Abstract
For someone on a food-restricted diet, food craving in response to food-paired cues may serve as a key behavioral transition point between abstinence and relapse to food taking 1. Food craving conceptualized in this way is akin to drug craving in response to drug-paired cues. A rich literature has been developed around understanding the behavioral and neurobiological determinants of drug craving; we and others have been focusing recently on translating techniques from basic addiction research to better understand addiction-like behaviors related to food 2-4.
As done in previous studies of drug craving, we examine sucrose craving behavior by utilizing a rat model of relapse. In this model, rats self-administer either drug or food in sessions over several days. In a session, lever responding delivers the reward along with a tone+light stimulus. Craving behavior is then operationally defined as responding in a subsequent session where the reward is not available. Rats will reliably respond for the tone+light stimulus, likely due to its acquired conditioned reinforcing properties 5. This behavior is sometimes referred to as sucrose seeking or cue reactivity. In the present discussion we will use the term "sucrose craving" to subsume both of these constructs.
In the past decade, we have focused on how the length of time following reward self-administration influences reward craving. Interestingly, rats increase responding for the reward-paired cue over the course of several weeks of a period of forced-abstinence. This "incubation of craving" is observed in rats that have self-administered either food or drugs of abuse 4,6. This time-dependent increase in craving we have identified in the animal model may have great potential relevance to human drug and food addiction behaviors. Here we present a protocol for assessing incubation of sucrose craving in rats. Variants of the procedure will be indicated where craving is assessed as responding for a discrete sucrose-paired cue following extinction of lever pressing within the sucrose self-administration context (Extinction without cues) or as responding for sucrose-paired cues in a general extinction context (Extinction with cues).
Keywords: Neuroscience, Issue 57, addiction, craving, cue-reactivity, extinction, reinstatement, relapse, sucrose seeking
Protocol
1. Animal Subjects
Male Long-Evans rats are 3 months old at the start of a study. Rats are Simonsen-derived (Gilroy, California, USA) bred in the Western Washington University vivarium and are housed individually on a 12-h reverse day/night cycle (lights off at 0700) with Purina Mills Inc. Mazuri Rodent Pellets (Gray Summit, MO, USA) and water available ad libitum.
All training and testing takes place between 0900-1300 with cohorts of rats always trained and tested at the same time daily.
Rats are weighed each Monday, Wednesday, and Friday for the duration of the study.
Immediately prior to the training phase, rats are deprived of water for approximately 20 h to encourage sucrose self-administration on the first day of training.
2. Behavioral Procedures
All operant behavioral procedures use standard operant conditioning chambers (Med Associates Inc., St. Albans, VT, USA) with an infusion pump (Razel Scientific Instruments, St. Albans, VT, USA) located on top of each operant conditioning chamber. Operant conditioning chambers are enclosed in sound-attenuating cabinets with ventilation fans (Western Washington University, USA). All experimental conditions and data collection are controlled by Med PC IV software (Med Associates Inc., St. Albans, VT, USA).
Operant conditioning chambers (30 x 20 x 24 cm) contain two levers (one stationary and one retractable), a tone generator, a white stimulus light above the retractable lever, and a red house light on the opposite wall. The infusion pump delivers sucrose into a reward receptacle to the right of the active lever. Levers are 10 cm from the grid floor. Four infrared photobeams crisscross each chamber. The front beams are each 10.5 cm from the side walls and the side beams each 6 cm from the side walls. Each beam is 4.5 cm above the stainless steel bar floor. The beams are set to count the number of complete breaks. The total number of beam breaks are recorded during Training and craving Testing.
With the exception of water restriction prior to the first Training session, rats are provided food and water ad libitum both in home cages and in the operant conditioning chambers. Chambers are in a dedicated test room that, as with the vivarium, is on a reverse light cycle. Rats are transported in home cages between the rooms on a cart that is covered with light blocking fabric. At the end of each session, rats are returned to home cages.
There are three phases to the typical experiment: Training, Forced Abstinence, and Testing. Testing is the session wherein craving is assessed. We assess craving in two general testing procedures, depending on the study. These two procedures are described in sections 2.3.1 and 2.3.2.
- Training
- In the typical experiment, rats spend 2 h/day for 10 consecutive days in operant conditioning chambers where they are allowed to press the retractable (active) lever for a 0.2 ml delivery of 10 % sucrose solution into the receptacle to the right of the lever.
- This response also activates a compound stimulus consisting of a tone (2 kHz, 15 dB over ambient noise) and the white light. The compound stimulus lasts for 5 s and is followed by a 40-s time out, during which presses on the active lever are recorded but have no programmed consequence.
