Abstract
Background:
Approach for surgical treatment of thoracolumbar tuberculosis has been controversial. The aim of present study is to compare the clinical, radiological and functional outcome of anterior versus posterior debridement and spinal fixation for the surgical treatment of thoracic and thoracolumbar tuberculosis.
Materials and Methods:
70 patients with spinal tuberculosis treated surgically between Jan 2001 and Dec 2006 were included in the study. Thirty four patients (group I) with mean age 34.9 years underwent anterior debridement, decompression and instrumentation by anterior transthoracic, transpleural and/or retroperitoneal diaphragm cutting approach. Thirty six patients (group II) with mean age of 33.6 years were operated by posterolateral (extracavitary) decompression and posterior instrumentation. Various parameters like blood loss, surgical time, levels of instrumentation, neurological recovery, and kyphosis improvement were compared. Fusion assessment was done as per Bridwell criteria. Functional outcome was assessed using Prolo scale. Mean followup was 26 months.
Results:
Mean surgical time in group I was 5 h 10 min versus 4 h 50 min in group II (P>0.05). Average blood loss in group I was 900 ml compared to 1100 ml in group II (P>0.05). In group I, the percentage immediate correction in kyphosis was 52.27% versus 72.80% in group II. Satisfactory bony fusion (grades I and II) was seen in 100% patients in group I versus 97.22% in group II. Three patients in group I needed prolonged immediate postoperative ICU support compared to one in group II. Injury to lung parenchyma was seen in one patient in group I while the anterior procedure had to be abandoned in one case due to pleural adhesions. Functional outcome (Prolo scale) in group II was good in 94.4% patients compared to 88.23% patients in group I.
Conclusion:
Though the anterior approach is an equally good method for debridement and stabilization, kyphus correction is better with posterior instrumentation and the posterior approach is associated with less morbidity and complications.
Keywords: Anterior approach, extracavitary approach, posterior approach, Pott's spine
INTRODUCTION
Approach for surgical treatment of thoracolumbar tuberculosis is always controversial. The goals of surgery in Pott's spine are adequate decompression, adequate debridement, maintenance and reinforcement of stability and correction and prevention of deformity. Traditionally, the anterior approach has been preferred throughout the spine to achieve these goals because the pathology of tuberculosis mainly affects the vertebral bodies and disc spaces, and the anterior approach allows direct access to the infected focus and is convenient for debriding infection and reconstructing the defect.1–3 In the thoracic and lumbar region, anterior instrumentation to provide bone stability may be tenuous because the concomitant osteoporosis associated with infection renders the vertebrae structurally weak and may prevent adequate fixation.4,5
A combined anterior plus posterior approach helps to overcome stability related drawbacks of anterior approach alone.4,6–9 However, it entails two surgeries (single event or staged) with additional morbidity.2,10,11 However, posterior or posterolateral2,12–14 approaches alone have also been described, where anterior and lateral column can be reached through extra pleural approach. Posterior approach has gained popularity in the last decade as it provides excellent exposure for circumferential spinal cord decompression and also allows posterior instrumentation to be extended for multiple levels above and below the level of pathology.
The selection of anterior versus posterior approach for surgical treatment of thoracolumbar tuberculosis is still a matter of debate. The aim of present study is to compare the clinical, radiological and functional outcome of anterior versus posterior debridement and spinal fixation for the surgical treatment of thoracic and thoracolumbar tuberculosis.
MATERIALS AND METHODS
Seventy patients with confirmed spinal tuberculosis (52 males:18 females, with mean age 34.3 years, range 18–56 years) were treated surgically between Jan 2005 to Dec 2009. All these patients were retrospectively analyzed and divided into two groups on the basis of surgical approach. Group I comprised 34 patients with mean age 34.9 years, (range 21–50 years), who underwent anterior debridement, decompression and spinal instrumentation by anterior transthoracic/transpleural approach for thoracic lesions or transthoracic retroperitoneal diaphragm cutting approach for thoracolumbar disease. Group II comprised 36 patients with mean age 33.6 years, (range 18–56 years), who were operated by posterolateral (extracavitory or extra pleural) debridement, decompression and reconstruction with cage and posterior instrumentation.
