Abstract
We have developed a microfluidic device that mimics the delivery and systemic clearance of drugs to heterogeneous three-dimensional tumor tissues in vitro. Nutrients delivered by vasculature fail to reach all parts of tumors, giving rise to heterogeneous microenvironments consisting of viable, quiescent and necrotic cell types. Many cancer drugs fail to effectively penetrate and treat all types of cells because of this heterogeneity. Monolayers of cancer cells do not mimic this heterogeneity, making it difficult to test cancer drugs with a suitable in vitro model. Our microfluidic devices were fabricated out of PDMS using soft lithography. Multicellular tumor spheroids, formed by the hanging drop method, were inserted and constrained into rectangular chambers on the device and maintained with continuous medium perfusion on one side. The rectangular shape of chambers on the device created linear gradients within tissue. Fluorescent stains were used to quantify the variability in apoptosis within tissue. Tumors on the device were treated with the fluorescent chemotherapeutic drug doxorubicin, time-lapse microscopy was used to monitor its diffusion into tissue, and the effective diffusion coefficient was estimated. The hanging drop method allowed quick formation of uniform spheroids from several cancer cell lines. The device enabled growth of spheroids for up to 3 days. Cells in proximity of flowing medium were minimally apoptotic and those far from the channel were more apoptotic, thereby accurately mimicking regions in tumors adjacent to blood vessels. The estimated value of the doxorubicin diffusion coefficient agreed with a previously reported value in human breast cancer. Because the penetration and retention of drugs in solid tumors affects their efficacy, we believe that this device is an important tool in understanding the behavior of drugs, and developing new cancer therapeutics.
Protocol
1. Device Fabrication
Replication of microfluidic features in elastomeric materials was based on the method described by Duffy et al.1
Mix elastomer (polydimethylsiloxane; PDMS) and curing agent from the Silicone Elastomer Kit (Dow Corning, Midland, MI) in a 9:1 weight ratio and pour over the master to form a 4 mm thick layer. Degas to remove air bubbles and cure the mixture at 60°C for 5 hours. Peel cured PDMS from the mold to obtain a stamp of flow features on the elastomer.
Punch holes for inlets and outlets using a 1.5 mm biopsy punch (Miltex, York, PA) mounted on a drill press. Pull out any debris.
Subject the features side of the stamp and a clean glass slide to oxygen plasma for 8 minutes in an oxygen plasma etcher. Bring the treated surfaces in contact immediately to form a bond between them. Maintain the assembly at 60°C on a slide warmer for at least 5 hours to strengthen the bond.
Connect 0.032" ID PTFE tubing (Cole Parmer, Vernon Hills, IL) to the inlets and outlets using an interface of male luer lock connectors attached to barbed female luer lock connectors (Qosina, Edgewood, NY).
Set up the flow assembly using shut off valves and a Y-connector (Upchurch Scientific, Oak Harbor, WA; Figure 1). Mount the device on to the microscope. Attach syringes to tubing using 20G 1.5" needles (BD Bioscience, Rockville, MD).
2. Formation of Uniform Spheroids
Spheroids were formed by the hanging drop method2,3.
Trypsinize cells under a sterile cell culture hood.
Centrifuge trypsinized cells at 2000 rpm for 5 minutes and resuspend in 6 ml fresh medium. Dilute the stock solution to a desired final concentration (Table 1).
Remove the cover of a 48 well plate and place it upside down in the sterilized hood. Circular regions corresponding to each well are apparent on the cover. Fill each well of the plate with 1 ml of sterilized water to maintain humidity. Put a 20 μl droplet of the dilute cell solution in each circular region using a micropipette. Carefully invert the cover and place it over the well plate, ensuring that the drops do not touch the edges of the wells.
Incubate the well plate at 37°C for a specified number of days (Table 1). A spheroid will form in each hanging drop.
3. Introduction of Spheroids into Device
Refer to Figure 1 and Table 2 for this section.
Sterilize the device by flushing with 70% ethanol introduced through the flow inlet. Subsequently, flush with PBS followed by HEPES buffered cell culture medium. Leave the medium syringe SF attached to the tubing.
Draw 2-3 spheroids into syringe SP, tap syringe to remove air bubbles, and attach to the packing inlet of the device.
