Abstract
Stem cell based therapies offer significant potential for the field of regenerative medicine. However, much remains to be understood regarding the in vivo kinetics of transplanted cells. A non-invasive method to repetitively monitor transplanted stem cells in vivo would allow investigators to directly monitor stem cell transplants and identify successful or unsuccessful engraftment outcomes.
A wide range of stem cells continues to be investigated for countless applications. This protocol focuses on 3 different stem cell populations: human embryonic kidney 293 (HEK293) cells, human mesenchymal stem cells (hMSC) and induced pluripotent stem (iPS) cells. HEK 293 cells are derived from human embryonic kidney cells grown in culture with sheared adenovirus 5 DNA. These cells are widely used in research because they are easily cultured, grow quickly and are easily transfected. hMSCs are found in adult marrow. These cells can be replicated as undifferentiated cells while maintaining multipotency or the potential to differentiate into a limited number of cell fates. hMSCs can differentiate to lineages of mesenchymal tissues, including osteoblasts, adipocytes, chondrocytes, tendon, muscle, and marrow stroma. iPS cells are genetically reprogrammed adult cells that have been modified to express genes and factors similar to defining properties of embryonic stem cells. These cells are pluripotent meaning they have the capacity to differentiate into all cell lineages 1. Both hMSCs and iPS cells have demonstrated tissue regenerative capacity in-vivo.
Magnetic resonance (MR) imaging together with the use of superparamagnetic iron oxide (SPIO) nanoparticle cell labels have proven effective for in vivo tracking of stem cells due to the near microscopic anatomical resolution, a longer blood half-life that permits longitudinal imaging and the high sensitivity for cell detection provided by MR imaging of SPIO nanoparticles 2-4. In addition, MR imaging with the use of SPIOs is clinically translatable. SPIOs are composed of an iron oxide core with a dextran, carboxydextran or starch surface coat that serves to contain the bioreactive iron core from plasma components. These agents create local magnetic field inhomogeneities that lead to a decreased signal on T2-weighted MR images 5. Unfortunately, SPIOs are no longer being manufactured. Second generation, ultrasmall SPIOs (USPIO), however, offer a viable alternative. Ferumoxytol (FerahemeTM) is one USPIO composed of a non-stoichiometric magnetite core surrounded by a polyglucose sorbitol carboxymethylether coat. The colloidal, particle size of ferumoxytol is 17-30 nm as determined by light scattering. The molecular weight is 750 kDa, and the relaxivity constant at 2T MRI field is 58.609 mM-1 sec-1 strength4. Ferumoxytol was recently FDA-approved as an iron supplement for treatment of iron deficiency in patients with renal failure 6. Our group has applied this agent in an “off label” use for cell labeling applications. Our technique demonstrates efficient labeling of stem cells with ferumoxytol that leads to significant MR signal effects of labeled cells on MR images. This technique may be applied for non-invasive monitoring of stem cell therapies in pre-clinical and clinical settings.
Protocol
1. Day 1
1) Plate cells
Plate hMSC in a T75 flask at a confluency of 80% at least 18-24 hours prior to labeling. Refer to Table 1 for instruction for alternate vessels.
2. Day 2
2) Prepare labeling solution. This preparation will label one (1) T75 flask at 80% confluency with a concentration of 400 μg Fe/ml. Refer to Table 1 for instruction for alternate vessels.
Create a solution (solution 1) by mixing 1 ml of serum-free media and 93.1 μl of ferumoxytol (stock: 30 mg/ml) in a 15 ml conical tube.
Create a second solution (solution 2) by mixing 1 ml of serum-free media and 7 μl of protamine sulfate (stock: 10 mg/ml) in a second 15 ml conical tube.
Allow each solution to rest for 5 minutes.
Add solutions 1 and 2 together. Gently mix the new labeling solution. Allow the solution to rest for 5 minutes to permit USPIO-protamine sulfate complexes to form.
Add 5 ml of serum-free media to the mixed 2 ml of SPIO/protamine sulfate solution for a final volume of 7 ml.
