Abstract
The modular domain structure of extracellular matrix (ECM) proteins and their genes has allowed extensive exon/domain shuffling during evolution to generate hundreds of ECM proteins. Many of these arose early during metazoan evolution and have been highly conserved ever since. Others have undergone duplication and divergence during evolution, and novel combinations of domains have evolved to generate new ECM proteins, particularly in the vertebrate lineage. The recent sequencing of several genomes has revealed many details of this conservation and evolution of ECM proteins to serve diverse functions in metazoa.
Introduction
ECM proteins are typically composed of multiple protein domains, and their gene structures were some of the first recognized to have arisen by exon shuffling (Engel, 1996; Patthy, 1999; Hohenester and Engel, 2002). Biochemical analyses of ECM proteins began in vertebrates. However, as cDNA and genomic sequences became available, it became increasingly evident that many ECM genes such as collagens and laminins are very ancient, and, in the last decade, as genomic sequences were determined for many metazoa, it was recognized that many ECM-encoding genes originated early in metazoan evolution. In particular, genomes of diverse bilaterian organisms (mammals, flies, worms, sea urchins, and ascidians) revealed a common set of ECM proteins shared by all bilateria (Hynes and Zhao, 2000; Whittaker et al., 2006; Huxley-Jones et al., 2007), which is consistent with the presence of common ECM structures such as basement membranes in all these organisms. Most recently, genomes of nonbilaterian eumetazoa and basal metazoa (see Box 1 and Fig. 1 for a summary of metazoan phylogeny), as well as unicellular relatives of metazoa, have allowed investigation of the origins of this common set of ECM proteins. Furthermore, the increasing amount of genomic information has allowed investigation of the elaboration, diversification, and specialization of ECM proteins in different evolutionary lineages to subserve differing functional roles. In this brief review, I will summarize our current understanding of the diversity and evolution of ECM proteins and attempt to relate them to the evolution of multicellularity and the subsequent evolution of metazoa.
Box 1. Outline of metazoan phylogeny
Taxon:
Any phylogenetic group, such as a phylum, class, genus, or species.
Clade:
A group of organisms that all share a common ancestor. Also applied to groups of proteins that are related by evolution and divergence.
Metazoa:
Multicellular animals.
Eumetazoa:
All metazoan animals apart from Porifera (sponges), Placozoa, and a few other obscure taxa. Within the eumetazoa there are two well defined clades of bilaterally symmetric animals; protostomes and deuterostomes, which are grouped together as Bilateria. Protostomes have two subdivisions: ecdysozoa, which include arthropods and nematodes; and lophotrochozoa, which include mollusks, annelids, flatworms, and others. Deuterostomes include echinoderms, hemichordates, protochordates, and chordates. Eumetazoa also include two additional clades: ctenophores (comb jellies) and cnidaria (Hydrazoa, sea anemones, jelly fish, and the like), which are traditionally viewed as radially symmetrical (but see Martindale et al., 2002; Ball et al., 2004). They are sometimes classed together as Radiata or Coelenterata; however, the phylogenetic relationships between ctenophores and cnidaria are not certain and there are, as yet, no complete genomic sequences available for ctenophores. The major eumetazoan phylogenetic divisions are outlined in Fig. 1; they all arose before the Cambrian era, >540 million years ago.
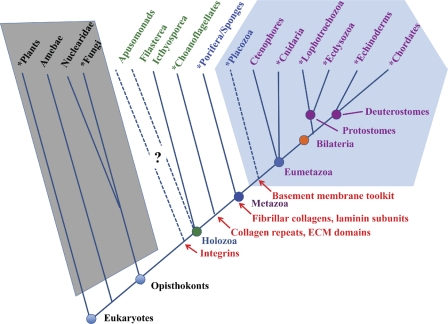
All eumetazoa have epithelial layers showing apical-basal polarity and underlain by basement membranes. Bilateria have three germ layers (ectoderm, endoderm, and mesoderm), whereas Radiata have two epithelial layers with limited interstitial cells among and between them. Two metazoan phyla (sometimes grouped as Parazoa) are basal to the eumetazoa and lack any obvious axes of symmetry: the Placozoa, which are flat, bilayered organisms with a very limited number of cell types (approximately four) and no obvious basement membranes or ECM; and Porifera, or sponges, in which most cells lack epithelial organization. Most sponges lack basement membranes, but interstitial ECM is present. The exact evolutionary relationships between the Parazoa and Eumetazoa remain incompletely defined. Most phylogenetic analyses place Placozoa closer than Porifera to the Eumetazoa, as shown in Fig. 1 (but see Schierwater et al., 2009), and, as we will see, analyses of the complement of ECM proteins conform with this conclusion.
Figure 1.
Eukaryotic phylogeny and the appearance of ECM proteins. The figure diagrams the major taxonomic divisions of eukaryotes (Box 1), and is based on published phylogenetic analyses (e.g., Philippe et al., 2005, 2009; Shalchian-Tabrizi et al., 2008; Pick et al., 2010) as well as on analyses of whole genome sequences (see main text). Taxa for which whole genome sequences are available are marked with asterisks. The diagram represents topologies of relationships, and the branch lengths are not intended to reflect accurate evolutionary distances. The relationships of apusomonads and placozoa are not well defined; their placement in the diagram is influenced in part by evidence from analyses of ECM-related genes (see main text). Taxa with the complement of ECM proteins typical of all bilateria are within the light blue hexagon. Taxa highlighted with green or blue text have some homologues of bilaterian ECM proteins or their receptors (see main text), but lack a complete set. The complexity of the known ECM protein sets in these organisms increases from left to right. In contrast, the taxa within the gray trapezoid show no evidence of any credible examples of metazoan ECM proteins. First known appearances of relevant genes/proteins or domains are marked in red. Note that taxa appearing to the right of (i.e., subsequent to) the origin of a given feature may have lost it; examples would be the absence of integrins in choanoflagellates sequenced to date and the absence of fibrillar collagens in Drosophila (in both cases, presumably by gene loss).
Major characteristics and categories of ECM proteins in metazoa
ECMs are, by definition, relatively or completely insoluble assemblies of proteins that form structures such as basement membranes, interstitial matrices, tendons, cartilage, bones, and teeth. The proteins that comprise these various ECMs are frequently large, with multiple characteristic domains specialized for protein interactions necessary for ECM assembly or for the recruitment of cells or other proteins (such as growth factors or cytokines) to the ECM (Hynes, 2009; Hynes and Naba, 2011; see Figs. 2 and 3 for illustration of domain structures). ECM proteins are frequently cross-linked by enzymatic and nonenzymatic reactions, further contributing to their insolubility. The large size, complexity, and insolubility of ECM proteins has made their analysis challenging, but the availability of complete genome sequences and their inferred complement of encoded proteins has made available reasonably reliable inventories of ECM proteins and allowed comparative analyses among species. These analyses have made clear that all bilaterian taxa share a common set of ECM proteins, with occasional examples of gene loss in certain lineages and many examples of taxon-specific elaborations based on the common set.