- A response on the inactive (stationary) lever has no programmed consequence, but presses are recorded. The numbers of beam breaks are recorded during Training and Testing. All response and locomotor measures are recorded as time courses (2 minute bins), but are usually reported as totals per session.
Forced Abstinence. Rats remain in home cages for the duration of forced abstinence. The typical forced abstinence periods we use are to have rats tested the day immediately following the final day of Training (Day 1 of forced abstinence) or 30 days following the final day of Training (Day 30 of forced abstinence).
- Testing
- Extinction without cues. In this Testing procedure, rats are first allowed to press the active lever in conditions identical to Training with the exception that the response has no consequences and the session duration is 1 h. Rats experience at least six of these extinction sessions on the Test day. Each extinction session is separated by a 5 minute period where the active lever is retracted and the house light is turned off. "Extinction" is indicated if active lever responding has been reduced to less than 20 presses/h. Some rats require a seventh session. The next session (after another 5 minute delay) is the Testing session where rats can then press for the tone+light cue that was initially paired with each delivery of sucrose during Training.
- Extinction with cues. In this abbreviated Testing procedure, rats are allowed to press the active lever in conditions identical to Training except that sucrose is not delivered.
Inactive lever pressing is used as a measure of lever discrimination. It is also, along with photobeam breaks, used as a measure of non-specific motor activation. Motoric vs. motivational changes complicate the interpretation of craving behavior. The inactive lever responding and photobeam break data are useful in clarifying motor vs. motivation contributions to craving, as are comparison groups in pharmacological studies where rats responding at a high rate for sucrose or another food are challenged with a potential craving affecting compound (e.g. 7,8).
3. Representative Manipulations to Affect Sucrose Craving after Variable Periods of Forced Abstinence
The general method described here provides a way to probe the behavioral and neurobiological determinants of craving after variable periods of forced abstinence. Described here are methods related to Representative Results provided in section 4.
Satiation. Some rats received bottles of 10 % sucrose in their home cages for the 17 h immediately prior to Testing 8. Rats in this study were tested using the Extinction without cues procedure.
Environmental Enrichment. During the forced-abstinence period, some rats remained in individual housing (Controls) while others were moved into a large (36 " width x 20 " depth x 40 " height; Quality Cage Products, Portland, OR, USA) cage with three cohorts (Enriched). The Enriched animals had several toys in their environment including PVC tubing and were also given novel toys every M,W,F 10. Rats in this study were tested using the Extinction without cues procedure.
Pharmacological challenge. On the Testing day, rats were pretreated with a dose of the dopamine D1 antagonist SCH 23390. Rats in this study were tested using the Extinction with cues procedure.
4. Representative Results:
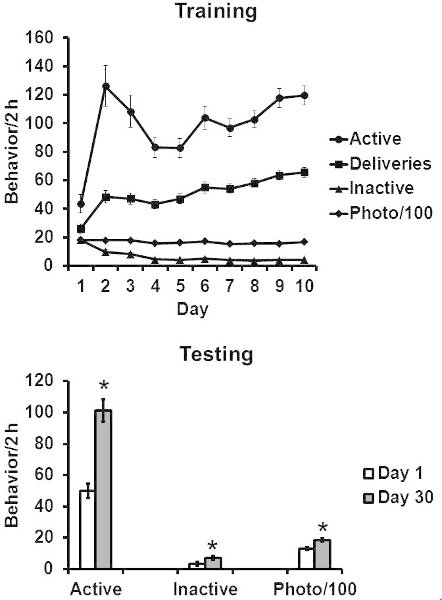 Figure 1 Representative Training and Testing data for an incubation of sucrose craving experiment.
Figure 1 Representative Training and Testing data for an incubation of sucrose craving experiment.
Data points indicate means ± SEMs. Group size for Figure 1a is 77 rats and for Figure 1b group sizes ranged from 11-12 per group. * significant difference from Day 1 group, p < 0.05. This figure is adapted from data in 8 with permission of Springer.
