Abstract
Dietary and medicinal uses of Panax notoginseng have been associated with reduced risk of cancer. This study was designed to investigate the profiles of P. notoginseng saponin extract (PNSE), the major bioactive ingredients in P. notoginseng (Burk.) F.H. Chen, by high-performance liquid chromatography, and, for the first time, the anticancer effect of PNSE in the human colon cancer cell line LoVo was further evaluated. The major saponins present in PNSE were ginsenosides Rg1 (31.1%) and Rb1 (34.4%), and the total content of the eight saponins identified (notoginsenoside R1, ginsenosides Rg1, Re, Rb1, Rc, and Rd, and isomeric ginsenosides Rb2 and Rb3) was 81.7%, indicating that it was a highly purified standardized saponin extract. Furthermore, PNSE was found to have a markedly cytotoxic effect and antiproliferative activity against the LoVo cell line in a dose- and time-dependent manner. Flow cytometry analysis demonstrated that PNSE caused cell cycle arrest at S phase. Moreover, PNSE was found to possess antioxidative capacities in the 1,1-diphenyl-2-picrylhydrazyl free radical scavenging assay and hydroxyl radical scavenging assay in vitro. Taken together, the present results suggest that naturally occurring PNSE may provide significant natural defense against human colon cancer.
Key Words: antioxidant activity, antitumor activity, ginsenoside, high-performance liquid chromatography, notoginsenoside, Panax notoginseng
Introduction
Colon cancer is a leading cause of cancer death in developed countries and represents almost 10% of all tumors with approximately 1 million new cases each year worldwide.1 It is also widely recognized that colon cancer is the third most common cancer in men (after lung and prostate cancers) and is the second to breast cancer in women.2 It is interesting that only 5–10% of the cases are due to genetic factors, whereas more than 70% are related to diet and lifestyle, suggesting that colon cancer rates could be substantially reduced by changes in dietary and lifestyle patterns.2 Epidemiological studies suggest that bioactive phytochemicals derived from plant-based dietary consumption play a significant role in reducing the incidence of colon cancer.3
Radix Notoginseng (Sanqi), the dried root of Panax notoginseng (Burk.) F.H. Chen, is commonly used as a medicine and dietary supplement for blood circulation, blood stasis removal, and pain alleviation in China and other Asian countries.4 In the United States and European countries, various P. notoginseng products are available on the market as dietary supplements.5 Thus, notoginseng tea or capsules are being sold as over-the-counter dietary supplements in the U.S. health food market. In China, P. notoginseng is classified as a functional food plant known worldwide and is equally reputed for its medicinal properties. Therefore, consumption of P. notoginseng is especially popular because of its health benefits, and its extracts are common botanical ingredients in dietary supplements and functional foods.6 However, apart from their application in trauma and bleeding treatments, to our knowledge, there is limited information about their potential as cancer chemopreventive agents or possible antitumor mechanisms.
Several recent studies have indicated that P. notoginseng can inhibit the growth of human hepatocarcinoma (SMMC-7721) cells,7 human prostate cancer (PC-3) cells,8 and breast cancer (MCF-7) cells9 through cell cycle arrest and the stimulation of apoptosis and that a series of triterpenoid saponins (Fig. 1A) are responsible for the anticancer effects. However, those studies on its anticancer properties focused largely on the crude extracts of P. notoginseng,5,7–9 and the saponins identified in the different crude extracts were fluctuant because of low purity.5,10–12 For this reason, much more research effort is needed to elucidate the antitumor profiles in a variety of cancer cell types and how P. notoginseng saponin extract (PNSE) influences cancer development and recurrence. It is also very necessary to evaluate the potential anticancer effect of a more purified saponin extract in order to understand the nature of PNSE contribution and its real application as a potential cancer chemopreventive agent.
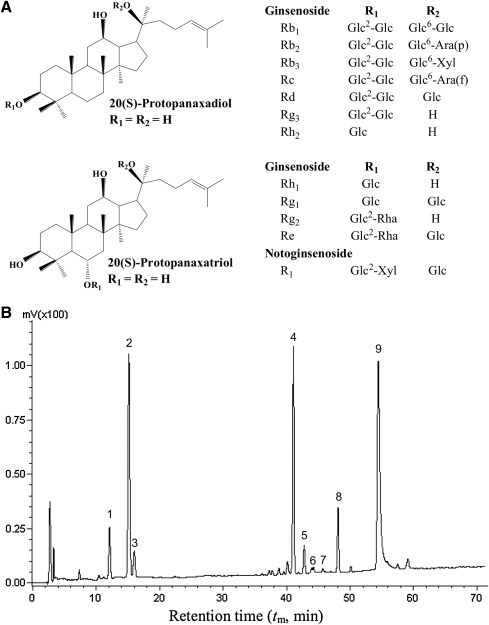
FIG. 1.
(A) Structures of triterpenoid saponins and their aglycone moieties and (B) the high-performance liquid chromatogram of the standardized saponin extract from Chinese P. notoginseng (Burk.) FH. Chen. Glc, β-d-glucose; Rha, α-l-rhamnose; Ara(p), α-l-arabinose (pyranose); Ara(f), α-l-arabinose (furanose); Xyl, β-d-xylose. The analysis was carried out as described in Materials and Methods. The peaks are identified as follows: peak 1, notoginsenoside R1; peak 2, ginsenoside Rg1; peak 3, Re; peak 4, Rb1; peak 5, Rc; peak 6, Rb2; peak 7, Rb3; peak 8, Rd; and peak 9, raloxifene (internal standard).