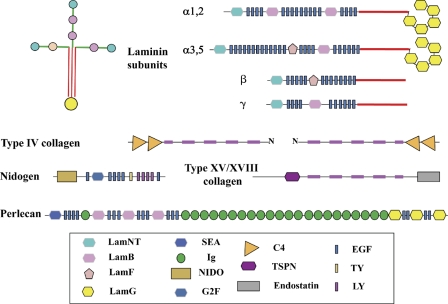
Figure 2.
The basement membrane toolkit. The figure shows the domain structures of the core proteins of metazoan basement membranes, based on domain predictions largely using SMART and Pfam domain definitions. All bilaterian clades encode this set of nine proteins in their genomes. There are typically two distinct laminin α subunits and one each of the laminin β and γ subunits. Each subunit has a characteristic domain organization. A laminin protomer is an αβγ trimer (shown at the top left) associated through coiled-coil domains in each subunit (red) and disulfide bonds (not depicted). Type IV collagen is a trimer of two homologous subunits, α1 and α2, usually adjacent in the genome in a head-to-head arrangement with a single promoter between the two genes. Signature pairs of C4 domains lie at the C termini of all type IV collagens, and the collagen segment (fuschia) is interrupted, allowing flexibility. Type IV collagen protomers associate through their N and C termini and through disulfide bonding to form a “chicken wire” network that provides structural strength to the basement membrane. Laminins bind to the collagen network and to nidogen. Perlecan, which is a complex heparan sulfate proteoglycan, is also incorporated into the basement membrane. Two other collagens, types XV and XVIII, are also associated with vertebrate basement membranes, and an orthologue is present in all bilaterian clades. The high degree of conservation of this “toolkit” over more than half a billion years testifies to the essentiality of basement membranes.
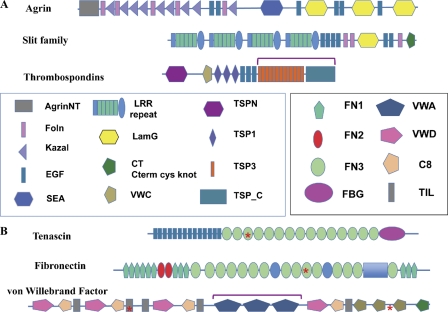
Figure 3.
Examples of ECM proteins. (A) Proteins common to all bilaterian clades: agrin, slits, and thrombospondins. The figure shows domain maps of vertebrate proteins. Agrin is involved in synapse organization and slit family members in axonal guidance, although both are also involved in other processes as well. Proteins with similar arrangements of domains are found in all bilaterian clades, although occasionally missing domains, at least according to current gene predictions. Thrombospondins are characterized by the C-terminal set of domains (bracketed), which can be associated with a variety of additional N-terminal domains (Bentley and Adams, 2010; Adams and Lawler, 2011). The particular set of domains shown (including TSPN, VWC, and TSP1 repeats) is that of so-called type A thrombospondins. Type A thrombospondins are found only in deuterostomes, but all bilateria encode examples of type B thrombospondins, which lack the TSPN, VWC, and TSP1 domains but often have EGF repeats and sometimes others. (B) Proteins found only in the chordate lineage: tenascin, fibronectin, and VWF. These proteins evolved in the deuterostome lineage (see main text) and exemplify different sorts of domain shuffling. Tenascins are built from ancient domains (EGF, FN3, and FBG) found in many proteins throughout metazoa (and even in lower organisms). However, the particular set of domains in tenascins appears first in Branchiostoma (amphioxus), and the family is expanded in vertebrates. Fibronectins are built from a mixture of ancient domains (FN3), more recent ones (FN2), and chordate-specific domains (FN1). The only true fibronectins are vertebrate-specific and, within that subphylum, highly conserved and essential. VWF is based on an ancient gene structure (mucins) altered by insertion of three VWA domains (bracketed), which incorporate many of the key functions of VWF in hemostasis in vertebrates. All three of these characteristic vertebrate ECM proteins contain RGD motifs (asterisks), which are sites for binding of integrins.
Basement membrane toolkit
Basement membranes are a characteristic feature of most metazoa, arguably an essential feature of tissue and epithelial organization, providing a locus for adhesion of epithelial cell layers and definition of basal-apical polarity of the cells (Fahey and Degnan, 2010). Studies, initially in vertebrates but more recently in invertebrates, have defined the major protein components of basement membranes (Fig. 2). All basement membranes are composed of a common set of interacting proteins (Yurchenco, 2011): a core network of cross-linked type IV collagen is associated with laminin (a trimer of related α, β, and γ subunits); nidogen, a laminin-binding glycoprotein; and perlecan, a very large and complex heparan sulfate proteoglycan. Strikingly, genes encoding this characteristic set of proteins, long-defined in vertebrates, were found in the genomes of two model protostomes, Caenorhabditis elegans (Hutter et al., 2000) and Drosophila melanogaster (Hynes and Zhao, 2000), when they were sequenced a little over a decade ago. The two homologous minor collagens XV and XVIII had also been observed to be associated with vertebrate basement membranes, although their functions were, and are, less clear. Genes encoding a collagen XV/XVIII orthologue were also found in both the fly and worm genomes. This set of 9–10 genes (2 laminin α, 1 laminin β, 1 laminin γ, 2 type IV collagen subunits, nidogen, perlecan, and 1–2 collagen XV/XVIII homologues; Fig. 2) has subsequently shown up in essentially every bilaterian genome sequenced, and we have called it the “basement membrane toolkit” (Whittaker et al., 2006). As is typical of most ECM proteins, the core constituents of basement membrane proteins are built from a set of well-defined protein domains (Fig. 2; Engel, 1996; Hohenester and Engel, 2002). This highly conserved set of genes has persisted in bilaterian genomes for well over half a billion years. This conservation indicates the essential nature of both this toolkit and the individual domains of its constituent proteins.
Fibrillar and other collagens
The most prevalent and earliest described collagens of vertebrates are those with long uninterrupted series of collagen repeats, typically ∼1,000 amino acids long. They comprise multiple repeats of the tripeptide unit Gly-X-Y, where X is frequently proline and Y is often hydroxyproline. This repeating amino acid structure allows collagen subunits to assemble into triple-helical protomers. A primordial exon (54 bp) encoding exactly six repeating Gly-X-Y tripeptides underwent duplications and modifications (such as deletions and fusions), always retaining the same phasing of introns, so that exons encoding collagen repeat units can be assembled in varying numbers and with other domains. In vertebrates, there are >40 collagen genes encoding diverse collagens (Ricard-Blum, 2011). Mammalian fibrillar collagens (11 genes) have collagen repeats flanked by characteristic noncollagenous domains at the N terminus and COLFI domains at the C terminus. In contrast, type IV collagen genes encode interrupted collagen repeats and a characteristic pair of C-terminal C4 domains (Fig. 2). Other vertebrate collagens have variations on these themes, with diverse arrays of collagen repeats with and without interruptions, interspersed with other ECM domains, such as FN3 and VWA domains (Ricard-Blum, 2011). We will discuss taxon-specific expansions of the collagen family later (see “Taxon-specific elaborations”).
