Abstract
Background:
India ranks second amongst the high-burden multi drug resistant tuberculosis (MDR-TB) countries, with an estimated incidence of 2.3% MDR-TB cases amongst the new cases and 17.2% amongst the previously treated cases. The diagnosis and treatment protocol for MDR-TB of the spine are not clearly established. We report outcome of a series of 15 cases of TB spine who were suspected to be therapeutically refractory cases (MDR-TB) on the basis of clinicoradiological failures of initial treatment.
Materials and Methods:
Fifteen cases of TB spine from C2 to L5 spine were suspected to be the cases of MDR-TB (therapeutically refractory cases) on the basis of failures of adequate clinicoradiological healing response at 5 months or more on antitubercular treatment (ATT). None of the patient was immunocompromised. Thirteen out of 15 patients had tissue samples sent for histopathology, culture and sensitivity, smear, BACTEC, and polymerase chain reaction (PCR). All patients were put on second line ATT and followed up fortnightly with regular liver and kidney function tests, erythrocyte sedimentation rate (ESR), and plain X-ray. Healing was documented as subjective improvement of symptoms, reduction in ESR, and observations on contrast enhanced magnetic resonance imaging (MRI) such as resolution of marrow edema, fatty replacement of bone marrow and resolution of abscesses. Ambiguous MRI observations in a few patients were resolved on positron emission tomography (PET) scan. Patients were monitored continuously for 2 years after stopping ATT.
Results:
We could demonstrate a positive culture in three cases. Two of them had multi drug resistance. We could achieve healing status in 13 out of 14 patients after starting second line drugs, one patient is still on treatment while other patient with no drug resistance is responding well on ATT.
Conclusions:
The suspicion of therapeutically refractory case is of paramount importance. Once suspected, surgery to procure tissue for diagnosis and culture is to be undertaken. The demonstration of drug resistance on culture may not be achieved in all TB spine cases and empiric drug regimen for MDR-TB is to be started. We have achieved the healed status with immunomodulation and second line ATT. The length of treatment needs to be monitored with MRI and PET scan.
Keywords: MRI, multi drug resistance, TB spine, therapeutically refractory disease
INTRODUCTION
Tuberculosis (TB) is a pandemic and is amongst the top 10 killer infectious diseases, second only to HIV. There are 9.4 million (range 8.9–9.9 million) cases of TB worldwide.1,2 The increasing number of immunocompromised population and longevity of human races with concomitant morbidity (diabetes, use of immune suppressive drugs in organ transplantation, and malignancy) has increased the occurrence of TB. The emergence of drug resistance has been reported ever since the first antitubercular drugs were introduced.3,4 The multi drug resistance for Mycobacterium tuberculosis is reported to be between 7 and 15% and it continues to increase manifold.5 According to an estimate, approximately 4,400,00 (range 390,000–510,000) cases of multi drug resistant TB (MDR-TB) emerged globally in 2008 amongst new TB cases.1,2 There are about half a million MDR-TB cases emerging every year amongst new and previously treated cases. Half of the MDR-TB cases are in India and China. India ranks second amongst the high burden MDR-TB countries, with an estimated 2.3% MDR-TB cases amongst new cases and 17.2% amongst previously treated cases.1
MDR-TB should be suspected when a patient of pulmonary TB continues to be sputum positive for acid-fast bacillus (AFB) at 5 months on CAT I/CAT II DOTS treatment.6 Osteoarticular TB is a paucibacillary disesase and the chances of demonstration of bacilli on smear vary from 10 to 30% and on culture vary from 25 to 75%.7 As spinal TB is a deep-seated lesion, it is difficult to define failures of drug treatment based on failure of conversion from AFB positive cases. The healing response which is observed on plain X-ray may be delayed by many months; hence, it is difficult to suspect an MDR-TB case. The diagnosis and treatment protocol for MDR-TB are not clearly established.8 We report a series of cases of TB spine, who were suspected to be cases of MDR-TB (therapeutically refractory cases) on the basis of clinicoradiological failures of treatment and treated by immune modulation and second line antitubercular therapy (ATT).