The objective of the present study was to characterize the purity and major chemical composition of a standardized PNSE, and the antioxidant activities of PNSE were also investigated. Furthermore, we used the human LoVo colon cancer cell line to assess the antiproliferative effect of PNSE. We found that PNSE caused cell cycle arrest at the S phase and induced an apoptotic response.
Materials and Methods
Cell lines, chemicals, and biochemicals
The human colon carcinoma cells LoVo and Caco-2 were obtained from the Cell Bank of the Chinese Academy of Science, Shanghai, China. IEC (human intestinal epithelial) cells were obtained from the Fourth Military Medical University, Xi'an, China. Notoginsenoside R1 (peak 1), ginsenoside Rg1 (peak 2), Re (peak 3), Rb1 (peak 4), Rc (peak 5), Rb2 (peak 6), Rb3 (peak 7), and Rd (peak 8) (98% pure, Fig. 1A) were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Triton X-100 and EDTA were the products of Sinopharm Chemical Reagent Co., Ltd. (Shanghai). High-performance liquid chromatography (HPLC)-grade acetonitrile and methanol were purchased from Tedia (Fairfield, OH, USA). 1,1-Diphenyl-2-picrylhydrazyl (DPPH), dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), RNase A, and propidium iodide (PI) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Deionized water was prepared using a Millipore (Bedford, MA, USA) Milli Q-Plus system. A stock solution of 5.0 mg/mL PNSE was prepared in DMSO and stored at −20°C until cell culture studies. All cell culture reagents were purchased from Sinopharm (Beijing). All other chemicals were of the highest grade available.
Plant material
The roots of P. notoginseng (Burk.) F.H. Chen were purchased in Yunnan, China and were identified according to the identification standard of the Pharmacopeia of the People's Republic of China. Voucher specimens of the harvested plant materials were deposited at the Key Laboratory of the Ministry of Education for Medicinal Resource and Natural Pharmaceutical Chemistry, Xi'an.
Isolation and purification of PNSE
The saponins were isolated and measured using the method described by Zheng et al.13 In brief, the dried powdered root of P. notoginseng (Burk.) F.H. Chen (10 g) was extracted 10 times in 70% alcohol and refluxed three times at 70°C for 90 min each time. After filtration, the filtrate was concentrated in vacuo to evaporate the solvents. The concentrated solution was subjected to D101 resin column chromatography, washed with deionized water, and eluted with 70% alcohol to give pure compounds. The purity of saponins measured by the method of vanillin colorimetry was up to 85%.
Preparation of PNSE standard solution
Stock standard solutions (5.0 mg/mL) were prepared by dissolving each saponin in water solution containing 60% methanol. Working solutions were further obtained by appropriate dilution of stock solutions with deionized water. A set of mixed standard working solutions containing ginsenosides Rg1, Rb1, Rb2, Rb3, Rc, Rd, and Re and notoginsenoside R1 were prepared for the establishment of calibration curves. The concentration of raloxifene as an internal standard was 50 μg/mL for all analytes. The sample solutions were filtered through a syringe filter (pore size, 0.22 μm) and were degassed using an ultrasonic bath for 2 min prior to use. All the solutions prepared were stored in the dark at 4°C until being used.
HPLC analysis for the chemical composition of PNSE
Analysis of the standardized saponin extract from P. notoginseng was carried out on a Shimadzu (Tokyo, Japan) LC-2010A HPLC system equipped with a quaternary gradient pump unit, a UV-Vis detector (190–700 nm), an autosampler (0.1–100 μL), and an XTerra-C18 (4.6 μm i.d. ×250 mm; film thickness, 5 μm; Waters, Milford, MA, USA) column; the column temperature was set at 35°C. The mobile phase A was acetonitrile, and the mobile phase B was KH2PO4-NaOH buffer (50 mM, pH 5.6) using the following gradient program: 81–80% B for 0–10 min, 80–75% B for 10–35 min, and 75–60% B for 35–70 min. The flow rate was 1.0 mL/min, and sample injection volume was 10 μL. The wavelength for ultraviolet detection was 203 nm.
Antioxidant activities of PNSE
The DPPH free radical scavenging activity of PNSE was measured according to the procedure described by Brand-Williams et al.14 In brief, 2.0 mL of ethanol (95%) and 1.0 mL of the purified saponins in ethanol at various concentrations (0.25–5 mg/mL) were mixed, and then 2.0 mL of DPPH· ethanol solution (0.2 mM) was added. The mixture was shaken vigorously and allowed to stand in the dark at room temperature for 30 min. The decrease in absorbance of the resulting solution was then measured at 517 nm. Background absorbance was quantified using ethanol (95%) instead of DPPH· solution, and subsequently the blank absorbency was obtained when saponin solution was replaced by ethanol (95%). Ascorbic acid (vitamin C [Vc]) was used as a positive control. All measurements were made in triplicate, and DPPH· scavenging activity was expressed as the inhibition percentage and was calculated using the following formula: DPPH· scavenging activity (%)=(1−[Absorbance of sample – Absorbance of background]/Absorbance of blank)×100. The percentage of scavenging activity was plotted against the sample concentration to obtain the 50% inhibition concentration.
The scavenging activity of PNSE against the hydroxyl radicals was measured by an improved Fenton-type reaction.15 In brief, 2.0 mL of 6 mM FeSO4 and 2.0 mL of PNSE sample solution at different concentrations (0.25–5 mg/mL) or Vc in ethanol were mixed, and the reaction was started by addition of 2.0 mL of 6 mM H2O2. After this mixture was reacted for 10 min, 2.0 mL of 6 mM salicylic acid was added and further reacted for 30 min. Finally, the absorbance was recorded at 510 nm, and the scavenging activity of PNSE was calculated according to the equation: scavenging activity on HO· (%)=(1 – [Absorbance of sample – Absorbance of blank]/Absorbance of control)×100.
