Abstract
Across the Americas and the Caribbean, nearly 561,000 slide-confirmed malaria infections were reported officially in 2008. The nine Amazonian countries accounted for 89% of these infections; Brazil and Peru alone contributed 56% and 7% of them, respectively. Local populations of the relatively neglected parasite P. vivax, which currently accounts for 77% of the regional malaria burden, are extremely diverse genetically and geographically structured. At a time when malaria elimination is placed on the public health agenda of several endemic countries, it remains unclear why malaria proved so difficult to control in areas of relatively low levels of transmission such as the Amazon Basin. We hypothesize that asymptomatic parasite carriage and massive environmental changes that affect vector abundance and behavior are major contributors to malaria transmission in epidemiologically diverse areas across the Amazon Basin. Here we review available data supporting this hypothesis and discuss their implications for current and future malaria intervention policies in the region. Given that locally generated scientific evidence is urgently required to support malaria control interventions in Amazonia, we briefly describe the aims of our current field-oriented malaria research in rural villages and gold-mining enclaves in Peru and a recently opened agricultural settlement in Brazil.
1. Introduction
After decades of systematic control and attempted eradication, malaria continues to keep a firm grip and remains endemic in 21 countries in the Americas and the Caribbean. Nearly 561,000 slide-confirmed infections, with 89 deaths, were reported by the Pan American Health Organization (PAHO) in 2008 (PAHO, 2009). Cases in the nine Amazonian countries (Bolivia, Brazil, Colombia, Ecuador, Guyana, French Guyana, Peru, Suriname, and Venezuela) accounted for 89.3% of these infections. These figures, which are derived from yearly reports from the Ministries of Health, are likely to be underestimated, due to limitations in the coverage of malaria notification and diagnosis in many malarious areas, but there is no consensus regarding the most appropriate strategy to adjust for subnotification. For example, the World Malaria Report 2008 (World Health Organization, 2008) derived estimates of global malaria burden by adjusting the reported number of malaria cases in 2006 for health facility reporting rates, care-seeking behavior for fever and the extent to which suspected cases are confirmed with laboratory tests. Using these criteria, the reported number of malaria cases in the Americas was multiplied by 2.6, with 2,000–3,000 malaria-related deaths in 2006. However, the resulting figures were considered by PAHO to overestimate the malaria burden in the Americas (PAHO, 2009), and no further attempts at adjusting reported numbers of malaria cases were made in subsequent years.
Although transmission of both Plasmodium falciparum and P. vivax occur across the Amazon Basin (with rare P. malariae infections), P. vivax currently accounts for 77% of the malaria burden in Amazonia (PAHO, 2009). As with many other tropical and mainly rural infectious diseases, malaria in the Americas disproportionately affects people in the lower socioeconomic strata, with difficult access to health care.
1.1 Malaria in Brazil
The annual incidence of malaria in Brazil increased >10-fold since 1970 following massive human migration and the establishment of frontier agricultural settlements and open mining enclaves in the Amazonian rainforest (Cruz Marques, 1987). Nearly 315,000 slide-confirmed infections were recorded in this country in 2008, 99.9% of them in the Amazon Basin; these figures represent 56.1% of all slide-confirmed malaria episodes diagnosed in the Americas and the Caribbean in 2008 (PAHO, 2009). The Program for Malaria Control in the Amazon Basin (known as PCMAM after the Portuguese acronym), launched in 1989 and financed with US$73 million from the World Bank (Barat, 2006), had a clear short-term impact: malaria morbidity decreased by 60% between 1989 and 1996 (Oliveira-Ferreira et al., 2010). The PCMAM strategy, focused on early diagnosis and treatment of malaria cases to reduce transmission and mortality, was proposed as being more cost-effective than widespread house spraying with residual insecticides (Akhavan et al., 1999). The existing network of malaria outposts was reinforced and expanded across Amazonia to provide early and free diagnosis (based on thick smear microscopy) and free treatment of slide-confirmed malaria episodes (with standardized drug regimens), while house spraying was gradually phased out (Roberts et al., 1997). However, the gains were not sustained over the next years, and the number of malaria cases increased by 34% between 1998 and 1999 (Loiola et al., 2002). Further periods of intensification of malaria control through early diagnosis and treatment once again resulted in substantial decreases in malaria incidence between 2000 and 2002 and after 2007 (Oliveira-Ferreira et al., 2010). DDT likely was critically important in reducing malaria incidence in South American after World War II (Loiola et al., 2002), and its use in fact was intensified in 2000 through the “Action Plan for Intensification of Malaria Control in the Amazon” established by the Brazilian government in concert with the WHO-initiated Roll Back Malaria initiative. Nonetheless, malaria has remained intractable to control efforts in Brazil and elsewhere in Amazonia. In 2009, the manufacture and use of DDT in Brazil was legally prohibited, despite well-articulated pleas to continue its use given lack of demonstrable human toxicity and environmental damage when use is limited to household spraying (Roberts et al., 2000).
Malaria transmission hotspots in Brazil, as in the rest of South America, are intermingled with areas of low or moderate risk (Figure 1). Most malaria transmission typically occurs in mining and logging camps and new farming settlements (da Silva-Nunes et al., 2008; de Castro et al., 2006; de Castro et al., 2007). These settlements not only induce massive environmental changes, such as deforestation (Figure 2), that alter vector biology and favor malaria transmission, but also cluster large number of non-immune migrants close to natural and man-made vector breeding sites (de Castro et al., 2007; Norris, 2004; Vittor et al., 2006; Vittor et al., 2009). Not surprisingly, malaria cases cluster in the sectors of most recent occupation in frontier settlements, where ongoing land clearing favors an increase in the abundance of the main malaria vector in the region, An. darlingi (da Silva et al., 2010).
Figure 1.