Open inlet valve VPin and close VFin. Open outlet valve VPout and close VFout. Hold the packing syringe vertical with the needle pointing downward; watch as spheroids settle to the bottom of the syringe into the luer lock connector of the needle. Push the plunger on the packing syringe and watch spheroids enter the tubing and flow into the device. Because only the packing outlet valve is open, a spheroid will enter the chamber of the device and be retained by posts at the back (Fig. 2).
Close inlet valve VPin and outlet valve VPout. Mount the syringe SF on a syringe pump. Open valve VFin and valve VFout. Flow medium into the device at 3 μl/min.
4. Introduction of Apoptosis Detecting Agent (CaspGLOW)
After packing chambers with spheroids, allow equilibration for up to 24 hours to establish nutrient gradients and microenvironments, before introducing apoptosis detecting or therapeutic agents. Because the tissues in chambers are in contact with impenetrable walls from top and bottom, there are no nutrient gradients along the thickness (0.15mm) of the tissue. After 24 hours of incubation, all cell layers along the thickness of the tissue are therefore equivalent and the only heterogeneity is in a direction away from the flow channel.
Shut off VFin, stop the syringe pump, remove syringe SF from the pump and replace it with a syringe having medium containing 0.25 μl/ml of CaspGLOW Red Active Caspase-3 marker (Red-DEVD-FMK; Biovision, Mountain View, CA).
Manually flush 0.7 ml of this solution through the device to ensure displacement of older medium. Mount the syringe on the pump, restart flow, and open VFin. Over a period of 5-8 hours, the apoptosis detecting agent diffuses into the tissue and fluoresces brighter in apoptotic regions. Apoptosis detecting agent at this concentration is maintained in all subsequent flow solutions into the device.
Note:
Tissue heterogeneity can be confirmed by obtaining linear intensity profiles of fluorescence as described in Section 6. The profiles should show spatial gradients in apoptosis, confirming that the tissue is heterogeneous with respect to cell viability.
CaspGLOW Green Active Caspase-3 marker (Fluorescein-DEVD-FMK; Biovision) can also be used to detect apoptosis. This marker contains the green fluorophore fluorescein instead of the red fluorophore rhodamine.
5. Introduction of Therapeutic Agent
Follow the procedure in steps 4.1-4.2 to introduce 10 μM doxorubicin hydrochloride (Dox; Sigma-Aldrich, St. Louis, MO) containing apoptosis detecting agent.
Continue flow of the therapeutic agent solution for a fixed period of time. Shut off valve VFin and turn off the syringe pump. Replace the treatment syringe with a fresh medium syringe containing apoptosis detecting agent. Allow flow of fresh medium for 24 –36 hours while continuously monitoring the tissue under a microscope.
6. Time-lapse Microscopy and Estimation of Drug Diffusivity Coefficients
For obtaining cleanest fluorescence data, acquire all images by focusing the microscope on the bottom layers of tissues. Because there are no nutrient gradients in the vertical direction (see Section 4), the bottom layers of cells are good representatives of all other layers above.
The entire acquisition process was automated using a customized script in IPLab (BD Bioscience, Rockville, MD). Acquire transmitted light and fluorescence images of the packed spheroid at 10x magnification every 30 minutes (Fig. 3A). Acquire an image of the background fluorescence prior to introduction of Dox. To accommodate for the large size of the chamber (1000 μm x 300 μm), acquire two adjoining images and tile them together4.
For mathematical estimation of drug diffusivity coefficients, the first step is to generate averaged linear intensity profiles of Dox fluorescence using ImageJ. Select a rectangular region of interest (ROI) encompassing the tissue in the chamber. Use the Plot Profile command to generate a profile of average intensities as a function of distance from the flow channel. Repeat for up to 3 different time points.
Obtain a background fluorescence image before introducing Dox to measure autofluorescence from the tissue. Subtract the average background fluorescence intensity from the obtained intensity profiles and normalize each profile by the corresponding maximum intensity to obtain
 (x,t)(Fig. 3B).
