Abstract
The current study examined the effects of gonadectomy (GDX) and subsequent testosterone treatment of male Long-Evans rats on an operant variable delay spatial alternation task (DSA). Gonadally-intact rats (intact-B), GDX rats receiving implants that delivered a physiological level of testosterone (GDX-T), and GDX rats receiving blank implants (GDX-B) were tested for 25 sessions on a DSA task with variable inter-trial delays ranging from 0 to 18 s. Acquisition of the DSA task was found to be enhanced following GDX in a time and delay dependent manner. Both the GDX-T and the intact-B rats had lower performance accuracies across delays initially, relative to GDX-B rats, and this deficit persisted into subsequent testing sessions at longer delays. The GDX-T and intact-B rats also had a tendency to commit more perseverative errors during the early testing sessions, with both groups persisting in pressing a lever which had not been associated with reinforcement for at least two consecutive trials. However, both the GDX-T and intact-B groups were able to achieve performance accuracy similar to that of the GDX-B rats by the final sessions of testing. Overall, these results suggest that castration of adult male rats enhances their acquisition of an operant DSA task.
Keywords: Gonadectomy, Working memory, Testosterone, Cognition, DSA
Introduction
Research suggests a role for sex steroids in cognition, but the extent and direction of this relationship is unclear. Human studies relating general cognitive processes in men to low testosterone levels, either following age related declines or androgen deprivation therapy (ADT), are inconsistent (see Alibhai and Mohamedali, 2010a; Cherrier et al., 2009; Driscoll and Resnick, 2007; Nelson et al., 2008). The relationship between testosterone and specific cognitive systems, including working memory, are also unclear (Alibhai et al., 2010b; Janowsky et al., 2000; Young et al., 2010).
Several lines of research suggest parallels between rodent and human brain systems, including prefrontal and hippocampal memory systems (Kesnar and Churchwell, 2011; Kesnar and Hopkins, 2006; Jones, 2002). A large body of research shows that the prefrontal cortex and hippocampus play important but distinct roles in working memory in humans, nonhuman primates and rodents (e.g. see Castner et al., 2004; D’Espostio, 2007; Kesnar et al., 2004). Although the rodent brain is not as anatomically or cognitively complex as the human or primate brain, rodent models can be useful for studying cognitive processes and brain function in a simpler system (Kesnar and Churchwell, 2011). Rodent studies have established an important role for gonadal steroids in the cognitive performance of adult rodents. To date, the majority of research has focused on the activational effects of 17β-estradiol on the performance of a variety of learning and memory tasks in females (see Frick, 2009; Korol, 2004; Luine, 2008). Conversely, relatively little attention has been paid to the potential activational effects of androgenic steroids on learning and memory in male rodents (see Van Haaren et al., 1990).
Research has provided evidence that testosterone affects performance accuracy of adult male rats in maze-based learning tasks, but the findings have not been consistent across studies. Performance in maze paradigms designed to tap reference memory (i.e. trial-independent memory, typically acquired with repeated training: Dudchneko, 2004; Frick et al., 1995) has been evaluated in several studies. GDX adult male rats acquired the location of a platform hidden in a static location in the Morris water maze (MWM) at the same rate as gonadally-intact rats (Isgor and Sengelaub, 1998; Mohaddes et al., 2009; Sandstrom et al., 2006; Spritzer et al., 2008). GDX also failed to affect overall reference memory error rate on a 12-arm radial maze (Gibbs and Johnson, 2008). However, GDX rats treated with testosterone at a physiological level (3.6 ng/ml serum: typical adult serum testosterone ranges from 0.59 to 4.0 ng/ml, Bimonte-Nelson et al., 2003; Chambers et al., 1991; Miller and Riegle, 1978; Moger, 1977; Wang et al., 1993) committed more reference memory errors than gonadally-intact rats in this same study (Gibbs and Johnson, 2008). Other research found that intra-hippocampal testosterone administration to gonadally-intact male rats, which would result in supra-physiological levels, dose-dependently impaired the acquisition of the location of a hidden platform in the MWM (Emamian et al., 2010; Moradpour et al., 2006; Naghdi et al., 2001; 2003). Still other studies failed to find any effects following peripheral treatment of gonadally intact rats with other androgenic compounds, including synthetic anabolic-androgenic steroids, on maze tasks designed to test reference memory (Clark et al., 1995; Smith et al., 1996), suggesting some specificity for the role of testosterone in these dose-dependent reference memory effects.
Most studies report a mnemonic-impairing effect of castration on maze tasks that tap working memory (i.e. trial dependent memory, typically of use for making a single response: Dudchneko, 2004; Frick et al., 1995). For instance, GDX male rats commit more working memory errors than gonadally-intact rats on both the 8-arm and 12-arm radial maze (Daniel et al., 2003; Gibbs and Johnson, 2008; Harrell et al., 1990; Spritzer et al., 2008). One study found that testosterone treated (4.59 ng/ml serum) rats committed fewer working memory errors in an 8-arm maze (Spritzer et al., 2011), while surprisingly, testosterone treatment (3.6 ng/ml serum) of GDX rats did not restore working memory performance on a 12-arm maze in another study (Gibbs and Johnson, 2008). Further, when working memory was assessed using a delayed-matching-to-place water maze task, performance following a 60 min delay was significantly impaired following GDX, with testosterone treatment (at a level not statistically different from intact controls) restoring performance accuracy to that of gonadally-intact male rats (Sandstrom et al., 2006). Together, these results suggest that GDX may not significantly affect acquisition on tasks which tap reference memory, whereas GDX may impair performance on maze tasks designed to test working memory.
Relatively little attention has been paid to the role of testosterone in the performance of behavioral tasks which rely heavily on the prefrontal cortex. Although the working memory components of the maze-based tasks discussed above have a prefrontal component, these tasks typically use long inter-trial delays (see Aggleton et al., 1995; Pontecorvo et al., 1996; Van Hest and Steckler, 1996), and rely heavily on the hippocampus for accurate performance (D’Hooge and De Deyn, 2001; Floresco et al., 1997; Goldman-Rakic, 1995; Jones, 2002). Conversely, operant tasks, which utilize short inter-trial delays, including both the delayed-matching-to sample and delayed alternation tasks, can selectively engage the prefrontal cortex (see Kesnar, 2005). For example, pharmacological inactivation or lesioning of the prefrontal cortex results in marked deficits on operant tasks with short inter-trial intervals. Deficits are measured following delays as short as 1–3 s (Chudasama and Muir, 1997; Harrison and Mair, 1996; Mair et al., 1998; Sloan et al., 2006; Van Haaren et al., 1985, 1988; Young et al., 1996). When longer inter-trial delays are included (typically in the 10–15 s range or longer) hippocampal disruption also can impair performance accuracy (Chudasama and Muir, 1997; Dunnett, 1985; Kirkby and Higgins, 1998; Izaki et al., 2008; Maruki et al., 2001). These results suggest that operant working memory tasks that incorporate short inter-trial delays are differentially sensitive to prefrontal disruption.
In a seminal study, performance accuracy on an operant delayed spatial alternation (DSA) task with variable inter-trial delays of 15, 30, and 60 s was impaired in GDX male rats (Van Hest et al., 1988). The response accuracy of gonadally-intact male rats increased more rapidly across 50 sessions of DSA testing than did that of GDX rats, an effect that was conserved across all three inter-trial delays. Consistent with this, acquisition of a T-maze alternation task with a short inter-trial delay of <10 s was also impaired following GDX (Kritzer et al., 2001). Performance of GDX rats was restored to that of gonadally-intact rats following treatment with physiological levels of testosterone (3–4 ng/ml blood) (Kritzer et al., 2001). Conversely, a separate study found no effect on the acquisition of a delayed matching to position T-maze task with inter-trial delays of <5 s in GDX male rats (Gibbs, 2005). Treatment with a supra-physiological level of testosterone (14.4 ng/ml serum) also failed to affect acquisition of GDX rats on this task, although the performance of the testosterone treated rats appeared to be less effected when longer inter-trial delays (30, 60, and 90 s) were used (Gibbs, 2005).
We have published a series of papers describing a detrimental role of chronic 17β-estradiol treatment on the performance of an operant DSA task in adult ovariectomized female rats (Neese et al., 2010a; Wang et al., 2008, 2009). Although ovariectomized vehicle treated and 17β-estradiol treated rats performed at similar levels during the initial sessions of testing, the performance accuracy of the 17β-estradiol treated rats did not improve at the same rate as that of the vehicle treated rats across subsequent testing sessions. Specifically, impairments were measured when inter-trial delays of 3, 6, and 9-s were imposed between opportunities to respond. Performance was, on average, equivalent between groups when either a 0-s delay (no delay) or an 18-s delay was imposed. As outlined above, this pattern of deficits is suggestive of a prefrontal cortical effect (Chudasama and Muir, 1997; Harrison and Mair, 1996; Mair et al., 1998; Sloan et al., 2006; Van Haaren et al., 1985, 1988; Young et al., 1996). The present study was conducted in order to determine what effect GDX and subsequent testosterone treatment had on the performance of male rats on this same operant DSA task.
Methods
Animals and exposure
Thirty-eight male Long-Evans rats, 18–20 weeks of age, were obtained from Harlan (Indianapolis, IN) and were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). Rats were housed in a temperature and humidity controlled room (22 °C, 40–55% humidity) on a 12-h reverse light–dark cycle (lights off at 8:30 am). All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Urbana-Champaign and were in accordance with the guidelines of the Public Health Service Policy on Humane Care and Use of Laboratory Animals (National Institutes of Health, 2002) and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2003).