MATERIALS AND METHODS
Fifteen cases of TB spine from C2 to L5 spine, who were suspected to be the cases of MDR-TB (therapeutically refractory case) on the basis of failure of adequate clinicoradiological healing response at 5 months or more, were studied. There were seven males and eight females with mean age 34.4 years (range 18–60 years). Mean duration of symptoms was 14 months (range 8–72 months) before the diagnosis of TB of spine could be made. The mean vertebral body affection on X-ray, kyphotic deformity, location of vertebral affection, and magnetic resonance imaging (MRI) observations were recorded. These cases were suspected to be therapeutically refractory on observing persistent worsening of lesion clinicoradiologically and by increase in deformity (n=9), persistently discharging sinus or ulcer (n=2), appearance of fresh lesion, or recurrence of previous lesion (n=4). Two of these 15 cases were diagnosed as TB of spine clinicoradiologically, while 13 had MRI observation when they were initially treated for TB spine. Three had histopathologic or fine needle aspiration cytology (FNAC) evidence of TB spine.
Twelve patients were on daily dosage regimen while three were treated by DOTS center. Twelve of the 15 patients were taken up for surgical debridement of the tubercular lesions where anterolateral decompression (n=10) was performed for dorsal and dorsolumbar spine lesion, while retroperitoneal anterior decompression was performed in two lumbar lesions. One patient required costotransversectomy for drainage of thoracic lesion and open abscess drainage from Petit's triangle for psoas abscess. In one patient, the pus from draining sinus of the scar of previous anterolateral decompression was obtained for culture and sensitivity. Two patients were treated by second line drugs without procuring the tissue. The tissue procured was submitted for AFB smear, BACTEC culture, polymerase chain reaction (PCR), and histopathologic examination. All were started on second line ATT once the tissue was sent. The drugs given were Rifampicin, INH, Ofloxacin, Ethiomamide, Cycloserine, and Inj. Kanamycin/Amikacin. Immunomodulation was done by administering Tab. Levamisole 2.5 mg/kg/day for 3 days at weekly interval; seven such cycles were repeated. Two doses of 0.1 ml Inj. BCG were given intradermally with a gap of 1 month, followed by injection DT deep intramuscularly. The liver function tests and kidney function tests were performed every 2 weeks for 2 months, followed by once in 3 months. These patients were evaluated by erythrocyte sedimentation rate (ESR) and plain X-ray every 3 months. At 1-year followup, contrast MR was done to evaluate the lesion for sign of healing and regression of abscess. The signs of healing on MRI were observed as resolution of bone edema, appearance of fatty degeneration, and resolution of paravertebral abscess. The radiological evidence of healing was recorded as remineralization of vertebral lesion, sharpening of disc margin, appearance of sclerosis, and reduction in paravertebral shadows. ATT was continued for the next 6 months. The lesions were again evaluated at 18 and 24 months. All those lesions where bony lesion had healed radiologically with persisting paraspinal abscess were evaluated by positron emission tomography (PET) scan (n=4). On PET scan, if no metabolic activity was present, the lesions were declared healed and ATT was stopped. These patients were followed up every 6 months for the next 2 years and were evaluated clinically and by ESR and plain X-rays. Eleven cases completed the 2 year followup. Two patients had followup of 1 year and two patients are still on treatment. None of the patient was immunocompromised. An informed consent was taken from all patients to enrol in study.
RESULTS
All patients presented with low backache and constitutional symptoms like low grade fever, weight loss, and malaise. Ten patients had no neurological deficits. Five patients had neurological deficit, with two of them having complete paraplegia while the other three had paraparesis. One patient had kyphus deformity of 30°. All patients were subjected to pretreatment MRI, which showed typically tubercular lesions with contiguous vertebral body involvement, relative preservation of disc space, subligamentous extension, large pre and paravertebral abscess, vertebral intraosseous abscess, and septate paravertebral shadow. Four patients had additional psoas abscess on MRI. The dorsal spine was involved in 10 cases, dorsolumbar junction in 2 cases, lumbar in 3, and lumbosacral in 3 cases. Two patients who were already on first line treatment, one for pulmonary Koch's and the other one for tubercular knee, developed newer lesion in spine. One patient had discharging sinus. On hematological examination, ESR was raised in all of the patients. One patient had submandibular lymphadenopathy from which the biopsy was taken to ascertain the diagnosis of TB [Figure 1]. AFB smear from lymph gland was full of bacilli. All patients were negative for HIV.
Figure 1.