The indications of surgery in both the groups were neurological deficit not responding to antituberculous chemotherapy for 4–6 weeks or significant kyphosis (>40° of segmental kyphosis) or instability (anteroposterior or lateral translation; >40° of segmental kyphosis).
Anterior surgery was done more frequently in the early part of the study period and posterior approach more often in the latter part of the study period. Specifically, anterior approach was avoided in patients with lesions above T5 (as instrumentation above T4 body is difficult), in patients with kyphosis of more than 60° (anterior only correction causes spinal lengthening), in patients with disease involving the posterior elements and in patients with a bad preoperative chest condition. The distribution of patients according to lesion level and involvement is shown in Table 1.
Table 1.
Distribution of the patients according to the lesion level and involvement
Plain radiography, computerized tomography (CT), and magnetic resonance imaging (MRI) studies had been conducted before surgery for all patients. All patients underwent four drug antituberculous chemotherapy (rifampicin, 15 mg/kg, maximum, 600 mg/day; and isoniazid, 6 mg/kg, maximum, 300 mg/day and ethambutol, 15 mg/kg, maximum 1000 mg/day and pyrazinamide, 25 mg/kg, maximum 1500 mg/day) before surgery for at least 3 weeks, except those who had established or recently developed progressive neurologic deficits necessitating urgent decompression (two patients in group I and three in group II). None of the patients in our study was HIV positive.
The operative technique for each group is as follows:
Group I (anterior approach)
All 34 patients underwent single-stage anterior radical debridement, decompression, autogenous bone grafting, and instrumentation. They were operated under general anesthesia with endotracheal intubation. Patients were placed in the right lateral decubitus position. A transthoracic intrapleural approach was used for the thoracic region and a transthoracic retroperitoneal diaphragm cutting approach was used for thoracolumbar region. Pus and necrotic tissue were removed as much as possible until normal bleeding bone was reached. Neural decompression was carried out with subtotal or complete corpectomy of the involved vertebrae. The titanium or Polyether ether ketone (PEEK) cages packed with autogenous rib or iliac crest grafts were used for reconstruction. Anterior instrumentation in the form of rod-screw construct was used following radical debridement and decompression in all patients [Figure 1]. None of these patients had undergone supplementary posterior instrumentation surgery.
Figure 1.
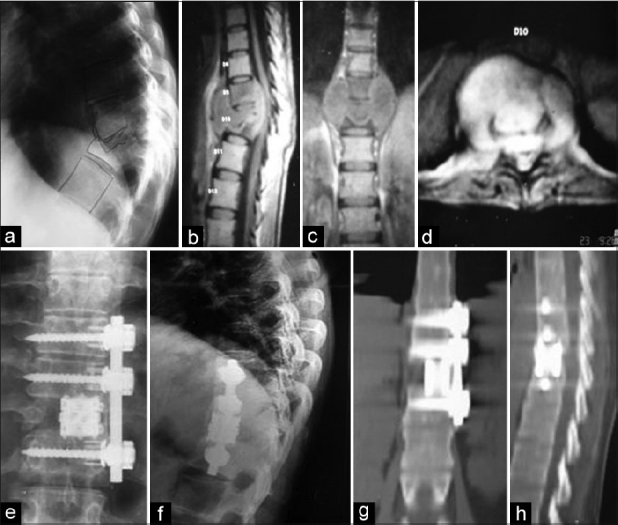
Preoperative lateral view (a) X-rays of a 26-year-old female with tuberculosis at D9–10 level with kyphosis. Sagittal (b), coronal (c) and axial (d) MRI images of the same patient show vertebral destruction and abscess formation with cord compression. This patient was treated by anterior approach. Postoperative X-ray (e and f) showing good decompression with reconstruction of defect with screw-rod and expandable cage construct. Postoperative CT images (g and h) showing solid bony union at 12 months
Group II (posterior approach)
All patients were operated under general anesthesia in prone position. A posterior midline approach was used in all patients. The posterolateral extra pleural approach was used to decompress the cord. The necrotic material within the body and disc was removed using curettes, and paraspinal abscess was drained. A titanium mesh cage filled with autograft was used from one side to reconstruct the defect. The spine was stabilized using transpedicular screw and rod system [Figure 2]. In cases of upper thoracic region, we preferred fusing as short a segment as possible. In the lower thoracic region or thoracolumbar junction, we preferred fusing at least two vertebras above and below the lesion. Anterior approach was not used for debridement.