Rats were single-housed in standard polycarbonate cages (45 × 24 × 20 cm) with corncob bedding. All rats were subject to isoflurane gas anesthesia prior to bilateral GDX or sham GDX (intact). Testis was excised through a small incision in the testis sac. A loop of surgical suture (Harvard Apparatus, #615527) was tied around the connective tissue on the anterior side of the testis prior to excision. The skin was then closed with wound clips (Becton Dickinson, #427631) and the area treated with bacitracin. At the time of surgery, rats were implanted with a Silastic capsule containing testosterone (GDX-T) or a blank capsule (GDX-B and intact-B). The final group sizes were: GDX-B, n=13; GDX-T, n=13; intact-B, n=12. The Silastic capsule was 5.5 cm in length (1.57 mm i.d., 3.18 mm o.d.). One end was plugged with 0.25 cm silicone and dried overnight before packing with testosterone (T1500, Sigma-Aldrich, St. Louis, MO) or left blank, after which the other end was plugged with silicone. Capsules were soaked in sterile saline at 37 °C overnight before insertion during surgery. Previous research has found implants of these specifications to produce stable plasma testosterone levels of about 2.5 ng/ml for at least 76 days (Damassa et al., 1976), a time point exceeding the length of the current study. Plasma testosterone levels for intact Long-Evans male rats of the age used in this study typically range from 2.0 to 5.0 ng/ml (Butler et al., 2001; Fentie et al., 2004; Keating and Tcholakian, 1979; Smith et al., 1992).
Soy is present in many rodent diets and has been found to influence the behavior of male rats in other studies (Lee et al., 2004; Lund et al., 2001). Because of the wide variability of soy content seen across lots of standard rodent chow (Brown and Setchell, 2001; Thigpen et al., 2004, 2007), the rats in this study were maintained on a low-soy diet (Harlan diet 2016, Madison, WI). Water was available ad libitum. Beginning one week after GDX surgery, wound clips were removed and rats were weighed daily and food was restricted to maintain them at 85% of their free-feeding body weights. During behavioral testing, rats were fed 1 h after the daily test session was completed. Operant training began two weeks following GDX and occurred once daily, six days/week during the dark phase of the light cycle.
Operant testing
Behavioral testing was conducted in standard automated operant chambers (Med Associates Inc., St. Albans, VT) housed in sound-attenuated wooden boxes (interior dimensions: 55.9 cm wide, 38.1 cm high, 35.6 cm deep). All of the test chambers had the same features and dimensions: 21.6 cm tall, with a 29.2 cm wide and 24.8 cm deep stainless-steel grid floor that rested just above a tray filled with corncob bedding. A pellet trough was centered 2.5 cm above the floor on the operant panel. A pair of retractable response levers and a pair of stimulus cue lamps, one above each lever, were positioned symmetrically on both sides of the pellet dispenser. The levers were 5.7 cm from the midline and 7.0 cm above the floor and the cue lights were located 5.7 cm above the levers. Each chamber also contained a Sonalert tone generator, a white noise generator, and a house light located on the back wall. Experimental contingencies were programmed using the Med-State behavioral programming language (Med-Associates, Ver-mont). Forty-five milligram soy-free purified rodent diet food pellets (5TUL, TestDiet, Richmond, IN) were used as reinforcers.
Response shaping and lever press training
Rats were trained to press the response levers by using an autoshaping program that has been used extensively, both by our group and by others for lever press training (e.g. Neese et al., 2010a, 2010b; Newland et al., 1986; Verma and Moghaddam, 1996; Widholm et al., 2001, 2003). At the beginning of the session, both response levers were extended into the chamber. A cue light above the right response lever was also illuminated according to a fixed-time 3-min schedule (FT-3 min), under which the cue light was illuminated for a 15 s duration every 3-min. Upon termination of the cue light, a reinforcer was delivered. Presses on either lever at any time during the autoshaping program resulted in immediate reinforcement. If a lever press occurred when the cue light was illuminated, a reinforcer was delivered and the cue light was immediately extinguished. The FT-3 cue illumination schedule remained in effect until a total of 10 lever presses had occurred, at which time delivery of reinforcers became contingent upon lever presses. Autoshaping sessions terminated after 60 min elapsed or 100 reinforcers were delivered, whichever occurred first. Criterion for this condition was set at 100 lever presses within a single session.
Following autoshaping, the rats were exposed to a continuous reinforcement (CRF) schedule in which the lever associated with reinforcement alternated following the delivery of every fifth reinforcer. The purpose of this schedule was to strengthen the recently acquired lever press response and to prevent the rats from developing a lever or side preference. Each session began with the randomly determined extension of either the right or left lever into the test chamber and the illumination of the cue light above the extended lever. Following five lever presses, the cue light was extinguished and the lever retracted. The previously unavailable lever was then extended and the corresponding cue light illuminated. This cycle of alternating levers terminated after 100 reinforcers were received or 60 min had elapsed. A performance criterion of 100 reinforcers for two consecutive sessions was established for this condition.
Training phases
After lever-press training, the rats were trained on two alternation tasks that have produced consistent and replicable behavior across a variety of treatment paradigms (e.g. Gendle et al., 2004; Neese et al., 2010a, b; Roegge et al., 2005; Widholm et al., 2004). The sequence began with cued alternation (CA) training in which a cue light indicated the correct lever on each trial. Each correct, cued lever press was associated with reinforcement. No delay was imposed between trials during CA training, although the response levers were retracted and extended between each trial (the time between retraction and extension of the levers was <0.15 s). Rats were trained to a criterion of one session above chance, defined as >60% correct presses. Next, a non-cued alternation (NCA) training task was presented where the cue light no longer indicated the correct lever. Both cue lights were illuminated when the levers were extended. Correct responses again consisted of alternating right and left lever presses, with the response levers again retracting and extending between presses. Each rat was tested for 10 consecutive sessions on the NCA training task.
Delayed spatial alternation (DSA) testing
The testing phase was a DSA task in which variable delays of 0, 3, 6, 9, or 18 s were imposed randomly between trials (see Neese et al., 2010a, 2010b; Roegge et al., 2005; Wang et al., 2008, 2009; Widholm et al., 2004). Following each lever press, levers were retracted, and again extended following one of the temporal delays listed above. At the beginning of each session a press on either lever was associated with reinforcement. After the initial press, the correct lever was always the lever opposite of the one on which the most recent “correct” lever press occurred. Delays were randomly balanced within each session such that any specific delay was not presented on more than three consecutive trials. There were 40 trials at each delay, with a total of 200 trials per session. Each rat was tested for 25 sessions, a testing schedule which we have used extensively (Neese et al., 2010a, 2010b; Wang et al., 2008, 2009).
Statistical analyses
The behavioral data were analyzed using SPSS for Windows, Version 18.0. Experimental group (GDX-T, GDX-B, or intact-B) was included in the analyses as the between subject factor and statistical significance was set at p < 0.05. Simple interaction effects were tested with one-way ANOVAs, and when appropriate, two-tailed Tukey post hoc tests were run for pair-wise comparisons (as no direction of effect was predicted). In order to describe effects which approached significance, but to not overstate these measured effects, p-values from Tukey analyses which were over 0.05 but less than 0.10 are discussed in terms of “marginal significance”.
For autoshaping and CRF training, sessions to criterion served as the primary measure of learning, and were analyzed independently via one-way ANOVA for experimental group. For CA, cumulative errors across all sessions served as the main measure of learning and were analyzed using between-subjects ANOVA for experimental group. For NCA, the overall proportion correct across the ten sessions served as the primary measure of learning, and was analyzed using a 3 (experimental group) × 10 (session) mixed ANOVA where session was a repeated measures factor. The latencies to respond following either a correct or an incorrect lever press during NCA training were analyzed separately using a 3 (experimental group) × 10 (session) mixed ANOVA where session was a repeated measures factor.
For DSA, the proportion correct across the 25 test sessions was first averaged across blocks of five test sessions to produce five 5-session test blocks. Proportion correct at each delay across the 25 test sessions was then analyzed using a mixed 3 (experimental group) × 5 (block) × 5 (delay) repeated measures ANOVA with block (1–5) and delay (0, 3, 6, 9, 18 s) serving as repeated measures factors. The latencies to lever press following either a correct or an incorrect response during DSA testing were analyzed separately using a 3 (experimental group) × 5 (block) mixed ANOVA where block was a repeated measures factor.
Error pattern analyses were also used to assess the rats’ tendency to repeat a correct or incorrect response. A “win-stay” error was defined as an incorrect response on the same lever that had been correct on the previous trial, whereby the rat responded correctly on the n – 1 trial, but incorrectly on the nth trail. A “lose-stay” error was defined as an incorrect lever press on the same lever that had been incorrect on the previous trial, whereby the rat responded incorrectly on the n – 1 trial as well as the nth trial. Lose-stay errors thus represent at least three consecutive presses on the same lever. Win-stay and lose-stay errors were analyzed separately using a mixed 3 (experimental group) × 5 (block) repeated measures ANOVA with block (1–5) serving as a repeated measures factor.
Results
Response shaping and lever press training
Experimental group did not influence sessions needed to acquire autoshaping or the continuous reinforcement schedule. The rats needed only 2 sessions to acquire the lever press response, with the exception of 1 rat from the intact-B group which required one additional testing session. All of the rats acquired the CRF schedule in 2 sessions.
Cued and non-cued alternation training
The GDX-B (mean ± SEM: 229.31 ± 23.43), GDX-T (279.38 ± 27.99), and intact-B (227.25 ± 14.42) groups had similar error rates during CA training. This was confirmed by a non-significant one-way ANOVA, F(2,35) = 1.661, p = 0.205.
The performances of the experimental groups also did not differ on proportion correct during NCA training. This was revealed by a nonsignificant experimental group × session ANOVA, F(18,315)=1.267, p = 0.245 (Fig. 1), and a nonsignificant main effect of experimental group, F(2,35) = 0.096, p = 0.909. A significant main effect of session was uncovered, F(9,315) = 126.71, p < 0.001. As Fig. 1 shows, the performances of all experimental groups improved across subsequent sessions of testing.
Fig. 1.