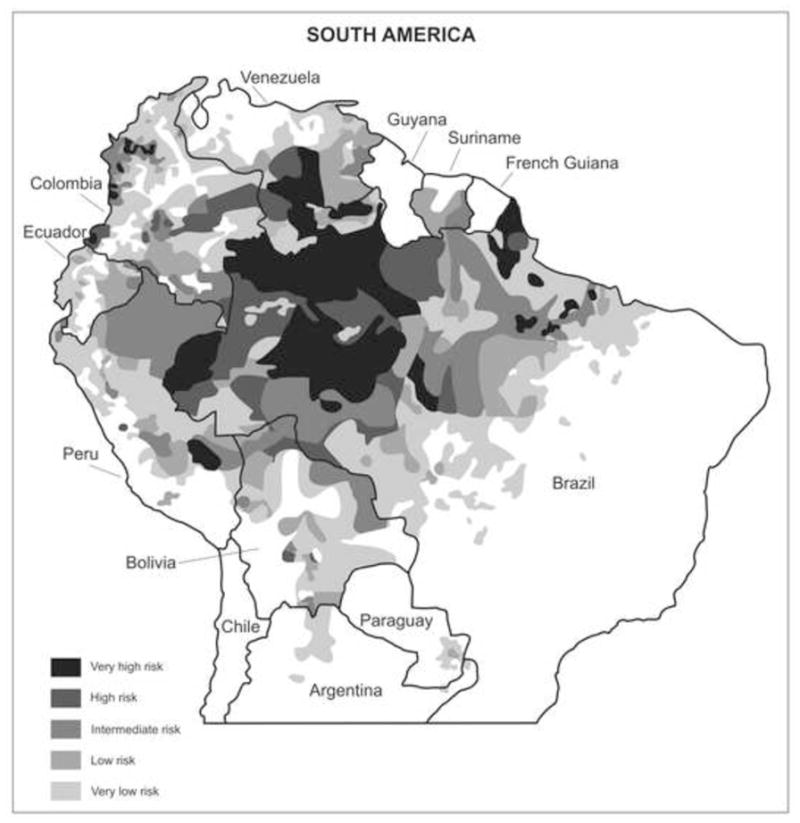
Map of South America showing the malaria-endemic areas with different shadding pattern according to transmission levels in 2008. Source of data: Pan American Health Organization, 2009. Report on the Situation of Malaria in the Americas, 2008. Washington, DC. Available at: http://new.paho.org/hq/index.php?option=com_content&task=view&id=2459&Itemid=2049
Figure 2.

Deforestation for slash-and-burn agriculture (left) and dwellings surrounded by rain forest (right) in the agricultural settlement of Remansinho, near Acrelândia, Acre, northwestern Brazil (Pictures by Marcelo U. Ferreira).
Recent data also reveal changes in the relative contribution of different malaria parasite species to the total malaria burden in Brazil. Whereas transmission of P. falciparum, which predominated between 1985 and 1990, decreased steadily, that of P. vivax maintained an upward trend throughout the 1990s. Plasmodium vivax now accounts for >80% of the malaria burden in Brazil (Oliveira-Ferreira et al., 2010), mirrored in Peru as well although with lower absolute incidence. Overall Plasmodium malariae transmission remains low and focal (Scopel et al., 2004; da Silva-Nunes et al., 2008; Ladeia-Andrade et al., 2009; da Silva et al., 2010), and this species accounts for less than 1% of all slide-confirmed malaria infections in Brazil (Oliveira-Ferreira et al., 2010). As in other parts of the world (Sattabongkot et al., 2004), the relatively limited success in reducing malaria incidence in Brazil over the past decade (Ferreira and Da Silva-Nunes, 2010) has substantially resulted from failure to prevent P. vivax infections, providing a compelling stimulus for malaria vivax-oriented research in Amazonia.
1.2 Malaria in Peru
Malaria in Peru is transmitted in diverse settings including coastal, mountainous and jungle regions, with an important epidemic beginning in the mid 1990s (Aramburu Guarda et al., 1999; Roper et al., 2000) and with nearly 36,900 cases recorded in 2008. Most transmission is recorded in Loreto and Madre de Dios Departments, both in the Amazon Basin (Figure 3). Ecological changes favored the spread of the competent vector Anopheles darlingi in Loreto in the early 1990s (Schoeler et al., 2003) and over the following years led to major outbreaks of malaria in rural villages surrounding Iquitos, a major port city with 450,000 inhabitants (Aramburu Guarda et al., 1999; Bautista et al., 2006; Roper et al., 2000; Roshanravan et al., 2003). Additional sociodemographic factors are also likely to be responsible for the reemergence of malaria as a public health problem in Loreto. A major livelihood of people living in the rural areas surrounding Iquitos is based on agriculture, fishing and timber extraction. Men and women farm small plots of land for sugar cane, fruit, harvest wood for charcoal, and raise livestock for sale. These activities often occur on land located away from permanent dwellings, resulting in a group of people who camp in the fields during the work week and return to their houses in villages on weekends. Such activities provide the opportunity for people from different malaria-endemic regions to congregate and admix parasites. If this hypothesis is correct, people with such epidemiological characteristics (rural travelers, immigrants) would be major targets for control strategies since this might be a major driver for mobilizing parasite diversity among populations.
Figure 3.