(x,t)(Fig. 3B).- The next step is to evaluate the effective diffusion coefficient D of Dox within tumor tissue. Dox diffusion can be represented by the following equation5
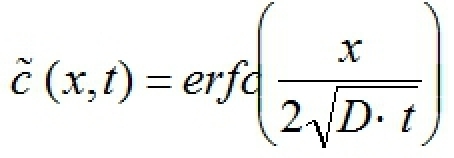 where erfc is the complementary error function, x is distance into the tissue from the channel, and t is time after introduction of Dox (refer to Fig. 3). Use the following iterative scheme at each time point considered:
where erfc is the complementary error function, x is distance into the tissue from the channel, and t is time after introduction of Dox (refer to Fig. 3). Use the following iterative scheme at each time point considered:
- Guess a value for D
- Calculate right side of the equation at each location x
- Calculate the sum of squared errors (residual) between the two sides
- Modify D to minimize the residual
Average the optimum values of D obtained at each time point to estimate the average effective diffusion coefficient of Dox in the tissue.
7. Representative Results:
The microfluidic devices provided 1mm x 0.3mm x 0.15mm optically accessible culture chambers for growth of three-dimensional tumor tissue. Multicellular tumor spheroids were flown into these chambers and were retained by two filter posts at the back. The hanging drop method allowed quick formation of spheroids of consistent size and shape from several cell lines. Spheroids were successfully grown on the device for up to 3 days. Growth in the chambers was associated with a reproducible modification of microenvironments within spheroids. Apoptosis occurred less in cells in proximity of the flow channel and higher deeper into the tissue. The device was used to estimate the diffusion coefficient of doxorubicin in tumor tissue. The obtained value of 8.75 x 10-7 cm2s-1 agrees with the value of 9.1 x 10-7 cm2s-1 reported previously6 in human breast cancer.
| Cell Line | Required Cell Concentration | Incubation Time |
| LS174T | 300 cells/μL | 2-3 days |
| T47D | 750 cells/μL | 3-4 days |
| MDA-MB-231 | 150 cells/μL | 5-6 days |
Table 1. Parameters for Hanging Drop Spheroids
| Part | Description |
| SF | Flow Syringe |
| SP | Packing Syringe |
| VFin | Flow Inlet Valve |
| VPin | Packing Inlet Valve |
| VFout | Flow Outlet Valve |
| VPout | Packing Outlet Valve |
Table 2. Syringes and Valves in Flow Setup
 Figure 1. Schematic of the flow setup VFin and VFout: Flow inlet and outlet
valves; VPin and VPout: Packing inlet and outlet valves; SF and SP: Flow and packing syringes. The
packing syringe and packing inlet and outlet valves are used when a spheroid is flown into the chamber. The flow syringe and flow inlet
and outlet valves are used to flow medium subsequently.
Figure 1. Schematic of the flow setup VFin and VFout: Flow inlet and outlet
valves; VPin and VPout: Packing inlet and outlet valves; SF and SP: Flow and packing syringes. The
packing syringe and packing inlet and outlet valves are used when a spheroid is flown into the chamber. The flow syringe and flow inlet
and outlet valves are used to flow medium subsequently.
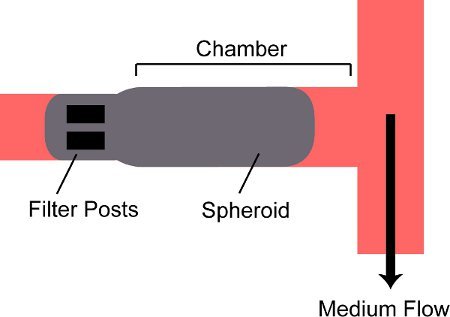 Figure 2. Schematic of a spheroid trapped in a chamber on the device. A spheroid is flowed into the chamber on the device and is blocked by two filter posts at the back of the chamber.
Figure 2. Schematic of a spheroid trapped in a chamber on the device. A spheroid is flowed into the chamber on the device and is blocked by two filter posts at the back of the chamber.