Cell culture
Cells were grown in Dulbecco's modified Eagle's medium (HyClone Laboratories, Inc., Logan, UT, USA) that was supplemented with l-glutamine, sodium pyruvate, 10% heat-inactivated fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin in 5% CO2 and 95% air.1,16,17 DMSO was used as the vehicle to deliver PNSE, and the final concentration of DMSO in all experiments was 0.1%. All experiments were done independently in triplicate per experimental point.
Analysis of cell viability
The colorimetric MTT assay was performed to assess cell viability.18,19 In brief, LoVo, Caco-2, and IEC cells (1×105) were seeded in 96-well plates. After 24 h (the cells were grown to 90% confluence), the cells were treated with serial concentrations (0, 100, 250, and 500 μg/mL) of PNSE in 1% DMSO for various periods of time (24 and 48 h). The final concentration of DMSO in culture medium was maintained at 0.1%,1,20 and fluorouracil (5-Fu) (250 μg/mL) was used as a positive control. After the exposure period, 10 μL of MTT (5 mg/mL) in phosphate-buffered saline (PBS) solution was added to each well at a final concentration of 0.5 mg/mL, and then the plate was further incubated for 4 h. MTT-containing media were removed, and 100 μL of a solution containing 10% sodium dodecyl sulfate (pH 4.8) plus 0.01 M HCl and 5% isobutyl alcohol was added to each well and mixed thoroughly to dissolve the formed crystal formazan. After incubation overnight at 37°C to ensure that all crystals were dissolved, the light absorption was measured at 570 nm using an enzyme-linked immunosorbent assay reader (model RT6000, Rayto, Guangdong, China). Viability was expressed as a percentage of absorbance of treated cells to that of controls.
Assay for lactate dehydrogenase
Leakage of lactate dehydrogenase (LDH) into the media indicating cell plasma membrane injury was detected using a commercial assay assay kit (Jiancheng BioEngineering, Nanjing, China)21 following the manufacturer's directions.
Cell cycle analysis using flow cytometry
The cell cycle was analyzed by flow cytometry.1,22 LoVo cells (1×106) were seeded in 6sixwell flat-bottomed plates, grown overnight until they reached 80% confluence, and then treated with vehicle alone (0.1% DMSO) or with PNSE (0, 100, or 500 μg/mL) for 48 h. After treatment, the detached cells in the medium were collected and combined with the remaining adherent cells that were detached by brief trypsinization (0.25% trypsin-EDTA, Sigma-Aldrich). Cell pellets were fixed in 70% ethanol, washed in PBS, resuspended in 1.0 mL of PBS containing 1.0 mg/mL RNase and 50 μg/mL PI, incubated in the dark for 30 min at room temperature, and analyzed with a GUAVA® easyCyte™ 8HT flow cytometer (Millipore).
Detection of apoptosis by flow cytometry
LoVo cells were treated exactly same as in cell cycle analyses described above, and apoptotic cells were quantified by the annexin V–fluorescein isothiocyanate (FITC)/PI double staining assay.1,21,22 Early and late apoptotic changes in LoVo cells were determined using an annexin V–FITC/PI apoptosis detection kit (BestBio, Shanghai) following the manufacturer's instructions. In brief, cells (1×106) were collected, washed twice with PBS, and suspended in 400 μL of binding buffer (adding 5 μL of annexin V–FITC and 10 μL of PI). Thereafter, the samples were incubated in the dark for 10 min at 2–8°C, and the samples were analyzed on the flow cytometer. The number of annexin V–FITC-positive and PI-positive of cells in each field was determined by counting the cells directly. All experiments were done independently at least three times per experimental point.
Statistical analysis
All data are mean±SD values of three independent experiments performed in triplicate. Statistical comparisons were performed by analysis of variance. Results were considered significant when P<.05.
Results
Composition of PNSE
An HPLC analysis was developed for determining the purity and composition of a standardized PNSE. As shown in Figure 1B and Table 1, the peaks were identified in the order of notoginsenoside R1 (11.2 min), ginsenoside Rg1 (15.2 min), Re (16.0 min), Rb1 (41.0 min), Rc (41.8 min), Rb2 (43.5 min), Rb3 (44.9 min). and Rd (48.1 min) by comparing the retention time of the unknown peaks with those of the standards. Here, the reproducibility was estimated analyzing the standards in triplicate (50 μg/mL for each analyte, Table 1). The results showed that all the coefficient of variation values were lower than 2.7% for the retention time and 4.2% for the corrected peak areas (Area/time), demonstrating good reproducibility. Good linearity of five calibration solutions from 10 to 100 μg/mL with correlation coefficient (r) values of 0.9993–0.9998 was obtained by regression analysis between Y (peak area ratio of the analytes with internal standard) and X (concentration, μg/mL), and the detection limits were lower or near 5.2 μg/mL (signal-to-noise ratio=3), which showed that this method was very sensitive.
Table 1.