3) Prepare cells for labeling
Aspirate the media from cells.
Gently wash cells once with 2-3 ml of pre-warmed Mg/Ca-free D-PBS or serum-free media to rinse away residual serum proteins and or other media components that could impair contrast agent uptake and labeling efficiency. Aspirate out the rinse fluid.
4) Label cells
Add the complete 7 ml of labeling solution to cells.
Place cells into an incubator (37°C/ 5% CO2) and allow the cells to incubate in the labeling solution for 4 hours.
Add 700 μl of FCS to the labeling solution of the cells to achieve a final serum concentration of 10%. Serum is added to re-establish the enriched environment of which the cells are accustomed, and to assist in minimizing cell death that could result from an abrupt shift to 24 hour exposure to a serum-free environment.
Allow the cells to incubate with the labeling solution for an additional 20 hours.
3. Day 3
5) Prepare labeled cells
Aspirate the labeling solution from cells.
Gently rinse cells with 2-3 ml of pre-warmed Mg/Ca-free D-PBS. Aspirate out the PBS. It is important to use Mg/Ca-free PBS as the Mg and Ca will impair the efficiency of Trypsin in the next step.
Add 2 ml of pre-warmed 0.05% Trypsin to the cells and tilt the flask back and forth to ensure the entire surface of the flask is covered with a thin layer of Trypsin. Place the cells in an incubator (37°C/ 5% CO2) and allow the cells to rest for 5-7 minutes or until cells begin to detach from the plate. It may be necessary to confirm detachment under a microscope. Gently tap the sides of the flask if necessary to facilitate cell-surface detachment.
Add 4 ml of pre-warmed complete media to the flask to neutralize the Trypsin. Gently rinse the flask by pipetting the Trypsin/media/cell solution up and down several times. Collect the entire solution in a 15 ml conical tube. Centrifuge the cell solution at 400 RCF for 5 minutes.
Carefully aspirate the supernatant without disturbing the cell pellet. Resuspend the cells in 5 ml of media (serum containing or serum-free). Centrifuge the cell solution at 400 RCF for 5 minutes.
Repeat the cell wash described in step 5.5. In total, cells will be rinsed three times to remove residual, free contrast agent in media and on the surface of cells.
Count cells at this point to attain the most accurate cell count, as cells will be lost during previous washing. Perform a viability test of cells. Cells are now ready for subsequent analysis and or experimental application. MR imaging can be performed with T1-w and T2-w parameters, because although ferumoxytol is a T2-w contrast agent, ferumoxytol also exhibits T1 effects. Representative T2-w MRI parameters that can be used to image ferumoxytol labeled cells include: FSE or SE_Multislice sequences with a TR of 2000-2500 ms and a TE of 60-80 ms. To acquire a T1-w image, decrease the TE value on a FSE or SE_Multislice sequence or use a GRE sequence with a high TR and low TE. Figure 4 demonstrates a potential in vivo application for labeled stem cell imaging.
4. Representative Results:
Labeled cells demonstrate a significant darkening or negative contrast effect on T2-weighted MR images and a brightening or positive contrast effect on T1-weighted MR images (Figure 2). Longitudinal MR imaging and viability tests performed 5 days post labeling demonstrated no significant MR signal and no significant impact on viability compared to unlabeled controls (data not shown).
Labeled stem cells can subsequently be differentiated into various cell types or intravenously injected for in vivo investigation.
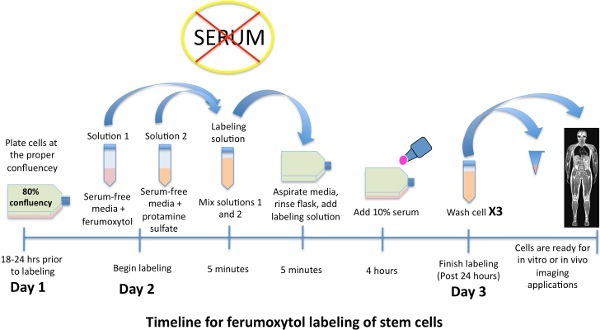 Figure 1. Schematic time line for labeling stem cells with iron oxide nanoparticle, ferumoxytol.