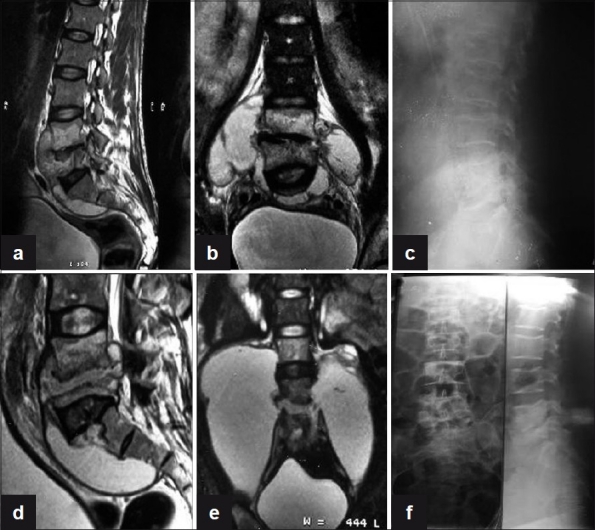
(a) Sagittal T2WI shows anterior subligamentous spread of large prevertebral collection with interosseous caseation seen in L4, L5 vertebra with endplate erosions and reduced disc height with discitis. (b) Coronal T2WI shows large paravertebral collections with interosseous caseation. (c) Plain X-ray lateral view of lumbosacral spine shows near complete obliteration of L4, L5 disc space with endplate erosions and loss of vertebral height at 3 months. (d, e) T2WI (Sagittal and coronal) show increase in the pre- and paravertebral collections with further loss of vertebral body height of L5 vertebra at 6 months. (f) Plain X-ray AP and lateral views of lumbosacral spine show sharpening of paradiscal cortical margins with better defined disc space and sclerosis of endplates with no paravertebral shadows on final healing
Out of the 15 cases, 10 were operated with anterolateral decompression while 2 cases were operated for debridement of lumbar lesion by anterior retroperitoneal approach. In one patient, costotransversectomy of D6 along with drainage of large abscess from Petit's triangle was done. Three patients had FNAC, PCR, histopathology (HPE), and smear, all positive for TB. Five patients had PCR and HPE positivity, whereas in three patients only smears were positive for Mycobacterium. In two of the patients, none of the above was positive and these were diagnosed as tubercular on clinicoradiological and MR imaging findings and continued on treatment despite no evidence of TB from lesion. The culture and sensitivity could be obtained for three patients; one patient showed resistance to Rifampicin and INH, while the second case was resistant to INH, Rifampicin, Streptomycin, Ethionamide, and Ofloxacin, and the third patient was sensitive to all first and second line drugs.
All 15 patients received initially first line treatment for spinal TB, whereas 2 were already on first line treatment. One with knee TB received treatment for 5 months and developed spinal lesion [Figure 2] and the second one received it for 7 months for pulmonary TB. After 15 months of first line of treatment, five patients had no improvement in symptoms, whereas four patients had worsening of the symptoms and developed a new lesion and two had recurrence of the previous lesion after full course of ATT. Thus, four patients were labeled as MDR-TB on worsening of the symptoms, five on showing no improvement with treatment, two on development of newer lesion on ATT, and two on recurrence of the previous lesion. On repeat MRI, none of the patients showed resolution of marrow edema, and replacement of marrow by fat was seen as bright signal on T1W1 and T2W1 and decrease or resolution of paravertebral collections.
Figure 2.
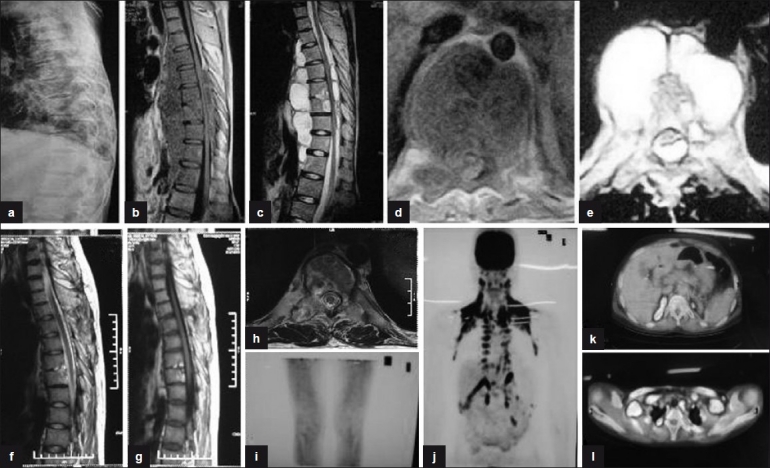
(a) Plain X-ray (pretreatment) lateral view shows reduction in disc space in D4, D5, D6, and in D9, D10, D11 with endplate erosions and fuzzy paradiscal margins. (b, c) Sagittal T1W and T2W (pretreatment) sections show paradiscal lesions with intraosseous caseation, preserved discs with discitis, anterior subligamentous spread of large prevertebral collection, anterior and posterior epidural spread of collection compressing the spinal cord. (d, e) Axial T1W and T2W sections show septate prevertebral collection, intraosseous caseation with large anterior and posterior epidural collections, and 80% canal encroachment. (f, g) Sagittal T1W and T2W (post-treatment) sections show patchy replacement of marrow by fat seen as bright T1 signal with near-complete resolution of collections and marrow edema. (j, k, l) Coronal and axial sections of PET scan show no FDG uptake in vertebral column at 18 months, suggestive of complete healing