As mentioned previously, type IV collagens have a pre-Cambrian origin. The same is true for fibrillar collagens. The fibrillar collagens assemble into the characteristic striated collagen fibrils of interstitial connective tissue matrices and provide structural strength to those ECMs. As such, they play crucial roles in the integrity of multicellular organisms. Fibrillar collagens are found in sponges, the most primitive metazoan phylum (Box 1 and Fig. 1). Three fibrillar collagen subclades (A, B, and C) arose before the eumetazoan radiation and are widespread, although not universal, in bilateria (Exposito et al., 2008, 2010; Heino et al., 2009). For example, Drosophila lacks any fibrillar collagens, which indicates the loss of the relevant genes in that lineage.
Proteoglycans
In addition to perlecan, vertebrate genomes encode many other proteoglycans, around three dozen in mammals. Many of these fall into two families (Merline et al., 2009; Schaefer and Schaefer 2010): one built of LRR domains and one, known as hyalectans, containing N-terminal IgV and LINK domains and C-terminal EGF-CLEC-CCP domain units, flanking a central section bearing attached glycosaminoglycans. In addition, a small family of proteins named SPOCKs or testicans are related to the ECM glycoprotein SPARC/osteonectin. The testicans, LRR repeat proteoglycans, and hyalectans have been reported only in chordates, and will be discussed later. Two membrane-bound families of proteoglycans—syndecans and glypicans (Couchman, 2010)—like perlecan, are found throughout bilateria (Ozbek et al., 2010).
ECM glycoproteins
Mammalian genomes encode around 200 further ECM glycoproteins distinct from collagens and proteoglycans (Hynes and Naba, 2011; Naba et al., 2011). These ECM glycoproteins are also built from characteristic arrays of domains of >50 different types (Figs. 2 and 3). Like the collagen repeats, these domains are typically encoded by single exons or groups of exons that have allowed shuffling during evolution of the exonic units encoding these domains to build a large variety of ECM proteins. Although the same domains can occur in many different proteins, including both ECM and non-ECM proteins, the domain composition, order, and number are characteristic of individual ECM proteins; that is, they are defined by their domain architectures. This is illustrated in Fig. 2, where the laminin subunits are clearly related to each other and share domains with nidogen and perlecan. Many of the mammalian and vertebrate ECM proteins are restricted to later-evolving taxa, as we will discuss. However, some of them are widespread in bilateria and a few more examples are shown in Fig. 3 A. These ancient ECM glycoproteins, like those of the basement membrane toolkit (Fig. 2), have been subject to strong selection since the divergence of bilateria >600 million years ago and must have fundamental functions.
Challenges of ECM phylogeny
Analyses of the evolution of ECM proteins present some challenges. As discussed, ECM proteins are large and complex, with multiple domains, which they share both among themselves and with many other proteins. Domains such as EGF, LRR, FN3, and Ig are widespread in many proteins encoded by metazoan genomes and do not themselves define ECM proteins. Therefore, simple Basic Local Alignment Search Tool (BLAST) or domain searches yield multiple partial homologues for most ECM proteins and can be misleading if not supplemented by analyses of domain composition. It is the patterns or arrangements of domains that are diagnostic of specific ECM proteins. However, because the genes are large, with many exons, they are frequently incomplete or interrupted in current databases of genomes, ESTs, cDNAs, and inferred proteins. Therefore, gene predictions for ECM proteins are significantly harder than for many other genes. Thorough analyses require high-quality genomic or cDNA sequences and, often, further annotation to yield complete and reliable ECM protein predictions. This has only become possible fairly recently for many taxa, but there has been an explosion of genomic information in recent years that has shed light on the origins of ECM proteins and, indeed, of ECM itself. These data have allowed extension of the comparative genomics of ECM beyond bilateria.
The genomes of Nematostella vectensis (starlet sea anemone; Putnam et al., 2007) and Hydra magnipapillata (Chapman et al., 2010) reveal that cnidaria share many but not the entire core set of ECM proteins found in bilateria. Some of these proteins had been described previously, based on cDNA cloning, but the completed genomes allow conclusions about what is absent as well as what is present (subject to the qualifications mentioned in the previous paragraph). The comparative analyses by Fahey and Degnan (2010) are particularly informative. They show clearly that N. vectensis encodes good homologues of most of the basement membrane toolkit: laminin (1α, 1β, and 1γ), nidogen, perlecan, and collagens (IV and XV/XVIII). H. magnipapillata also encodes these proteins, and cnidaria encode examples of all three fibrillar collagen clades. They also encode homologues of fibrillins and thrombospondins (Fig. 3 A), as well as some other ECM proteins. Also conserved across eumetazoa are cellular receptors for ECM proteins: integrins, which bind many ECM proteins; dystroglycan, which binds laminin and agrin; and CD36, which binds thrombospondins, as well as the membrane proteoglycans, syndecan, and glypican (Hynes and Zhao, 2000; Huhtala et al., 2005; Ewan et al., 2005; Whittaker et al., 2006; Knack et al., 2008; Ozbek et al., 2010). Therefore, it appears that all eumetazoan genomes encode a common set of ECM proteins, although data for ctenophores are sparse. Individual taxa may lack some of this set but it is clear that the common ancestor of eumetazoa had a reasonably complex repertoire of ECM proteins that has been largely conserved throughout subsequent evolution.
Evolution of ECM in basal metazoa
Given this strong conservation of a core set of ECM proteins in all eumetazoa, it is of obvious interest to ask when the genes encoding these proteins arose during evolution and to attempt to correlate their emergence with the acquisition of novel morphological and developmental features. The taxa closest to Metazoa are the Placozoa and the Porifera (sponges). Genomes from these two phyla have recently been completed: the placozoan Trichoplax adhaerens (Srivastava et al., 2008) and the demosponge Amphimedon queenslandica (Srivastava et al., 2010). These genomes have proven quite informative concerning the origins of ECM proteins (see also Fahey and Degnan, 2010 and Ozbek et al., 2010). As mentioned earlier, neither organism has any true basement membranes. However, the T. adhaerens genome encodes reasonably good orthologues of type IV collagen (two subunits); laminin α, β and γ subunits; and nidogen and perlecan—essentially the entire basement membrane toolkit apart from type XV/XVIII collagen. This is a surprising result given the reported absence of basement membranes in T. adhaerens and it suggests that T. adhaerens has the ingredients to make a basement membrane. Perhaps there are stages in the T. adhaerens life cycle where basement membranes are assembled or perhaps some other protein is needed for coassembly or as a cell-surface receptor. T. adhaerens does encode potential laminin receptors, including dystroglycan as well as an integrin, although the homology of the latter with subclasses of bilaterian integrins has not yet been explored. It will be of interest to determine the biosynthetic patterns and distributions of the basement membrane proteins and these potential receptors in T. adhaerens.