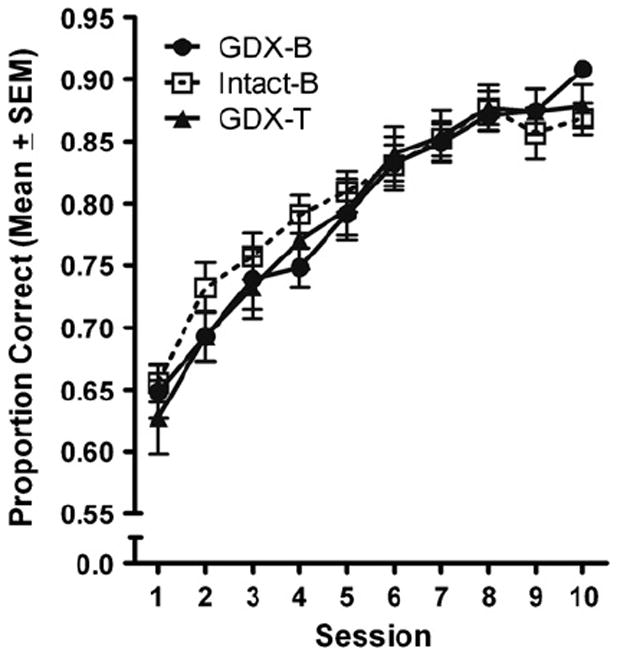
Proportion correct across 10 sessions of NCA training. No effect of experimental group was measured.
The latencies to respond following either a correct or an incorrect lever press during NCA training were subtly effected by experimental group. These main effects approached significance as revealed by repeated measures ANOVA for latencies following a correct press, F(2,35) = 2.944, p = 0.066, or following an incorrect press, F(2,35) = 3.015, p = 0.062. As Figs. 2A and B show, overall, latencies to respond tended to be longer in GDX-B rats. A main effect of session was also uncovered for latencies following either a correct, F(9,315) = 8.567, p < 0.001, or an incorrect lever press F(9, 315) = 13.832, p < 0.001. Latencies following a correct response tended to increase across training sessions, while latencies following an incorrect response tended to decrease across training sessions.
Fig. 2.
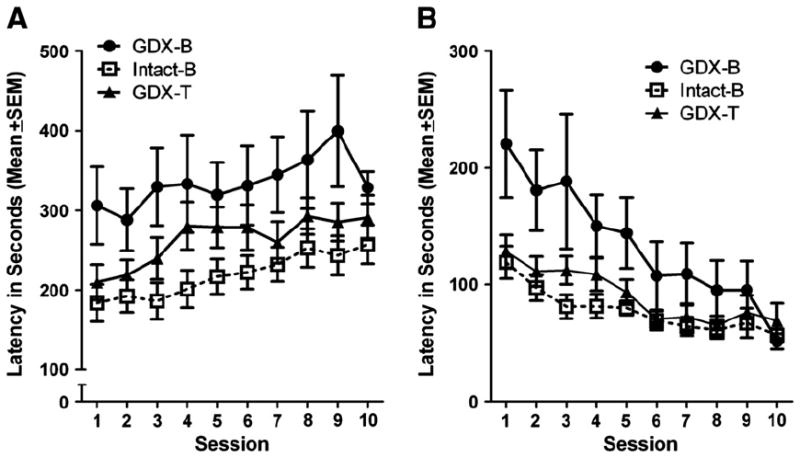
(A) Latency to lever press following a correct lever press. GDX-B rats tended to take longer to respond. (B) Latency to lever press following an incorrect lever press. GDX-B rats tended to take longer to respond.
Delayed spatial alternation
Proportion correct
Performance during the DSA testing sessions differed significantly by experimental group. This was revealed by a significant block × delay × experimental group interaction, F(32,650) = 1.939, p = 0.047. A significant block × delay interaction was also uncovered, F(16,560)=27.438, p < 0.001. As Fig. 3 shows, at each delay, the performance of all experimental groups improved across subsequent blocks of testing. For ease of comparison, the group effects are listed below, sorted by delay.
Fig. 3.
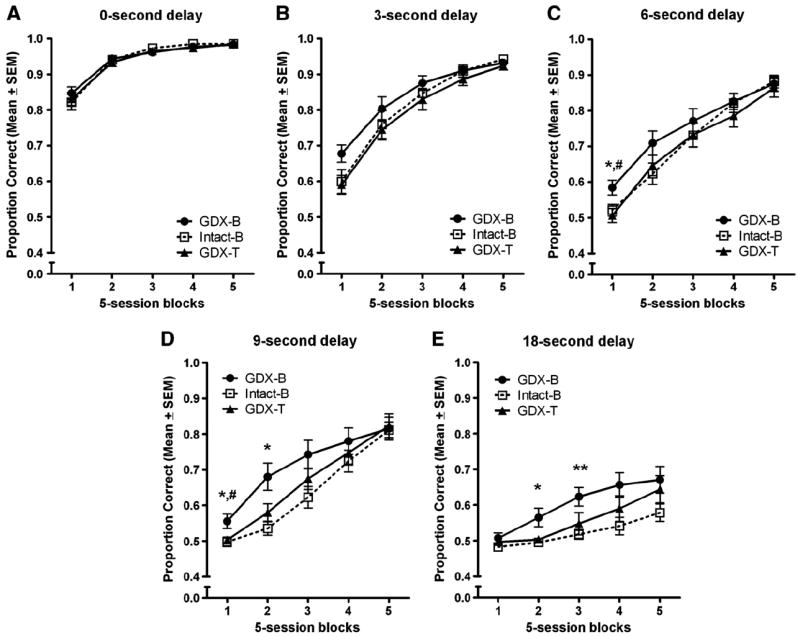
Proportion correct across 5-day blocks of testing, sorted by inter-trial delay. (A) 0-second delay. No effect of experimental group was measured. (B) 3-second delay. No effect of experimental group was measured. (C) 6-second delay. 1st block of testing: GDX-B rats performed better than did the *GDX-T and #intact-B rats. (D) 9-second delay. 1st block: GDX-B rats performed better than did the *GDX-T and #intact-B rats. 2nd block: GDX-B rats performed better than did the *GDX-T and *intact-B rats. (E) 18-second delay. 2nd block: GDX-B rats performed better than did the *GDX-T and *intact-B rats. 3rd block: GDX-B rats performed better than did the **intact-B rats. (*,**p < 0.05, #p < 0.10).
0-second delay
As Fig. 3A shows, the performances of the experimental groups did not differ when no delay was imposed between opportunities to press. This lack of effect is consistent with the equivalent performances across experimental groups during NCA training.
3-second delay
Follow-up analyses uncovered a marginally significant simple effect of experimental group in the 1st block of testing, F(2,35) 3.024, p = 0.061. The GDX-B group tended to perform better in this block of testing (Fig. 3B). Comparisons between experimental groups for blocks 2–5 of testing did not approach significance.
6-second delay
Follow-up analyses at the 6-s delay uncovered a significant simple effect of experimental group in the 1st block of testing, F(2,35) = 4.801, p = 0.014. Tukey post hoc analyses found the GDX-B group to perform better than the GDX-T group (p = 0.016) and marginally better than the intact-B group (p = 0.070) (Fig. 1C).
9-second delay
At the 9-s delay, follow-up analyses uncovered significant simple effects of experimental group in the 1st [F(2,53) = 4.076, p = 0.026] and 2nd [F(2,35) = 6.565, p = 0.004] blocks of testing. Tukey post hoc analyses found that, during the 1st block of testing, the GDX-B group performed better than the intact-B (p = 0.041) and marginally better than the GDX-T (p = 0.057) groups (Fig. 1D). In the 2nd block of testing, the GDX-B group performed significantly better than both the intact-B (p = 0.033) and the GDX-T (p = 0.048) groups. Follow-up analysis of simple effects for experimental group in the 3rd block of testing revealed an effect that approached statistical significance, F(2,35) = 3.072, p = 0.059 (Fig. 1D).
18-second delay
In contrast to the shorter delays, at the 18-s delay, the performance accuracy of all experimental groups was statistically equivalent in the 1st block of testing (Fig. 1E). However, follow-up analyses of simple effects of experimental group revealed significant effects in the 2nd [F(2,35) = 5.186, p = 0.011] and 3rd [F(2,35) = 4.556, p = 0.017] blocks of testing. In the 2nd block of testing, Tukey post hoc analyses found the GDX-B group to perform better than did both the GDX-T (p = 0.033) and the intact-B (p = 0.017) groups (Fig. 1E). In the 3rd block of testing, post hoc analyses found the GDX-B group to perform better than did the intact-B group, p = 0.017. Lastly, a simple main effect of experimental group that approached significance was measured in the 4th block of testing, F(2,35) = 3.101, p = 0.058 (Fig. 1E).
Error patterns
Experimental group influenced the type of errors committed during DSA testing. This was revealed by a significant block × experimental group interaction for lose-stay errors, F(8,140) = 3.094, p = 0.013. Follow-up analyses of simple effects for experimental group found a significant difference in the 1st [F(2,35) = 3.661, p = 0.036] and 2nd [F(2,35) = 4.612, p = 0.017] blocks of testing. Tukey post hoc analyses found the GDX-B group to commit fewer lose-stay errors than did the GDX-T (p = 0.062) and intact-B (p = 0.066) groups in the 1st block of testing (Fig. 4). Post hoc analyses also found the GDX-B group to commit fewer lose-stay errors than did the intact-B group (p = 0.016) in the 2nd block of testing (Fig. 4). A follow-up analysis for simple effects of experimental group also revealed an effect in the 3rd block of testing that approached significance, F(2,35) = 2.697, p = 0.081. No significant main effect of experimental group, F(2,35) = 1.719, p = 0.194, or experimental group × block interaction, F(8,140) = 0.550, p = 0.729, was observed for win-stay errors. Finally, a main effect of block was uncovered for both lose-stay, F (4,140) = 265.664, p < 0.001, and win-stay errors, F(4,140) = 149.073, p < 0.001. All experimental groups committed fewer errors of both types across subsequent blocks of testing.
