Abstract
Introduction
In proliferative diabetic retinopathy (PDR), vascular endothelial growth factor (VEGF) and connective tissue growth factor (CTGF) may cause blindness by neovascularisation followed by fibrosis of the retina. It has previously been shown that a shift in the balance between levels of CTGF and VEGF in the eye is associated with this angiofibrotic switch. This study investigated whether anti-VEGF agents induce accelerated fibrosis in patients with PDR, as predicted by this model.
Methods
CTGF and VEGF levels were measured by ELISA in 52 vitreous samples of PDR patients, of which 24 patients had received intravitreal bevacizumab 1 week to 3 months before vitrectomy, and were correlated with the degree of vitreoretinal fibrosis as determined clinically and intra-operatively.
Results
CTGF correlated positively, and VEGF correlated negatively with the degree of fibrosis. The CTGF/VEGF ratio was the strongest predictor of fibrosis. Clinically, increased fibrosis was observed after intravitreal bevacizumab.
Conclusions
These results confirm that the CTGF/VEGF ratio is a strong predictor of vitreoretinal fibrosis in PDR, and show that intravitreal anti-VEGF treatment causes increased fibrosis in PDR patients. These findings provide strong support for the model that the balance of CTGF and VEGF determines the angiofibrotic switch, and identify CTGF as a possible therapeutic target in the clinical management of PDR.
Keywords: Angiogenesis, choroid, CTGF, diabetic retinopathy, drugs, fibrosis, imaging, macula, retina, VEGF, vitreous
Proliferative diabetic retinopathy (PDR) is a major cause of blindness. PDR is characterised by neovascularisation due to retinal ischaemia, and subsequent vitreoretinal fibrosis.1 PDR is a wound healing-like response, in which newly formed vessels may progress to form fibrovascular tissue, which can cause traction on the retina, resulting in retinal detachment and blindness. At some point during this process there is a transition from angiogenesis to fibrosis, which we have introduced as the angiofibrotic switch.2 The mechanisms underlying this switch have not been fully elucidated.
Several growth factors play a role in the different phases of PDR. The pro-angiogenic cytokine vascular endothelial growth factor (VEGF) is considered to be the primary factor involved in neovascularisation in PDR.3 A possible causal factor of fibrosis in PDR is connective tissue growth factor (CTGF). CTGF, a member of the CCN family of matricellular proteins, is also known as CCN2. It is a cytokine with profibrotic activity in various other organs and diseases, and is associated with fibrosis in vitreoretinal disorders, including diabetic retinopathy.2 4 VEGF and CTGF have been shown to interact. VEGF can induce CTGF expression,5–7 and CTGF can inhibit VEGF-induced angiogenesis by CTGF–VEGF protein binding.8 9
We recently investigated the correlation of vitreous CTGF levels with angiogenesis, fibrosis and VEGF levels in PDR, and demonstrated that the ratio between CTGF and VEGF levels is a strong predictor of the angiofibrotic switch in PDR.2 This would imply that increasing CTGF levels and/or decreasing VEGF levels shift the balance between CTGF and VEGF causing the angiofibrotic switch that drives fibrosis.
Intravitreal administration of anti-VEGF antibodies, most commonly bevacizumab and ranibizumab, has become increasingly important in the treatment of PDR. Bevacizumab (Avastin; Genentech, San Francisco, California, USA) is a full-length recombinant humanised anti-VEGF monoclonal antibody, approved by the US Food and Drug Administration for the treatment of colorectal cancer.10 In the eye, bevacizumab is used off-label for neovascular age-related macular degeneration11 and for diabetic macular oedema.12 13 Furthermore, intravitreal injections of bevacizumab have been used as an adjunct to vitreoretinal surgery in PDR. It facilitates surgery by reducing iris and retinal neovascularisation,14 thereby limiting intraoperative bleeding from new vessels.15 Bevacizumab also reduces early postoperative vitreous cavity haemorrhage.16 17 In cases of tractional retinal detachment (TRD), bevacizumab minimises bleeding during the peeling of fibrovascular membranes.18
However, bevacizumab may increase the risk of fibrotic complications. Progression or development of TRD shortly after bevacizumab has been reported.19 Also, a fibrotic switch has been observed in diabetic fibrovascular proliferative membranes after bevacizumab.20
Our model suggests a critical balance between VEGF and CTGF as a determinant of the disease course in PDR, in particular the angiofibrotic switch. It predicts that a reduction in VEGF levels, by causing a shift in the CTGF/VEGF balance in favour of CTGF, leads to accelerated fibrosis. In the present study, we aimed to confirm our previously observed correlation of the CTGF/VEGF ratio with fibrosis in an independent cohort of patients with PDR. In addition, we investigated whether anti-VEGF treatment leads to increased fibrosis in PDR patients, as predicted by our model that the balance of CTGF and VEGF in the vitreous determines the clinical course of PDR.