Retention Time, Limit of Detection, and Regression Analysis of the Proposed High-Performance Liquid Chromatography Method
| |
|
Regression equationb |
|
|
|
|---|---|---|---|---|---|
| Analyte | tR(min)a | a | b | r | LOD (μg/mL)c |
| R1 | 11.192 | 0.00295 | 0.00526 | 0.9993 | 5.8 |
| Rg1 | 15.168 | 0.05357 | 0.00914 | 0.9998 | 5.2 |
| Re | 16.019 | −0.00763 | 0.00902 | 0.9997 | 6.3 |
| Rb1 | 41.036 | 0.08204 | 0.01146 | 0.9996 | 5.4 |
| Rc | 41.808 | 0.00162 | 0.00498 | 0.9994 | 5.9 |
| Rb2 | 43.505 | 0.00076 | 0.01754 | 0.9998 | 7.2 |
| Rb3 | 44.850 | −0.00078 | 0.01623 | 0.9996 | 7.9 |
| Rd | 48.047 | 0.02042 | 0.00860 | 0.9997 | 5.7 |
tR, retention time. The mobile phase A consisted of acetonitrile, and the mobile phase B was 50 mM KH2PO4-NaOH buffer (pH 5.6) in a gradient program of 81–80% B for 0–10 min, 80–75% B for 10–35 min, and 75–60% B for 35–70 min. The injection volume was 10 μL.
Y=a+bX, where Y and X are the peak area ratio of the analytes to the internal standard (raloxifene) and the concentration of the analyte (10–100 μg/mL), respectively.
The limit of detection (LOD) corresponds to concentrations giving a signal-to-noise ratio of 3.
A typical chromatogram of the saponins in purified PNSE is shown in Figure 1B. It was found that the notoginsenoside R1 (peak 1), ginsenoside Rg1 (peak 2), Re (peak 3), Rb1 (peak 4), Rc (peak 5), Rb2 (peak 6), Rb3 (peak 7), and Rd (peak 8) in the purified PNSE sample could still be separated at baseline and the component saponins could be identified by the migration time of analytes compared with the standard mixture solution. The results showed that PNSE was composed of notoginsenoside R1 (peak 1), ginsenoside Rg1 (peak 2), Re (peak 3), Rb1 (peak 4), Rc (peak 5), Rb2 (peak 6), Rb3 (peak 7), and Rd (peak 8) in the percentages of 5.31%, 31.1%, 3.40%, 34.4%, 0.17%, 0.01%, 0.06%, and 7.30% (grams per 100 g of dry sample), respectively. Rg1 and Rb1 were the predominant saponins in PNSE, accounting for 65.5%, and the purity of PNSE is above 81.7%, indicating that it is a standardized saponin extract with high purity.
PNSE scavenging of hydroxyl radical and DPPH radicals
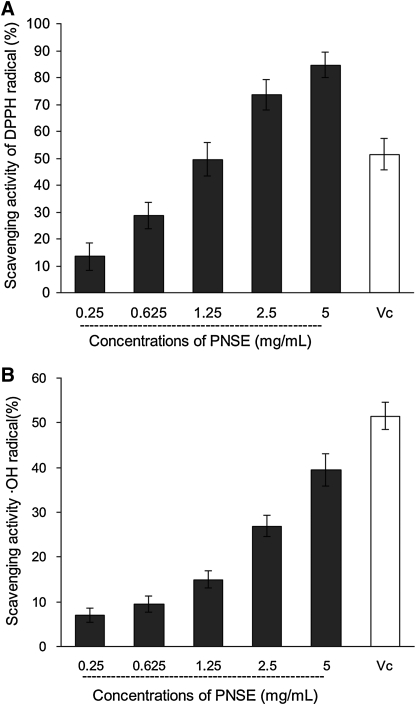
In vivo, HO· easily crosses cell membranes at specific sites and reacts with most biomolecules, resulting in tissue damage and cell death. Thus, HO· scavenging assessment is of great importance for evaluating the potency of antioxidants.23 Therefore, we examined the HO· scavenging activity of PNSE in vitro (Fig. 2A). As expected, PNSE in the range of 0.25–5 mg/mL dose-dependently scavenged HO· with a 50% effective concentration of 1.20 mg/mL, although it was much weaker than the tested value of 51.4% for the reference antioxidant Vc at 0.625 mg/mL. This test indicated that PNSE possessed a definite scavenging activity.
FIG. 2.
Radical scavenging activity of P. notoginseng saponin extract (PNSE) against (A) hydroxyl radical (HO·) and (B) 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical in vitro. Ascorbic acid (vitamin C [Vc]) at the concentration of 0.625 mg/mL was used as a reference antioxidant. Data are mean±SD values (n=3).
It is well known that the model system of scavenging DPPH· is a simple method to evaluate the antioxidative activity of antioxidants.24,25 In this study, DPPH· scavenging activity of PNSE was further investigated, as shown in Figure 2B. PNSE exhibited dose-dependent scavenging effects against DPPH·. At 0.25 mg/mL, the scavenging ability of PNSE against DPPH· was 13.5%, and at 5 mg/mL it was 84.7%, but the scavenging ability of Vc was 51.4% at 0.625 mg/mL. These results indicate that the tested saponins have modest DPPH· scavenging activity.