At the last followup, all patients had clinical improvement in symptoms such as a decrease or complete resolution of pain and neurological improvement. ESR was decreased in all patients. We had achieved complete healing of lesions in 13 cases, with 2 cases still on followup. On MRI, decrease or resolution of paravertebral collections and replacement of marrow by fat were seen as bright signal on T1W1 and T2W1. Nine patients showed resolution of lesion at 18–24 months while four had persistent activity, and PET scan was performed to clarify ambiguous MRI picture where marrow edema was persistent. In two patients, there was no biological activity on PET scan, and hence ATT was stopped (there was no 18F-FDG uptake in these patients), while two patients had persistent uptake and second line ATT was continued for 30 months. One patient continued to have persistent vertebral disease as was shown in her PET scan and another patient had healed vertebral disease, but persistent activity in his psoas [Figure 3] as was demonstrated on PET scan. ATT was further continued for 6 months in these two patients and second line drugs were stopped once the contrast MRI showed healed lesion in one and healed psoas abscess on ultrasonography.
Figure 3.
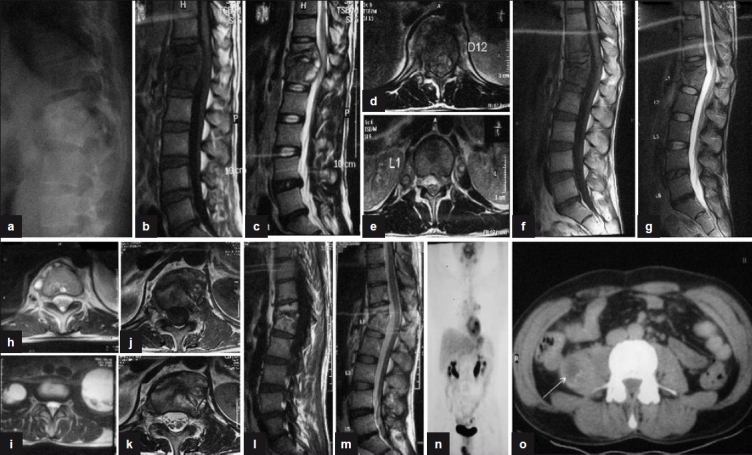
(a) Plain X-ray lateral view shows anterior wedge collapse of D12 and L1 vetebra with complete obliteration of disc space. (b, c) Sagittal T1W and T2W sections show paradiscal lesions with intraosseous caseation, preserved discs with anterior subligamentous spread of prevertebral collection, and anterior epidural spread of collection compressing the spinal cord. (d, e) T2WI (axial) of D12 and L1 show septate prevertebral collection, intraosseous caseation with large anterior epidural collection, and 60% canal encroachment. (f, g) Sagittal T1WI and T2WI show persistent intraosseous caseation with decrease in epidural collection. (h, i) T2WI (axial) shows persistent septate collections with bilateral psoas collections at 18 months. (j, k) T1WI and T2WI (axial) show near complete resolution of paravertebral collections with residual marrow edema and no epidural extension at 24 months. (l, m) Sagittal T1WI and T2WI show fatty replacement of marrow in D12 and L1 vertebra seen as bright T1 signal with near complete resolution of collections and marrow edema at 30 months. (n, o) Coronal and axial PET scan shows persistent low grade activity in right psoas with no FDG uptake in the vertebra involved
One patient [Figure 4], whose pus from a discharging sinus was sent for culture and sensitivity, was found to have drug resistance to RCin, INH, Streptomycin, Ethionamide, and Ofloxacin. This patient responded well to second line drugs and has received 18 months of second line ATT. The ATT will continue for another 1 year in this case. The last case was an underweight, anemic young girl who had lumbosacral TB with bilateral psoas abscess. She was previously on a poorly devised ATT regimen for 4 months when she developed a new lesion (dorsal spine) while on ATT. She underwent a middorsal costotransversectomy which revealed thick pus, while drainage from Petit's triangle gave thin watery (suggestive of responsive TB lesion) pus. The patient was given IV alimentation and started on second line ATT. Fortunately, the BACTEC culture report revealed the Mycobacterium to be sensitive to all first line drugs despite the previous poor regimen. The girl was switched on to first line ATT, and on her last followup, she had shown significant clinical improvement in general symptoms and is still on followup.