Materials and methods
Patients
Vitreous samples were collected from 52 consecutive patients with PDR undergoing vitrectomy. The main exclusion criteria were significant ocular co-morbidity, previous vitrectomy, and ocular surgery within 3 months of vitrectomy. Twenty-eight patients with PDR had not been treated with anti-VEGF at any time (PDR group). Seventeen PDR patients had received a single intravitreal injection of 1.25 mg bevacizumab as adjunct within 1 week before vitrectomy (bevacizumab 1 group). A group of seven patients had received bevacizumab and were operated at least 4 weeks later (bevacizumab 2 group). All but six patients (three PDR and three bevacizumab 1) had previous panretinal photocoagulation laser treatment. The study was conducted according to the tenets of the Declaration of Helsinki and informed consent was obtained from all patients. The Institutional Review Board of the Academic Medical Center at the University of Amsterdam approved the study.
Preoperative ophthalmic and ultrasound examinations, patient files and peri-perative observations allowed grading of fibrosis, determination of the presence of haemorrhage and type of diabetes with a standardised form. Fibrosis was graded 0 when there was no fibrosis, 1 when preretinal membranes were found as in epiretinal membrane/macular pucker, 2 when white preretinal fibrotic membranes were found with limited extension into the vitreous, and 3 when abundant white membranes were found reaching into the vitreous body.
Sample collection and ELISA
Undiluted vitreous samples (0.5–1 ml) were obtained by using a vitrectome at the start of a three-port pars plana vitrectomy with the infusion line in position but not opened. The vitreous was transferred to sterile Eppendorf tubes and immediately frozen in dry ice in duplicate. The samples were kept at −80°C until assayed.
After thawing, vitreous samples were centrifuged at 20 000g for 15 min at 4°C, and supernatant was collected. Concentrations of VEGF165 were determined by the Quantikine ELISA assay according to the manufacturer's protocol (R&D Systems, Minneapolis, Minnesota, USA). Concentrations of CTGF were determined by sandwich ELISA, using two distinct monoclonal antibodies specifically recognising the N-terminal part of the CTGF protein (FibroGen, San Francisco, California, USA), as described previously.21 Purified recombinant human CTGF (FibroGen) was used as standard.
Statistical analysis
The growth factor levels in vitreous were tested for normal distribution using histograms and the Shapiro–Wilk test. VEGF levels showed a left skewed distribution, and were log10 transformed when appropriate. Differences in the degree of fibrosis were assessed using the χ2 test. Differences in growth factor levels were assessed by the non-parametric Mann–Whitney U test. Correlations were expressed as Spearman's correlation coefficient; a ρ value of 0.5 or higher was considered relevant. Univariate and multiple ordinal logistic regression analyses were performed with the degree of fibrosis as dependent variable, and outcomes were expressed as OR with a 95% CI. A two-tailed p value less than 0.05 was considered to indicate statistical differences. All analyses were carried out using PASW Statistics (V.18) software (SPSS, Chicago, Illinois, USA).