PNSE inhibited cell proliferation in human colon cancer cells
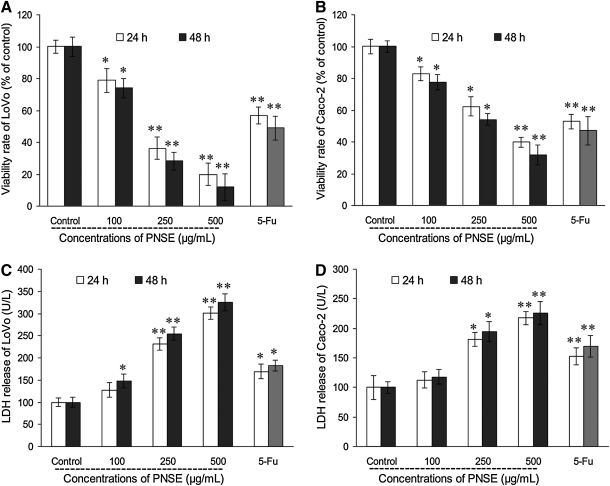
Cell proliferation inhibitory activity of PNSE in colon cancer cells was examined in LoVo and Caco-2 cells. As shown in Figure 3A and B, the exposure of LoVo and Caco-2 cells to PNSE for 24 h and 48 h significantly inhibited the cell growth in a dose- and time-dependent manner. The 50% inhibition concentrations of PNSE were 166.8 μg/mL for LoVo cells and 276.9 μg/mL for Caco-2 cells after 48 h of treatment. In contrast, no proliferation inhibitory effect of PNSE on IEC normal human intestinal epithelial cells was observed at the tested concentrations (data not shown). As shown in Figure 3A, growth inhibition was significantly improved by 21.2% (P<.05 vs. control) for PNSE at 100 μg/mL in 24 h. Treatment with PNSE at the higher doses of 250 μg/mL and 500 μg/mL for 48 h further enhanced cellular growth inhibition by 71.8% and 88.1%, respectively, in comparison with the untreated control group (P<.01). However, the cell viability in the presence of a final concentration of 0.1% DMSO vehicle alone was not affected (data not shown). As can be seen in Figure 3B, treatment with PNSE at 500 μg/mL for 48 h also led to a 68.1% increase in cellular growth inhibition compared with the untreated control group (P<.01), but LoVo cells were found to be more sensitive to PNSE than Caco-2 cells. It is noteworthy that 250 μg/mL PNSE showed a 20.8% greater cellular growth inhibition in LoVo cells than the same concentration of the reference compound 5-Fu (51%) (P<.01). The present results suggested that PNSE is a superior antitumor agent to 5-Fu.
FIG. 3.
Inhibitory and cytotoxic effects of PNSE on LoVo and Caco-2 human colon cancer cells. Cells were treated with PNSE at concentration of 0 (untreated control), 100, 250, and 500 μg/mL in complete medium for 24 and 48 h, and authentic fluorouracil (5-Fu) (250 μg/mL) was used as a positive control. The cell proliferation of (A) LoVo and (B) Caco-2 cells was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, and cytotoxic effects on (C) LoVo and (D) Caco-2 cells were measured by lactate dehydrogenase (LDH) assay as described in Materials and Methods, respectively. Data are mean±SD values (n=3). Asterisks denote a significant difference compared with the control group: *P<.05, **P<.01.
Cytotoxicity of PNSE in LoVo and Caco-2 cells
LDH release into the media is usually used as an index of the integrity of cell membrane or necrosis in response to antitumor activity.20 In this study, PNSE-induced toxicity in LoVo and Caco-2 cells was also evaluated based on its effect on LDH leakage into media. As shown in Figure 3C, exposure of LoVo cells to 100 μg/mL PNSE caused a 1.2-fold increase of LDH leakage (P<.05). The treatment with PNSE at 250 and 500 μg/mL resulted in a further increase in cytotoxicity, as reflected by the 130.5% and 201.5% increase in LDH leakage (P<.01), respectively. Consistent with the antiproliferation results, PNSE at an equal concentration was more effective than 250 μg/mL 5-Fu (69.5%) (P<.05). Treatment of Caco-2 cells with PNSE at 250 and 500 μg/mL caused an increase in cytotoxicity by 81.5% and 117.5%, respectively (P<.05 and P<.01, respectively, vs. control, Fig. 3D). Taken together, PNSE was also found to have significant cytotoxic effects in the colon cancer cells LoVo and Caco-2, indicating that the PNSE-induced inhibition of colon cancer cell growth was accompanied by the induction of cell membrane injury. This sensitivity of LoVo cancer cells to PNSE led to further examination on the mechanism of its antiproliferative effects.
Regulatory effect of PNSE on cell cycle arrest
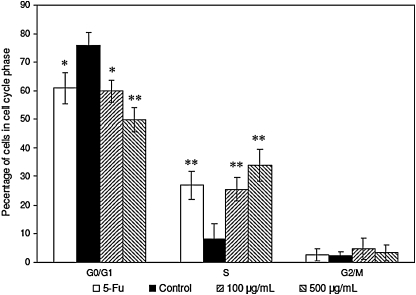
To further investigate the mechanisms leading to loss of cell proliferation by PNSE, we tested the effect of PNSE on cell cycle progression using flow cytometry. Figure 4 shows the effects of PNSE on the different phases of the cell cycle. A dose-dependent increase in S-phase cell population along with a compensatory decrease of cells in G0/G1 was observed after 48 h of treatment. As depicted in Figure 4, flow cytometry analysis of untreated cells indicated that approximately 75.7%, 8.3%, and 2.3% of the cells were distributed among the G0/G1, S, and G2/M phases, respectively. Compared with vehicle-treated cells (control), PNSE at 100 and 500 μg/mL decreased the cell population in the G0/G1 phase by 59.8% (P<.05) and 49.9% (P<.01), respectively. The decrease was accompanied by a concomitant increase in the cell population in the S phase of 25.5% (P<.01) and 34.0% (P<.01) by 100 and 500 μg/mL PNSE, respectively, meaning that after treatment for 48 h, the S-phase–arrested LoVo cells were unable to proceed into the G2/M phase (Fig. 4). However, no significant cell cycle blockage was observed at the G2/M phase in the tested cell lines (P>.05). Similarly, the reference compound 5-Fu at 250 μg/mL also increased the S-phase population by 26.9% (P<.01 vs. vehicle control), concomitant with a compensated decrease in the proportion of G1-phase cells (60.9%) (P<.01). The results demonstrated that PNSE arrested LoVo cell cycle progression at S phase, resulting in the growth inhibition of the cancer cells.