Fig. 4.
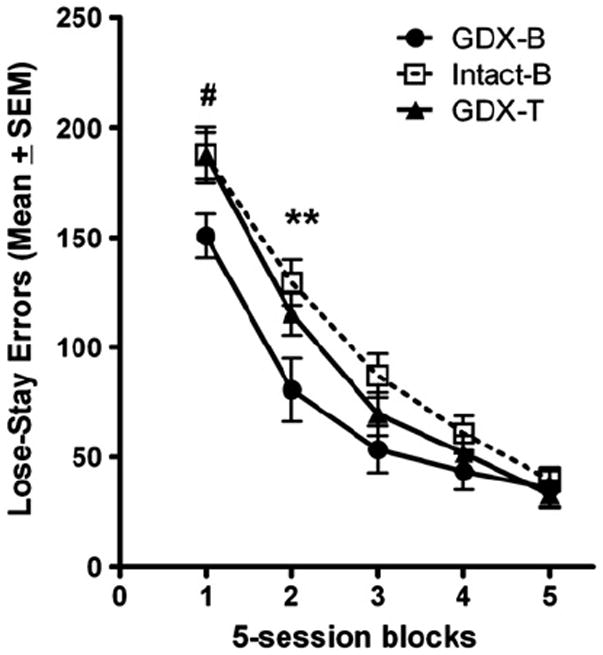
Lose-stay errors committed across five 5-day blocks of testing. 1st block: GDX-B rats committed more lose-stay errors than both the #GDX-T and #intact-B rats. 2nd block: GDX-B rats committed fewer lose-stay errors than did the **intact-B rats. (**p < 0.05, #p < 0.10).
Latencies to lever press
Similar to NCA training, latencies between lever presses were affected by experimental group. Repeated measures ANOVA found a main effect for experimental group which approached significance for latencies to respond following a correct lever press, F(2,35) = 2.796, p = 0.075. As Fig. 5A shows, the latencies in the GDX-B group tended to be longer than those in both the GDX-T and intact-B groups. No significant effects of experimental group were measured in the latencies to respond following an incorrect lever press (Fig. 5B).
Fig. 5.
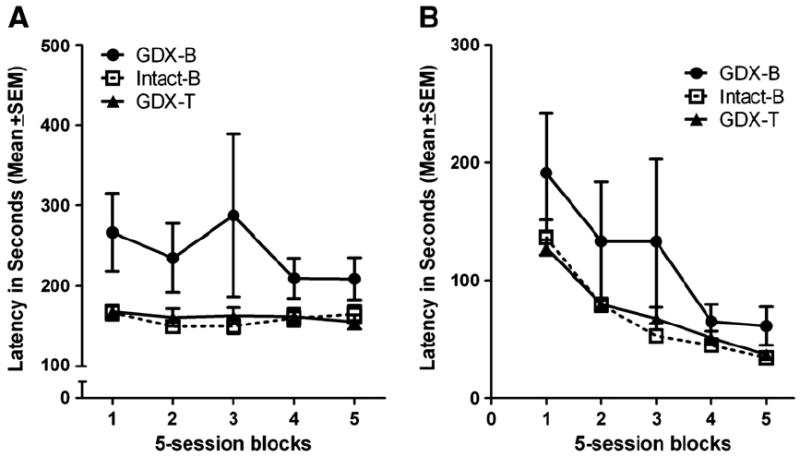
(A) Latency to lever press following a correct lever press. GDX-B rats tended to take longer to respond. (B) Latency to lever press following an incorrect lever press. No effect of experimental group was found.
Discussion
Initial acquisition of a variable delay operant DSA task was enhanced by GDX in male rats. Performances on the training phases prior to DSA testing were not influenced by GDX or subsequent testosterone treatment to GDX rats, but when variable inter-trial delays were instituted, performance accuracy of the GDX-T and intact-B groups was impaired in comparison to that of the GDX-B group. The performance deficit measured in both the GDX-T and intact-B groups was time and delay dependent, present only during the initial block of testing at the shorter delays, but extending into later blocks of testing at the two longest delays. The impairment in acquisition suggests a disruption in working memory because the deficit became progressively worse at longer delays (Pontecorvo et al., 1996; Van Hest and Steckler, 1996). Further, deficits were measured at both short and longer inter-trial delays, suggesting an influence of testosterone on both the prefrontal cortex and hippocampus (Chudasama and Muir, 1997; Dunnett, 1985; Kirkby and Higgins, 1998; Harrison and Mair, 1996; Izaki et al., 2008; Mair et al., 1998; Maruki et al., 2001; Sloan et al., 2006; Van Haaren et al., 1985, 1988; Young et al., 1996). It is important to note that there were subtle differences between the performance of the GDX-T and intact-B groups. Research has shown that plasma testosterone levels fluctuate throughout the day in intact male rats (2–5 fold: Bartke et al., 1973), which may explain some of the differences measured between these 2 groups.
Error-pattern analyses
Analysis of the error patterns found both the GDX-T and intact-B groups to commit more “lose-stay” errors. A lose-stay error is a response to a lever which had not been associated with reinforcement for at least two consecutive trials and is reflective of response perseveration (see Martinez et al., 1988; Zornetzer et al., 1982). These results are not surprising as gonadally-intact male rats tend to make more perseverative responses than female rats in an operant autoshaping task following the reversal of experimental contingencies (i.e. they continue pressing a lever no longer associated with reinforcement: Van Haaren et al., 1987). Further, testosterone treatment of gonadally-intact rats or mice also leads to an increase in perseverative responding following a change in reinforcement contingencies on both an operant discrimination task and a runway test (Archer, 1977; Thompson and Wright, 1979), and on reversal-learning trials in the MWM (Spritzer et al., 2011). On the DSA task used here, the tendency for both the GDX-T and intact-B groups to commit repetitive strings of incorrect responses was responsible for slower acquisition of the task.
The fact that both GDX-T and intact-B rats committed more perseverative errors than GDX-B rats suggests a role for acetylcholine (ACh) in the performance deficit measured here. Disruptions in ACh are associated with response perseveration in both rodents (Cabrera et al., 2006; Dalley et al., 2004; Ragozzino et al., 2002) and humans (see McNamara and Albert, 2004). Research in animal models suggests that testosterone can modulate the cholinergic system (see Mitsushima, 2010), in a brain region specific manner. One study reported a GDX induced change in choline acetlytransferase (ChAT) immunoreactive fibers in the male rat hippocampus, with testosterone treatment (7.5 mg/ml serum) reversing this effect (Nakamura et al., 2002). Conversely, no GDX associated change in ChAT immunoreactivity was measured in the frontal lobe of male rats (Kritzer, 2003). Behaviorally, scopolamine resulted in a greater disruption of response rate and a larger increase in error rate on an operant repeated acquisition task in GDX rats treated with a physiological dose of testosterone than in untreated GDX rats (Leonard et al., 2007). In a subsequent study, donepezil [an acetylcholinesterase (AChE) inhibitor] resulted in a more potent disruption of response rate and increased error rates to a greater extent in GDX rats than intact or testosterone (supraphysiological — 20.3 ng/ml serum) treated rats (Leonard et al., 2010). GDX also resulted in an increase in AChE in the hippocampus and prefrontal cortex in this study. These studies suggest that testosterone may suppress cholinergic activity in brain regions implicated in memory, and this could be an underlying mechanism responsible for the increase in perseverative responses in both the GDX-T and intact-B groups measured here.
Latencies to lever press
The latencies to respond following a lever press were also influenced by GDX. During NCA training, and to a lesser extent during DSA testing, the GDX-B group tended to respond more slowly than both the GDX-T and intact-B groups. Latencies to respond can be indicative of many things, including such non-mnemonic factors as attention, impulsivity, and/or motivation to complete a task (Carli and Samanin, 1992, 2000; Fujiwara et al., 2009; Kondrad and Burk, 2004). It is unlikely that the increased latencies in GDX rats were due to an attentional deficit, as this group had longer latencies to respond, yet actually performed better on the DSA task. This conclusion is supported by a previous study in which GDX failed to alter attentional processing in male rats (Johnson and Burk, 2006). Rather, it appears that both the GDX-T and intact-B rats responded more quickly when given the opportunity, a response pattern suggested by other operant studies which found gonadally-intact male rats to respond faster than females when reinforcement is associated with a lever press (Heinsbroek et al., 1987; Van Haaren et al., 1987; Van Hest et al., 1989).
Because DSA is an appetitive task, a GDX-induced change in motivation to work for food could potentially influence performance. Previous research shows that GDX produces a significant decrease in body weight and food intake in male rats, and that testosterone treatment can dose-dependently reverse this effect (Chai et al., 1999; Gentry and Wade, 1976). From this research it appears that androgens stimulate food intake, a factor which could affect the motivation to complete appetitively reinforced tasks. It is important to note that the GDX-B rats tended to take longer to complete the task (as indicated by latencies to respond), yet this group performed better on this task overall. Although both the GDX-T and intact-B groups may have been more motivated to lever press for food, this “motivation” failed to improve performance of the DSA task.
Previous research has established a performance enhancing effect in GDX male rats in other behavioral paradigms, including both avoidance and consummatory conflict tasks (Mora et al., 1983; Svensson et al., 2000). Although acquisition of these tasks is reliant upon a mnemonic process, inhibitory control is also important for accurate performance. In line with this, it is important to note that an inhibitory control deficit could play a role in the impaired acquisition of both the GDX-T and intact-B rats on the DSA task, as impulsive-type behaviors have been found to disrupt working memory performance on other operant tasks (Bizot and Thiebot, 1996; Dellu-Hagedorn, 2006; Reading and Dunnett, 1991). A difference in inhibitory control between the GDX-T and intact-B rats could theoretically be a reason for both the shorter response latencies and the perseverative responding measured in this group, but it would require further testing to confirm this.