Figure 2.
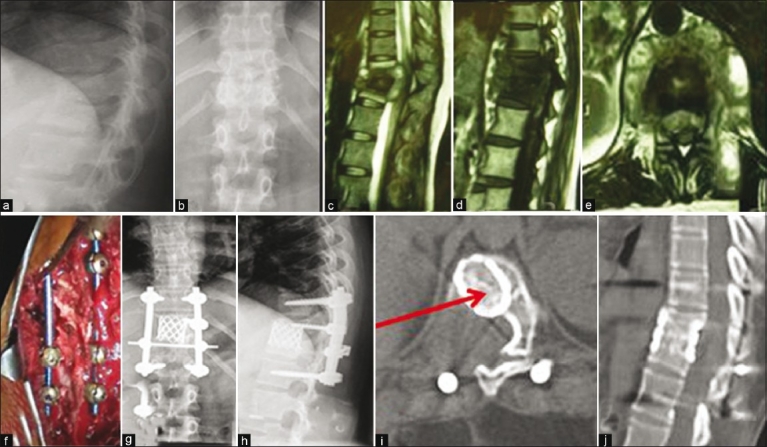
Preoperative lateral (a) and anteroposterior view X-rays (b) a 32-year-old female with tuberculosis of D11–12. Sagittal T2 WI (c) and T1WI (d) and axial T2WI (e) MRI images show active tuberculosis with abscess formation and cord compression. This patient was treated by posterior extrapleural approach with pedicular screw-rod fixation (f) Postoperative X-rays (g and h) of the same patient show good decompression and kyphosis correction. At 9months followup, solid bony fusion was seen on computed tomography axial and sagittal reconstruction (i and j)
Various surgical parameters like blood loss, surgical time, levels of instrumentation were compared between both the groups.
All but 7 patients were given standard antituberculous chemotherapy for a total of 12 months: Four drugs (isoniazid, rifampicin, pyrazinamide and ethambutol) for 3 months, three drugs (isoniazid, rifampicin and ethambutol) for 3 months and two drugs (isoniazid and rifampicin) for 6 months. Besides this, intravenous antibiotic drug, a 3rd generation cephalosporin, was given for 5–7 days to all patients after surgery. Four patients in group I and three patients in group II were found to have multi drug resistant tuberculosis and were treated with second line antituberculous treatment (ATT). All patients were immobilized in a rigid external orthosis for 12–16 weeks after surgery.
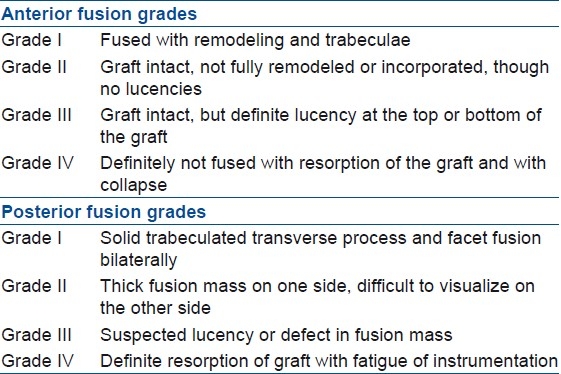
Immediately post surgery, routine lateral and anteroposterior radiographs were obtained to assess the extent of decompression and placement of graft and instrumentation. All patients were seen at 1, 3, 6, 9, and 12 months after surgery and were followed up annually thereafter. At each followup evaluation, plain radiographic studies were obtained in standing position to determine the fusion status, development or progression of deformity after surgery, and instrumentation failure. The erythrocyte sedimentation rate (ESR) and C-reactive protein were measured to determine the presence of active disease. Clinical examination was also performed at each followup visit. The clinical and radiological evidences of successful fusion were defined as absence of local pain and tenderness over the site of fusion, abnormal motion, loss of correction and instrumentation failure, and presence of trabecular bone bridging between the grafts and the vertebrae. Patients were also evaluated for radiological parameters like improvement in local kyphosis. Final fusion assessment was done according to Bridwell15 criteria [Table 2]. Neurological deficit was graded according to Frankel system. Pain was also assessed according to the following scale: Severe, moderate, mild, and no pain. Functional outcome was assessed according to Prolo scale.16
Table 2.