Results
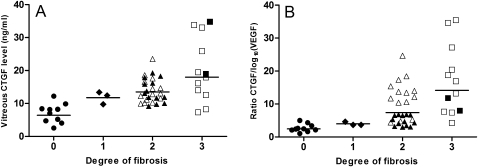
CTGF levels correlated with the degree of fibrosis (figure 1), with a Spearman's ρ value of 0.6 (p<0.001). VEGF levels correlated negatively with fibrosis (ρ −0.5; p=0.001). The ratio of CTGF/log10(VEGF) levels (CTGF/VEGF ratio) had an even stronger correlation with the degree of fibrosis (ρ 0.7; p<0.001; figure 1). VEGF and CTGF levels did not correlate (p>0.05). Also, CTGF and VEGF levels did not correlate with age, gender, diabetes type or vitreous haemorrhage (all p>0.05). Patient characteristics and vitreous CTGF and VEGF levels are presented in table 1.
Figure 1.
Vitreous connective tissue growth factor (CTGF) levels (A) and CTGF/log10 (vascular endothelial growth factor; VEGF) ratio (B) per degree of fibrosis of the retina. Filled symbols: proliferative diabetic retinopathy group. Open symbols: bevacizumab group; means of each group are also presented. (A) Vitreous CTGF levels and degree of fibrosis correlated significantly (Spearman's ρ 0.6, p<0.001). (B) The CTGF/log10(VEGF) ratio and degree of fibrosis correlated significantly (Spearman's ρ 0.7, p<0.001).
Table 1.
Patient characteristics and data
| Patient characteristics (n=52) | Subcategory | ||
| Anti-VEGF treatment | None | PDR group | n=28 |
| <1 week before surgery | Bevacizumab 1 group | n=17 | |
| >4 weeks before surgery | Bevacizumab 2 group | n=7 | |
| Age (mean±SD) | 53.2±14.2 years | ||
| Gender | Male | n=32 | |
| Female | n=20 | ||
| Diabetes type | Type I | n=19 | |
| Type II | n=33 | ||
| Degree of fibrosis | No fibrosis (0) | n=10 | |
| Only few preretinal membranes (1) | n=3 | ||
| Some proliferative membranes (2) | n=27 | ||
| Abundant proliferative membranes (3) | n=12 | ||
| Haemorrhage | No haemorrhage (0) | n=8 | |
| Haemorrhage (1) | n=44 | ||
| CTGF (geometric mean, 95% CI) | 12.4 ng/ml (10.8–14.2) | ||
| VEGF (geometric mean, 95% CI) | 121.9 pg/ml (66.4–223.9) | ||
| Ratio CTGF/log10(VEGF) (geometric mean, 95% CI) | 6.7 (5.3–8.4) | ||
CTGF, connective tissue growth factor; PDR, proliferative diabetic retinopathy; VEGF, vascular endothelial growth factor.
Univariate ordinal regression analysis to determine the strongest predictor of fibrosis of the retina showed that both CTGF levels and VEGF levels were significantly associated with the degree of fibrosis: CTGF associated positively and VEGF associated negatively with fibrosis (table 2). Furthermore, a significant association with fibrosis was found for the CTGF/VEGF ratio. In a multivariate model with both CTGF and VEGF levels as predictors of fibrosis, similar associations with the degree of fibrosis were found (table 2).
Table 2.
Predictors of fibrosis of the retina in 52 diabetes patients
| Variable | Contrast | Degree of fibrosis | |
| OR (95% CI) | p Value | ||
| Univariate ordinal regression | |||
| CTGF | Per unit increase | 1.38 (1.19 to 1.61) | <0.001 |
| VEGF | Per 10-fold increase | 0.35 (0.19 to 0.67) | 0.001 |
| Ratio CTGF/log10(VEGF) | Per unit increase | 1.27 (1.12 to 1.43) | <0.001 |
| Multiple ordinal regression* | |||
| CTGF | Per unit increase | 1.37 (1.16 to 1.60) | <0.001 |
| VEGF | Per 10-fold increase | 0.37 (0.18 to 0.78) | 0.009 |
CTGF and VEGF in model.
CTGF, connective tissue growth factor; VEGF, vascular endothelial growth factor.