Figure 4.
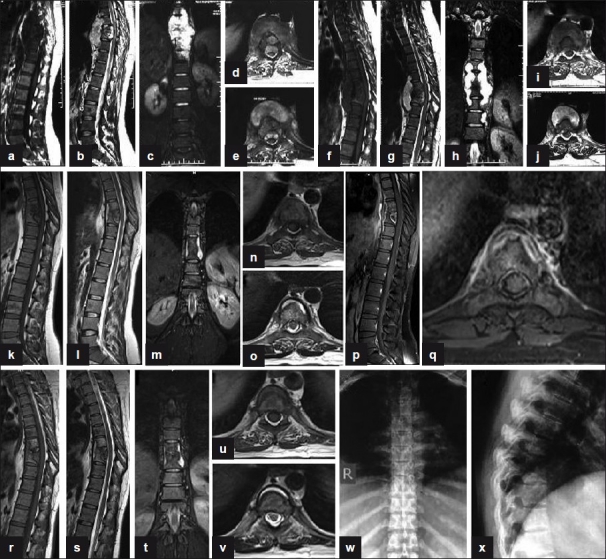
(a, b) Sagittal T1W and T2W (preoperative) sections show paradiscal lesions with intraosseous caseation, preserved discs with discitis, anterior subligamentous spread of large prevertebral collection, anterior epidural spread of collection compressing the spinal cord. (c) Coronal T2W section shows large paravertebal collection. (d, e) Axial T1W and T2W sections show septate prevertebral collection, intraosseous caseation with large anterior epidural collections, and 80% canal encroachment. (f, g) Sagittal T1W and T2W (4-month post-op) sections show persistent collections and further destruction of D7, D8, and D9 vertebrae. (h) Coronal T2W section shows persistent large paravertebal collection. (i, j) Axial T1W and T2W sections show persistent septate loculated collections and anterior epidural extension. (k, l, m, n, o) MR sagittal, coronal, and axial (at 9 months of second line ATT) sections show significant resolution of collections with persistence of a thin rim of prevertebral collections with preserved discs and near-complete resolution of anterior epidural abscess. (p, q) Post-Gd-DTPA contrast sagittal and axial T1W sections show a thin rim of paravertebral abscess with complete resolution of anterior epidural abscess. (r, s, t, u, v) MR sagittal, coronal, and axial (at 14 months of second line ATT) sections show significant resolution of collections with patchy replacement of bone marrow by fat in D8, D9 vertebrae. A thin rim of anterior prevertebral collection is seen. (w, x) Plain X-ray AP and lateral views (at 18 months of second-line ATT) show sharpening and sclerosis of paradiscal margins with no significant paravertebral shadows suggestive of healing
DISCUSSION
Drug resistance has a recorded history as old as the development of antibiotics.3,4 The bacteria undergo mutation when exposed to newer antibiotics and it occurs more if the drugs are exposed in subclinical doses.9–11 Drug resistance to antitubercular drugs has also been reported as early as 1948.3,4 When a patient of TB develops resistance to many drugs, it poses a serious health threat.
The patient may show a poor response to treatment due to non compliance or a faulty drug regimen in drug combination, dosage duration, or spurious drugs. Drug resistance is one of the causes of failure of treatment.12 It could be because of infection by an atypical organism in an immunocompromised patient or may be acquired. When an organism develops resistance to one drug, it is called monoresistance, while resistance to two or more drugs is called polyresistance. If a patient develops resistance to Rifampicin and Isoniazid, it is called as multi drug resistance (MDR-TB).8,13 Extensive drug resistance is labeled when organisms are resistant to Rcin and INH (MDR-TB) and also to an injectable aminoglycoside (other than Streptomycin) and a fluoroquinolone.8
Drug resistance is a manmade problem13 and arises as a consequence of inappropriate treatment or by exposure to a case with MDR-TB or due to co-infection with HIV or in an immunocompromised state due to socioeconomic deprivation or in a patient on anticancer therapy or in patients with comorbidities such as diabetes.