Bridwell criteria15
RESULTS
The mean duration between surgery and onset of symptoms was 10.2 months (range 5–14 months) in group I and 9.7 months (range 6–13 months) in group II. The distribution of lesions was almost similar in both the groups [Table 1]. The mean surgical time in group I (anterior group) was 5 h 10 min (range 3 h 45 min–7 h 30 min), while in group II (posterior group) it was 4 h 50 min (3 h 50 min–6 h 30 min) (P>0.05). Average blood loss in group I was 900 ml (500–1000 ml), while it was 1100 ml (700–1800 ml) in group II (P>0.05). The mean fusion levels were 2.9 (range 2–6) in group I and 4.4 (range 3–8) in group II. Mean followup period was 26 months (range 12–72 months).
Eighteen patients were classified as Frankel type C, 12 as Frankel type D, and 4 as Frankel grade E before surgery in group I. After surgery, out of 18 patients with Frankel C, 10 patients improved to Frankel E, 6 patients improved to Frankel D and 2 patients remained as Frankel C, at final followup. Out of 12 patients with Frankel D, 10 patients improved to Frankel E, 1 remained as Frankel D while 1 worsened to Frankel C. All except one patient with Frankel E had no worsening at final followup [Table 3]. One patient had complete paraplegia which recovered to Frankel B at final followup.
Table 3.
Neurological recovery in group I (anterior) and group II (posterior)
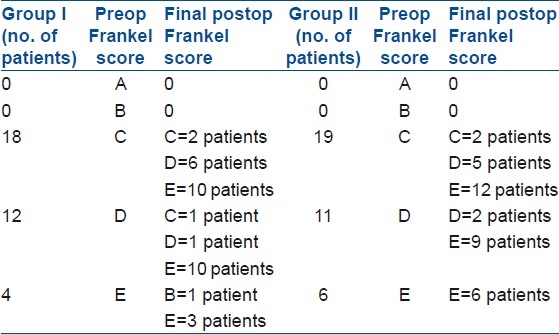
In group II, 19 patients were classified as Frankel type C, 11 as Frankel type D, and 6 as Frankel grade E before surgery. After surgery, out of 19 patients with Frankel C, 12 patients improved to Frankel E, 5 patients improved to Frankel D and 2 patients remained as Frankel C, at final followup. Out of 11 patients with Frankel D, 9 patients improved to Frankel E and 2 remained as Frankel D. All patients with Frankel E had no worsening at final followup.
In group I (anterior group), mean preoperative local kyphosis in the thoracic and thoracolumbar spine (T1–L1) was 44.6° (25°–58°), which was corrected to a mean of 21.3° (14°–26°) in the immediate postoperative radiographs. The percentage immediate correction was 52.3%. There was an average loss of correction of 2.8° at final followup. In group II (posterior group), the mean preoperative kyphosis 74.6° (48°–86°) was corrected to a mean of 20.3° (14°–28°) in the immediate postoperative radiographs. The percentage immediate correction was 72.8%, which was statistically significant when compared with group I (P≤0.001). There was an average loss of correction of 2.2° at final followup in group II.
According to Bridwell criteria [Table 2],15 all the patients in group I (anterior group) had grade I (definite) fusion in 70.6% (n=24) and grade II (probably) fusion in 29.4% (n=10), while in group II patients, grade I fusion was seen in 72.2% (n=26), grade II fusion in 25% (n=9) and grade III (probably not) in 2.8% (n=1) of patients. Functional outcome (Prolo scale) in group II was graded as good in 34 patients (94.4%) and fair in 2 patients (5.5%). On the other hand, 30 patients (88.3%) in group I had a good functional outcome, 3 patients (8.8%) had a fair outcome and 1 patient (2.9%) had a poor outcome.