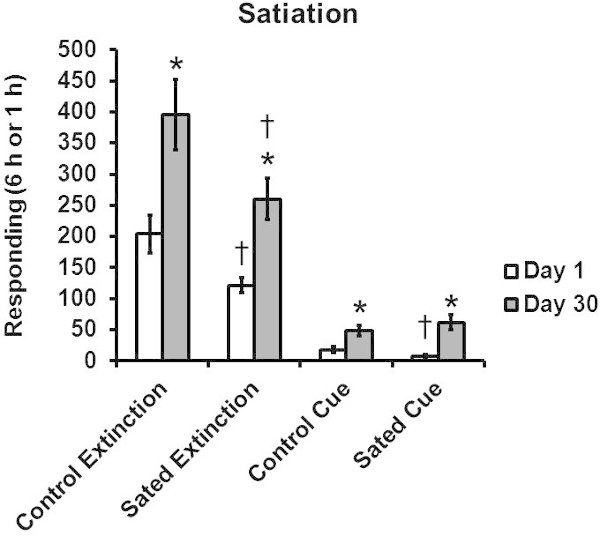 Figure 2 Effect of sucrose satiation on sucrose craving after 1 or 30 days of forced abstinence. Group sizes are 8-9 per group. * significant difference from Day 1 group, significant difference from Control group, p < 0.05. This figure is adapted from 9 with permission of Elsevier.
Figure 2 Effect of sucrose satiation on sucrose craving after 1 or 30 days of forced abstinence. Group sizes are 8-9 per group. * significant difference from Day 1 group, significant difference from Control group, p < 0.05. This figure is adapted from 9 with permission of Elsevier.
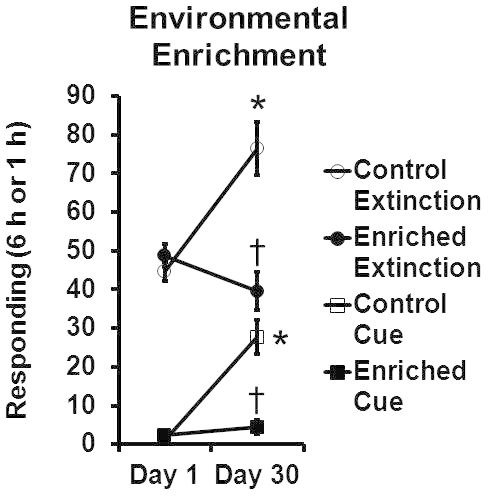 Figure 3 One month of environmental enrichment reduced sucrose craving. Group sizes are each 8 rats. * significant difference from Day 1, significant difference from Control group, p < 0.05. This figure is adapted from 10 with permission of Wolters Kluwer Health.
Figure 3 One month of environmental enrichment reduced sucrose craving. Group sizes are each 8 rats. * significant difference from Day 1, significant difference from Control group, p < 0.05. This figure is adapted from 10 with permission of Wolters Kluwer Health.
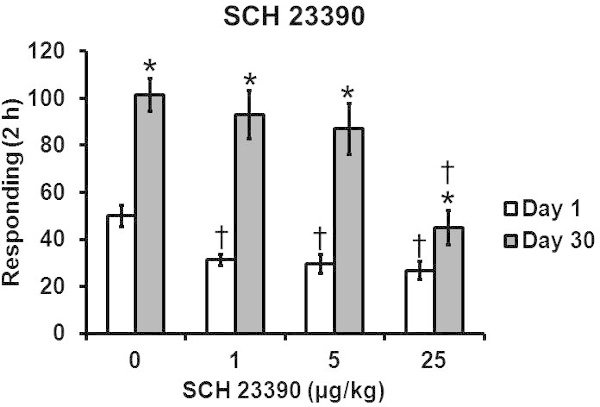 Figure 4 Systemic SCH 23390 decreased sucrose craving more effectively after one day of forced abstinence. Group sizes are 8-12 per group. * significant difference from Day 1 group, significant difference from 0 dose group, p < 0.05. This figure is adapted from 8 with permission of Springer.
Figure 4 Systemic SCH 23390 decreased sucrose craving more effectively after one day of forced abstinence. Group sizes are 8-12 per group. * significant difference from Day 1 group, significant difference from 0 dose group, p < 0.05. This figure is adapted from 8 with permission of Springer.
Figure 1 indicates representative Training and Testing data for rats self-administering sucrose (Figure 1 TOP) and then responding in Extinction with the sucrose-paired cue available (Figure 1 BOTTOM). As shown, rats respond selectively on the active lever during Training and during Testing. Interestingly, inactive responding and locomotor activity show some incubation, although the effect is most robust on active lever responding.
Figure 2 shows the effect of satiation with sucrose on sucrose craving after 1 or 30 days of forced abstinence. Satiation reduced Extinction without cues responding after either period of forced abstinence, but only reduced responding for the sucrose cue alone in rats that had only one day of forced abstinence.
As shown in Figure 3, environmental enrichment reduced incubated Extinction without cue responding and responding for the sucrose cue alone behaviors to levels found after only one day of forced abstinence.
Figure 4 depicts an abstinence-dependent effect of SCH 23390 on Extinction with cues responding. SCH 23390 was most effective at reducing sucrose craving after 1, vs. 30, days of forced abstinence.