Subanalysis of PDR patients and bevacizumab patients showed that the latter had a significantly higher degree of fibrosis (p<0.001, χ2 test) and higher CTGF levels (p=0.021, Mann–Whitney U test) compared with the other PDR patients.
We further reviewed the files of the seven PDR patients who received bevacizumab and were operated at least 4 weeks later (the bevacizumab 2 group). The median time interval between bevacizumab injection and vitrectomy was 11 weeks (range 4–26 weeks). The degree of fibrosis had remained stable after bevacizumab in two patients, who were already planned for surgery because of retinal traction before bevacizumab. However, the degree of fibrosis increased in five patients. In these patients, the development or increase of fibrosis after bevacizumab was the indication to perform vitrectomy. Overall, the degree of fibrosis increased significantly after bevacizumab (p=0.038, Wilcoxon signed rank test).
Follow-up
Patients had a mean follow-up of 20.4 months (±13.0) after vitrectomy. Two of 17 patients in the bevacizumab 1 group developed proliferative vitreoretinopathy and had a TRD at 6 weeks or 4 months after vitrectomy and delamination, respectively. Both patients had grade 3 fibrosis at the time of operation, and significantly higher vitreous CTGF levels compared with all other bevacizumab 1 patients (p=0.027) and the highest CTGF/VEGF ratios of 39.1 and 40, respectively (compare with figure 1). Interestingly, another patient in the PDR group with a CTGF level comparable to these two bevacizumab 1 patients and grade 3 fibrosis but with a low CTGF/VEGF ratio of 3.3 did not develop proliferative vitreoretinopathy or retinal detachment.
Discussion
In the present study, we have confirmed our previous findings2 in an independent group of 52 patients with diabetes that CTGF (CCN2) levels are strongly correlated with the degree of vitreoretinal fibrosis in PDR. In addition, we confirmed that the CTGF/VEGF ratio is the strongest predictor of fibrosis in PDR patients, including those treated with intravitreal anti-VEGF antibodies.
Retrospectively, five out of seven patients who received bevacizumab and were operated at least 4 weeks later (the bevacizumab 2 group), showed an increased degree of fibrosis after bevacizumab, ie, developed retinal fibrosis or progressed to TRD and therefore needed vitreoretinal surgery. In the 17 patients in the bevacizumab 1 group who were operated within 1 week after injection, progression of fibrosis was not observed, which may be due to the short time interval between bevacizumab injection and vitrectomy. This suggests that the chance that fibrosis develops or progresses increases with time, possibly due to prolonged high CTGF/VEGF ratios, which only gradually decline after anti-VEGF treatment.
The post-bevacizumab fibrotic phenomenon has been observed previously in PDR as well as in age-related macular degeneration.20 22–25 In a series of 211 patients with PDR, Arevalo et al19 found progression or development of TRD after bevacizumab injection in 11 (5.2%) patients. In this prospective study, bevacizumab was administered as an adjunct for vitrectomy and TRD was noted after a mean of 13 days after bevacizumab. Patients who did not develop TRD had their vitrectomies earlier. The authors could not discern whether TRD happened by natural history or by rapid neovascular involution with accelerated fibrosis as a response to decreased VEGF levels. Based on our present findings, it can be stated that decreased VEGF levels combined with elevated CTGF levels may well have accelerated the fibrotic response in these patients.
Is it possible that high CTGF levels combined with low VEGF levels after anti-VEGF treatment also increase the risk of fibrotic complications after vitrectomy? During surgery, fibrovascular membranes are peeled and the vitreous, and thereby the pool of growth factors, is removed. However, in the time interval between bevacizumab and vitrectomy VEGF but not CTGF is inactivated, and thus CTGF may well induce fibrotic responses in the retina or the vitreoretinal surface. As an example, two patients in the bevacizumab 1 group developed TRD after vitrectomy. These cases had the two highest CTGF levels and CTGF/VEGF ratios that we encountered. In comparison, a PDR patient with comparable CTGF levels and degree of fibrosis, but without anti-VEGF treatment and therefore a lower CTGF/VEGF ratio, did not develop fibrotic complications postoperatively. This suggests that a high CTGF level in combination with low levels of VEGF after bevacizumab, even for a short period of time, is a risk factor for the development of (late) postoperative fibrotic complications.