Three patients in group I needed prolonged immediate postoperative ICU support as compared to one in group II. Injury to lung parenchyma was seen in one patient in group I, while the anterior procedure had to be abandoned in one case due to pleural adhesions. Later, the patient was turned prone and posterolateral decompression with posterior instrumentation was carried out.
DISCUSSION
Anterior approach is considered the gold standard17 for debridement and decompression in Pott's spine, which was popularized by Hodgson18 in 1960. Advocates of the traditional anterior approach1–3 cite the ability to directly access the disease pathology and perform decompression, less muscle dissection and the ability to place a large graft under compressive load for fusion. Spinal instability is likely to increase after surgical decompression in the immediate postoperative period. The bone graft does not give initial stability and graft related complications occur more often when the span of the graft exceeds a two-disc space.10,19–21 Anterior instrumentation in tuberculous spondylitis is a relatively new concept.1 Oga et al.22 evaluated the adherence capacity of Mycobacterium tuberculosis to stainless steel and concluded that adherence was negligible and the use of implants in regions with active tuberculosis infection may be safe. Several studies19–21,23,24 have demonstrated that treatment of active tuberculosis spondylitis with anterior instrumentation along with anterior debridement and fusion provides a high and effective rate of deformity correction and maintenance. However, there may be associated lung scarring secondary to old/active pulmonary tuberculosis, which may preclude the anterior approach. One case in our series also needed to be abandoned due to extensive pleural adhesions. Besides this, there are also issues regarding stability of anterior instrumentation as concomitant inflammation associated with infection may not provide adequate fixation.5 Anterior instrumentation is usually appropriate to prevent deterioration of the kyphus during treatment.2
Posterior instrumentation has been reported to be quite effective in preventing graft related complications and progression of kyphosis. The main advantage of posterior instrumentation is that it can provide good fixation through posterior elements as the disease pathology is anterior. Posterior fixation also helps in correcting pre-existing kyphosis effectively.8–11,25 Posterior instrumentation with anterior decompression and fusion can be performed in one or two stages. There is a decrease in the incidence of recurrence of infection and revision surgery with combined approaches as compared with a single approach.6 However, if performed in one stage, the procedure has more morbidity. When anterior decompression and bone grafting is performed as a first stage procedure, there is a risk of graft slippage and neural deterioration while waiting for second stage stabilization. In the second stage, only in situ stabilization will be performed. When the posterior procedure is performed first, it will be only in situ stabilization followed by second-stage decompression, so kyphus correction will be minimal.10
Posterior approach utilizing only extra pleural approach, as described by Jain et al.,2 is an effective option. Extra pleural approach allows decompression of spinal cord under direct vision and also putting structural support anteriorly. This is then supplemented with a stable posterior instrumentation, which has the multilevel flexibility to be extended above and below if needed. The pattern of neurological recovery is almost the same in both the groups, demonstrating adequate decompression through posterior approach alone. Also, the fusion rate is similar in both the groups. Since the approach to the vertebral body is extra pleural, respiratory function is not compromised and this approach can be used in patients with concomitant pulmonary tuberculosis and compromised pulmonary reserve,2 where the anterior approach is contraindicated. Four (11.76%) cases in group I needed prolonged ICU support and lung injury as compared to 1 (2.78%) patient in group II, who needed postoperative ICU support because of excessive bleeding.
Poor sagittal spinal correction has been documented following anterior approach alone.26 While anterior instrumentation may prevent progression of kyphosis during treatment,2 it is not so effective in correcting pre-existing kyphosis. Addition of posterior instrumentation has shown to improve correction of sagittal alignment.2,7–10,25 Reported kyphosis correction ranges from initial 30°–35° to 15°–18° postoperatively, with 2°–3° loss of correction with an average followup of 45 months. In our series also, the kyphosis correction was significantly better with posterior approach alone.