Relationship to previous rodent research
The mnemonic enhancing effect we found in GDX rats stands in contrast to other studies which found performance deficits in GDX rats on water- and radial-maze tasks which engage a rat’s natural foraging strategy and tap working memory (Daniel et al., 2003; Gibbs and Johnson, 2008; Harrell et al., 1990; Kritzer et al., 2001; Sandstrom et al., 2006; Spritzer et al., 2008, 2011). Testosterone treatment often restores performance to gonadally-intact levels (Sandstrom et al., 2006; Spritzer et al., 2011). A detrimental effect of GDX was also measured on a T-maze alternation task, which is procedurally similar to the operant alternation task used here (Kritzer et al., 2001). As discussed, there are many procedural differences between maze-based and operant-based working memory tasks, (Aggleton et al., 1995; Pontecorvo et al., 1996; Van Hest and Steckler, 1996), making it difficult to directly compare the results of this study with those of maze-based tasks (see also Porter et al., 2000; Steckler et al., 1998). Operant-based tasks do not require the animal to remember movement through space, but rather to remember the location of response levers. Thus, a search process strongly dependent on spatial perception is not required to attain the food reinforcer (see also Mellgren and Elsmore, 1991), a difference which may explain why our findings were contrary to those measured in the T-maze (Kritzer et al., 2001). That said, our results are in agreement with at least one maze-based study that measured perseverative behavior in GDX rats treated with testosterone in the MWM (Spritzer et al., 2011).
It is important to note that the current findings also differ from those of a previous study which found a GDX-induced impairment on the performance of an operant DSA task (Van Hest et al., 1988). In that study, the response accuracy of gonadally-intact male rats improved more rapidly across 50 sessions of testing than did that of GDX male rats when variable delays of 15, 30, and 60 s were imposed between opportunities to respond. Further, gonadally-intact rats learned the task at a faster rate regardless of delay. Equivalent deficits across all delay intervals may be indicative of a non-mnemonic process, such as motivation or possibly a motor impairment, rather than a working memory impairment, per se (see Pontecorvo et al., 1996; Van Hest and Steckler, 1996). Additionally, Van Hest et al. imposed three inter-trial delays, two of which were much longer than the inter-trial delays used in the current study, and they did not include 0-s (no delay) trials. Performance accuracy can be altered by the length of other inter-trial intervals and by the inclusion of trials with no delay (Honig and Wasserman, 1981: Honig, 1987; Van Hest and Steckler, 1996; White and Bunnell-McKenzie, 1985). The incorporation by Van Hest et al. of a 4 s “time out” following an incorrect response may also play a role in these differences, as this negative consequence influences both performance accuracy and rate of acquisition in some operant matching to sample studies (Ferster and Appel, 1961; Zimmerman and Ferster, 1963). Other methodological factors which may have contributed to these differences in outcomes include differences in housing conditions (Ferdman et al., 2007; Frick et al., 2003) and rat strain (Andrews et al., 1995; Lindner and Schallert, 1988).
The delays used in this study where chosen in order to determine if GDX had a dissociable impact on working memory performance as mediated by the prefrontal cortex or hippocampus. As discussed, all groups were able to accurately complete the task when no delay was imposed between opportunities to press, as evidenced by the similar learning curves displayed in Fig. 3A. Conversely, performance deficits occurred in the initial testing block for the shorter delays in the GDX-T and intact-B groups, suggestive of prefrontal cortical dysfunction (Chudasama and Muir, 1997; Harrison and Mair, 1996; Mair et al., 1998; Sloan et al., 2006; Van Haaren et al., 1985, 1988; Young et al., 1996). Interestingly, all groups initially performed near chance when the 18-s delay was imposed, but the GDX-T and intact-B groups exhibited a deficit in acquisition in later blocks, suggestive of hippocampal dysfunction (Chudasama and Muir, 1997; Dunnett, 1985; Kirkby and Higgins, 1998; Izaki et al., 2008; Maruki et al., 2001). The temporal delay in the emergence of this effect suggests a unique contribution of the hippocampus on this deficit. The reasons for this are not known at this time. Overall, these data suggest the contribution of multiple brain regions in the delayed acquisition of a working memory task as measured here.
Contribution of light/dark cycle
The rats in this study were tested during the dark portion of a reverse light–dark cycle, during the rat’s normal active period, whereas in other studies the animals are most often tested during the light phase of the cycle (e.g. Daniel et al., 2003; Sandstrom et al., 2006). Time of testing during the light–dark cycle can influence behavioral outcomes. Specifically, when maintained on a reverse light–dark cycle, older rats tended to perform better on a Morris water maze task, while the opposite was true for younger rats (Winocur and Hasher, 1999, 2004). Old rats also performed better on an operant delayed alternation task when tested in the early part of the dark cycle vs. later in the dark cycle (Winocur and Hasher, 1999, 2004). Due to the complex nature of light/dark cycle and behavioral performance, it is difficult to predict to what degree testing during the dark phase of the cycle may have affected the performance of the rats used in this study.
Relationship to 17β-estradiol effects on DSA testing
We have previously reported a detrimental effect of 17β-estradiol treatment on the performance of ovariectomized female rats on the same DSA task used here (Neese et al., 2010a; Wang et al., 2008, 2009). In those studies, 17β-estradiol was found to impair acquisition of the DSA task in later blocks of testing, specifically following the 3-, 6-, and 9-s inter-trial delays. The deficit was unrelated to any specific pattern of error, and persisted until the cessation of 25-sessions of testing. Although the deficits measured here were limited to early blocks of testing and error patterns indicated perservative responding, the effects uncovered here could potentially be mediated by the conversion of testosterone to 17β-estradiol. Importantly, 17β-estradiol can influence the performance of male rats on some behavioral tasks (see Foy et al., 2008; Packard, 1998). Although 17β-estradiol produced an effect similar that of testosterone in GDX rats in a short delay T-maze alternation task (Kritzer et al., 2001), results were not equivalent in a short delay matching to position T-maze task (Gibbs, 2005). In accordance, an important area for future research should aim to determine the effects of 17β-estradiol and the testosterone derivative dihydrotestosterone (DHT) on the performance of male rats on this operant DSA task.
Importance to human research
A few studies have addressed the relationship between testosterone and working memory in men, and the results of these studies are mixed (Alibhai et al., 2010b; Janowsky et al., 2000; Young et al., 2010). The results reported here were subtle and short-lived but seem to indicate that a loss of circulating testosterone may result in a short-term improvement in working memory. Our data also support a role for testosterone in perseverative behavior (see also Spritzer et al., 2011), a behavioral pattern which can result in impairments on working memory tasks. Interestingly, a human study also related poorer performance on tests of executive function, specifically set-shifting, in aging men with higher testosterone levels (Martin et al., 2007). Importantly, some studies are beginning to suggest a curvilinear relationship in which “optimal” levels of testosterone may result in a positive working memory impact (see Matousek and Sherwin, 2010), a dose-dependent effect which we did not investigate in the current study. As this is the first animal study to report a deficit on a short-delay operant alternation task, more research is needed to fully understand this effect, and how it relates to working memory processing in men.
Summary and conclusions
In conclusion, we found GDX of adult male rats to result in an enhancement in the acquisition of a variable delay operant DSA task relative to gonadally-intact and GDX testosterone-treated rats. Following GDX, male rats performed better at shorter delays during the initial block of testing than did both GDX-T and intact-B male rats, an effect which persisted into later blocks at longer delays. Both the GDX-T and intact-B rats also committed more perseverative errors during the earlier blocks of testing, which explains the lower performance accuracy in these two groups. These deficits were dependent upon the presentation of a delay between opportunities to press, and could be reflective of a working memory deficit and/or a deficit in inhibitory control. Future research should include longer delays (i.e. 30 and 60 s) in order to clarify the influence of testosterone on performance accuracy in this operant DSA task as it relates to a previously published study (Van Hest et al., 1988), and to determine if these deficits would persist into later blocks following longer inter-trial delays.
Acknowledgments
This research was supported by the National Institute on Aging Grant P01 AG024387 (SLS). Steven Neese also received support from the National Institute of Environmental Health Sciences Grant T32 ES007326.