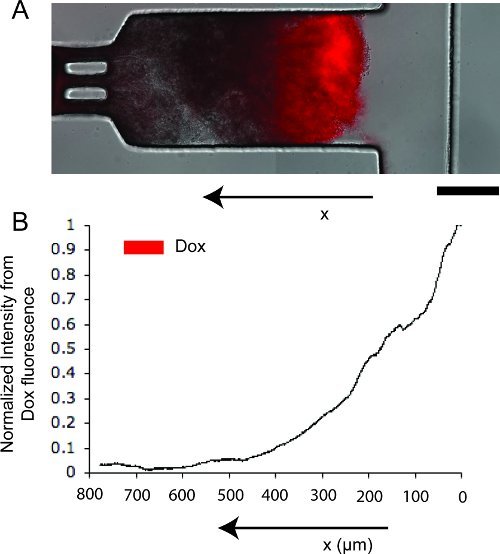 Figure 3. Doxorubicin diffusion in tumor tissue on the device. A. Merged transmitted light and red fluorescent image of tissue, showing the location and concentration of doxorubicin (in red). Scale bar represents 250 μm. B. Normalized linear intensity profile from doxorubicin fluorescence.
Figure 3. Doxorubicin diffusion in tumor tissue on the device. A. Merged transmitted light and red fluorescent image of tissue, showing the location and concentration of doxorubicin (in red). Scale bar represents 250 μm. B. Normalized linear intensity profile from doxorubicin fluorescence.
Discussion
The vasculature in tumors is sparse and ill developed7,8. There are regions located far (>100 μm) from the blood vessels that are inaccessible to nutrients and drugs supplied though the vasculature9. The resulting heterogeneous microenvironment contributes to the limited efficacy of many chemotherapeutics10. The microfluidic device developed here recreates a heterogeneous tumor microenvironment characterized by proliferating, quiescent and apoptotic or necrotic cells, in vitro. Cells in proximity of the channel are viable, but diffusion limitations give rise to nutritionally deficient apoptotic regions far from the channel. The flow channel represents a blood vessel and the spheroid represents a small region within a tumor adjacent to a blood vessel.
While introducing spheroids into the device, ensure that the punched inlet hole is clean and free of debris, because any obstruction to flow of tissue will result in failure to fill the chamber, or a broken spheroid. The packing syringe is operated manually during the process of introducing spheroids. The flow rate must be maintained as low as possible to avoid pushing the spheroid through the posts at the back of the chamber. As with all microfluidic devices, certain measures must be taken to avoid bubbles in the system, especially when switching syringes. Cutting a small piece of tubing each time a syringe is switched will help avoid bubbles.
Modifications to the device to allow use of multiple chambers and multiple treatments in a single experiment are ongoing. We believe that these devices can be used to understand the behavior of current cancer therapeutics in solid tumors. They can also be used as easy to construct drug-screening platforms to develop new cancer therapies.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by the National Institute of Health grant # 1R01CA120825-01A1, the Collaborative Biomedical Research (CBR) Program at the University of Massachusetts Amherst, and the Isenberg Scholarship for Bhushan J. Toley. We gratefully acknowledge the valuable contribution of James Schafer, the videographer, narrator, and editor of this video.
References
- Duffy DC, McDonald JC, Schueller OJ, Whitesides GM. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane) Anal. Chem. 1998;70:4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol. Bioeng. 2003;83:173–180. doi: 10.1002/bit.10655. [DOI] [PubMed] [Google Scholar]
- Timmins NE, Nielsen LK. Generation of multicellular tumor spheroids by the hanging-drop method. Methods. Mol. Med. 2007;140:141–151. doi: 10.1007/978-1-59745-443-8_8. [DOI] [PubMed] [Google Scholar]
- Kasinskas RW, Forbes NS. Salmonella typhimurium specifically chemotax and proliferate in heterogeneous tumor tissue in vitro. Biotechnol. Bioeng. 2006;94:710–721. doi: 10.1002/bit.20883. [DOI] [PubMed] [Google Scholar]
- Walsh CL. A multipurpose microfluidic device designed to mimic microenvironment gradients and develop targeted cancer therapeutics. Lab. Chip. 2009;9:545–554. doi: 10.1039/b810571e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankelma J, Fernandez Luque R, Dekker H, Schinkel W, Pinedo HM. A mathematical model of drug transport in human breast cancer. Microvasc. Res. 2000;59:149–161. doi: 10.1006/mvre.1999.2218. [DOI] [PubMed] [Google Scholar]
- Less JR, Skalak TC, Sevick EM, Jain RK. Microvascular architecture in a mammary carcinoma: branching patterns and vessel dimensions. Cancer. Res. 1991;51:265–273. [PubMed] [Google Scholar]
- Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer. Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Cancer. 1955;9:539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat. Rev. Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]