FIG. 4.
Effects of PNSE on LoVo cell cycle distribution by flow cytometry analysis. The cells treated with 5-Fu (250 μg/mL) or PNSE (0, 100, or 500 μg/mL) in complete medium for 48 h were collected and stained with propidium iodide after fixation by 70% ethanol. Following flow cytometry, cell cycle distribution was analyzed using the FlowJo (Tree Star, Inc., Ashland, OR, USA) program as described in Materials and Methods. Data are mean±SD values (n=3). Asterisks denote a significant difference compared with the control group: *P<.05, **P<.01.
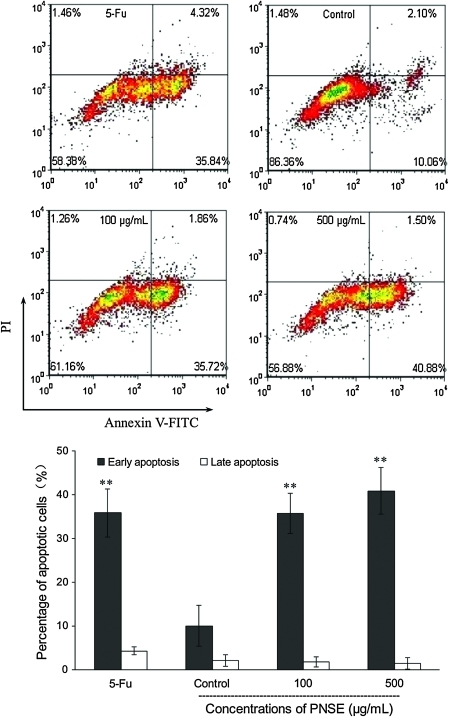
PNSE-induced apoptosis in LoVo cells
In order to determine the extent to which apoptosis contributed to the PNSE-induced growth inhibition, we studied the effects of PNSE on cellular apoptosis in LoVo colon cancer cells after a 48-h treatment. The apoptotic cells were quantified by the annexin V–FITC /PI double staining assay. Viable cells (FITC-negative) and early apoptotic cells (FITC-positive) were PI-negative, whereas late apoptotic and necrotic cells were PI-positive and FITC-positive. The percentage of apoptotic cells was calculated from the ratio of late apoptotic or necrotic cells to viable or early apoptotic cells. As shown in Figure 5, at 100 μg/mL PNSE, the apoptotic cell population is very close to that of the 5-Fu-treated cells. The significant cell apoptotic rate (35.7%) (P<.01) was initially observed using 100 μg/mL PNSE and compared with the untreated control cells (10.1%) (P<.01), and a dose-dependent effect was further observed in the treatment with 500 μg/mL PNSE (P<.01), where PNSE increased the apoptotic cell population to 40.9% from that of the control cells (P<.01). In agreement with cell cycle analysis, PNSE was shown to strongly induce apoptosis in LoVo cells.
FIG. 5.
Apoptotic effects of PNSE in LoVo cells. The cells treated with 5-Fu (250 μg/mL) or PNSE (0, 100, or 500 μg/mL) in complete medium for 48 h were collected and stained with fluorescein isothiocyanate (FITC)–conjugated annexin V and propidium iodide (PI). Following flow cytometry, the percentage of apoptotic cells was calculated as the ratio of early to late apoptotic cells using FCS-Express Software (De Novo Software, Inc., Los Angeles, CA, USA). Data are mean±SD values (n=3). Asterisks denote a significant difference compared with the control group: *P<.05, **P<.01. Color images available online at www.liebertonline.com/jmf
Discussion
P. notoginseng, a close relative of American ginseng, has a very distinct saponin profile compared with that of the well-investigated ginseng.26 The main bioactive compounds in P. notoginseng are dammarane-type triterpenoid saponins, including a set of ginsenosides present in ginseng. However, the oleanane-type saponins, present in Asian ginseng and American ginseng, are not found in notoginseng.26 In this work, the HPLC identification results clearly showed that the major saponins present in PNSE were ginsenosides Rg1 and Rb1, accounting for up to 65.5% of PNSE, followed by 7.30% Rd, 5.31% notoginsenoside R1, 3.40% Re, 0.17% Rc, 0.06% Rb3, and 0.01% Rb2 (Fig. 1B). All of the active saponins identified are derivatives of 20(S)-protopanaxadiol and 20(S)-protopanaxatriol (Fig. 1A), and the total content of the eight saponins was 81.7%, indicating that PNSE is a high-purity saponin preparation.
Ginseng reportedly has potential chemopreventative effects relevant to colon cancer, which are believed to be due to the ginsenosides, for which antitumor effects have been well documented in a few types of human cancers.5,7–9,26 However, to the best of our knowledge, little research has addressed the anticancer activities of P. notoginseng. It is of interest that a few previous articles have described the antiproliferative properties of the crude saponin extracts from P. notoginseng against various cancer cells.5,7–9 Here, our attention has been further focused on the anticancer properties of a standardized PNSE, isolated from P. notoginseng for application in cancer prevention. We demonstrated for the first time that the tested PNSE exhibited potent antiproliferative and cytotoxic effects, cell cycle arrest, and induction of apoptosis, indicating that the saponin ingredients are responsible for the bioactivity of P. notoginseng.