In contrast, the A. queenslandica genome encodes homologues of all three laminin subunits, albeit with imperfect matches in domain composition (Fahey and Degnan, 2010), but does not encode any of the other proteins of the basement membrane toolkit, which is consistent with the absence of basement membranes in demosponges. The more complete set of basement membrane proteins encoded by T. adhaerens as compared with A. queenslandica is consistent with a closer evolutionary relationship of Placozoa with eumetazoa, as shown in Fig. 1. However, it should be noted that sponges are diverse, with four distinguishable clades (Gazave et al., 2010), one of which, homoscleromorphs, has been reported to have basement membranes. Indeed, type IV collagen cDNA has been isolated from Pseudocorticium jarrei, a homoscleromorph sponge (Boute et al., 1996). Thus, it remains plausible that some sponges may express the basement membrane toolkit and assemble basement membranes, an obvious topic for future investigations.
The T. adhaerens genome also encodes many other candidate ECM glycoproteins, including a homologue of B-type thrombospondins (although in the current genome assembly, the gene may be fused with another) and a partial match with agrin. The genome includes many genes with known ECM domains in unusual combinations not seen in eumetazoa. Some of these inferred proteins include predicted transmembrane domains and may, in fact, be surface glycoproteins rather than true ECM proteins. In contrast with sponges, there is little evidence for collagens other than type IV in T. adhaerens. However, it is clear that this simple organism with only four known cell types has elaborated large numbers of genes encoding multiple ECM domains. The elaboration of ECM proteins appears further developed in Placozoa than in the sponge species analyzed to date. Further comparative analyses of the T. adhaerens genome and those of sponges should shed further light on the evolution of diverse combinations of extracellular domains in these simple metazoan animals.
Hints of earlier evolution of ECM domains in unicellular organisms
There is widespread agreement that choanoflagellates are the closest unicellular relatives of metazoa (King et al., 2003, 2008). Their characteristic cellular organization, with a collar of actin-based filopodia surrounding a single apical flagellum, is similar to that of choanocytes, the feeding cells of sponges. The complete genome of Monosiga brevicollis (King et al., 2008) and the partial one of Salpingoeca rosetta (Broad Institute Origins of Multicellularity Initiative; http://www.broadinstitute.org/annotation/genome/multicellularity_project/MultiHome.html) have revealed that these two choanoflagellates encode several proteins previously considered to be specific to metazoa. These include homologues of the cell–cell adhesion receptor cadherins. The presence of some integrin domains in choanoflagellates might also suggest a role in ECM-mediated adhesion, but there are no true integrins. There are a few genes encoding α integrin repeats, but none of them looks like a fully developed integrin subunit, and there is no evidence for any β subunits (King et al., 2008). Furthermore, neither genome encodes any of the proteins of the basement membrane toolkit. Although there are several proteins that include one or more laminin domains, only one approaches eumetazoan (or Placozoan or sponge) laminin subunits in the complexity of domain organization. However, it lacks some domains and is not a true orthologue, and there is no evidence for laminin αβγ heterotrimers. Also, collagen IV, nidogen, and perlecan all appear to be absent (King et al., 2008; unpublished data). Both choanoflagellate species encode several proteins with collagen repeats and others with COLFI domains, but so far never in the same protein, which indicates that they lack true fibrillar collagens. Both choanoflagellate species do encode a protein with multiple collagen repeats and VWA domains. This is superficially reminiscent of certain vertebrate collagens, but the matches in domain architecture are not at all good (unpublished data).
Thus it appears that choanoflagellates do encode several characteristic ECM domains, but, to date, no true matches with bilaterian ECM proteins have been found (King et al., 2008; Ozbek et al., 2010; unpublished data). The unusual VWA collagen may represent an early ECM protein, and it has been suggested that there is a putative fibrillin-like protein encoded in each genome (Ozbek et al., 2010). However, these proposed fibrillin-like proteins consist solely of EGF repeats, lack the TGF-β–binding TB domains of fibrillins, and have transmembrane domains, so their homology with fibrillins is not at all close (unpublished data). Fibrillins and the homologous latent transforming growth factor β-binding proteins (LTBPs) are involved in binding and regulating TGF-β family members but, to date, appear to be eumetazoan in origin (Robertson et al., 2011); placozoa, sponges, and choanoflagellates do not have the TB domain. In fact, M. brevicollis does not actually encode very many ECM-type proteins, and many known ECM domains, which play important roles in conserved bilaterian ECM proteins (compare Figs. 2 and 3), appear to be absent from the genome. There are also very few Ig family domains and only one or two copies of several other ECM domains, all of which are, in contrast, extremely prevalent in the T. adhaerens genome (unpublished data).
In conclusion, at this point it is clear that choanoflagellate genomes contain some domains typical of ECM proteins (LamNT, LamG, FN3, VWA, EGF, COLFI, and collagen repeats) but do not appear to have assembled them into the characteristic arrangements of domains seen in metazoan ECM proteins. They also lack many other ECM domains. Most choanoflagellates are unicellular, although S. rosetta does have a colonial phase. The transition to multicellularity therefore seems to have involved both considerable shuffling of preexisting domains (King et al., 2008) as well as evolution of many new ones.
The taxon that contains metazoa and choanoflagellates as well as fungi and several other unicellular relatives is called the opisthokonts. Although fungi contain no credible homologues of ECM proteins (or integrins), several of the other opisthokonts do encode some integrin subunits (Shalchian-Tabrizi et al., 2008; Sebé-Pedrós et al., 2010), but so far there have been no reports of ECM proteins. One additional unicellular organism that encodes an integrin β subunit but, so far, no α subunits (Thecamonas trahens formerly known as Amastigomonas sp.), is an apusomonad (Sebé-Pedrós et al., 2010). This group is of uncertain phylogenetic position, but the shared integrin subunit suggests a relationship with the other unicellular organisms discussed here (compare Fig. 1). The presence of integrin homologues of unknown function in these unicellular opisthokonts suggests that integrins may have been lost in the choanoflagellate lineage. Why these unicellular organisms encode integrins is unclear. One possibility is that the integrins function in phagocytosis, as has been suggested for the cadherins in choanoflagellates (King et al., 2008). It will be of considerable interest to see the entire genomes of representatives of these unicellular taxa and to investigate the expression and functions of their integrins and whether or not there are any ECM ligands.