The MDR-TB can be diagnosed only if it is suspected. The pulmonary TB lesion in view of high oxygen concentration has abundant M. tuberculosis organism. When a case of pulmonary TB continues to remain sputum positive under CAT I or RNTCP DOTS treatment at 5 months or under CAT II at 4 months, it is labeled as a suspected case of MDR-TB.8 History of previous drug treatment or repeated defaulters or a patient who has converted to sputum negative and then again becomes sputum positive also raise suspicion for MDR-TB cases.8 The problem with spinal TB is that it is a paucibacillary disease and chances of demonstrating Mycobacterium on smear and culture vary between 25 and 75% in a virgin case.5,7 The chances of the culture showing growth and demonstrating culture positivity are very low. Spinal TB is a deep-seated lesion where the tissue cannot be procured repeatedly; hence, it is difficult to suspect and diagnose drug resistance.14,15 So, our suspicion has to be clinical and radiological, which is not reliable. The radiological sign of a lesion responding to drug treatment also lags behind by 2 months. It takes 2–3 months to show a discernible remineralization in an area of spine where there is no overlap of lung shadow.16 Hence, the radiological predictors are unreliable. MRI observation demonstrates inflammatory activity, and therefore MRI done at 2-3 months even in a responsive TB spine lesion would show increased destruction.17 In such cases, if a patient does not show clinical improvement or there is deterioration of spinal deformity or a new lesion appears or ulcer/sinus fails to heal or if wounds undergo dehiscence after surgery at 5 months, they should be considered as therapeutically refractory or suspected MDR-TB cases. MDR-TB can only be labeled once drug resistance is demonstrated after culture. It is prudent we label them as therapeutically refractory TB spine.8 The MRI finding shows deterioration in the post-treatment period for around 2–4 months. However, if MRI shows a deteriorating picture at 5-6 months of treatment, we should suspect a therapeutically refractory case. We could demonstrate drug resistance in two cases only. There was one case where the Mycobacterium cultured was sensitive to all primary line drugs, which was already suspected from the thin and watery consistency of the intraoperative pus collection.
Once therapeutically refractory spinal TB is suspected, the patient should be put on immunomodulation.18–20 Immune potentiation does occur on a regimen which we have followed.20 The surgical debridement of the lesion will reduce the disease load and give adequate tissues to be sent for AFB smear, culture, and sensitivity histology and PCR to ascertain the diagnosis. The tissue for culture and sensitivity for Mycobacterium should be sent to laboratories that follow quality assurance protocols and have national/international accreditation. It is recommended to send the samples to at least two such laboratories and results of the better laboratory should be relied on. If results are contradictory, then either the results of the better laboratory should be relied on or the result with the worst case scenario should be entertained.8 The tissue should also be sent for PCR and histology to reduce the risk of false diagnosis in case the culture and sensitivity does not show growth of the bacteria.
The difficulty with spinal TB is failure to get growth of tubercular organism on culture. We could get positive culture in 3 out of 13 reports for culture and sensitivity. In one sample, the resistance was for Rcin and INH, while in another case it was resistant to five drugs (Rcin, INH, Ethionamide, streptomycin, and Ofloxacin) and the third one was sensitive to all the drugs. In rest of the cases, the diagnosis was confirmed on histological evidence as all tissues showed evidence of granulomatous osteomyelitis. This raises a question as to how should these cases which have not shown any growth be treated. When the culture report was awaited, we had started these patients on second line regimen. The drugs selected should be based on a drug susceptibility pattern of the community which may not be available in most of the low-resource countries, particularly for spinal TB. The complete drug-o-gram should be prepared before starting any new drug.6 One should use drugs which have never been used. Pyrazinamide is usually added even if used before because resistance to this drug is rare. Preferably five (at least four) new drugs should be added that include a fluoroquinolone and one injectable drug. Bactericidal drugs should be preferred. One should use a drug that has never been used. One should never add a single drug to a failing regimen. The drugs should be given in a single dose and in a daily dosage regimen. One should not give intermittent therapy. The compliance of drug intake is very important and patient should be counseled not to stop treatment even if discomfort persists or appears.
Since in a spinal lesion we could not monitor them by conversion of culture report, we evaluated the lesions by MRI-based observations, and on regression of a lesion at 1 year, we continued three to four drugs in a continuation phase. We continued again for another year, and on observing resolution of lesion on MRI, the drugs were stopped and patients were clearly monitored for 2 years.