References
- Aggleton JP, Neave N, Nagle S, Sahgal A. A comparison of the effects of medial prefrontal, cingulated cortex, and cingulum bundle lesions of spatial memory: evidence of a double dissociation between frontal and cingulum bundle contribution. J Neurosci. 1995;15:7270–7281. doi: 10.1523/JNEUROSCI.15-11-07270.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alibhai SMH, Mohamedali HZ. Cardiac and cognitive effects of androgen deprivation therapy: are they real? Curr Oncol. 2010a;17:S55–S64. doi: 10.3747/co.v17i0.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alibhai SMH, Mahmoud S, Hussain F, Naglie G, Tannock I, Tomlinosn G, Fleshner N, Krahn M, Warde P, Klotz L, Breunis H, Leach M, Canning SD. Levels of sex hormones have limited effect on cognition in older men with or without prostate cancer. Crit Rev Oncol Hematol. 2010b;73:167–175. doi: 10.1016/j.critrevonc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Andrews JS, Jansen JH, Linders S, Princen A, Broekkamp CL. Performance of four different rat strains in the autoshaping, two-object discrimination, and swim maze tests of learning and memory. Physiol Behav. 1995;57:785–790. doi: 10.1016/0031-9384(94)00336-x. [DOI] [PubMed] [Google Scholar]
- Archer J. Testosterone and persistence in mice. Anim Behav. 1977;25:479–488. doi: 10.1016/0003-3472(77)90023-9. [DOI] [PubMed] [Google Scholar]
- Bartke A, Steele RE, Musto N, Caldwell BV. Fluctuations in plasma testosterone in adult male rats and mice. Endocrinology. 1973;92:1223–1228. doi: 10.1210/endo-92-4-1223. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Nelson ME, Eckman CB, Barber J, Scott TY, Granholm AC. Testosterone, but not nonaromatizable dihydrotestosterone, improves working memory and alters nerve growth factor levels in aged male rats. Exp Neurol. 2003;181:301–312. doi: 10.1016/s0014-4886(03)00061-x. [DOI] [PubMed] [Google Scholar]
- Bizot JC, Thiebot MH. Impulsivity as a confounding factor in certain animal tests of cognitive function. Cogn Brain Res. 1996;3:243–250. doi: 10.1016/0926-6410(96)00010-9. [DOI] [PubMed] [Google Scholar]
- Brown NM, Setchell KD. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab Invest. 2001;81:735–747. doi: 10.1038/labinvest.3780282. [DOI] [PubMed] [Google Scholar]
- Butler PC, Mills RH, Bloch GJ. Inhibition of lordosis behavior in male and female rats by androgens and progesterone. Horm Behav. 2001;40:384–395. doi: 10.1006/hbeh.2001.1703. [DOI] [PubMed] [Google Scholar]
- Cabrera SM, Chavez CM, Corley SR, Kitto MR, Butt AE. Selective lesions of the nucleus basalis magnocellularis impair cognitive flexibility. Behav Neurosci. 2006;120:298–306. doi: 10.1037/0735-7044.120.2.298. [DOI] [PubMed] [Google Scholar]
- Carli M, Samanin R. Serotonin2 receptor agonists and serotonergic anorectic drugs affect rats’ performance differently in a five-choice serial reaction time task. Psychopharmacology. 1992;106:228–234. doi: 10.1007/BF02801977. [DOI] [PubMed] [Google Scholar]
- Carli M, Samanin R. The 5-HT(1A) receptor agonist 8-OH-DPAT reduces rats’ accuracy of attentional performance and enhances impulsive responding in a five-choice serial reaction time task: role of presynaptic 5-HT(1A) receptors. Psychopharmacology. 2000;149:259–268. doi: 10.1007/s002139900368. [DOI] [PubMed] [Google Scholar]
- Castner SA, Goldman-Rakic PS, Williams GV. Animals models of working memory: insights for targeting cognitive dysfunction in schizophrenia. Psychopharmacology. 2004;174:111–125. doi: 10.1007/s00213-003-1710-9. [DOI] [PubMed] [Google Scholar]
- Chai JK, Blaha V, Meguid MM, Lavinao A, Yang ZJ, Varma M. Use of orchiectomy and testosterone replacement to explore meal number-to-meal size relationship in male rats. Am J Physiol. 1999;276:R1366–R1373. doi: 10.1152/ajpregu.1999.276.5.R1366. [DOI] [PubMed] [Google Scholar]
- Chambers KC, Thornton JE, Roselli CE. Age-related deficits in brain androgen binding and metabolism, testosterone and sexual behavior of male rats. Neurobiol Aging. 1991;12:123–130. doi: 10.1016/0197-4580(91)90050-t. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Aubin S, Higano CS. Cognitive and mood changes in men undergoing intermittent combined androgen blockade for non-metastatic prostate cancer. Psychooncology. 2009;18:237–247. doi: 10.1002/pon.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Muir JL. A behavioural analysis of the delayed non-matching to position task: the effects of scopolamine, lesions of the fornix and of the prelimbic region on mediating behaviours by rats. Psychopharmacology. 1997;134:73–82. doi: 10.1007/s002130050427. [DOI] [PubMed] [Google Scholar]
- Clark AS, Mitre MC, Brinck-Johnsen T. Anabolic–androgenic steroid and adrenal steroid effects on hippocampal plasticity. Brain Res. 1995;679:64–71. doi: 10.1016/0006-8993(95)00202-2. [DOI] [PubMed] [Google Scholar]
- D’Espostio M. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci. 2007;29:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Bouger P, Chudasama Y, Cardinal RN, Robbins TW. Cortical cholinergic function and deficits in visual attentional performance in rats following 192 IgG-saporin-induced lesions of the medial prefrontal cortex. Cereb Cortex. 2004;14:922–932. doi: 10.1093/cercor/bhh052. [DOI] [PubMed] [Google Scholar]
- Damassa DA, Kobashigawa D, Smith ER, Davidson JM. Negative feedback control of LH by testosterone: a quantitative study in male rats. Endocrinology. 1976;99:736–742. doi: 10.1210/endo-99-3-736. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Winsauer PJ, Moerschbaecher JM. Castration in rats impairs performance during acquisition of a working memory task and exacerbates deficits in working memory produced by scopolamine and mecamylamine. Psychopharmacology. 2003;170:294–300. doi: 10.1007/s00213-003-1537-4. [DOI] [PubMed] [Google Scholar]
- Dellu-Hagedorn F. Relationship between impulsivity, hyperactivity and working memory: a differential analysis in the rat. Behav Brain Funct. 2006;2 doi: 10.1186/1744-9081-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Resnick SM. Testosterone and cognition in normal aging and Alzheimer’s disease: an update. Curr Alzheimer Res. 2007;4:33–45. doi: 10.2174/156720507779939878. [DOI] [PubMed] [Google Scholar]
- Dudchneko PA. An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev. 2004;2:699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Dunnett SB. Comparative effects of cholinergic drugs and lesions of nucleus basalis or fimbria-fornix on delayed matching in rats. Psychopharmacology. 1985;87:357–363. doi: 10.1007/BF00432721. [DOI] [PubMed] [Google Scholar]
- Emamian S, Naghdi N, Sepehri H, Jananshahi M, Sadeghi Y, Choopani S. Learning impairment caused by intra-CA1 microinjection of testosterone increases the number of astrocytes. Behav Brain Res. 2010;208:30–37. doi: 10.1016/j.bbr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Fentie IH, Greenwood MM, Wyss JM, Clark JT. Age-related decreases in gonadal hormones in Long-Evans rats: relationship to rise in arterial pressure. Endocrine. 2004;25:15–22. doi: 10.1385/ENDO:25:1:15. [DOI] [PubMed] [Google Scholar]
- Ferdman N, Murmu RP, Bock J, Braun K, Leshem M. Weaning age, social isolation, and gender, interact to determine adult explorative and social behavior, and dendritic and spine morphology in prefrontal cortex of rats. Behav Brain Res. 2007;180:174–182. doi: 10.1016/j.bbr.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Ferster CB, Appel JB. Punishment of S delta responding in matching to sample by time out from positive reinforcement. J Exp Anal Behav. 1961;4:45–56. doi: 10.1901/jeab.1961.4-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, pre-frontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy MR, Baudry M, Diaz Brinton R, Thompson RF. Estrogen and hippocampal plasticity in rodent models. J Alzheimers Dis. 2008;15:589–603. doi: 10.3233/jad-2008-15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurbiol Aging. 1995;16:149–160. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- Frick KM, Stearns NA, Pan JY, Berger-Sweeney J. Effects of environmental enrichment on spatial memory and neurochemistry in middle-aged mice. Learn Mem. 2003;10:187–198. doi: 10.1101/lm.50703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara A, Lino M, Sasaki M, Hironaka N, Wakasa Y. A new behavioral test for assessment of drugs on attentional performance and its validity in cynomolgus monkeys. J Toxiciol Sci. 2009;34:183–190. doi: 10.2131/jts.34.183. [DOI] [PubMed] [Google Scholar]
- Gendle MH, Strawderman MS, Mactutus CF, Booze RM, Levitsky DA, Strupp BJ. Prenatal cocaine exposure does not alter working memory in adult rats. Neurotoxicol Teratol. 2004;26:319–329. doi: 10.1016/j.ntt.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Gentry RT, Wade GN. Androgenic control of food intake and body weight in male rats. J Comp Physiol Psychol. 1976;90:18–25. doi: 10.1037/h0077264. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm Behav. 2005;48:268–277. doi: 10.1016/j.yhbeh.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology. 2008;149:3176–3183. doi: 10.1210/en.2007-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Architecture of the prefrontal cortex and the central executive. Ann N Y Acad Sci. 1995;15:71–83. doi: 10.1111/j.1749-6632.1995.tb38132.x. [DOI] [PubMed] [Google Scholar]
- Harrell LE, Goyal M, Parsons DS, Peagler A. The effect of gonadal steroids on the behavioral and biochemical effects of hippocampal sympathetic ingrowth. Physiol Behav. 1990;48:507–513. doi: 10.1016/0031-9384(90)90291-b. [DOI] [PubMed] [Google Scholar]
- Harrison LM, Mair RG. A comparison of the effects of frontal cortical and thalamic lesions of measures of spatial learning and memory in the rat. Behav Brain Res. 1996;75:195–206. doi: 10.1016/0166-4328(96)00173-8. [DOI] [PubMed] [Google Scholar]