Though anterior approach is a favored method for debridement and decompression as the lesion is situated anteriorly, there is an increased morbidity related to the approach (transthoracic, transpleural). The posterior/posterolateral approach (extracavitory approach) gives a reasonable access to the lateral and anterior aspects of the cord for an equally good decompression of the cord.2 Better functional outcome and significantly better sagittal plane and kyphosis correction by the posterior approach are strong pointers favoring the posterior approach.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Benli T, Kaya A, Acaroglu E. Anterior instrumentation in tuberculous spondylitis: Is it effective and safe? Clin Orthop Relat Res. 2007;460:108–16. doi: 10.1097/BLO.0b013e318065b70d. [DOI] [PubMed] [Google Scholar]
- 2.Jain AK, Dhammi IK, Prashad B, Sinha S, Mishra P. Simultaneous anterior decompression and posterior instrumentation of the tuberculous spine using an anterolateral extrapleural approach. J Bone Joint Surg Br. 2008;90:1477–81. doi: 10.1302/0301-620X.90B11.20972. [DOI] [PubMed] [Google Scholar]
- 3.Hodgson AR, Stock FE, Fang HS, Ong GB. Anterior spinal fusion: The operative approach and pathological findings in 412 patients with Pott's disease of the spine. Br J Surg. 1960;48:172–8. doi: 10.1002/bjs.18004820819. [DOI] [PubMed] [Google Scholar]
- 4.Hee HT, Majd ME, Holt RT, Pienkowski D. Better treatment of vertebral osteomyelitis using posterior stabilization and titanium mesh cages. J Spinal Disord Tech. 2002;15:149–56. doi: 10.1097/00024720-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Krodel A, Kruger A, Lohscheidt K, Pfahler M, Refior HJ. Anterior debridement.fusion, and extrafocal stabilization in the treatment of osteomyelitis of spine. J spinal Disord. 1999;8:304–9. [PubMed] [Google Scholar]
- 6.Fukuta S, Miyamoto K, Masuda T, Hosoe H, Kodama H, Nishimoto H, et al. Two stage (Posterior and anterior) surgical treatment using posterior spinal instrumentation for pyogenic and tuberculotic spondylitis. Spine (Phila Pa 1976) 2003;28:E302–8. doi: 10.1097/01.BRS.0000083318.40123.5E. [DOI] [PubMed] [Google Scholar]
- 7.Laheri VJ, Badhe NP, Dewnany GT. Single stage decompression, anterior interbody fusion and posterior instrumentation for tuberculous kyphosis of the dorso-lumbar spine. Spinal Cord. 2001;39:429–36. doi: 10.1038/sj.sc.3101185. [DOI] [PubMed] [Google Scholar]
- 8.Moon MS. Combined posterior instrumentation and anterior interbody fusion for active tuberculous kyphosis of the thoraco-lumbar spine. Curr Orthopaedics. 1991;5:177–9. [Google Scholar]
- 9.Moon MS, Woo YK, Lee KS, Ha KY, Kim SS, Sun DH. Posterior instrumentation and anterior interbody fusion for tuberculous kyphosis of dorsal and lumbar spines. Spine (Phila Pa 1976) 1995;20:1910–6. doi: 10.1097/00007632-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Jain AK, Dhammi IK. Tuberculosis of the Spine: A Review. Clin Orthop Relat Res. 2007;460:39–49. doi: 10.1097/BLO.0b013e318065b7c3. [DOI] [PubMed] [Google Scholar]
- 11.Güven O, Kumano K, Yalçin S, Karaham M, Tsuji S. A single stage posterior approach and rigid fixation for preventing kyphosis in the treatment of spinal tuberculosis. Spine (Phila Pa 1976) 1994;19:1039–43. doi: 10.1097/00007632-199405000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Louw JA. Spinal tuberculosis with neurological deficit: Treatment with anterior vascularised rib grafts, posterior osteotomies and fusion. J Bone Joint Surg Br. 1990;72:686–93. doi: 10.1302/0301-620X.72B4.2380228. [DOI] [PubMed] [Google Scholar]