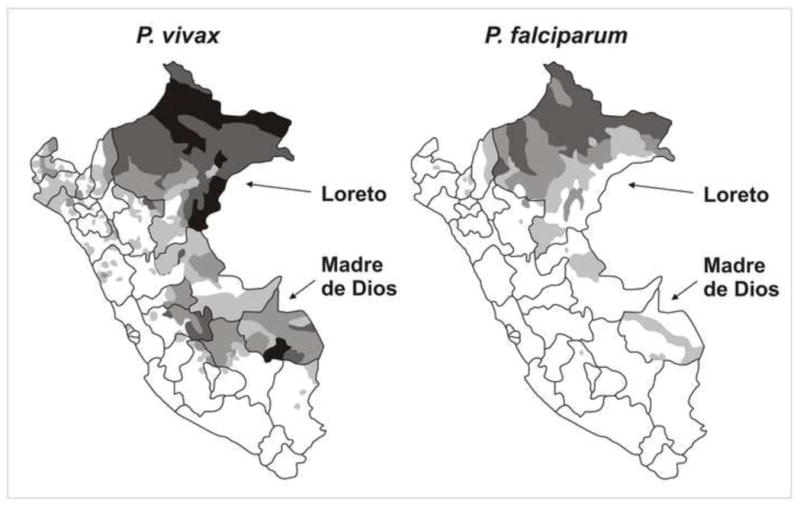
Map of Peru showing the areas with highest levels of malaria transmission, the Departments of Loreto (where one of our field sites is located) and Madre de Dios (where our second Peruvian field site is located). The different shadding patterns (as in Figure 1) reflect different transmission levels in 2008. Source of data: Ministry of Health of Peru, 2009.
Since the late 1990s, malaria-naïve migrants, mostly from the Andean region of Peru, have been attracted to mining sites across the Madre de Dios river and its tributaries (Figure 4). Most confirmed malaria cases in the area, all due to P. vivax, came from Huepetuhe, the large gold mining camp located in the southwest of the Madre de Dios Department in southeastern Peru. Malaria-endemic Madre de Dios Department is also currently affected by massive migration and drastic environmental changes induced by the construction of the Transoceanic Highway, which connects Amazonian Brazil to the Pacific Coast of Peru.
Figure 4.

Gold-mining enclave surrounding the rain forest in the Department of Madre de Dios, Peru (Picture by Marta Moreno).
1.3 Transmission dynamics of Plasmodium vivax
Populations of Plasmodium vivax, the predominant local malaria parasite, are extremely diverse genetically and geographically structured in the Amazon Basin of both Brazil and Peru, with significant genetic divergence between populations separated by relatively short distances (Ferreira et al., 2007; Orjuela-Sánchez et al., 2010; Van den Eede et al., 2010). When comparing sympatric parasites of each species, much more microsatellite diversity is found in P. vivax than in P. falciparum populations from Amazonian Brazil (Ferreira et al., 2007; Orjuela-Sánchez et al., 2009a). The latter populations display a fast haplotype replacement rate over time, as revealed by genotyping parasites recovered from the same community over 12–24 months of follow-up (Ferreira et al., 2007; Orjuela-Sánchez et al., 2009b; Van den Eede et al., 2011). Such a large spatial-temporal genetic diversity may severely delay the development of the variant-specific component of naturally acquired immunity to P. vivax in Amazonians (Bastos et al., 2007; Souza-Silva et al., 2010). However, such an extensive genetic diversity paradoxically coexists with low meiotic recombination rates, with a strong linkage disequilibrium between genetic markers that map to different chromosomes (Ferreira et al., 2007; Orjuela-Sánchez et al., 2009b; Van den Eede et al., 2010; Van den Eede et al., 2011) and a slow decay in linkage disequilibrium with increasing physical distance between markers along the same chromosome (Orjuela-Sánchez et al., 2010).
Plasmodium vivax recurrences are often seen, in both Brazil and Peru, 1–6 months after treatment with standard doses of chloroquine and primaquine (Orjuela-Sánchez et al., 2009b; da Silva et al, 2010; Van den Eede et al., 2011). Because malaria in the Amazon Basin is transmitted year-round, recrudescences, relapses, and new infections may all originate these recurrences. Multilocus microsatellite typing was recently used to compare haplotypes in pairs of consecutive P. vivax infections diagnosed in cohorts of rural Amazonians in Brazil and Peru; in all studies, haplotypes that had not been detected in primary infections predominated in recurrences, consistent with new infections or relapses due to the reactivation of heterologous hypnozoites (Orjuela-Sánchez et al., 2009b; Van den Eede et al., 2011). Whether emerging resistance to chloroquine (de Santana Filho et al.2007) and perhaps to primaquine (the only drugs currently in use to treat P. vivax infections) plays a major role in post-treatment late recurrences in these countries remains unclear (da Silva-Nunes and Ferreira, 2010); subtherapeutic primaquine doses, however, are commonly used and clearly associated with relapses (Duarte et al., 2001).
2. Asymptomatic parasite carriage
At a time when malaria elimination is again on the public health agenda of several endemic countries (Feachem and Sabot, 2008; Mendis et al., 2009), it remains unclear why malaria has proved so difficult to control across the Amazon Basin, where transmission rates remain far below those recorded in tropical Africa. To provide scientific evidence that can be translated into effective interventions for malaria control in the region, we are currently investigating the clinical and public health significance of asymptomatic parasite carriage. We hypothesize that asymptomatic infections represent a major source of infective gametocytes for the increased vector population found in recently deforested areas across the Amazon Basin.