- Heinsbroek RP, Van Haaren F, Zantvoord F, Van de Poll NE. Sex differences in response rates during random ratio acquisition: effects of gonadectomy. Physiol Behav. 1987;39:269–272. doi: 10.1016/0031-9384(87)90020-5. [DOI] [PubMed] [Google Scholar]
- Honig WK. Memory interval distribution effects in pigeons. Anim Learn Behav. 1987;15:6–14. [Google Scholar]
- Honig WK, Wasserman EA. Performance of pigeons on delayed simple and conditional discriminations under equivalent training procedures. Learn Motiv. 1981;12:149–170. [Google Scholar]
- Isgor C, Sengelaub DR. Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 pyramidal cell morphology in rats. Horm Behav. 1998;34:183–198. doi: 10.1006/hbeh.1998.1477. [DOI] [PubMed] [Google Scholar]
- Izaki Y, Takita M, Akema T. Specific role of the posterior dorsal hippocampus-prefrontal cortex in short-term working memory. Eur J Neurosci. 2008;27:3029–3034. doi: 10.1111/j.1460-9568.2008.06284.x. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Chavez B, Orwoll E. Sex steroids modify working memory. J Cogn Neurosci. 2000;12:407–414. doi: 10.1162/089892900562228. [DOI] [PubMed] [Google Scholar]
- Johnson RT, Burk JA. Effects of gonadectomy and androgen supplementation on attention in male rats. Neurobiol Learn Mem. 2006;85:219–227. doi: 10.1016/j.nlm.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Jones MW. A comparative review of rodent prefrontal cortex and working memory. Curr Mol Med. 2002;2:639–647. doi: 10.2174/1566524023361989. [DOI] [PubMed] [Google Scholar]
- Keating RJ, Tcholakian RK. In vivo patterns of circulating steroids in adult male rats. I. Variations in testosterone during 24- and 48-hour standard and reverse light/dark cycles. Endocrinology. 1979;104:184–188. doi: 10.1210/endo-104-1-184. [DOI] [PubMed] [Google Scholar]
- Kesnar RP. Temporal processing of information: the role of the medial prefrontal cortex and hippocampus: theoretical comment on Gilmartin and McEchron (2005) Behav Neurosci. 2005;119:1705–1709. doi: 10.1037/0735-7044.119.6.1705. [DOI] [PubMed] [Google Scholar]
- Kesnar RP, Churchwell JC. An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem. 2011;96:417–431. doi: 10.1016/j.nlm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Kesnar RP, Hopkins RO. Mnemonic function in the hippocampus: a comparison between animals and humans. Biol Psychol. 2006;73:3–18. doi: 10.1016/j.biopsycho.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Kesnar RP, Lee I, Gilbert P. A behavioral assessment of hippocampal function based on a subregional analysis. Rev Neurosci. 2004;15:333–351. doi: 10.1515/revneuro.2004.15.5.333. [DOI] [PubMed] [Google Scholar]
- Kirkby DL, Higgins GA. Characterization of perforant path lesions in rodent models of memory and attention. Eur J Neurosci. 1998;10:823–838. doi: 10.1046/j.1460-9568.1998.00087.x. [DOI] [PubMed] [Google Scholar]
- Kondrad RL, Burk JA. Transient disruption of attentional performance following escalating amphetamine administration in rats. Psychopharmacology. 2004;175:436–442. doi: 10.1007/s00213-004-1857-z. [DOI] [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82:309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kritzer MF. Long-term gonadectomy affects the density of tyrosine hydroxylase but not dopamine-beta-hydroxylase-, choline acetyltranferase- or serotonin-immunoreactive axons in the medial prefrontal cortices of adult male rats. Cereb Cortex. 2003;13:282–296. doi: 10.1093/cercor/13.3.282. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, McLaughlin PJ, Smirlis T, Robinson JK. Gonadectomy impairs T-maze acquisition in adult male rats. Horm Behav. 2001;39:167–174. doi: 10.1006/hbeh.2001.1645. [DOI] [PubMed] [Google Scholar]
- Lee YB, Lee HJ, Won MH, Hwang IK, Kang TC, Lee JY, Nam SY, Kim KS, Kim E, Cheon SH, Sohn HS. Soy isoflavones improve spatial delayed matching-to-place performance and reduce cholinergic neuron loss in elderly male rats. J Nutr. 2004;134:1827–1831. doi: 10.1093/jn/134.7.1827. [DOI] [PubMed] [Google Scholar]
- Leonard ST, Moerschbaecher JM, Winsauer PJ. Testosterone potentiates scopolamine-induced disruptions of nonspatial learning in gonadectomized male rats. Exp Clin Psychopharmacol. 2007;15:48–57. doi: 10.1037/1064-1297.15.1.48. [DOI] [PubMed] [Google Scholar]
- Leonard ST, Hearn JK, Catling AD, Winsauer PJ. Gonadal hormones modulate the potency of the disruptive effects of donepezil in male rats responding under a nonspatial operant learning and performance task. Behav Pharmacol. 2010;21:121–134. doi: 10.1097/FBP.0b013e328337be3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner MD, Schallert T. Aging and atropine effects on spatial navigation in the Morris water task. Behav Neurosci. 1988;102:621–634. doi: 10.1037//0735-7044.102.5.621. [DOI] [PubMed] [Google Scholar]
- Luine VN. Sex steroids and cognitive function. J Neuroendocrinol. 2008;20:866–872. doi: 10.1111/j.1365-2826.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- Lund TD, West TW, Tian LY, Bu LH, Simmons DL, Setchell KD, Aldercreutz H, Lephart ED. Visual spatial memory is enhanced in female rats (but inhibited in males) by dietary soy phytoestrogens. BMC Neuroci. 2001;2 doi: 10.1186/1471-2202-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair RG, Burk JA, Porter MV. Lesions of the frontal cortex, hippocampus, and intralaminar nuclei have distinct effects on remembering in rats. Behav Neurosci. 1998;112:772–792. doi: 10.1037//0735-7044.112.4.772. [DOI] [PubMed] [Google Scholar]
- Martin DM, Wittert G, Burns NR, Haren MT, Sugarman R. Testosterone and cognitive function in ageing men: data from the Florey Adelaide Male Ageing Study (FAMAS) Maturitas. 2007;57:182–194. doi: 10.1016/j.maturitas.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Martinez SL, Jr, Shulteis G, Janak PH, Weinberger SB. Behavioral assessment of forgetting in aged rodents and its relationship to peripheral sympathetic function. Neurbiol Aging. 1988;9:697–708. doi: 10.1016/s0197-4580(88)80135-0. [DOI] [PubMed] [Google Scholar]
- Maruki K, Izaki Y, Hori K, Nomura M, Yamauchi T. Effects of rat ventral and dorsal hippocampus temporal inactivation on delayed alternation task. Brain Res. 2001;895:273–276. doi: 10.1016/s0006-8993(01)02084-4. [DOI] [PubMed] [Google Scholar]
- Matousek RH, Sherwin BB. Sex steroid hormones and cognitive functioning in healthy, older men. Horm Behav. 2010;57:352–359. doi: 10.1016/j.yhbeh.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara P, Albert ML. Neuropharmacology of verbal perseveration. Semin Speech Lang. 2004;25:309–321. doi: 10.1055/s-2004-837244. [DOI] [PubMed] [Google Scholar]
- Mellgren RL, Elsmore TF. Extinction of operant behavior: an analysis based on foraging considerations. Anim Learn Behav. 1991;19:317–325. [Google Scholar]
- Miller AE, Riegle GD. Serum testosterone and testicular response to HCG in young and aged male rats. J Gerontol. 1978;33:197–203. doi: 10.1093/geronj/33.2.197. [DOI] [PubMed] [Google Scholar]
- Mitsushima D. Sex steroids and acetylcholine release in the hippocampus. Vitam Horm. 2010;82:263–277. doi: 10.1016/S0083-6729(10)82014-X. [DOI] [PubMed] [Google Scholar]
- Moger Serum 5alpha-androstane-3alpha, 17beta-diol, androsterone, and testosterone concentrations in the male rat. Influence of age and gonadotropin stimulation. Endocrinology. 1977;100:1027–1032. doi: 10.1210/endo-100-4-1027. [DOI] [PubMed] [Google Scholar]
- Mohaddes G, Naghdi N, Khamnei S, Khatami S, Haeri A. Effect of spatial learning on hippocampal testosterone in intact and castrated male rats. Iran Biomed J. 2009;13:49–58. [PubMed] [Google Scholar]
- Mora S, Nasello AG, Mandelli-Lopes M, Diaz-Veliz G. LHRH and rat avoidance behavior: influence of castration and testosterone. Physiol Behav. 1983;30:19–22. doi: 10.1016/0031-9384(83)90032-x. [DOI] [PubMed] [Google Scholar]
- Moradpour F, Naghdi N, Fathollahi Y. Anastrozole improved testosterone-induced impairment acquisition of spatial learning and memory in the hippocampal CA1 region in adult male rats. Behav Brain Res. 2006;175:223–232. doi: 10.1016/j.bbr.2006.08.037. [DOI] [PubMed] [Google Scholar]
- Naghdi N, Nafisy N, Majlessi N. The effects of intrahippocampal testosterone and flutamide on spatial localization in the Morris water maze. Brain Res. 2001;897:44–51. doi: 10.1016/s0006-8993(00)03261-3. [DOI] [PubMed] [Google Scholar]
- Naghdi N, Oryan S, Etemadi R. The study of spatial memory in adult male rats with injection of testosterone enanthate and flutamide into the basolateral nucle-aus of the amygdala in Morris water maze. Brain Res. 2003;972:1–8. doi: 10.1016/s0006-8993(03)02227-3. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Fujita H, Kawata M. Effects of gonadectomy on immunoreactivity for choline acetyltransferase in the cortex, hippocampus, and basal forebrain of adult male rats. Neuroscience. 2002;109:473–485. doi: 10.1016/s0306-4522(01)00513-9. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Public Health Service Policy on Humane Care and Use of Laboratory Animals. NIH/Office of Laboratory Animal Welfare; Rockville, MD: 2002. [Google Scholar]
- National Research Council Institute for Laboratory Animals Research. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. National Academy Press; Washington, D. C.: 2003. [PubMed] [Google Scholar]