The tubercular lesions were considered healed on followup contrast enhanced MRI on observing complete loss of marrow edema, resolution of paravertebral collections and replacement of vertebral body marrow by fat as seen as increased signal on T1-weighted images.21,22,23,24 Gillams et al.24 concluded that a high signal intensity rim on T1 weighed images at the edge of lesion was suggestive of healing, and reconstituted marrow was fatty and appeared to have equal intensity at both T1 and T2 phases. They also added that progressive reduction and complete disappearance of gadolinium enhancement of bone lesions and of soft tissue is highly suggestive of healing lesion. FDG PET shows increased uptake in tuberculosis. But FDG PET also shows increased uptake in neoplastic as well as other granulomatous diseases like sarcoidosis, histoplasmosis, aspergillosis, and coccidioidomycosis. PET cannot be used to differentiate between neoplastic and nonneoplastic causes of increased uptake.25,26 Hence in patients with ambiguous MRI findings or enhancing areas on MRI after full clinical resolution of the symptoms and completion of ATT increased uptake on FDG PET corroborates presence of active infection. FDG PET provides a quantitative measurement of the absolute fraction of the injected dose reaching a tissue called the Standard Uptake Value (SUV). SUV values are raised in cases of active tuberculosis.
The optimal duration of ATT has still not been defined even for MDR pulmonary TB.8 It is said to be 18-24 months after sputum conversion. Since it is not possible to demonstrate bacteriological conversion in a spinal lesion, these lesions may be evaluated by contrast MRI, and in cases of persistence of abscess, evaluation by PET scan. Pawar et al.27 have suggested predictors of good clinical outcome as progressive improvement in clinical picture at 6 months and radiological improvement in successive X-rays. All these patients who have resistance to three or less drugs and requiring four or less second line drugs with continued compliance of drug intake will respond well.
CONCLUSION
Therapeutically refractory (MDR-TB) spinal TB is a man made problem due to inappropriate drug regimen taken irregularly. It is better to prevent than to treat. The suspicion of therapeutically refractory case is of paramount importance. Once suspected, surgery to procure tissue for diagnosis and culture is to be undertaken. We have to use a proper combination of drugs till the lesion heals as this is the last chance for the patient to effectively control disease activity.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.The World Health Organization/International Union Against Tuberculosis and Lung Disease (WHO/UNION) Global Project on Anti-Tuberculosis Drug Resistance Surveillance 2002–2007 Anti-Tuberculosis Drug Resistance In The World: Fourth Global Report [Google Scholar]
- 2.Park K. 21st ed. Jabalpur, India: M/s Banarsidas Bhanot Publishers; 2011. Park textbook of preventive and social medicine; p. 164. [Google Scholar]
- 3.Croften J, Mitchison D. Streptomycin resistence in pulmonary tuberculosis. Br Med J. 1948;2:1009–15. doi: 10.1136/bmj.2.4588.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pyle MM. Relative numbers of resistant tubercle bacilli in sputum of patients before and during treatment with streptomycin. Proc Mayo Clin. 1947;22:465–73. [PubMed] [Google Scholar]
- 5.Tuli SM. Challenge of therapeutically refractory and multidrug resistant tuberculosis in orthopaedic practice. Indian J Orthop. 2002;36:211–3. [Google Scholar]
- 6.4th ed. WHO. Treatment of tuberculosis: Guidelines. WHO/HTM/TB/2009.420. [Google Scholar]
- 7.Jain AK. Tuberculosis of the spine: A fresh look at an old disease. J Bone Joint Surg Br. 2010;92- B:905–13. doi: 10.1302/0301-620X.92B7.24668. [DOI] [PubMed] [Google Scholar]
- 8.Multidrug and extensively drug-resistant TB (M/XDR-TB): global report on surveillance and response. 2010 [Google Scholar]
- 9.Dooley SW, Simone PM. Clinical tuberculosis. London: Chapman and Hall; 1994. The extent and management of drug-resistant tuberculosis: The American experience; pp. 171–89. [Google Scholar]