- 13.Lee TC, Lu K, Yang LC, Huang HY, Liang CL. Transpedicular instrumentation as an adjunct in the treatment of thoracolumbar and lumbar spine tuberculosis with early state bone destruction. J Neurosurg. 1999;91(2 Suppl):163–9. doi: 10.3171/spi.1999.91.2.0163. [DOI] [PubMed] [Google Scholar]
- 14.Jain AK, Aggarwal A, Dhammi IK, Aggarwal PK, Singh S. Extrapleural anterolateral decompression in tuberculosis of the dorsal spine. J Bone Joint Surg Br. 2004;86:1027–31. doi: 10.1302/0301-620x.86b7.14546. [DOI] [PubMed] [Google Scholar]
- 15.Bridwell KH, Lenke LG, McEnery KW, Baldus C, Blanke K. Anterior fresh frozen structural allografts in the thoracic and lumbar spine.Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine (Phila Pa 1976) 1995;20:1410–8. [PubMed] [Google Scholar]
- 16.Prolo DJ, Oklund SA, Butcher M. Toward uniformity in evaluating results of lumbar spine operations.A paradigm applied to posterior lumbar interbody fusions. Spine (Phila Pa 1976) 1986;11:601–6. doi: 10.1097/00007632-198607000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Tuli SM. Tuberculosis of the Spine: A Historical Review. Clin Orthop Relat Res. 2007;460:29–38. doi: 10.1097/BLO.0b013e318065b75e. [DOI] [PubMed] [Google Scholar]
- 18.Hodgson AR, Stock FE, Fang HS, Ong GB. Anterior spinal fusion: The operative approach and pathological findings in 412 patients with Pott's disease of the spine. Br J Surg. 1960;48:172–8. doi: 10.1002/bjs.18004820819. [DOI] [PubMed] [Google Scholar]
- 19.Rajasekaran S, Soundarapandian S. Progression of kyphosis in tuberculosis of the spine treated by anterior arthrodesis. J Bone Joint Surg Am. 1989;71:1314–23. [PubMed] [Google Scholar]
- 20.Chen WJ, Chen CH, Shih CH. Surgical treatment of tuberculosis spondylitis: 50 patients followed for 2–8 years. Acta Orthop Scand. 1995;66:137–42. doi: 10.3109/17453679508995507. [DOI] [PubMed] [Google Scholar]
- 21.Chen WJ, Wu CC, Jung CH, Chen LH, Niu CC, Lai PL. Combined anterior and posterior surgeries in the treatment of spinal tuberculous spondylitis. Clin Orthop Relat Res. 2002;398:50–9. doi: 10.1097/00003086-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Oga M, Arizono T, Takasita M, Sugioka Y. Evaluation of the risk of instrumentation as a foreign body in spinal tuberculosis: Clinical and biologic study. Spine (Phila Pa 1976) 1993;18:1890–4. doi: 10.1097/00007632-199310000-00028. [DOI] [PubMed] [Google Scholar]
- 23.Ozdemir HM, Us AK, Ogün T. The role of anterior spinal instrumentation and allograft fibula for the treatment of pott disease. Spine (Phila Pa 1976) 2003;28:474–9. doi: 10.1097/01.BRS.0000048666.17934.17. [DOI] [PubMed] [Google Scholar]
- 24.Christodoulou AG, Givissis P, Karataglis D, Symeonidis PD, Pournaras J. Treatment of tuberulosis spondylitis with anterior stabilization and titanium cage. Clin Orthop Relat Res. 2006;444:60–5. doi: 10.1097/01.blo.0000201175.87635.28. [DOI] [PubMed] [Google Scholar]
- 25.Sundararaj GD, Behera S, Ravi V, Venkatesh K, Cherian VM, Lee V. Role of posterior stabilisation in the management of tuberculosis of the dorsal and lumbar spine. J Bone Joint Surg Br. 2003;85:100–6. doi: 10.1302/0301-620x.85b1.13300. [DOI] [PubMed] [Google Scholar]
- 26.Calderone RR, Thomas JC, Jr, Haye W, Abeles D. Outcome assessment in spinal infections. Orthop Clin North Am. 1996;27:201–5. [PubMed] [Google Scholar]