Taxon-specific elaborations
As for most other categories of genes and proteins, there is a steady increase in the complexity of the “matrisome,” the set of proteins contributing to the ECM, as one ascends the tree of life. This increase comprises several different processes. There are notable examples of taxon-specific elaborations of the matrisome, both by duplication and divergence of existing genes as well as by the addition of new domains, including domains not observed at all in the genomes of earlier taxa. In this section, we will consider some examples to illustrate these processes.
Basement membranes.
As discussed earlier, essentially all eumetazoan genomes studied to date encode a set of proteins that make up basement membranes (Fig. 2). This core basement membrane toolkit is found in placozoa, cnidaria, protostomes, and invertebrate deuterostomes with very little change, and appears sufficient for assembly of all the basement membranes of all these organisms. However, vertebrates encode multiple paralogs of most of these proteins; only perlecan remains a unique gene/protein in vertebrate genomes. Mammals have multiple laminin subunits: three pairs of type IV collagen subunits, both collagen XV and collagen XVIII, and two nidogens. This expansion is consistent with the two whole genome duplications that have occurred during the evolution of vertebrates. These paralogs have undergone divergence, both in structure and in patterns of expression. For example, among the duplicated laminin subunits (6α, 3β, and 3γ), some have altered patterns of domains and assemble into trimeric laminin protomers with different shapes (Yurchenco, 2011), and the three type IV collagen gene pairs are differentially expressed during development and in different tissues. Thus, the basement membranes of vertebrate tissues differ from one another and, although we do not yet understand the full implications of this divergence, it is clear that it contributes to the increased complexity of vertebrates.
Collagens.
The collagen gene family offers many examples of taxon-specific divergence to suit particular purposes. Although the three clades of fibrillar collagens have an ancient origin before the divergence of eumetazoa (Exposito et al., 2008, 2010; Heino et al., 2009), individual lineages have expanded the set in different ways. Again, vertebrates provide some prime examples. Each of the three clades has expanded (to give a total of 11 fibrillar collagen genes), and individual members of each clade have become specialized for different functions; one from each clade of collagens is expressed selectively in notochord, cartilage, and bone (Wada et al., 2006). Vertebrate genomes also encode complex collagens with additional ECM domains, such as VWA and FN3. These are not newly developed domains; both are widespread and found in many other genes (Fig. 3; Whittaker and Hynes, 2002), and VWA domains do occur in collagen genes of unknown function in H. magnipapillata (Zhang et al., 2007) and, as mentioned, in choanoflagellates. There are several specialized vertebrate collagens incorporating VWA and FN3 domains. These include FACIT collagens, which form side branches on collagen fibrils; and collagens VI and VII, which assemble into short fibrils connecting basement membranes to underlying interstitial ECM in locations such as the skin (for review see Ricard-Blum, 2011). The inclusion of these extra domains confers additional interaction capabilities on these collagens, allowing assembly of higher-order structures important for the organisms.
Another example comes from sponges. They encode a family of short-chain collagens (∼120 Gly-X-Y repeats) called spongins, which form exoskeletons (familiar in the form of bath sponges). Spongins have a C-terminal domain distantly related to that of type IV collagens, and appear to have diverged from those basement membrane collagens before the parazoa/metazoa split (Aouacheria et al., 2006). Relatives of spongins are found in other invertebrates, although not in ecdysozoa or vertebrates, the spongin genes presumably having been lost in those lineages. The nematode, C. elegans, is one such ecdysozoan. The genome of this worm instead encodes a large number (>160) of collagen genes (Hutter et al., 2000; Myllyharju and Kivirikko, 2004). These encode short collagen chains (∼50 collagen Gly-X-Y repeats), which form the cuticle of the worm, a structure that undergoes remodeling at each larval molt. Different sets of cuticle collagen genes are expressed at different times. This is therefore a nematode-specific expansion of this family of specialized collagens for a taxon-specific ECM function, the cuticle. In contrast, flies (also ecdysozoans), which have a chitin-based exoskeleton, have entirely dispensed with fibrillar collagens and have lost those genes as well.
Deuterostomes and vertebrates.
The structure of collagen genes, built of multiple exons with common codon phasing, allows exon shuffling to generate the diverse collagens discussed above. Similarly, most ECM domains are encoded as exonic units, and that has allowed exon shuffling to develop new genes encoding ECM proteins with novel domain architectures. Examples of the evolution of novel ECM gene and protein architectures are particularly prevalent in the deuterostome lineage leading to vertebrates (Fig. 4). Whereas invertebrates of the protostome and deuterostome clades have similar sets of ECM proteins (aside from occasional taxon-specific expansions as discussed for collagens), vertebrates have a significantly expanded set of ECM proteins encoding diverse and novel ECM proteins. Thus, although deuterostome sea urchins share most of their ECM proteins with the protostome taxa of flies and nematodes, they lack many ECM genes found in vertebrates (see Whittaker et al., 2006; Huxley-Jones et al., 2007; and Ozbek et al., 2010 for more complete lists). We have already mentioned the large increase in number of collagen genes, both by duplication and divergence (e.g., fibrillar collagens) and by the development of novel domain architectures. Vertebrates also encode several families of proteoglycans (LRR-repeat PGs, hyalectans, and testicans), all of which are absent from the sea urchin genome and from protostomes and cnidaria. The hyalectans include the novel LINK domain, which is not found in protostomes or cnidaria and only twice in sea urchins (and then not in a context like that in hyalectans). This domain binds to hyaluronic acid, a high molecular weight glycosaminoglycan, and allows proteoglycans to assemble into multiprotein aggregates, which is important for the structure of cartilage but also for other ECMs. Many other vertebrate-specific ECM proteins are also probably involved in assembly and function of the major structural ECMs that define vertebrates. However, there are also many novel vertebrate ECM proteins whose functions do not appear obviously linked to cartilage, bones, or teeth.
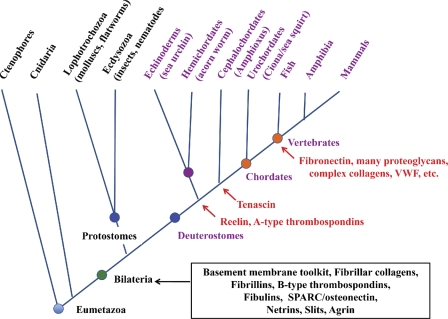
Figure 4.
Deuterostome phylogeny and elaboration of ECM proteins. The figure diagrams deuterostome evolution according to the same principles outlined in Fig. 1. The core set of ECM proteins encoded in the genomes of all bilateria is boxed in black. Many of these proteins are also found in cnidaria (see main text). The main taxa of the deuterostome lineage and their relationships as currently understood are indicated with representative animals noted. The first reported appearances of particular proteins are marked in red.