2.1 Asymptomatic malaria in frontier settlements
Unstable and hypo- or mesoendemic malaria transmission usually seen in Amazonia typically fails to elicit the status of clinical immunity seen among adults exposed to holoendemic malaria in rural Africa. The entire population is vulnerable to infection and nearly all slide-positive infections are followed by clinical disease (Camargo et al., 1999; Camargo et al., 1996; Prata et al., 1988). However, the longstanding dogma that immunity rarely develops in areas of low malaria endemicity (MacDonald, 1956, 1957) has been challenged by studies of Javanese transmigrants in Papua New Guinea (Baird et al., 1991) and, in more recent observations, in frontier settlements and riverine communities across the Amazon Basin of Brazil (Alves et al., 2002; da Silva et al., 2010; da Silva-Nunes et al., 2008; Ladeia-Andrade et al., 2009; Suarez-Mutis et al., 2007), where subclinical infections with very low parasite loads, most of them detected only by polymerase chain reaction (PCR), are quite common. After five to eight years of continuous exposure to low to moderate levels of malaria transmission, the prevalence or incidence of both infection and disease decreases steadily, suggesting that Amazonian populations acquire not only anti-disease immunity but also some degree of anti-parasite immunity (Ladeia-Andrade et al., 2009). Native and migrant populations exposed to malaria seem to develop immunity, but the immunological correlates of clinical protection remain largely unexplored. Asymptomatic malaria parasite carriage is also commonly seen among inhabitants of rural villages surrounding Iquitos, in Peru, with very recent exposure to epidemic malaria (Branch et al., 2005; Parekh et al., 2007; Roshanravan et al., 2003).
2.2 A role for aggressive active case detection?
Malaria infections across the Amazon Basin are routinely identified through active or passive case detection (ACD and PCD, respectively). According to the classical definitions from the malaria eradication era (Pampana, 1963), cases are found through PCD when febrile subjects visiting malaria diagnosis outposts have a blood sample tested positive for malaria parasites. ACD implies periodic visits to households, with collection of thick blood smears from every person having had fever since the last visit (Najera, 2001). A major limitation of ACD and PCD is that both strategies target only symptomatic infections (classically defined as “fever cases”); as a result, asymptomatic infections go undetected and untreated (Coura et al., 2006), extending the average duration of parasite carriage to around 270 days (Freeman et al., 1999). Because the clinical spectrum of symptomatic malaria in semi-immune Amazonians ranges from a very mild illness to a full-blown disease with periodic fever paroxysms, ACD and PCD face a heterogeneous disease in which cyclical paroxysms with fever, chills and profuse sweating are not necessarily prominent features (da Silva-Nunes and Ferreira, 2007).
The periodic population-wide screening for malaria parasites, irrespective of any clinical symptoms, is known as aggressive active case detection (AACD; Macauley, 2005), mass blood survey or mass blood examination (Pampana, 1963). Alternatively, AACD may be carried out during the epidemiological investigation of a focus, targeting persons living in the neighborhood of a positive case (Pampana, 1963). Although AACD can potentially detect asymptomatic infections and supplement ACD and PCD in areas where the sources of infection cannot be sufficiently screened by using fever as a criterion (Pampana, 1963), whether AACD should be implemented as a public health strategy in Amazonia remains a matter of debate (Alves et al., 2002; Alves et al., 2005; Coura et al., 2006; da Silva et al., 2010; da Silva-Nunes et al., 2008; Ladeia-Andrade et al., 2009; Macauley, 2005; Roshanravan et al., 2003; Vinetz and Gilman, 2002). To evaluate the cost-effectiveness of population-wide AACD, the relative role of asymptomatic infections in maintaining malaria transmission in Amazonia must be quantified. Mathematical models identified asymptomatic infections as a crucial target for malaria eradication efforts in Africa (Aguas et al., 2008), but no similar analysis are available for other endemic areas.
2.3 Gametocyte carriage and infectivity in asymptomatic infections
The prevalence and average duration of gametocytemia in asymptomatic infections remain largely unknown. Little is known on infectiousness of asymptomatic carriers of low-grade parasitemias (Alves et al., 2002; Alves et al., 2005). In addition, it remains unclear whether people living in malaria-endemic regions develop immunity against the sexual stages of malaria parasites that infect mosquitoes, the so-called naturally acquired transmission-blocking immunity. If this were demonstrated to be the case, the presence of naturally acquired transmission-blocking antibodies would provide the basis of novel approaches to the development of malaria transmission-blocking strategies. We have experimentally infected local Anopheles darlingi mosquitoes with Plasmodium vivax directly obtained from malaria patients in the Iquitos region of Peru and found that only about half of patients with acute P. vivax malaria were able to infect mosquitoes (this observation has held up with more than 200 such feedings using blood from acute vivax malaria patients; JMV, unpublished observations). There was no relationship between the presence of gametocytes (the transmission stages) and infectivity of mosquitoes, suggesting that some clinical factor was impairing parasite transmission from humans to mosquitoes. Based on these results, we hypothesize that patients who inefficiently infect mosquitoes have developed acquired immunity in the form of antibodies against protein antigens expressed by the transmission stages of the malaria parasite.
2.4 Diagnostic challenges
Laboratory methods appropriate for large-scale use, such as microscopy and rapid diagnostic tests (RDTs), are not sensitive enough to detect low-grade, asymptomatic infections. A highly sensitive PCR-based diagnosis, which is between 2.7-fold and 8.6-fold more sensitive than conventional microscopy in detecting malaria parasites in apparently healthy Amazonians (da Silva et al., 2010), has been suggested as a public health tool for AACD in Peru (Roshanravan et al., 2003), but its large-scale use remains constrained by its high cost and complexity. RDTs are not a feasible alternative alternative to PCR for population-based screening of asymptomatic infections because of low parasitemia during asymptomatic infection. Most commercially available RDT kits use the Histidine Rich Protein 2 (HRP2) as the target antigen (Moody, 2002) and antibodies to HRP2 may also cross react with another member of the HRP gene family, HRP3. Our recent evaluation of RDTs revealed a substantial number of malaria parasites in Peru and Colombia with a deletion of the HRP2 and HRP3 genes. In Peru, the HRP2 and HRP3 genes were missing in 41% and 70% isolates of P. falciparum, respectively; both genes had been deleted in 22% of these isolates (Gamboa et al., 2010). Similar studies are underway in Brazil. These deletions of HRP2 and HRP3 may compromise the ability of RDTs based on these antigens to detect the presence of parasites in patient samples, especially in asymptomatic carriers of low-grade parasitemias.