- 10.Cardoso RF, Cooksey RC, Morlock GP, Barco P, Cecon L, Forestiero F, et al. Screening and Characterization of Mutations in isoniazid-resistant Mycobacterium tuberculosis isolates obtained in Brazil. Antimicrob Agents Chemother. 2004;48:3373–81. doi: 10.1128/AAC.48.9.3373-3381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heym B, Alzari PM, Honore N, Cole ST. Missense mutations in the catalase-peroxidase gene,katG, are associated with isoniazid resistance in Mycobacterium tuberculosis. Mol Microbiol. 1995;15:235–45. doi: 10.1111/j.1365-2958.1995.tb02238.x. [DOI] [PubMed] [Google Scholar]
- 12.Loddenkemper R, Sagebiel D, Brende A. Strategies against multidrug-resistant tuberculosis. Eur Respir J. 2002;36(Suppl):66–7. doi: 10.1183/09031936.02.00401302. [DOI] [PubMed] [Google Scholar]
- 13.Davis HL. Probability distribution of drug-resistant mutants in unselected populations of Mycobacterium tuberculosis. Appl Microbiol. 1990;20:810–4. doi: 10.1128/am.20.5.810-814.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain AK, Jena SK, Singh MP, Dhammi IK, Ramachadran VG, Dev G. Evaluation of clinico-radiological, bacteriological, serological, molecular and histological diagnosis of osteoarticular tuberculosis. Indian J Orthop. 2008;42:173–7. doi: 10.4103/0019-5413.40253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuli SM. Tuberculosis of the spine: A historical review. Clin Orthop. 2007;460:29–38. doi: 10.1097/BLO.0b013e318065b75e. [DOI] [PubMed] [Google Scholar]
- 16.Desai SS. Early diagnosis of spinal tuberculosis by MRI. J Bone Joint Surg Br. 1994;76:863–9. [PubMed] [Google Scholar]
- 17.Tuli SM. 4th ed. New Delhi: Jaypee Brothers Medical Publishers; 2010. Tuberculosis of the skeletal system; pp. 220–226. [Google Scholar]
- 18.Immunomodulation in multi-drug resistant and non-responsive cases of skeletal tuberculosis. Orthop Today. 2001;3:147–9. [Google Scholar]
- 19.Tuli SM. Preliminary observation of the effect of immunomodulation in multidrug resistant cases of osteo-articular tuberculosis. Indian J Orthop. 1999;33:83–5. [Google Scholar]
- 20.Arora A. Basic science of host immunity in osteoarticular tuberculosis – A clinical study. Indian J Orthop. 2006;40:1–15. [Google Scholar]
- 21.Sharif HS, Clark DC, Aabed MY, Haddad MC, al Deeb SM, Yaqub B, et al. Granulomatous spinal infections: MR imaging. Radiology. 1990;177:101–7. doi: 10.1148/radiology.177.1.2399306. [DOI] [PubMed] [Google Scholar]
- 22.Smith AS, Weinstein MA, Mizushima A, Coughlin B, Hayden SP, Lakin MM, et al. MR imaging characteristics of tuberculous spondylitis vs.vertebral osteomyelitis. AJR Am J Roentgenol. 1989;153:399–405. doi: 10.2214/ajr.153.2.399. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman EB, Crosier JH, Cremin BJ. Imaging in children with spinal tuberculosis.A comparison of radiography, computed tomography and magnetic resonance imaging. J Bone Joint Surg Br. 1993;75:233–9. doi: 10.1302/0301-620X.75B2.8444943. [DOI] [PubMed] [Google Scholar]
- 24.Gillams AR, Chaddha B, Carter AP. MR appearances of the temporal evolution and resolution of infectious spondylitis. AJR Am J Roentgenol. 1996;166:903–7. doi: 10.2214/ajr.166.4.8610571. [DOI] [PubMed] [Google Scholar]
- 25.Pitman AG, Hicks RJ, Binns DS, Ware RE, Kalff V, McKenzie AF, et al. Performance of sodium iodide based 18F-fluorodeoxyglucose positron emission tomography in the characterization of indeterminate pulmonary nodules or masses. Br J Radiol. 2002;75:114–21. doi: 10.1259/bjr.75.890.750114. [DOI] [PubMed] [Google Scholar]
- 26.Kostakoglu L, Agress H, Jr, Goldsmith SJ. Clinical role of FDG PET in evaluation of cancer patients. Radio Graphics. 2003;23:315–40. doi: 10.1148/rg.232025705. [DOI] [PubMed] [Google Scholar]
- 27.Pawar UM, Kundnani V, Agashe V, Nene A, Nene A. Multidrug-resistant tuberculosis of the spine--is it the beginning of the end? A study of twenty-five culture proven multidrug-resistant tuberculosis spine patients. Spine (Phila Pa 1976) 2009;34:E806–10. doi: 10.1097/BRS.0b013e3181af7797. [DOI] [PubMed] [Google Scholar]