- Neese SL, Korol DL, Katzenellenbogen JA, Schantz SL. Impact of estrogen receptor alpha and beta agonists on delayed alternation in middle-aged rats. Horm Behav. 2010a;58:878–890. doi: 10.1016/j.yhbeh.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neese SL, Wang VC, Doerge DR, Woodling KA, Andrade JE, Helferich WG, Korol DL, Schantz SL. Impact of dietary genistein on aging and executive function in rats. Neurotoxicol Teratol. 2010b;32:200–211. doi: 10.1016/j.ntt.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CJ, Lee JS, Gaboa MC, Roth AJ. Cognitive effects of hormone therapy in men with prostate cancer: a review. Cancer. 2008;113:1097–1106. doi: 10.1002/cncr.23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland MC, Ng WW, Baggs RB, Gentry GD, Weiss B, Miller RK. Operant behavior in transition reflects neonatal exposure to cadmium. Teratology. 1986;34:231–241. doi: 10.1002/tera.1420340302. [DOI] [PubMed] [Google Scholar]
- Packard MG. Posttraining estrogen and memory modulation. Horm Behav. 1998;34:126–139. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- Pontecorvo MJ, Sahgal A, Stecker T. Further developments in the measurement of working memory in rodents. Brain Res Cogn Res. 1996;3:205–213. doi: 10.1016/0926-6410(96)00007-9. [DOI] [PubMed] [Google Scholar]
- Porter MC, Burk JA, Mair RG. A comparison of the effects of hippocampal or prefrontal cortical lesions in three versions of delayed non-matching-to-sample based on positional or spatial cues. Behav Brain Res. 2000;109:69–81. doi: 10.1016/s0166-4328(99)00161-8. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Jih J, Tzavos A. Involvement of the dorsomedial striatum in behavioral flexibility: role of muscarinic cholinergic receptors. Brain Res. 2002;25:205–214. doi: 10.1016/s0006-8993(02)03287-0. [DOI] [PubMed] [Google Scholar]
- Reading PJ, Dunnett SB. Response inhibition on a delayed matching to position task induces by amphetamine, nicotine and age. Psychopharmacology. 1991;104:137–139. doi: 10.1007/BF02244568. [DOI] [PubMed] [Google Scholar]
- Roegge CS, Widholm JJ, Engeseth NJ, Wang X, Brosch KO, Seegal RF, Schantz SL. Delayed spatial alternation impairments in adult rats following dietary n-6 deficiency during development. Neurotoxicol Teratol. 2005;27:485–495. doi: 10.1016/j.ntt.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Kim JH, Wasserman MA. Testosterone modulated performance on a spatial working memory task in male rats. Horm Behav. 2006;50:18–26. doi: 10.1016/j.yhbeh.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Sloan HL, Good M, Dunnett SB. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behav Brain Res. 2006;171:116–126. doi: 10.1016/j.bbr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Smith ER, Stefanick ML, Clark JT, Davidson JM. Hormones and sexual behavior in relationship to aging in male rats. Horm Behav. 1992;26:110–135. doi: 10.1016/0018-506x(92)90035-t. [DOI] [PubMed] [Google Scholar]
- Smith ST, Stackman RW, Clark AS. Spatial working memory is preserved in rats treated with anabolic–androgenic steroids. Brain Res. 1996;737:313–316. doi: 10.1016/0006-8993(96)00932-8. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Gill M, Weinberg A, Galea LA. Castration differentially affects working and reference memory in male rats. Arch Sex Behav. 2008;37:19–29. doi: 10.1007/s10508-007-9264-2. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Daviau ED, Coneeny MK, Engelman SM, Prince WT, Rodrigez-Wisdom KN. Effects of testosterone on spatial learning and memory in adult male rats. Horm Behav. 2011;59:484–496. doi: 10.1016/j.yhbeh.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler T, Drinkenburg WH, Sahgal A, Aggleton JP. Recognition memory in rats — I. Concepts and classification. Prog Neurobiol. 1998;54:289–311. doi: 10.1016/s0301-0082(97)00060-9. [DOI] [PubMed] [Google Scholar]
- Svensson AL, Soderplam B, Engel JA. Gonadectomy enhances shock-induced behavioral inhibition in adult male rats: implications for impulsive behavior. Pharmacol Biochem Behav. 2000;65:731–736. doi: 10.1016/s0091-3057(99)00266-x. [DOI] [PubMed] [Google Scholar]
- Thigpen JE, Setchell KD, Saunders HE, Haseman JK, Grant MG, Forsythe DB. Selecting the appropriate rodent diet for endocrine disruptor research and testing studies. I L A R J. 2004;45:410–416. doi: 10.1093/ilar.45.4.401. [DOI] [PubMed] [Google Scholar]
- Thigpen JE, Setchell KD, Padilla-Banks E, Haseman JK, Saunders HE, Caviness GF, Kissling GE, Grant MG, Forsythe DB. Variations in phytoestrogen content between different mill dates of the same diet produces significant differences in the time of vaginal opening in CD-1 mice and F344 rats but not in CD Sprague–Dawley rats. Environ Health Perspect. 2007;115:1717–1726. doi: 10.1289/ehp.10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WR, Wright JS. “Persistence” in mice: effects of testosterone. Physiol Psychol. 1979;7:291–294. [Google Scholar]
- Van Haaren F, De Bruin JP, Heinsbroek RP, Van de Poll NE. Delayed spatial response alternation: effects of delay-interval duration and lesions of the medial prefrontal cortex on response accuracy of male and female Wistar rats. Behav Brain Res. 1985;18:41–49. doi: 10.1016/0166-4328(85)90167-6. [DOI] [PubMed] [Google Scholar]
- Van Haaren F, Van Hest A, Van de Poll N. Acquisition and reversal of a discriminated autoshaped response in male and female rats: effects of long or short and fixed or variable intertrial interval durations. Learn Motiv. 1987;18:220–233. [Google Scholar]
- Van Haaren F, Van Zijderveld G, Van Hest A, De Bruin JP, Van Eden CG, Van de Poll NE. Acquisition of conditional associations and operant delayed spatial response alternation: effects of lesions in the medial prefrontal cortex. Behav Neurosci. 1988;102:481–488. doi: 10.1037//0735-7044.102.4.481. [DOI] [PubMed] [Google Scholar]
- Van Haaren F, Van Hest A, Heinsbroek RP. Behavioral differences between male and female rats: effects of gonadal hormones on learning and memory. Neurosci Biobehav Rev. 1990;14:23–33. doi: 10.1016/s0149-7634(05)80157-5. [DOI] [PubMed] [Google Scholar]
- Van Hest A, Steckler T. Effects of procedural parameters on response accuracy: lessons from delayed (non-)matching procedures in animals. Brain Res Cog Brain Res. 1996;3:193–203. doi: 10.1016/0926-6410(96)00006-7. [DOI] [PubMed] [Google Scholar]
- Van Hest A, Van Kempen M, Van Haaren F, Van de Poll NE. Memory in male and female Wistar rats: effects of gonadectomy, and stimulus presentations during the delay interval. Behav Brain Res. 1988;29:103–110. doi: 10.1016/0166-4328(88)90057-5. [DOI] [PubMed] [Google Scholar]
- Van Hest A, Van Haaren F, Van de Poll NE. Perseverative responding in male and female Wistar rats: effect of gonadal hormones. Horm Behav. 1989;23:57–67. doi: 10.1016/0018-506x(89)90074-3. [DOI] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci. 1996;16:373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Leung A, Sinha-Hikim AP. Reproductive aging in the male brown-Norway rat: a model for the human. Endocrinology. 1993;133:2773–2781. doi: 10.1210/endo.133.6.8243304. [DOI] [PubMed] [Google Scholar]
- Wang VC, Sable HK, Ju Y, Allred CD, Helferich WG, Korol DL, Schantz SL. Effects of chronic estradiol treatment on delayed spatial alternation and differential reinforcement of low rates of responding. Behav Neurosci. 2008;122:794–804. doi: 10.1037/a0012513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VC, Neese SL, Korol DL, Schantz SL. Chronic estradiol replacement impairs performance on an operant delayed spatial alternation task in young, middle-aged, and old rats. Horm Behav. 2009;56:382–390. doi: 10.1016/j.yhbeh.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KG, Bunnell-McKenzie J. Potentiation of delayed matching with variable delays. Anim Learn Behav. 1985;13:397–402. [Google Scholar]
- Widholm JJ, Clarkson GB, Strupp BJ, Crofton KM, Seegal RF, Schantz SL. Spatial reversal learning in Aroclor 1254-exposed rats: Sex-specific deficits in associative ability and inhibitory control. Toxicol Appl Pharmacol. 2001;174:188–198. doi: 10.1006/taap.2001.9199. [DOI] [PubMed] [Google Scholar]
- Widholm JJ, Seo BW, Strupp BJ, Seegal RF, Schantz SL. Effects of perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin on spatial and visual reversal learning in rats. Neurotoxicol Teratol. 2003;25:459–471. doi: 10.1016/s0892-0362(03)00014-x. [DOI] [PubMed] [Google Scholar]
- Widholm JJ, Villareal S, Seegal RF, Schantz SL. Spatial alternation deficits following developmental exposure to Aroclor 1254 and/or methylmercury in rats. Toxicol Sci. 2004;82:577–589. doi: 10.1093/toxsci/kfh290. [DOI] [PubMed] [Google Scholar]
- Winocur G, Hasher L. Aging and time-of-day effects on cognition in rats. Behav Neurosci. 1999;113:991–997. doi: 10.1037//0735-7044.113.5.991. [DOI] [PubMed] [Google Scholar]
- Winocur G, Hasher L. Age and time-of-day effects on learning and memory in a non-matching-to-sample test. Neurbiol Aging. 2004;25:1107–1115. doi: 10.1016/j.neurobiolaging.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Young HL, Stevens AA, Converse E, Mair RG. A comparison of temporal decay in place memory tasks in rats (Rattus norvegicus) with lesions affecting thalamus, frontal cortex, or the hippocampal system. Behav Neurosci. 1996;110:1244–1260. doi: 10.1037//0735-7044.110.6.1244. [DOI] [PubMed] [Google Scholar]
- Young LA, Neiss MB, Samuels MH, Roselli CE, Janowsky JS. Cognition is not modified by large but temporary changes in sex hormones in men. J Clin Endocrinol Metab. 2010;95:280–288. doi: 10.1210/jc.2009-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman J, Ferster CB. Intermittent punishment of S delta responding in matching to sample. J Exp Anal Behav. 1963;6:349–356. doi: 10.1901/jeab.1963.6-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zornetzer SF, Thompson R, Rogers J. Rapid forgetting aged rats. Behav Neural Biol. 1982;36:49–60. doi: 10.1016/s0163-1047(82)90234-5. [DOI] [PubMed] [Google Scholar]