2.5 Asymptomatic infections and risk of subsequent clinical disease
Recent evidence suggests that long-term asymptomatic carriage of malaria parasites protects against subsequent disease in Tanzania (Bereczky et al., 2007) and Senegal (Males et al., 2008), possibly by reducing the risk of superinfection with more virulent strains. These results imply that eradicating asymptomatic infections can increase the risk of clinical malaria over the next transmission season. The biological bases for this phenomenon remain largely unknown, but data from experimental rodent malaria models suggest that ongoing blood-stage infection, once a minimum parasite load is reached, may arrest the development of subsequently inoculated sporozoites in the liver. Such an inhibition of superinfection seems to be mediated by the iron regulatory hormone hepcidin, produced in response to blood-stage parasitemia (Portugal et al., 2011). Because of their major public health implications, these findings require validation, with more extensive adjustment for potential confounders such as cumulative exposure to malaria and acquired immunity (Gosling, 2008), in epidemiologically diverse endemic settings, such as the Amazon Basin.
3. Vector biology and control
One of the main contributors to continued malaria transmission in the Amazon is deforestation that alters breeding sites (Vittor et al., 2006, 2009; Monteiro de Barros et al., 2011), and settlement by susceptible humans near new frontier agricultural and open mining projects in rainforests (da Silva-Nunes et al., 2008; de Castro et al., 2006). Another important factor is the presence of asymptomatic parasite carriers, a potential source of gametocytes for infecting mosquitoes (Alves et al., 2005). The other critical piece of this transmission picture is the species of local malaria vector and its ecology, behavior, and population structure (de Castro et al., 2006; Lounibos and Conn, 2000; Vittor et al., 2009).
3.1 Local vector species
The World Health Organization’s strategic framework for vector control calls for “an evidence-based decision-making approach which involves the adaptation of strategies and interventions to local vector ecology, epidemiology and resources that are guided by operational research and subject to routine monitoring and evaluation.” However, malaria vector biology remains understudied in Amazonia, and much less is known about species other than Anopheles darlingi that contribute to the maintenance of local transmission (but see Zimmerman et al., 2006; Galardo et al., 2007, 2009). In particular, species misidentification remains a serious issue in Amazonian vector biology (Marrelli et al., 2005; Sallum et al., 2008; Matson et al., 2008; Cienfuegos et al., 2011). By combining male genitalia analysis, progeny broods, and molecular sequences, puzzling anomalies can be resolved (Ruiz et al., 2005; Sallum et al., 2008).
Anopheles (Nyssorhynchus) darlingi is the most anthropophilic and efficient malaria vector in Latin America, capable of transmitting P. falciparum, P. vivax and P. malariae (Galardo et al., 2007; Lourenco-de-Oliveira et al., 1989; Magris et al., 2007). It is broadly distributed in the Neotropics, and has recently been detected for the first time in eastern Panamá (Loaiza et al., 2009). The larval habitats of An. darlingi are typically natural water bodies, particularly slow-moving margins of streams or rivers with shaded, clear water, or lagoons that remain following flooding (reviewed in Charlwood 1996; Rejmánková et al., 1999; Sinka et al., 2010). In addition, An. darlingi larvae can be found in human-modified habitats, such as fish ponds (Lounibos and Conn, 2000; Vittor et al., 2009).
In much of Loreto Department in Amazonian Peru, An. darlingi has become synonymous with malaria transmission, but only since its spectacular emergence around Iquitos in the 1990s (Aramburu Guarda et al., 1999; Fernández et al., 1996; Roberts et al., 1997) and subsequent spread through much of Loreto (Schoeler et al., 2003). Whereas in 1991, several hundred malaria cases were reported in Loreto (Roper et al., 2000), by 1997, the number had risen to tens of thousands (Schoeler et al., 2003). The region therefore faced epidemic malaria, mainly linked to deforestation and the invasion of this aggressive vector (Aramburu Guarda et al., 1999; Vittor et al., 2006). Currently, in sites around Iquitos, as well as in nearby Puerto Almendra (Turell et al., 2008), An. darlingi is the primary vector, transmitting mainly P. vivax.
In the mining camps in southeastern Amazonian Peru, limited available data suggest a more complex picture. In Madre de Dios Department, near the Peru-Bolivia-Brazil border, adult An. darlingi was the most abundant species captured biting humans indoors and outdoors in two of four localities; however, An. benarrochi was the most abundant in the other two localities (Tineo et al., 2003). In a newer study in the same region, An. benarrochi is hypothesized to be an important regional vector (Flores-Mendoza et al., 2004). An. benarrochi has been reported as a primary vector in western Loreto and Ucayali (Aramburu Guarda et al., 1999; Fernández et al., 1996; Schoeler et al., 2003). Despite being much more abundant in West Loreto and Ucayali than An. darlingi (71% vs. 24%), An. benarrochi was infected with both P. falciparum and P. vivax at much lower rates (Flores-Mendoza et al., 2004), suggesting that An. benarrochi is less competent. These data are consistent with a recent study in southern Colombia that described a new species, An. benarrochi B (Ruiz et al., 2005), and found that despite its anthropophily and high prevalence (66.1% of 2,445 anophelines tested), no An. benarrochi B were positive for Plasmodium by ELISA (Quinones et al., 2006). Furthermore, by comparing ITS2 sequences and male genitalia of voucher specimens Quinones et al.(2006) determined that both An. benarrochi s.s. and An. benarrochi B occur in Peru. Detection of a morphological variant and ITS2 PCR-RFLP differences in An. benarrochi collected from Loreto and Ucayali in Peru (Matson et al., 2008) may support these findings. It is critically important to accurately identify the main vector(s) in the mining areas of Madre de Dios Department to evaluate the risk of malaria transmission and the effectiveness of current control methods.