Anti-VEGF therapy is directed at the angiogenic stimulus for retinal neovascularisation and its introduction has made a significant advance in the management of PDR. However, retinal fibrosis can develop or progress as a consequence. Therefore, the development of adjuvant therapy targeted against pathways other than the angiogenic pathway may be required in combination with anti-VEGF therapy. Our present study and other studies in various organs and conditions4 26 have identified CTGF and in particular the CTGF/VEGF ratio in vitreous as markers of fibroproliferative disease. Targeting of the fibrotic pathway with anti-CTGF therapy may be an attractive option in combination with anti-VEGF treatment to prevent the angiofibrotic switch in PDR patients and other diseases with ocular angiogenesis.
In conclusion, we confirmed that CTGF levels and in particular the CTGF/VEGF ratio in vitreous are associated with vitreoretinal fibrosis in PDR. These findings support our working model that the balance of VEGF and CTGF in the vitreous in PDR drives the course of the disease, in particular the angiofibrotic switch. As predicted by this model, anti-VEGF therapy shifts the CTGF/VEGF ratio causing the angiofibrotic switch, which may lead to harmful accelerated fibrosis in PDR.
Acknowledgments
The authors gratefully acknowledge the careful preparation of the manuscript by Monique Arendse.
Footnotes
Funding: This study was financially supported by grant 2005.00.042 from the Diabetes Fonds Nederland.
Competing interests: Until 1 September 2009, RG received research grants and salary from FibroGen Inc., San Francisco, California, USA. The other authors have nothing to declare.
Patient consent: Obtained.
Ethics approval: Ethics approval was obtained from the Institutional Review Board of the Academic Medical Center.
Contributors: RJVG carried out the experiments and wrote the first version of the manuscript, SYLO, HST and MM provided the clinical samples, RG provided the CTGF measurement technology, IK was responsible for the day-to-day laboratory supervision of the study and edited the manuscript, CJFVVN was responsible for the logistic laboratory supervision and editing of the manuscript, and ROS designed the study and headed the overall clinical supervision.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data available on request from the corresponding author Prof. dr. Reinier O. Schlingemann: r.o.schlingemann@amc.uva.nl.
References
- 1.Fong DS, Aiello LP, Ferris FL, 3rd, et al. Diabetic retinopathy. Diabetes Care 2004;27:2540–53 [DOI] [PubMed] [Google Scholar]
- 2.Kuiper EJ, van Nieuwenhoven FA, de Smet MD, et al. The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PLoS One 2008;3:e2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witmer AN, Vrensen GF, Van Noorden CJ, et al. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res 2003;22:1–29 [DOI] [PubMed] [Google Scholar]
- 4.Leask A, Parapuram SK, Shi-Wen X, et al. Connective tissue growth factor (CTGF, CCN2) gene regulation: a potent clinical bio-marker of fibroproliferative disease? J Cell Commun Signal 2009;3:89–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He S, Jin ML, Worpel V, et al. A role for connective tissue growth factor in the pathogenesis of choroidal neovascularization. Arch Ophthalmol 2003;121:1283–8 [DOI] [PubMed] [Google Scholar]
- 6.Kuiper EJ, Hughes JM, Van Geest RJ, et al. Effect of VEGF-A on expression of profibrotic growth factor and extracellular matrix genes in the retina. Invest Ophthalmol Vis Sci 2007;48:4267–76 [DOI] [PubMed] [Google Scholar]
- 7.Suzuma K, Naruse K, Suzuma I, et al. Vascular endothelial growth factor induces expression of connective tissue growth factor via KDR, Flt1, and phosphatidylinositol 3-kinase-akt-dependent pathways in retinal vascular cells. J Biol Chem 2000;275:40725–31 [DOI] [PubMed] [Google Scholar]