Among the ECM proteins missing from sea urchins as well as other invertebrates are tenascin, fibronectin, and von Willebrand factor (VWF; Whittaker et al., 2006). All three proteins comprise novel assemblages of domains in combinations not found in other ECM proteins (Fig. 3 B), and they serve to illustrate some issues common to the many other vertebrate-specific ECM proteins. Tenascins include multiple EGF and FN3 domains and a single C-terminal FBG domain. All of these domains are ancient in origin, but the combination is only found in deuterostomes. The sea urchin genome does not encode a tenascin, but those of Branchiostoma floridae (amphioxus, lancelet, cephalochordate; Putnam et al., 2008), Ciona intestinalis, and Ciona savignyi (sea squirts, ascidians, tunicates, urochordates; Dehal et al., 2002) all do, and all vertebrates encode multiple tenascins (Tucker and Chiquet-Ehrismann, 2009; Chiquet-Ehrismann and Tucker, 2011). The different vertebrate tenascins are differentially expressed in various ECMs, including those in the central nervous system (CNS) and during inflammatory and carcinogenic processes, and, given their association with disease states, clearly play important roles in vertebrates (Chiquet-Ehrismann and Tucker, 2011). Fibronectin appeared even later in the deuterostome lineage. In contrast with tenascins, fibronectin does include novel domains; although FN3 domains are ancient in origin, FN2 and FN1 domains are much more recent developments largely confined to chordates. The structure of vertebrate fibronectin is highly conserved in the entire vertebrate subphylum—once assembled, this gene appears to have been under strong selection—and it is essential for life in every species tested. Ascidians do encode a fibronectin-related gene (Tucker and Chiquet-Ehrismann, 2009) with all three fibronectin domains (FN1, -2, and -3), but it lacks key features (domains and motifs) of fibronectin structure and function, has additional domains not found in vertebrate fibronectins, and is best viewed as a proto-fibronectin (unpublished data). VWF is the final vertebrate ECM protein we will discuss in this context. This gene appears conserved in mammals, birds, amphibians, and fish (and presumably other vertebrates). As for fibronectin, there appears to be a proto-VWF in ascidians with similar domains but differentially arranged and including additional domains (unpublished data). VWF is a key protein in hemostasis, being responsible for platelet adhesion under high-shear conditions such as those in arterioles (Sadler, 2009; Bergmeier and Hynes, 2012). So, its function would appear to be necessary only in vertebrates. Its domain structure reveals that it is related to mucins, which are found in many invertebrates; the key innovation is the inclusion of a set of three VWA domains that are involved in binding to collagen (as in certain integrins) and to the cell-surface receptor, GPIb/V/IX, on platelets.
These three proteins, as well as the collagens, exemplify the role of domain shuffling and the addition of novel domains to ECM proteins to confer novel functions. For VWF, it is plausible to infer the novel functions from our knowledge of its hemostatic role in mammals. What could be the novel functions that selected for the evolution of tenascins and fibronectin in vertebrates? It could be that they are necessary for the development of vertebrate-specific structural ECMs like cartilage (as for some collagens and proteoglycans), but tenascin and fibronectin do not have obvious roles in such ECMs. Another possibility is neural crest migration, a key feature of vertebrate development; both tenascin C and fibronectin are strongly expressed in neural crest, and fibronectin has been functionally implicated in this migration as well as in condensation of somites (Hynes 1990), another vertebrate synapomorphy. Development and function of an endothelium-lined vasculature and high-pressure circulation is also a specialization of vertebrates. Fibronectin clearly plays a role there, and tenascins are expressed in the vertebrate CNS (Chiquet-Ehrismann and Tucker, 2011), as are many other ECM proteins, including both the pan-eumetazoan proteins, laminins, netrins, slits, and agrin, as well as later-evolving proteins (e.g., reelin and thrombospondin-1) and vertebrate-specific proteins such as proteoglycans and SCO-spondin (Barros et al., 2011).
Concluding remarks
The recent completion of genome sequences for cnidaria has shown that the common set of ECM proteins already known to be shared by all bilaterian taxa originated before the eumetazoan radiation >600 million years ago, and that many of these proteins have been conserved ever since, which indicates the importance of ECM for metazoan life. Furthermore, genome sequences of basal metazoa have shown that placozoans have many of the same proteins, most notably including the basement membrane toolkit. Sponges have a somewhat simpler repertoire of ECM proteins, and the demosponge for which genomic information is available lacks the basement membrane toolkit. Thus, it appears that, with respect to ECM content, placozoa are closer to eumetazoa than are demosponges, although information on other sponge clades will be of future interest. With the core of eumetazoan ECM proteins as a point of reference, one can ask when these proteins arose in premetazoan organisms and how the repertoire has been expanded in higher-order taxa.
Genomes of the closest unicellular relatives of metazoa—choanoflagellates—encode some domains characteristic of ECM proteins but appear not to have organized them in the combinations and patterns typical of metazoan ECM proteins. Choanoflagellates also lack ECM receptors such as integrins. However, some other unicellular opisthokonts do encode integrins, although no metazoan-type ECM proteins have yet been detected. Therefore, assembly of complex domain structures in ECM proteins seem to have accompanied the acquisition of multicellularity, with placozoa showing extensive elaboration of novel ECM proteins with domain combinations not reported elsewhere. The core set of ECM proteins has shown multiple taxon-specific expansions to meet particular needs. This is particularly evident in the deuterostome lineage leading to chordates and vertebrates. These taxa have greatly expanded the repertoire of ECM proteins both by gene duplication and divergence, and by the evolution of novel ECM proteins incorporating novel arrangements of old domains as well as the occasional addition of new ones. Evolution of this diverse set of ECM proteins has been enabled by their modular protein structures, with individual domains encoded as exonic units allowing shuffling during evolution.
Acknowledgments
I would like to thank Charlie Whittaker and Sebastian Hoersch of the Swanson Biotechnology Bioinformatics Facility in the Koch Institute and Alexandra Naba of my own laboratory for their collaborations on annotation of ECM proteins.
I would like to thank the Howard Hughes Medical Institute for financial support.