Figure 1. Schematic time line for labeling stem cells with iron oxide nanoparticle, ferumoxytol.
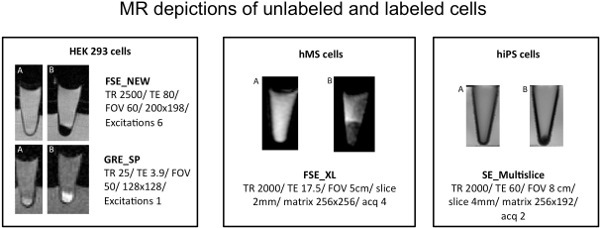 Figure 2. MR images of sagittal cross sections thrrough eppendorf tubes with cells in a cell pellet. A) Unlabeled controls. B) Labeled cells demonstrating a significant T2 or negative contrast agent effect on T2-weighted FSE or SE_Multislice imaging and a T1 or positive contrast agent effect on the T1-weighted GRE sequence.
Figure 2. MR images of sagittal cross sections thrrough eppendorf tubes with cells in a cell pellet. A) Unlabeled controls. B) Labeled cells demonstrating a significant T2 or negative contrast agent effect on T2-weighted FSE or SE_Multislice imaging and a T1 or positive contrast agent effect on the T1-weighted GRE sequence.
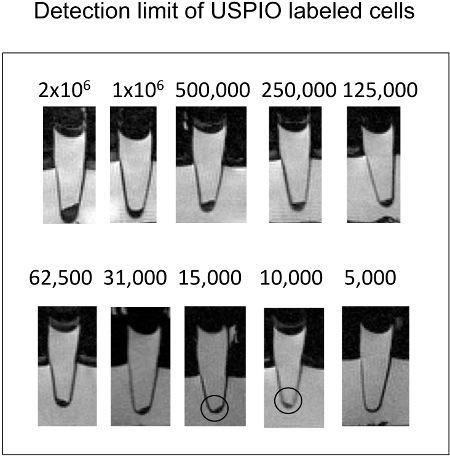 Figure 3. Sagittal cross sections of eppendorf tubes with cells in cell pellets. As little as 10,000 ferumoxytol labeled cells can be detected via T2-w MR imaging.
Figure 3. Sagittal cross sections of eppendorf tubes with cells in cell pellets. As little as 10,000 ferumoxytol labeled cells can be detected via T2-w MR imaging.
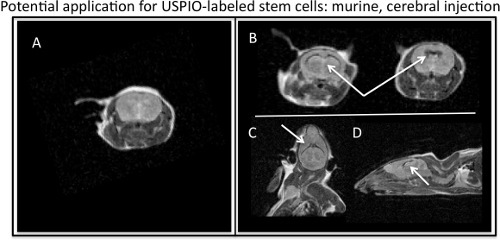 Figure 4. MR visualization of ferumoxytol-labeled stem cells injected into murine cerebral ventricles. A) Axial, MR image of murine barin with no injection of USPIO-labeled stem cells. B) Axial, C) coronal, and D) sagittal MR images depicting the T2, negative contrast effect of USPIO-labeled stem cells (white arrows) injected in a murine brain.
Figure 4. MR visualization of ferumoxytol-labeled stem cells injected into murine cerebral ventricles. A) Axial, MR image of murine barin with no injection of USPIO-labeled stem cells. B) Axial, C) coronal, and D) sagittal MR images depicting the T2, negative contrast effect of USPIO-labeled stem cells (white arrows) injected in a murine brain.
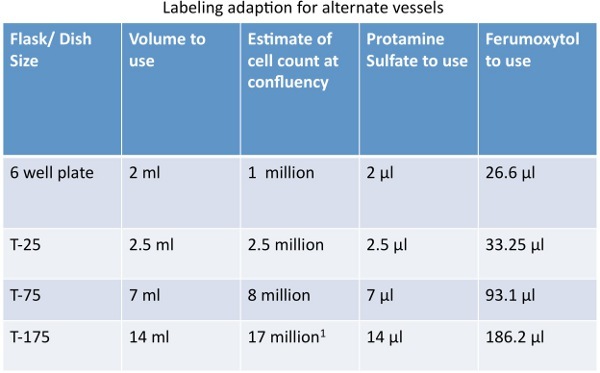 Table 1. Quantity adaptions for labeling cells in alternate vessels.