It is well known that the increased proliferation and decreased apoptosis are two major processes that contribute to the progression of tumor cell growth. Cytotoxicity, a common preliminary method, is helpful to detect whether tested compounds have potential antineoplastic abilities.27 The cytotoxic effect of the crude saponin extracts from P. notoginseng has been previously described in hepatocarcinoma (SMMC-7721), prostate cancer (PC-3). and breast cancer (MCF-7) cell lines.5,7–9 In this study, our results showed that a standardized PNSE significantly induced both dose- and time-dependent effects on cell growth and proliferation as assessed by MTT assay. We also found that PNSE exhibited potent cytotoxicity against LoVo human colon cancer cells as estimated by LDH leakage into media. Hence, these data suggested that PNSE might be a strong antitumor agent, superior to 5-Fu, and that these saponins might effectively reduce the malignancy and suppress the generation potential of cancer cells.
It has been reported that some anticancer agents cause growth inhibition through interfering with the processes of the cell cycle or by causing cell death (apoptosis).27 On the one hand, cell cycle is under strict regulation in the cell with numerous control points that allow correct progression through the different phases. In this sense, a delay in progression of the G0/G1, S, or G2/M cell cycle phases would constitute a cellular defense mechanism to allow action of the DNA repair systems.27 On the other hand, apoptosis is considered to be a physiologically important process that functions to eliminate undesired cells during development and homeostasis of multicellular organisms. Therefore, to determine whether cell cycle arrest or apoptosis is involved in growth inhibition, we examined cell cycle phase distribution and induction of apoptosis of the PNSE-treated cells by flow cytometry. Our results indicated that at the concentration of 100 μg/mL PNSE, the inhibition of proliferation appeared to be the result of inhibition of cell cycle progression because PNSE blocked proliferation of tumor cells by arresting the cells in the S phase of the cell cycle. It was of interest that this inhibitory effect was associated with the induction of apoptosis. Our results are in agreement with previous reports describing the antiproliferative and apoptosis-inducing effect of Panax ginseng on hepatocarcinoma (SMMC-7721), prostate cancer (PC-3) and breast cancer (MCF-7) cell lines,5,7–9 where the cell cycle blockage occurred in S phase or G2/M phase.
There is growing evidence indicating that reactive oxygen species are involved in both the initiation and progression of cancer.27 In this sense, the cancer chemopreventive properties of antioxidants are generally believed to be due to their ability to scavenge endogenous reactive oxygen species.27 The crude P. notoginseng saponin extracts have been previously reported to possess antioxidant properties in a variety of experimental systems.14,15 However, very little is known about the free radical scavenging activity of triterpenoids. Here, our results indicated that the purified PNSE exhibited significant free radical scavenging capacity as assessed by HO· and DPPH· chemical assays, and the profound inhibitory effects of PNSE against LoVo cell growth have also been achieved using a standardized saponin extract with high purity. Dietary supplementation of naturally occurring standardized plant-based extracts has been prevalent in cancer therapy for a long time. Here we show the potential interest of a standardized saponin preparation as an alternative source of anticancer ingredients on human colon cancer cells.
In conclusion, our results clearly demonstrated that naturally occurring PNSE exerted antioxidant effects, inhibited cell growth, arrested the cell cycle at S phase, and induced apoptosis in LoVo human colon cancer cells. Our findings provide new insight into the anticarcinogenic action of PNSE and support the hypothesis that the PNSE may be considered as a valuable food ingredient or medicine for use as a cancer chemotherapeutic or chemopreventive agent. To our knowledge, this is the first report of the effects of PNSE on human colon cancer cells.
Acknowledgments
This study was supported by grants C30972054, C20802091, and C31171678 from the National Natural Science Foundation of China.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Jaramillo S. Lopez S. Varela LM. Odriguez-Arcos R. Jimenez A. Abia R. Guillen R. Muriana FJG. The flavonol isorhamnetin exhibits cytotoxic effects on human colon cancer cells. J Agric Food Chem. 2010;58:10869–10875. doi: 10.1021/jf102669p. [DOI] [PubMed] [Google Scholar]
- 2.Duijnhoven FJB. De Mesquita HB. Ferrari P. Jenab M. Boshuizen HC. Ros MM. Casagrande C. Bingham SA. Khaw KT. Key TJ. Allen NE. Boffetta P. Slimani N. Rinaldi S. Gallo V. Norat T. Riboli E. Fruit, vegetables, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2009;89:1441–1452. doi: 10.3945/ajcn.2008.27120. [DOI] [PubMed] [Google Scholar]