Anopheles oswaldoi has been identified as a malaria vector in Brazil (de Arruda et al., 1986; de Oliveira-Ferreira et al., 1990), playing a major role in malaria transmission in the northwestern state of Acre, bordering with Bolivia and Peru (Branquinho et al., 1993). However, the species originally identified as An. oswaldoi s.s. in Acre is now believed to be one of two new species in the An. oswaldoi or An. benarrochi complexes (Sallum et al., 2008). An. oswaldoi was described as a complex (Flores-Mendoza et al., 2004; Marrelli et al., 1999) and one of the member species is a vector in Colombia (Quinones et al., 2006; Rodriguez et al., 2009). An. deaneorum, a member of the Albitarsis complex (Klein et al., 1991a; Rosa-Freitas, 1989; Wilkerson et al., 1995a, 1995b) was also implicated, though it was, and remains, much less abundant (Branquinho et al., 1993). This species is competent for P. falciparum and P. vivax in a laboratory setting (Klein et al., 1991a, 1991b, 1991c), and it has been found infected in field studies (Branquinho et al., 1993), assuming this identification is confirmed (Sallum et al., 2008). Because the anopheline collections for Branquinho et al.’s 1993 study were done in 1990–1991, a long time ago in a dynamic frontier malaria area (da Silva-Nunes et al., 2008), we hypothesize that major shifts in species composition have occurred in this region.
3.2 Environmental change and vector populations
As a settlement becomes stable, there is generally a plateau of malaria cases (de Castro et al. 2006). However, with subsequent expansion and additional habitat alteration, a new cycle of increased mosquito-human contact can be initiated. Deforestation and other anthropogenic activities often create diversity in larval habitats, resulting in changes in mosquito species composition and increased abundance (Povoa et al., 2003; Tadei et al., 1998; Vittor et al., 2006; Moutinho et al., 2011). In the areas studied by Tadei et al. (1998), anopheline abundance was estimated to be approximately five times greater in disturbed compared with undisturbed habitats. The significant correlation between deforestation and human biting rates of An. darlingi along the Iquitos-Nauta road (Vittor et al., 2006, 2009) is an important finding; breeding sites of An. darlingi were detected in areas with 24.1% forest cover, in contrast to 41% coverage areas where this species was absent (Vittor et al., 2009). A new study that analyzed multiple forested and deforested sites along a river in Roraima state, Brazil, found the highest abundance of An. darlingi larvae in the forested-deforested transition zones during the dry season, and in natural microdams where the river current was obstructed (Monteiro de Barros et al., 2011). Shade and nearby human dwellings were also important correlates. Taken together, these data suggest that An. darlingi is most strongly associated with some forest cover in diverse types of habitats significantly altered by human activities, especially in rural settings (Moutinho et al., 2011).
The unprecedented invasion into the Iquitos region of An. darlingi resulted in major increases in its abundance in most localities (Aramburu Guarda et al., 1999). It also bore the molecular signature of an invasive species, with limited diversity, as seen in (Pinedo-Cancino et al., 2006) using RAPDs, and subsequently confirmed in some localities by microsatellite analysis (Mirabello et al., 2008). We postulate that a similar pattern of An. darlingi expansion, and increased malaria transmission, will take place in the rural settlements near Iquitos and in the mining camps in Madre de Dios Department, both in Peru, as well as in the recently colonized agricultural projects in Amazonian Brazil (Moutinho et al., 2011). A better understanding of the dispersal of species into newly altered habitats is vital to reduce human-vector contact.
4. A role for insecticide-treated bednets (ITNs) for malaria control in Amazonia?
Long-lasting insecticide-treated nets (ITNs) have emerged in the 1990s as one of the great hopes for controlling malaria worldwide. Their efficacy has been clearly demonstrated in different endemic areas, especially in Africa, where they reduce anemia incidence and overall mortality (Curtis et al., 2006). In a systematic literature review of Plasmodium falciparum endemic settings, it is assumed the impact of IRS is equal to that of ITNs on reducing malaria-attributable mortality in children (Eisele et al, 2010). When ITNs are provided free of charge and a high population coverage is achieved, their impact on malaria transmission in Africa is comparable to that in the best house spraying projects (Lengeler and Snow, 1996). In a review paper of Pluess and collaborators (2010), some limited data suggested that ITNs give better protection than IRS in unstable areas, but more trials are needed to compare the effects of ITNs with IRS, as well as to quantify their combined effects. In this review, ITNs appeared to provide better protection against any infection compared to IRS in India (Misra, 1999). In another review, using the Entomological Inoculation Rate (EIR) to assess the impact of vector control on malaria transmission, two different studies with separate ITN and IRS intervention groups, (in Tanzania and Solomon Islands) showed that in the second year of the Tanzania study, EIR was 90% lower in the ITN community and 93% lower in the IRS community, relative to the community without intervention, and that the ITN and IRS effects were not significantly different. In contrast, in the Solomon Islands study, EIR was 94% lower in the ITN community and 56% lower in the IRS community (Shaukat et al., 2010).
Nevertheless, Zimmerman and Voorham concluded, in their review published more than a decade ago, that “it would be premature to use insecticide-impregnated mosquito nets or other materials as a major component of an integrated malaria control program in the Americas at this time” and called for well-designed large-scale trials in this region (Zimmerman and Voorham, 1997)(see also (Kroeger et al., 1995; Kroeger et al., 1997)). This is partly because the biting behavior of Neotropical malaria vectors, especially An. darlingi, is predominantly exophagic (feeds outdoors) and often unimodal, during the early evening (Vittor et al., 2006; Voorham, 2002; Zimmerman and Voorham, 1997); but see (Moreno et al., 2007; Rosa-Freitas et al., 1992) when human activity peaks and people are generally not under mosquito nets (Loaiza et al., 2008). An. darlingi can present a marked early biting behavior and high outdoor-to-indoor biting ratio (Tadei et al., 1998) in some areas, although a late biting behavior and indoor preference have also been described (Rozendaal, 1989). Very little is currently known about the biting behavior of other malaria vectors in Amazonia (Sinka et al., 2010).