- 8.Inoki I, Shiomi T, Hashimoto G, et al. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J 2002;16:219–21 [DOI] [PubMed] [Google Scholar]
- 9.Jang HS, Kim HJ, Kim JM, et al. A novel ex vivo angiogenesis assay based on electroporation-mediated delivery of naked plasmid DNA to skeletal muscle. Mol Ther 2004;9:464–74 [DOI] [PubMed] [Google Scholar]
- 10.Marshall J. The role of bevacizumab as first-line therapy for colon cancer. Semin Oncol 2005;32:S43–7 [DOI] [PubMed] [Google Scholar]
- 11.Martin DF, Maguire MG, Ying GS, et al. ; CATT Research Group Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364:1897–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology 2010;117:1078–86 [DOI] [PubMed] [Google Scholar]
- 13.Soheilian M, Ramezani A, Obudi A, et al. Randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus macular photocoagulation in diabetic macular edema. Ophthalmology 2009;116:1142–50 [DOI] [PubMed] [Google Scholar]
- 14.Avery RL, Pearlman J, Peramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology 2006;113:1695–715 [DOI] [PubMed] [Google Scholar]
- 15.di Lauro R, De Ruggiero P, di Lauro R, et al. Intravitreal bevacizumab for surgical treatment of severe proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2010;248:785–91 [DOI] [PubMed] [Google Scholar]
- 16.Ahmadieh H, Shoeibi N, Entezari M, et al. Intravitreal bevacizumab for prevention of early postvitrectomy hemorrhage in diabetic patients: a randomized clinical trial. Ophthalmology 2009;116:1943–8 [DOI] [PubMed] [Google Scholar]
- 17.Smith JM, Steel DH. Anti-vascular endothelial growth factor for prevention of postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy. Cochrane Database Syst Rev 2011;5:CD008214. [DOI] [PubMed] [Google Scholar]
- 18.Chen E, Park CH. Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina 2006;26:699–700 [DOI] [PubMed] [Google Scholar]
- 19.Arevalo JF, Maia M, Flynn HW, Jr, et al. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol 2008;92:213–16 [DOI] [PubMed] [Google Scholar]
- 20.El-Sabagh HA, Abdelghaffar W, Labib AM, et al. Preoperative intravitreal bevacizumab use as an adjuvant to diabetic vitrectomy: histopathologic findings and clinical implications. Ophthalmology 2011;118:636–41 [DOI] [PubMed] [Google Scholar]
- 21.Kuiper EJ, de Smet MD, van Meurs JC, et al. Association of connective tissue growth factor with fibrosis in vitreoretinal disorders in the human eye. Arch Ophthalmol 2006;124:1457–62 [DOI] [PubMed] [Google Scholar]
- 22.Batman C, Ozdamar Y. The relation between bevacizumab injection and the formation of subretinal fibrosis in diabetic patients with panretinal photocoagulation. Ophthalmic Surg Lasers Imaging 2010;41:190–5 [DOI] [PubMed] [Google Scholar]
- 23.Hwang JC, Del Priore LV, Freund KB, et al. Development of subretinal fibrosis after anti-VEGF treatment in neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging 2011;42:6–11 [DOI] [PubMed] [Google Scholar]
- 24.Wu L, Arvalo JF, Roca JA, et al. Comparison of two doses of intravitreal bevacizumab (Avastin) for treatment of macular edema secondary to branch retinal vein occlusion: results from the Pan-American Collaborative Retina Study Group at 6 months of follow-up. Retina 2008;28:212–19 [DOI] [PubMed] [Google Scholar]
- 25.Wu L, Martinez-Castellanos MA, Quiroz-Mercado H, et al. Twelve-month safety of intravitreal injections of bevacizumab (Avastin): results of the Pan-American Collaborative retina study group (PACORES). Graefes Arch Clin Exp Ophthalmol 2008;246:81–7 [DOI] [PubMed] [Google Scholar]
- 26.Leask A. Trial by CCN2: a standardized test for fibroproliferative disease? J Cell Commun Signal 2009;3:87–8 [DOI] [PMC free article] [PubMed] [Google Scholar]