing mouse lines. Wang et al. used a Foxg1-Cre knockin line, which is active at the anterior neural ridge, neural epithelia and adjacent frontal nasal ectoderm at E8.75 (Wang et al.,2011b). In contrast to our findings, LOF β-catenin mutants using Foxg1-Cre line resulted in agenesis of the mxBA1-derived maxillary structures due to anterior neural ridge defect at early developmental stages (Wang et al.,2011b). Williams and colleagues used a transgenic line that appeared to have an even earlier and broader Cre activity including frontal nasal ectoderm and pharyngeal ectoderm at E8.5 (Reid et al.,2011). A LOF in their studies resulted in agenesis of both mxPA1 and mdPA1 and a GOF resulted in early lethal phenotype. Unlike these Cre lines, the Pitx1/Cre line used in our study is restricted to the pharyngeal arch epithelium, but not in the frontal nasal epithelium. Furthermore, the Pitx1/Cre transgene has a later onset of Cre activity at E9.0 (Olson et al.,2006). The unique spatiotemporal properties of the Pitx1/Cre line enable us to uncover a previously unknown asymmetric function of epithelial β-catenin during patterning of upper and lower jaw structures. Taken together, these studies suggest that development of the lower jaw depends on continuous epithelial β-catenin activity while temporal early activation and late attenuation of epithelial β-catenin is required for normal upper jaw development.
Our studies indicate that epithelial β-catenin controls expression, either directly or indirectly, of other epithelial signaling molecules including Fgf8, Bmp4, Shh, Wnt5a, and Edn1 (Figs. 5, 8). Concerted changes of these epithelial signals are likely responsible for mesenchymal cell proliferation and survival. Mesenchymal cell phenotypes may also influence the behavior of epithelial cells (Sheehy et al.,2010). Consistent with the notion that epithelial β-catenin might be involved in the specification of the PA1, a small portion of distal mxPA1 mesenchyme in β-cateGOF mutants assumed mdPA1-like molecular features, including expression of Dlx5 and Hand2 genes (Beverdam et al.,2002; Depew et al.,2002; Sato et al.,2008; Fig. 7H,I). Recent studies indicate that Notch antagonizes β-catenin activities within a cell (Sanders et al.,2009), and Notch signaling promotes dorsal identity of the PAs (Zuniga et al.,2010). Together, these findings suggest that genetic modifications of Wnt/β-catenin signaling activity may be involved in deploying localized epithelial signals as well as species-specific patterning of the vertebrate jaw.
EXPERIMENTAL PROCEDURES
Mice
All animal studies were performed according to protocols reviewed and approved by the Institutional Animal Care and Use Committee at the Children's Hospital Boston. Generation of the genetic modified mouse lines were reported previously: β-catex2-6 (Brault et al.,2001), β-catex3 (Harada et al.,1999), Pitx1/Cre transgenic mice (Olson et al.,2006), and the R26RlacZ (Soriano,1999).
Gene Expression and Histology Analyses
Whole-mount RNA in situ hybridization with digoxigenin-UTP-labeled RNA probes was performed as previously described (Guo et al.,2011). Histology and X-gal staining were preformed using essentially the same method as reported previously (Li et al.,2002,2003; Guo et al.,2011; Wang et al.,2011a). Edn1 plasmid was a gift from Dr. Hiroki Kurihara and Dr. Jeong Kyo Yoon (Sato et al.,2008; Jin et al.,2011).
RNA Isolation and Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction
Total RNA was isolated from tissue dissected from the first pharyngeal arch of E10.5 embryos (wild-type littermate controls and mutants). cDNA was synthesized using the AccuScript High Fidelity 1st strand cDNA Synthesis Kit. Real-time polymerase chain reaction (PCR) was performed as described in the manufacturer's protocol on an Applied Biosystems StepOnePlus. The mRNA levels of Dlx5, Dlx6, Edn1, Endra, and Hand2 were normalized using β-actin. PCR was performed using the primers 5′-TCT CTA GGA CTG ACG CAA ACA-3′ and 5′-GTT ACA CGC CAT AGG GTC GC-3′ for Dlx5, 5′-AAA ACG ACA GTG ATC GAA AAC GG-3′ and 5′-AGT CTG CTG AAA GCG ATG GTT-3′ for Dlx6, 5′-GCA CCG GAG CTG AGA ATG G-3′ and 5′-GTG GCA GAA GTA GAC ACA CTC-3′ for Edn1, 5′-TAC AAG GGC GAG CTG CAT AG-3′ and 5′-GGC GTG CTG ATT TCC AAA GG-3′ for Ednra, 5′-GCA GGA CTC AGA GCA TCA ACA-3′ and 5′-AGG TAG GCG ATG TAT CTG GTG-3′ for Hand2, 5′-TCG TCG ACA ACG GCT CCG GCA TGT-3′ and 5′-CCA GCC AGG TCC AGA CGC AGG AT-3′ for β-actin. Statistical significance was determined by Student's t-test with P < 0.05.