Surprisingly, no large community-based randomized trials of ITNs have been carried out in the Americas since the 1997 review of Zimmerman and Voorham. The fact that the use of ITNs alone does not adequately reduce malaria rates in every region (but see Charlwood et al., 2005), may undermine their efficacy to impact malaria transmission throughout the Amazon Basin (Alexander et al., 2005; Harvey et al., 2008; Hill et al., 2007). One decade after the publication of Zimmerman and Voorham’s review, we are still waiting for “well-conceived, large-scale trials at the community or regional level” that are “based on a thorough understanding of the dynamics of malaria transmission in the areas involved” (Zimmerman and Voorham, 1997). ITNs are currently recommended for use against malaria transmission by An. darlingi in Amazonian French Guiana (Girod et al., 2008) and, since 1999, the Peruvian Ministry of Health has been distributing ITNs in the Amazonian region for use against biting mosquitoes with some success (Harvey et al., 2008). In Brazil, only recently (2007–2010) has the Ministry of Health started to freely distribute ITNs in malaria-endemic areas. There are two studies in the Amazon region showing evidence of the effectiveness of the ITNs in this area. One in Amazonian Colombia showed that impregnated nets were associated with more than a 50% reduction in malaria relative to no net use (Alexander et al, 2005), although the advantage of impregnated over non-impregnated nets was not statistically significant. Furthermore, a recent randomized trial, in the Amazonas State of Venezuela, of lambdacyhalothrin-versus placebo-treated nets, found a protective efficacy of 55% (Magris et al, 2007). However, variation in terms of vector species (Tadei & Dutary-Tacher, 2000) and possibly of human behavior, mean that optimal policies may vary within the Amazon region.
4.1 Assessing ITN-based interventions
To predict the effectiveness of ITN-based malaria control strategies, it is critically important to monitor and evaluate peak anopheline biting activities for variation and plasticity of seasonal and temporal change, particularly in relation to ecosystem transformations that can dramatically increase malaria transmission (Gil et al., 2007; Moreno et al., 2007; Vittor et al., 2006). One of the main problems of the invasion of new vector species, such as An. darlingi into Iquitos, Peru, is the potential introduction and spread of genes conferring insecticide resistance, which could reduce the effectiveness of current vector control measures. Resistance to pyrethroids is increasing due to overuse and the lack of alternative insecticides (Chareonviriyaphap et al., 2003; Chouaibou et al., 2006; Singh et al., 2011). Unfortunately, the genetic potential to develop multiple insecticide resistance is common in Anopheles spp.(Casimiro et al., 2006; Chareonviriyaphap et al., 2003; Hargreaves et al., 2003). Periodic surveillance of insecticide susceptibility is necessary for adequate management of vector strategies but this is completely absent in Amazonia. Furthermore, in areas where different Anopheles species coexist, resistance evaluation is more complicated since each species may respond differently to the same insecticide (Hargreaves et al., 2003). An. darlingi resistance to DDT was first reported in Colombia (Suarez et al., 1990), and a recent paper shows some Colombian populations of An. nuneztovari resistant to pyrethroids and organophosphates (Fonseca-Gonzalez et al., 2009). Overall, there is a serious lack of accurate and current information about susceptibility levels in Amazonian malaria vectors.
One way to monitor the effectiveness of ITNs or insecticides (in use as indoor residual spray locally (e.g. Santos et al., 2007), in Amazonian Brazil) on the size of anopheline populations is to measure the effective population size (Ne), or simply put, the number of reproductive females, at distinctive temporal points (Pinto et al., 2002; Pinto et al., 2003). This measure is indirect, in that it depends on temporal variation in allele frequencies (i.e., genetic drift) and population size (Wondji et al., 2005). Based on the heterogeneous, but generally small Ne estimates of An. darlingi (92 – 202 females, in Amazonian Brazil, (Conn et al., 2006; Scarpassa and Conn, 2007); around Iquitos, 8 −1,786 females (Mirabello et al., 2008), there could be large fluctuations in this species in Peru and Brazil (Taylor et al., 1993). Diversity measures (observed and expected heterozygosities, mean number of alleles per locus, allelic and genotypic frequencies, and levels of differentiation between populations) are also informative in deducing any effects of ITNs and (or) insecticide in comparisons before and after applications, and also among local and regional populations (Wondji et al., 2005). The estimates of gene flow, especially among anopheline populations in malaria endemic areas, can provide baseline information for the tracking of genes that confer insecticide resistance (Lehmann et al., 2003).
We anticipate that successful vector control for malaria in Amazonian Peru and Brazil can be accomplished with the accurate incrimination of the local primary malaria vector species, the assessment of the impact of ecological changes (deforestation and mining) on their population structure and behavior, and the evaluation of ITNs or insecticide treatment to control An. darlingi and other vectors.
5. Setting the stage for applied malaria research in Amazonia
There has been an explosion of information from large scale genomic, proteomic and gene expression profiling of multiple Plasmodium species. The whole genome sequence of Homo sapiens and the major malaria-transmitting vector mosquito, Anopheles gambiae have been published. A major challenge confronting the malaria community is how to apply such knowledge towards the amelioration of malaria at the field level, in the real world setting. There is an emerging consensus that “public health needs to be evidence-based if it is to be done correctly” (Eriksson, 2000), but translating scientific evidence into public health interventions may be particularly challenging.