Footnotes
Abbreviations used in this paper:
- LRR
- leucine-rich repeat
- VWF
- von Willebrand factor
References
- Adams J.C., Lawler J. 2011. The thrombospondins. Cold Spring Harb. Perspect. Biol. 3:a009712 10.1101/cshperspect.a009712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouacheria A., Geourjon C., Aghajari N., Navratil V., Deléage G., Lethias C., Exposito J.-Y. 2006. Insights into early extracellular matrix evolution: spongin short chain collagen-related proteins are homologous to basement membrane type IV collagens and form a novel family widely distributed in invertebrates. Mol. Biol. Evol. 23:2288–2302 10.1093/molbev/msl100 [DOI] [PubMed] [Google Scholar]
- Ball E.E., Hayward D.C., Saint R., Miller D.J. 2004. A simple plan—cnidarians and the origins of developmental mechanisms. Nat. Rev. Genet. 5:567–577 10.1038/nrg1402 [DOI] [PubMed] [Google Scholar]
- Barros C.S., Franco S.J., Müller U. 2011. Extracellular matrix: functions in the nervous system. Cold Spring Harb. Perspect. Biol. 3:a005108 10.1101/cshperspect.a005108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley A.A., Adams J.C. 2010. The evolution of thrombospondins and their ligand-binding activities. Mol. Biol. Evol. 27:2187–2197 10.1093/molbev/msq107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmeier W., Hynes R.O. 2012. Extracellular matrix proteins in hemostasis and thrombosis. Cold Spring Harb. Perspect. Biol. 4:a005132 10.1101/cshperspect.a005132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boute N., Exposito J.Y., Boury-Esnault N., Vacelet J., Noro N., Miyazaki K., Yoshizato K., Garrone R. 1996. Type IV collagen in sponges, the missing link in basement membrane ubiquity. Biol. Cell. 88:37–44 10.1016/S0248-4900(97)86829-3 [DOI] [PubMed] [Google Scholar]
- Chapman J.A., Kirkness E.F., Simakov O., Hampson S.E., Mitros T., Weinmaier T., Rattei T., Balasubramanian P.G., Borman J., Busam D., et al. 2010. The dynamic genome of Hydra. Nature. 464:592–596 10.1038/nature08830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Tucker R.P. 2011. Tenascins and the importance of adhesion modulation. Cold Spring Harb. Perspect. Biol. 3:a004960 10.1101/cshperspect.a004960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchman J.R. 2010. Transmembrane signaling proteoglycans. Annu. Rev. Cell Dev. Biol. 26:89–114 10.1146/annurev-cellbio-100109-104126 [DOI] [PubMed] [Google Scholar]
- Dehal P., Satou Y., Campbell R.K., Chapman J., Degnan B., De Tomaso A., Davidson B., Di Gregorio A., Gelpke M., Goodstein D.M., et al. 2002. The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science. 298:2157–2167 10.1126/science.1080049 [DOI] [PubMed] [Google Scholar]
- Engel J. 1996. Domain organizations of modular extracellular matrix proteins and their evolution. Matrix Biol. 15:295–299 10.1016/S0945-053X(96)90130-4 [DOI] [PubMed] [Google Scholar]
- Ewan R., Huxley-Jones J., Mould A.P., Humphries M.J., Robertson D.L., Boot-Handford R.P. 2005. The integrins of the urochordate Ciona intestinalis provide novel insights into the molecular evolution of the vertebrate integrin family. BMC Evol. Biol. 5:31 10.1186/1471-2148-5-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exposito J.-Y., Larroux C., Cluzel C., Valcourt U., Lethias C., Degnan B.M. 2008. Demosponge and sea anemone fibrillar collagen diversity reveals the early emergence of A/C clades and the maintenance of the modular structure of type V/XI collagens from sponge to human. J. Biol. Chem. 283:28226–28235 10.1074/jbc.M804573200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exposito J.-Y., Valcourt U., Cluzel C., Lethias C. 2010. The fibrillar collagen family. Int. J. Mol. Sci. 11:407–426 10.3390/ijms11020407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey B., Degnan B.M. 2010. Origin of animal epithelia: insights from the sponge genome. Evol. Dev. 12:601–617 10.1111/j.1525-142X.2010.00445.x [DOI] [PubMed] [Google Scholar]
- Gazave E., Lapébie P., Renard E., Vacelet J., Rocher C., Ereskovsky A.V., Lavrov D.V., Borchiellini C. 2010. Molecular phylogeny restores the supra-generic subdivision of homoscleromorph sponges (Porifera, Homoscleromorpha). PLoS ONE. 5:e14290 10.1371/journal.pone.0014290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino J., Huhtala M., Käpylä J., Johnson M.S. 2009. Evolution of collagen-based adhesion systems. Int. J. Biochem. Cell Biol. 41:341–348 10.1016/j.biocel.2008.08.021 [DOI] [PubMed] [Google Scholar]
- Hohenester E., Engel J. 2002. Domain structure and organisation in extracellular matrix proteins. Matrix Biol. 21:115–128 10.1016/S0945-053X(01)00191-3 [DOI] [PubMed] [Google Scholar]
- Huhtala M., Heino J., Casciari D., de Luise A., Johnson M.S. 2005. Integrin evolution: insights from ascidian and teleost fish genomes. Matrix Biol. 24:83–95 10.1016/j.matbio.2005.01.003 [DOI] [PubMed] [Google Scholar]
- Hutter H., Vogel B.E., Plenefisch J.D., Norris C.R., Proenca R.B., Spieth J., Guo C., Mastwal S., Zhu X., Scheel J., Hedgecock E.M. 2000. Conservation and novelty in the evolution of cell adhesion and extracellular matrix genes. Science. 287:989–994 10.1126/science.287.5455.989 [DOI] [PubMed] [Google Scholar]
- Huxley-Jones J., Robertson D.L., Boot-Handford R.P. 2007. On the origins of the extracellular matrix in vertebrates. Matrix Biol. 26:2–11 10.1016/j.matbio.2006.09.008 [DOI] [PubMed] [Google Scholar]
- Hynes R.O. 1990. Fibronectins. Springer-Verlag, New York. 546 pp. [Google Scholar]
- Hynes R.O. 2009. The extracellular matrix: not just pretty fibrils. Science. 326:1216–1219 10.1126/science.1176009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R.O., Naba A. 2011. Overview of the matrisome—an inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 4:a004903 10.1101/cshperspect.a004903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R.O., Zhao Q. 2000. The evolution of cell adhesion. J. Cell Biol. 150:F89–F96 10.1083/jcb.150.2.F89 [DOI] [PubMed] [Google Scholar]
- King N., Hittinger C.T., Carroll S.B. 2003. Evolution of key cell signaling and adhesion protein families predates animal origins. Science. 301:361–363 10.1126/science.1083853 [DOI] [PubMed] [Google Scholar]