- 3.Davis CD. Milner JA. Gastrointestinal microflora, food components and colon cancer prevention. J Nutr Biochem. 2009;20:743–752. doi: 10.1016/j.jnutbio.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jia Y. Li ZY. Zhang HG. Li HB. Liu Y. Li XH. Panax notoginseng saponins decrease cholesterol ester via up-regulating ATP-binding cassette transporter A1 in foam cells. J Ethnopharmacol. 2010;132:297–302. doi: 10.1016/j.jep.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 5.Sun S. Wang CZ. Tong R. Li XL. Fishbein A. Wang Q. He TC. Du W. Yuan CS. Effects of steaming the root of Panax notoginseng on chemical composition and anticancer activities. Food Chem. 2010;118:307–314. [Google Scholar]
- 6.Ruan JQ. Leong WI. Yan R. Wang YT. Characterization of metabolism and in vitro permeability study of notoginsenoside R1 from radix notoginseng. J Agric Food Chem. 2010;58:5770–5776. doi: 10.1021/jf1005885. [DOI] [PubMed] [Google Scholar]
- 7.Shang XL. Fu HQ. Liu J. Li XD. Inhibitory effects on human hepatocarcinoma cells with Panax notoginseng saponins [in Chinese] Chin J Clin Rehabil. 2006;10:121–124. [Google Scholar]
- 8.Chung VQ. Tattersall M. Cheung AHT. Interactions of a herbal combination that inhibits growth of prostate cancer cells. Cancer Chemother Pharmacol. 2004;53:384–390. doi: 10.1007/s00280-003-0746-1. [DOI] [PubMed] [Google Scholar]
- 9.Ng TB. Pharmacological activity of sanchi ginseng (Panax notoginseng) J Pharm Pharmacol. 2006;58:1007–1019. doi: 10.1211/jpp.58.8.0001. [DOI] [PubMed] [Google Scholar]
- 10.Xie GX. Qiu YP. Qiu MF. Gao XF. Liu YM. Jia W. Analysis of dencichine in Panax notoginseng by gas chromatography-mass spectrometry with ethyl chloroformate derivatization. J Pharmaceut Biomed. 2007;43:920–925. doi: 10.1016/j.jpba.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Wan JB. Lai CM. Li SP. Lee MY. Kong LY. Wang YT. Simultaneous determination of nine saponins from Panax notoginseng using HPLC and pressurized liquid extraction. J Pharm Biomed Anal. 2006;41:274–279. doi: 10.1016/j.jpba.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 12.Wang SF. Ye S. Cheng YY. Separation and on-line concentration of saponins from Panax notoginseng by micellar electrokinetic chromatography. J Chromatogr A. 2006;1109:279–284. doi: 10.1016/j.chroma.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 13.Zheng M. Qu LH. Lou YJ. Studies on separation and purification of total arasaponin. J Mod Appl Pharm. 2007;24:118–120. [Google Scholar]
- 14.Brand-Williams W. Cuvelier ME. Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. [Google Scholar]
- 15.Sminoff N. Cumbes QJ. Hyroxyl radical scavenging activity of compatible solutes. Phytochemistry. 1989;28:1057–1060. [Google Scholar]
- 16.Lüpertz R. Wätjen W. Kahl R. Chovolou Y. Dose- and time-dependent effects of doxorubicin on cytotoxicity, cell cycle and apoptotic cell death in human colon cancer cells. Toxicology. 2010;271:115–121. doi: 10.1016/j.tox.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Goh D. Lee YH. Ong ES. Inhibitory effects of a chemically standardized extract from Scutellaria barbata in human colon cancer cell lines, LoVo. J Agric Food Chem. 2005;53:8197–8204. doi: 10.1021/jf051506+. [DOI] [PubMed] [Google Scholar]
- 18.Yang XB. Zhao Y. He NW. Croft KD. Isolation, characterization, and immunological effects of α-galacto-oligosaccharides from a new source, the herb Lycopus lucidus Turcz. J Agric Food Chem. 2010;58:8253–8258. doi: 10.1021/jf101217f. [DOI] [PubMed] [Google Scholar]
- 19.Carvalho M. Silva BM. Silva R. Valentao P. Andrade PB. Bastos ML. First report on Cydonia oblonga Miller anticancer potential: differential antiproliferative effect against human kidney and colon cancer cells. J Agric Food Chem. 2010;58:3366–3370. doi: 10.1021/jf903836k. [DOI] [PubMed] [Google Scholar]
- 20.Zheng YQ. Xin YW. Shi XN. Guo YH. Cytotoxicity of Monascus pigments and their derivatives to human cancer cells. J Agric Food Chem. 2010;58:9523–9528. doi: 10.1021/jf102128t. [DOI] [PubMed] [Google Scholar]
- 21.Nzaramba MN. Reddivari L. Bamberg JB. Miller JC., Jr Anti-proliferative activity and cytotoxicity of Solanum jamesii tuber extracts on human colon and prostate cancer cells in vitro. J Agric Food Chem. 2009;57:8308–8315. doi: 10.1021/jf901567k. [DOI] [PubMed] [Google Scholar]
- 22.Jin CY. Choi YH. Moon DO. Park C. Park YM. Jeong SC. Heo MS. Lee TH. Lee JD. Kim GY. Induction of G2/M arrest and apoptosis in human gastric epithelial AGS cells by aqueous extract of Agaricus blazei. Oncol Rep. 2006;16:1349–1355. [PubMed] [Google Scholar]
- 23.Yang JX. Guo J. Yuan JF. In vitro antioxidant properties of rutin. LWT Food Sci Technol. 2008;41:1060–1066. [Google Scholar]
- 24.Yuan YV. Bone DE. Carrington MF. Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem. 2005;91:485–494. doi: 10.1016/j.fct.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Mariod AA. Ibrahim RM. Ismail M. Ismail N. Antioxidant activity and phenolic content of phenolic rich fractions obtained from black cumin (Nigella sativa) seedcake. Food Chem. 2009;116:306–312. [Google Scholar]
- 26.Wang CZ. Yuan CS. Potential role of ginseng in the treatment of colorectal cancer. Am J Chin Med. 2008;36:1019–1028. doi: 10.1142/S0192415X08006545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allouche Y. Warleta F. Campos M. Sanchez-Quesada C. Uceda M. Beltran G. Gaforio JJ. Antioxidant, antiproliferative, and pro-apoptotic capacities of pentacyclic triterpenes found in the skin of olives on MCF-7 human breast cancer cells and their effects on DNA damage. J Agric Food Chem. 2011;59:121–130. doi: 10.1021/jf102319y. [DOI] [PubMed] [Google Scholar]