Table 1. Quantity adaptions for labeling cells in alternate vessels.
Discussion
Improving the efficacy of stem cell engraftments is critical for the advancement of regenerative medicine. A non-invasive visualization technique for stem cells in vivo significantly enhances our ability to understand mechanisms that lead to successful engraftment outcomes. Magnetic labeling for MR visualization, such as the procedure we have demonstrated, allows in vivo tracking of stem cells with MR imaging. Magnetically labeled stem cells have previously been transplanted into target tissues and have been visualized up to weeks with MR imaging 7. Long -term labeling and longitudinal imaging is possible due to the lysosomal storage of the iron oxide nanoparticles within cells 8. Challenges, however, do correspond with long term monitoring of iron labeled cells. One challenge associated with longitudinal imaging of iron labeled cells is that as healthy, viable cells proliferate the contrast agent is distributed to daughter cells. Distribution of the contrast agent to daughter cells is uneven and results in a marked dilution effect of the contrast agent over time. A second challenge associated with long term monitoring of iron labeled cells is differentiating between originally labeled, viable cells from resident phagocytes, such as macrophages, that may have phagocytosed released contrast agent from originally labeled, dead cells 4. Our group has identified differences in long-term signal kinetics of viable and apoptotic stem cell transplants, which had been labeled with iron oxide nanoparticles other than ferumoxytol 9-11. Future studies are required to determine whether these principles also apply to ferumoxytol-labeled cell transplants.
While in this study the potential effects ferumoxytol labeling could exhibit on the differentiation capacity of stem cells was not examined, previous studies have demonstrated an SPIO dose dependent effect on differentiation capacity and SPIO doses that do not impair differentiation potential, suggesting, a ferumoxtyol dose that does not impair the differentiation capacity of stem cells can also be determined 12, 13.
Large SPIOs (hydrodynamic diameter > 50 nm) have been applied previously for cell tracking purposes following stem cell labeling via various labeling techniques including simple incubation and transfection with electroporation or transfection agents such as protamine, lipofectin and poly-L-lysine (PLL) 3, 13-16.,Although not always necessary for cell labeling with SPIOs, transfection methods for cell labeling have proven beneficial for facilitating labeling and increasing labeling efficiency 15. SPIOs which have been used for cell labeling, however, are no longer being produced for commercial reasons. Ultrasmall SPIO (USPIO, hydrodynamic diameter < 50 nm) such as ferumoxytol, on the other hand are still being produced but require the aid of a transfection agent to achieve efficient cellular uptake 8. Protamine sulfate is a clinically applicable transfection agent that forms complexes with USPIOs to facilitate phagocytic uptake of the contrast agent by stem cells 17. The combination of FDA-approved protamine sulfate with the FDA-approved USPIO, ferumoxytol, provides the potential for clinical translation of stem cell tracking techniques via off-label use of this agent. Of note, ferumoxytol has demonstrated an excellent safety profile in clinical trials 18. Direct visualization of the transplanted cells via a technique such as the one demonstrated would allow for a better understanding of the factors that promote or impair successful stem cell transplantations and help to identify the most promising techniques for clinical applications. With the use of clinically applicable cell markers and imaging equipment, this imaging technique is in principle, readily accessible to patients who undergo stem cell transplants.
Disclosures
All contributors to this study offer no disclosures.
Acknowledgments
This work was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases: 3R01AR054458-02S2.