- King N., Westbrook M.J., Young S.L., Kuo A., Abedin M., Chapman J., Fairclough S., Hellsten U., Isogai Y., Letunic I., et al. 2008. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature. 451:783–788 10.1038/nature06617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knack B.A., Iguchi A., Shinzato C., Hayward D.C., Ball E.E., Miller D.J. 2008. Unexpected diversity of cnidarian integrins: expression during coral gastrulation. BMC Evol. Biol. 8:136 10.1186/1471-2148-8-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale M.Q., Finnerty J.R., Henry J.Q. 2002. The Radiata and the evolutionary origins of the bilaterian body plan. Mol. Phylogenet. Evol. 24:358–365 10.1016/S1055-7903(02)00208-7 [DOI] [PubMed] [Google Scholar]
- Merline R., Schaefer R.M., Schaefer L. 2009. The matricellular functions of small leucine-rich proteoglycans (SLRPs). J Cell Commun Signal. 3:323–335 10.1007/s12079-009-0066-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllyharju J., Kivirikko K.I. 2004. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 20:33–43 10.1016/j.tig.2003.11.004 [DOI] [PubMed] [Google Scholar]
- Naba A., Clauser K.R., Hoersch S., Liu H., Carr S.A., Hynes R.O. 2011. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell. Proteomics. 10.1074/mcp.M111.014647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbek S., Balasubramanian P.G., Chiquet-Ehrismann R., Tucker R.P., Adams J.C. 2010. The evolution of extracellular matrix. Mol. Biol. Cell. 21:4300–4305 10.1091/mbc.E10-03-0251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patthy L. 1999. Genome evolution and the evolution of exon-shuffling—a review. Gene. 238:103–114 10.1016/S0378-1119(99)00228-0 [DOI] [PubMed] [Google Scholar]
- Philippe H., Lartillot N., Brinkmann H. 2005. Multigene analyses of bilaterian animals corroborate the monophyly of Ecdysozoa, Lophotrochozoa, and Protostomia. Mol. Biol. Evol. 22:1246–1253 10.1093/molbev/msi111 [DOI] [PubMed] [Google Scholar]
- Philippe H., Derelle R., Lopez P., Pick K., Borchiellini C., Boury-Esnault N., Vacelet J., Renard E., Houliston E., Quéinnec E., et al. 2009. Phylogenomics revives traditional views on deep animal relationships. Curr. Biol. 19:706–712 10.1016/j.cub.2009.02.052 [DOI] [PubMed] [Google Scholar]
- Pick K.S., Philippe H., Schreiber F., Erpenbeck D., Jackson D.J., Wrede P., Wiens M., Alié A., Morgenstern B., Manuel M., Wörheide G. 2010. Improved phylogenomic taxon sampling noticeably affects nonbilaterian relationships. Mol. Biol. Evol. 27:1983–1987 10.1093/molbev/msq089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam N.H., Srivastava M., Hellsten U., Dirks B., Chapman J., Salamov A., Terry A., Shapiro H., Lindquist E., Kapitonov V.V., et al. 2007. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 317:86–94 10.1126/science.1139158 [DOI] [PubMed] [Google Scholar]
- Putnam N.H., Butts T., Ferrier D.E.K., Furlong R.F., Hellsten U., Kawashima T., Robinson-Rechavi M., Shoguchi E., Terry A., Yu J.-K., et al. 2008. The amphioxus genome and the evolution of the chordate karyotype. Nature. 453:1064–1071 10.1038/nature06967 [DOI] [PubMed] [Google Scholar]
- Ricard-Blum S. 2011. The collagen family. Cold Spring Harb. Perspect. Biol. 3:a004978 10.1101/cshperspect.a004978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson I., Jensen S., Handford P. 2011. TB domain proteins: evolutionary insights into the multifaceted roles of fibrillins and LTBPs. Biochem. J. 433:263–276 10.1042/BJ20101320 [DOI] [PubMed] [Google Scholar]
- Sadler J.E. 2009. von Willebrand factor assembly and secretion. J. Thromb. Haemost. 7:24–27 10.1111/j.1538-7836.2009.03375.x [DOI] [PubMed] [Google Scholar]
- Schaefer L., Schaefer R.M. 2010. Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res. 339:237–246 10.1007/s00441-009-0821-y [DOI] [PubMed] [Google Scholar]
- Schierwater B., Eitel M., Jakob W., Osigus H.-J., Hadrys H., Dellaporta S.L., Kolokotronis S.-O., Desalle R. 2009. Concatenated analysis sheds light on early metazoan evolution and fuels a modern “urmetazoon” hypothesis. PLoS Biol. 7:e20 10.1371/journal.pbio.1000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebé-Pedrós A., Roger A.J., Lang F.B., King N., Ruiz-Trillo I. 2010. Ancient origin of the integrin-mediated adhesion and signaling machinery. Proc. Natl. Acad. Sci. USA. 107:10142–10147 10.1073/pnas.1002257107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalchian-Tabrizi K., Minge M.A., Espelund M., Orr R., Ruden T., Jakobsen K.S., Cavalier-Smith T. 2008. Multigene phylogeny of choanozoa and the origin of animals. PLoS ONE. 3:e2098 10.1371/journal.pone.0002098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava M., Begovic E., Chapman J., Putnam N.H., Hellsten U., Kawashima T., Kuo A., Mitros T., Salamov A., Carpenter M.L., et al. 2008. The Trichoplax genome and the nature of placozoans. Nature. 454:955–960 10.1038/nature07191 [DOI] [PubMed] [Google Scholar]
- Srivastava M., Simakov O., Chapman J., Fahey B., Gauthier M.E.A., Mitros T., Richards G.S., Conaco C., Dacre M., Hellsten U., et al. 2010. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature. 466:720–726 10.1038/nature09201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R.P., Chiquet-Ehrismann R. 2009. Evidence for the evolution of tenascin and fibronectin early in the chordate lineage. Int. J. Biochem. Cell Biol. 41:424–434 10.1016/j.biocel.2008.08.003 [DOI] [PubMed] [Google Scholar]
- Wada H., Okuyama M., Satoh N., Zhang S. 2006. Molecular evolution of fibrillar collagen in chordates, with implications for the evolution of vertebrate skeletons and chordate phylogeny. Evol. Dev. 8:370–377 10.1111/j.1525-142X.2006.00109.x [DOI] [PubMed] [Google Scholar]
- Whittaker C.A., Hynes R.O. 2002. Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol. Biol. Cell. 13:3369–3387 10.1091/mbc.E02-05-0259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker C.A., Bergeron K.-F., Whittle J., Brandhorst B.P., Burke R.D., Hynes R.O. 2006. The echinoderm adhesome. Dev. Biol. 300:252–266 10.1016/j.ydbio.2006.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco P.D. 2011. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 3:a004911 10.1101/cshperspect.a004911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Boot-Handford R.P., Huxley-Jones J., Forse L.N., Mould A.P., Robertson D.L., Lili, Athiyal M., Sarras M.P., Jr 2007. The collagens of hydra provide insight into the evolution of metazoan extracellular matrices. J. Biol. Chem. 282:6792–6802 10.1074/jbc.M607528200 [DOI] [PubMed] [Google Scholar]