Immunohistochemistry
Cryostat sagittal sections (10 mm) of staged embryos were used for immunohistochemical analyses. Room-temperature slides were washed for 10 min in phosphate buffered saline (PBS), placed in blocking solution (0.1% Tween 20 and 5% goat serum in PBS) for 1 hr at room temperature, and then incubated with anti-β-catenin antibody (BD Transduction Laboratory) in blocking solution for 3 hr at room temperature. Sections were washed 3 times for 10 min each in PBST (PBS with 0.1% Tween 20) and incubated with Cy2-conjugated secondary antibody (Jackson ImmunoResearch, Inc.) in blocking solution for 2 hr at room temperature. These stained sections were counterstained with DAPI (4′,6-diamidine-2-phenylidole-dihydrochloride) and imagined using an Olympus SZX16 fluorescent microscope with a DP71 digital camera.
Alcian Blue Cartilage Staining and Alizarin Red Bone Staining
The cartilage and bone staining were performed as reported (Li et al.,2003). Briefly, staged embryos were collected and fixed in 95% ethanol for more than 24 hr before staining for cartilage with Alcian blue (15–30 mg in 80% ethanol and 20% glacial acetic acid) for 2 days. Bones of the E16.5 embryos were stained with 75 μg/ml Alizarin red and 1% potassium hydroxide overnight. All stained embryos were cleared in glycerol before photo imaging using an Olympus M15 dissecting microscope.
Acknowledgments
We very much appreciate the comments and suggestions from Dr. John Rubenstein, and plasmid gifts from Dr. Hiroki Kurihara and Dr. Jeong Kyo Yoon. X.K.L. is a ChangJiang Scholar. X.L. was funded by the NIH/NIDCR.
REFERENCES
- Abzhanov A, Protas M, Grant BR, Grant PR, Tabin CJ. Bmp4 and morphological variation of beaks in Darwin's finches. Science. 2004;305:1462–1465. doi: 10.1126/science.1098095. [DOI] [PubMed] [Google Scholar]
- Beverdam A, Merlo GR, Paleari L, Mantero S, Genova F, Barbieri O, Janvier P, Levi G. Jaw transformation with gain of symmetry after Dlx5/Dlx6 inactivation: mirror of the past? Genesis. 2002;34:221–227. doi: 10.1002/gene.10156. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Brito JM, Teillet MA, Le Douarin NM. An early role for sonic hedgehog from foregut endoderm in jaw development: ensuring neural crest cell survival. Proc Natl Acad Sci U S A. 2006;103:11607–11612. doi: 10.1073/pnas.0604751103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito JM, Teillet MA, Le Douarin NM. Induction of mirror-image supernumerary jaws in chicken mandibular mesenchyme by Sonic Hedgehog-producing cells. Development. 2008;135:2311–2319. doi: 10.1242/dev.019125. [DOI] [PubMed] [Google Scholar]
- Brugmann SA, Goodnough LH, Gregorieff A, Leucht P, ten Berge D, Fuerer C, Clevers H, Nusse R, Helms JA. Wnt signaling mediates regional specification in the vertebrate face. Development. 2007;134:3283–3295. doi: 10.1242/dev.005132. [DOI] [PubMed] [Google Scholar]
- Charite J, McFadden DG, Merlo G, Levi G, Clouthier DE, Yanagisawa M, Richardson JA, Olson EN. Role of Dlx6 in regulation of an endothelin-1-dependent, dHAND branchial arch enhancer. Genes Dev. 2001;15:3039–3049. doi: 10.1101/gad.931701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couly G, Creuzet S, Bennaceur S, Vincent C, Le Douarin NM. Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development. 2002;129:1061–1073. doi: 10.1242/dev.129.4.1061. [DOI] [PubMed] [Google Scholar]
- Depew MJ, Simpson CA. 21st century neontology and the comparative development of the vertebrate skull. Dev Dyn. 2006;235:1256–1291. doi: 10.1002/dvdy.20796. [DOI] [PubMed] [Google Scholar]
- Depew MJ, Lufkin T, Rubenstein JL. Specification of jaw subdivisions by Dlx genes. Science. 2002;298:381–385. doi: 10.1126/science.1075703. [DOI] [PubMed] [Google Scholar]
- Depew MJ, Simpson CA, Morasso M, Rubenstein JL. Reassessing the Dlx code: the genetic regulation of branchial arch skeletal pattern and development. J Anat. 2005;207:501–561. doi: 10.1111/j.1469-7580.2005.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Castro MI, Marcelle C, Bronner-Fraser M. Ectodermal Wnt function as a neural crest inducer. Science. 2002;297:848–851. doi: 10.1126/science.1070824. [DOI] [PubMed] [Google Scholar]
- Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of {beta}-catenin in mice. Genes Dev. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Sun Y, Zhou B, Adam RM, Li X, Pu WT, Morrow BE, Moon A, Li X. A Tbx1-Six1/Eya1-Fgf8 genetic pathway controls mammalian cardiovascular and craniofacial morphogenesis. J Clin Invest. 2011;121:1585–1595. doi: 10.1172/JCI44630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Xiong W, Wang Y, Li L, Liu C, Yamagami T, Taketo MM, Zhou C, Chen Y. Epithelial Wnt/beta-catenin signaling regulates palatal shelf fusion through regulation of Tgfbeta3 expression. Dev Biol. 2011;350:511–519. doi: 10.1016/j.ydbio.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms JA, Schneider RA. Cranial skeletal biology. Nature. 2003;423:326–331. doi: 10.1038/nature01656. [DOI] [PubMed] [Google Scholar]
- Hobmayer B, Rentzsch F, Kuhn K, Happel CM, von Laue CC, Snyder P, Rothbacher U, Holstein TW. WNT signalling molecules act in axis formation in the diploblastic metazoan Hydra. Nature. 2000;407:186–189. doi: 10.1038/35025063. [DOI] [PubMed] [Google Scholar]
- Hu D, Marcucio RS. A SHH-responsive signaling center in the forebrain regulates craniofacial morphogenesis via the facial ectoderm. Development. 2009;136:107–116. doi: 10.1242/dev.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Marcucio RS, Helms JA. A zone of frontonasal ectoderm regulates patterning and growth in the face. Development. 2003;130:1749–1758. doi: 10.1242/dev.00397. [DOI] [PubMed] [Google Scholar]
- Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18:937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J, Li X, McEvilly RJ, Rosenfeld MG, Lufkin T, Rubenstein JL. Dlx genes pattern mammalian jaw primordium by regulating both lower jaw-specific and upper jaw-specific genetic programs. Development. 2008;135:2905–2916. doi: 10.1242/dev.019778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YR, Turcotte TJ, Crocker AL, Han XH, Yoon JK. The canonical Wnt signaling activator, R-spondin2, regulates craniofacial patterning and morphogenesis within the branchial arch through ectodermal-mesenchymal interaction. Dev Biol. 2011;352:1–13. doi: 10.1016/j.ydbio.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys DN, Lewis DL, Selegue JE, Pearson BJ, Goodrich LV, Johnson RL, Gates J, Scott MP, Carroll SB. Recruitment of a hedgehog regulatory circuit in butterfly eyespot evolution. Science. 1999;283:532–534. doi: 10.1126/science.283.5401.532. [DOI] [PubMed] [Google Scholar]
- Kuratani S. Craniofacial development and the evolution of the vertebrates: the old problems on a new background. Zoolog Sci. 2005;22:1–19. doi: 10.2108/zsj.22.1. [DOI] [PubMed] [Google Scholar]
- Kusserow A, Pang K, Sturm C, Hrouda M, Lentfer J, Schmidt HA, Technau U, von Haeseler A, Hobmayer B, Martindale MQ, Holstein TW. Unexpected complexity of the Wnt gene family in a sea anemone. Nature. 2005;433:156–160. doi: 10.1038/nature03158. [DOI] [PubMed] [Google Scholar]
- Lan Y, Jiang R. Sonic hedgehog signaling regulates reciprocal epithelial-mesenchymal interactions controlling palatal outgrowth. Development. 2009;136:1387–1396. doi: 10.1242/dev.028167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Perissi V, Liu F, Rose DW, Rosenfeld MG. Tissue-specific regulation of retinal and pituitary precursor cell proliferation. Science. 2002;297:1180–1183. doi: 10.1126/science.1073263. [DOI] [PubMed] [Google Scholar]
- Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, Nigam SK, Aggarwal AK, Maas R, Rose DW, Rosenfeld MG. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426:247–254. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- Liu W, Selever J, Murali D, Sun X, Brugger SM, Ma L, Schwartz RJ, Maxson R, Furuta Y, Martin JF. Threshold-specific requirements for Bmp4 in mandibular development. Dev Biol. 2005;283:282–293. doi: 10.1016/j.ydbio.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Mallarino R, Grant PR, Grant BR, Herrel A, Kuo WP, Abzhanov A. Two developmental modules establish 3D beak-shape variation in Darwin's finches. Proc Natl Acad Sci U S A. 2011;108:4057–4062. doi: 10.1073/pnas.1011480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BL, Kimelman D. Wnt signaling and the evolution of embryonic posterior development. Curr Biol. 2009;19:R215–R219. doi: 10.1016/j.cub.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoux M, Rijli FM. Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development. 2010;137:2605–2621. doi: 10.1242/dev.040048. [DOI] [PubMed] [Google Scholar]
- Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, Wu W, Taketo MM, Kemler R, Grosschedl R, Rose D, Li X, Rosenfeld MG. Homeodomain-mediated beta-catenin-dependent switching events dictate cell-lineage determination. Cell. 2006;125:593–605. doi: 10.1016/j.cell.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Pang K, Ryan JF, Mullikin JC, Baxevanis AD, Martindale MQ. Genomic insights into Wnt signaling in an early diverging metazoan, the ctenophore Mnemiopsis leidyi. Evodevo. 2010;1:10. doi: 10.1186/2041-9139-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CP, Reddien PW. Wnt signaling and the polarity of the primary body axis. Cell. 2009;139:1056–1068. doi: 10.1016/j.cell.2009.11.035. [DOI] [PubMed] [Google Scholar]
- Reid BS, Yang H, Melvin VS, Taketo MM, Williams T. Ectodermal Wnt/beta-catenin signaling shapes the mouse face. Dev Biol. 2011;349:261–269. doi: 10.1016/j.ydbio.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards GS, Degnan BM. The dawn of developmental signaling in the metazoa. Cold Spring Harb Symp Quant Biol. 2009;74:81–90. doi: 10.1101/sqb.2009.74.028. [DOI] [PubMed] [Google Scholar]
- Sanders PG, Munoz-Descalzo S, Balayo T, Wirtz-Peitz F, Hayward P, Arias AM. Ligand-independent traffic of Notch buffers activated Armadillo in Drosophila. PLoS Biol. 2009;7:e1000169. doi: 10.1371/journal.pbio.1000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santagati F, Rijli FM. Cranial neural crest and the building of the vertebrate head. Nat Rev Neurosci. 2003;4:806–818. doi: 10.1038/nrn1221. [DOI] [PubMed] [Google Scholar]
- Sato T, Kurihara Y, Asai R, Kawamura Y, Tonami K, Uchijima Y, Heude E, Ekker M, Levi G, Kurihara H. An endothelin-1 switch specifies maxillomandibular identity. Proc Natl Acad Sci U S A. 2008;105:18806–18811. doi: 10.1073/pnas.0807345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider RA, Helms JA. The cellular and molecular origins of beak morphology. Science. 2003;299:565–568. doi: 10.1126/science.1077827. [DOI] [PubMed] [Google Scholar]
- Sheehy NT, Cordes KR, White MP, Ivey KN, Srivastava D. The neural crest-enriched microRNA miR-452 regulates epithelial-mesenchymal signaling in the first pharyngeal arch. Development. 2010;137:4307–4316. doi: 10.1242/dev.052647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Trumpp A, Depew MJ, Rubenstein JL, Bishop JM, Martin GR. Cre-mediated gene inactivation demonstrates that FGF8 is required for cell survival and patterning of the first branchial arch. Genes Dev. 1999;13:3136–3148. doi: 10.1101/gad.13.23.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Gargollo P, Guo C, Tang T, Mingin G, Sun Y, Li X. Six1 and Eya1 are critical regulators of peri-cloacal mesenchymal progenitors during genitourinary tract development. Dev Biol. 2011a;360:186–194. doi: 10.1016/j.ydbio.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Song L, Zhou CJ. The canonical Wnt/beta-catenin signaling pathway regulates Fgf signaling for early facial development. Dev Biol. 2011b;349:250–260. doi: 10.1016/j.ydbio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Wikramanayake AH, Hong M, Lee PN, Pang K, Byrum CA, Bince JM, Xu R, Martindale MQ. An ancient role for nuclear beta-catenin in the evolution of axial polarity and germ layer segregation. Nature. 2003;426:446–450. doi: 10.1038/nature02113. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Young NM, Chong HJ, Hu D, Hallgrimsson B, Marcucio RS. Quantitative analyses link modulation of sonic hedgehog signaling to continuous variation in facial growth and shape. Development. 2010;137:3405–3409. doi: 10.1242/dev.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga E, Stellabotte F, Crump JG. Jagged-Notch signaling ensures dorsal skeletal identity in the vertebrate face. Development. 2010;137:1843–1852. doi: 10.1242/dev.049056. [DOI] [PMC free article] [PubMed] [Google Scholar]