References
- Narsinh KH, Plews J, Wu JC. Comparison of human induced pluripotent and embryonic stem cells: fraternal or identical twins? Mol Ther. 2011;19:635–638. doi: 10.1038/mt.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulte JW. In vivo MRI cell tracking: clinical studies. AJR. Am. J. Roentgenol. 2009;193:314–325. doi: 10.2214/AJR.09.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning TD, Boddington S, Daldrup-Link HE. Labeling hESCs and hMSCs with Iron Oxide Nanoparticles for Non-Invasive in vivo Tracking with MR Imaging. J. Vis. Exp. 2008;(13):e685–e685. doi: 10.3791/685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallheden T, Nannmark U, Lorentzon M. In vivo MR imaging of magnetically labeled human embryonic stem cells. Life. Sci. 2006;79:999–1006. doi: 10.1016/j.lfs.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Jung CW, Jacobs P. Physical and chemical properties of superparamagnetic iron oxide MR contrast agents: ferumoxides, ferumoxtran, ferumoxsil. Magn. Reson. Imaging. 1995;13:661–674. doi: 10.1016/0730-725x(95)00024-b. [DOI] [PubMed] [Google Scholar]
- Coyne DW. Ferumoxytol for treatment of iron deficiency anemia in patients with chronic kidney disease. Expert. Opin. Pharmacother. 2009;10:2563–2568. doi: 10.1517/14656560903224998. [DOI] [PubMed] [Google Scholar]
- Li Z, Suzuki Y, Huang M. Comparison of reporter gene and iron particle labeling for tracking fate of human embryonic stem cells and differentiated endothelial cells in living subjects. Stem Cells. 2008;26:864–873. doi: 10.1634/stemcells.2007-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz S, Bonaterra G, Rudelius M. Capacity of human monocytes to phagocytose approved iron oxide MR contrast agents in vitro. Eur. Radiol. 2004;14:1851–1858. doi: 10.1007/s00330-004-2405-2. [DOI] [PubMed] [Google Scholar]
- Nedopil A, Klenk C, Kim C. MR signal characteristics of viable and apoptotic human mesenchymal stem cells in matrix-associated stem cell implants for treatment of osteoarthritis. Invest. Radiol. 2010;45:634–640. doi: 10.1097/RLI.0b013e3181ed566c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraitchman DL, Heldman AW, Atalar E. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107:2290–2293. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]
- Stuckey DJ, Carr CA, Martin-Rendon E. Iron particles for noninvasive monitoring of bone marrow stromal cell engraftment into, and isolation of viable engrafted donor cells from, the heart. Stem Cells. 2006;24:1968–1975. doi: 10.1634/stemcells.2006-0074. [DOI] [PubMed] [Google Scholar]
- Henning TD, Sutton EJ, Kim A. The influence of ferucarbotran on the chondrogenesis of human mesenchymal stem cells. Contrast. Media. Mol. Imaging. 2009;4:165–173. doi: 10.1002/cmmi.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbab AS, Yocum GT, Kalish H. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood. 2004;104:1217–1223. doi: 10.1182/blood-2004-02-0655. [DOI] [PubMed] [Google Scholar]
- Nedopil AJ, Mandrussow LG, Daldrup-Link HE. Implantation of Ferumoxides Labeled Human Mesenchymal Stem Cells in Cartilage Defects. J. Vis. Exp. 2010;(38):e1793–e1793. doi: 10.3791/1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbab AS, Yocum GT, Wilson LB. Comparison of transfection agents in forming complexes with ferumoxides, cell labeling efficiency, and cellular viability. Mol Imaging. 2004;3:24–32. doi: 10.1162/15353500200403190. [DOI] [PubMed] [Google Scholar]
- Babic M, Horak D, Trchova M. Poly(L-lysine)-modified iron oxide nanoparticles for stem cell labeling. Bioconjug Chem. 2008;19:740–750. doi: 10.1021/bc700410z. [DOI] [PubMed] [Google Scholar]
- Golovko DM, T Henning, Bauer JS. Accelerated stem cell labeling with ferucarbotran and protamine. Eur. Radiol. 2010;20:640–648. doi: 10.1007/s00330-009-1585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Cohen MH, Rieves D. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am. J. Hematol. 2010;85:315–319. doi: 10.1002/ajh.21656. [DOI] [PubMed] [Google Scholar]