Discussion
The procedures we describe here could be identified as reinstatement procedures, although this is technically inaccurate as lever pressing for the cue is not extinguished prior to the craving test session. In a typical reinstatement procedure, responding with reward-paired cues is extinguished and then "reinstated" with administration of a non-contingent presentation of the reward (priming-induced reinstatement) or a stressor such as footshock (stress-induced reinstatement) 11. The extinction of lever pressing alone followed by access to the cue (item 2.3.1) is the closest approximation to reinstatement. Nevertheless, the procedures we describe allow measurement of goal-directed responding for a sucrose-paired cue. Operationally, this behavior is the cue-enhanced "craving" behavior that may be relevant to relapse behaviors 4,12-14.
There are some practical advantages of the Extinction with cues procedure (item 2.3.2) over the Extinction without cues followed by access to the cue procedure (item 2.3.1). With the latter approach, some rats do not extinguish to criterion in six sessions and this requires an extra extinction session. In contrast, the 2 h Extinction with cues procedure provides a predictable time frame for assessment of craving and is therefore more amenable to designs where exposure to the self-administration context should be kept consistent across subjects and conditions. An example of such a study would be one where brain tissue is collected immediately at the end of a craving session for subsequent assessment of immediate-early gene expression.
A limitation of the Extinction with cues procedure is that contextual and discrete cues are presented together. Therefore interpretation of the contribution of contextual vs. discrete cues to craving behavior and brain molecular changes will be confounded. This could be especially important in the molecular studies as evidence exists for a dissociation between the neural substrates of context vs. discrete cue-mediated learning 15,16.
Finally, a general limitation of a forced abstinence procedure regarding repeated testing designs is that "abstinence" matters. Responding for the cue increases over time. Therefore, this model does not allow for meaningful repeated testing of subjects, unless the time between tests is taken into account.
In conclusion, the incubation of sucrose craving procedure provides a model of time-dependent increases in craving behaviors that may contribute to the intractable nature of addiction. Generally speaking, the model has reasonable predictive validity, in part due to having the well-validated reinstatement model at its core 4,17,18. Using the incubation of sucrose craving model to probe the behavioral and neurobiological determinants of incubation may yield insight into novel treatment approaches for addiction.
Disclosures
All procedures followed the guidelines outlined in the "Principles of Laboratory Animal Care" (NIH publication no. 86-23) and were approved by the Western Washington University Institutional Animal Care and Use Committee.
Acknowledgments
Production of this manuscript and video was supported by National Institute on Drug Abuse/National Institutes of Health grant R15 DA016285-02, the Western Washington University Biomedical Research Activities in Neuroscience Initiative, and Western Washington University.
References
- Sobik L, Hutchison K, Craighead L. Cue-elicited craving for food: a fresh approach to the study of binge eating. Appetite. 2005;44:253–2561. doi: 10.1016/j.appet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Geiger BM. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 2009;159:1193–1199. doi: 10.1016/j.neuroscience.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW. In: Craving In Animal Models of Drug Addiction. Olmstead C, editor. New York: Humana Press; 2011. pp. 311–336. [Google Scholar]
- Grimm JW, Kruzich PJ, See RE. Contingent access to stimuli associated with cocaine self-administration is required for reinstatement of drug-seeking behavior. Psychobiology. 2000;28:383–386. [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur. J. Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J. Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW. Effects of systemic or nucleus accumbens-directed dopamine D1 receptor antagonism on sucrose seeking in rats. Psychopharmacology (Berl) 2011. [DOI] [PMC free article] [PubMed]
- Grimm JW, Fyall AM, Osincup DP. Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiol Behav. 2005;84:73–79. doi: 10.1016/j.physbeh.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW. Environmental enrichment attenuates cue-induced reinstatement of sucrose seeking in rats. Behav Pharmacol. 2008;19:777–785. doi: 10.1097/FBP.0b013e32831c3b18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Childress AR. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt AN. Neural Correlates of Food Addiction. Arch. Gen. Psychiatry. 2011. [DOI] [PMC free article] [PubMed]
- Markou A. Animal models of drug craving. Psychopharmacology (Berl) 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Holland PC, Bouton ME. Hippocampus and context in classical conditioning. Curr. Opin. Neurobiol. 1999;9:195–202. doi: 10.1016/s0959-4388(99)80027-0. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt DA, Brown ZJ, Erb S. A Procedure for Studying the Footshock-Induced Reinstatement of Cocaine Seeking in Laboratory Rats. J. Vis. Exp. 2011. [DOI] [PMC free article] [PubMed]