Currently available tools and interventions may contribute to bringing down the overall malaria burden in Amazonia, but locally generated evidence is urgently needed to address major gaps in existing interventions. The external validity of well-designed controlled trials in Africa and Asia may be surprisingly limited when interventions deal with diseases with relatively complex causal pathways. For example, the varying biting behavior of local malaria vectors is a clear source of effect modification that affects the generalizability of ITN efficacy trials (Kroeger et al., 1999). Patterns of antimalarial drug resistance are also clearly regional, and a given therapy that has proven to be highly effective in some endemic settings may fail in others. Additional examples of major gaps in the malaria research agenda are the need for improved strategies to identify and treat asymptomatic reservoirs of disease and to monitor and prevent the emergence of resistance to several pesticides, especially pyrethroids, in the vector population. To address these and other gaps in our understanding of the scientific basis for malaria control, we designed field-oriented studies in areas with diverse epidemiology across the Amazon Basin of Brazil and Peru. The field sites include: (a) Remansinho, a typical frontier agricultural settlement in Amazonian Brazil with endemic malaria transmission, (b) rural villages close to Iquitos, a major city in Peru that became recently exposed to epidemic malaria, and (c) gold-mining enclaves near Puerto Maldonado, in Madre de Dios, Peru, with explosive malaria outbreaks due to P. vivax. Research in these sites is carried out in close collaboration with the malaria control teams of the Ministry of Health of both countries, with the aim of enhancing the partnership between researchers and decision-makers to face the challenges in malaria control in Amazonia.
Highlights.
Transmission of malaria continues but is declining in the Americas
Mortality rates are low with fewer than 600,000 annual cases regionally
Amazonian countries are the main malaria-endemic regions
Asymptomatic parasite carriage is common in Amazonia
Environmental change affecting vector abundance contributes to malaria transmission
Acknowledgments
We thank Cassiano P. Nunes for drawings. Work by the authors described in this paper has been supported by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH; U19 AI089681), United States of America, and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; 09/52729-9), Brazil. M.U.F. receives a research scholarship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguas R, White LJ, Snow RW, Gomes MG. Prospects for malaria eradication in sub-Saharan Africa. PLoS One. 2008;3:e1767. doi: 10.1371/journal.pone.0001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhavan D, Musgrove P, Abrantes A, d’A Gusmão R. Cost-effective malaria control in Brazil. Cost-effectiveness of a Malaria Control Program in the Amazon Basin of Brazil, 1988–1996. Soc Sci Med. 1999;49:1385–1399. doi: 10.1016/s0277-9536(99)00214-2. [DOI] [PubMed] [Google Scholar]
- Alexander N, Rodriguez M, Perez L, Caicedo JC, Cruz J, Prieto G, Arroyo JA, Cotacio MC, Suarez M, FDLH, Hall AJ. Case-control study of mosquito nets against malaria in the Amazon region of Colombia. Am J Trop Med Hyg. 2005;73:140–148. [PubMed] [Google Scholar]
- Alves FP, Durlacher RR, Menezes MJ, Krieger H, Silva LH, Camargo EP. High prevalence of asymptomatic Plasmodium vivax and Plasmodium falciparum infections in native Amazonian populations. Am J Trop Med Hyg. 2002;66:641–648. doi: 10.4269/ajtmh.2002.66.641. [DOI] [PubMed] [Google Scholar]
- Alves FP, Gil LH, Marrelli MT, Ribolla PE, Camargo EP, Da Silva LH. Asymptomatic carriers of Plasmodium spp. as infection source for malaria vector mosquitoes in the Brazilian Amazon. J Med Entomol. 2005;42:777–779. doi: 10.1093/jmedent/42.5.777. [DOI] [PubMed] [Google Scholar]
- Aramburu Guarda J, Ramal Asayag C, Witzig R. Malaria reemergence in the Peruvian Amazon region. Emerg Infect Dis. 1999;5:209–215. doi: 10.3201/eid0502.990204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird JK, Jones TR, Danudirgo EW, Annis BA, Bangs MJ, Basri H, Purnomo, Masbar S. Age-dependent acquired protection against Plasmodium falciparum in people having two years exposure to hyperendemic malaria. Am J Trop Med Hyg. 1991;45:65–76. doi: 10.4269/ajtmh.1991.45.65. [DOI] [PubMed] [Google Scholar]
- Barat LM. Four malaria success stories: how malaria burden was successfully reduced in Brazil, Eritrea, India, and Vietnam. Am J Trop Med Hyg. 2006;74:12–16. [PubMed] [Google Scholar]
- Bastos MS, da Silva-Nunes M, Malafronte RS, Hoffmann EHE, Wunderlich G, Moraes SL, Ferreira MU. Antigenic polymorphism and naturally acquired antibodies to Plasmodium vivax merozoite surface protein 1 in rural Amazonians. Clin Vaccine Immunol. 2007;14:1249–1259. doi: 10.1128/CVI.00243-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista CT, Chan AS, Ryan JR, Calampa C, Roper MH, Hightower AW, Magill AJ. Epidemiology and spatial analysis of malaria in the Northern Peruvian Amazon. Am J Trop Med Hyg. 2006;75:1216–1222. [PubMed] [Google Scholar]
- Bereczky S, Liljander A, Rooth I, Faraja L, Granath F, Montgomery SM, Farnert A. Multiclonal asymptomatic Plasmodium falciparum infections predict a reduced risk of malaria disease in a Tanzanian population. Microbes Infect. 2007;9:103–110. doi: 10.1016/j.micinf.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Branch O, Casapia WM, Gamboa DV, Hernandez JN, Alava FF, Roncal N, Alvarez E, Perez EJ, Gotuzzo E. Clustered local transmission and asymptomatic Plasmodium falciparum and Plasmodium vivax malaria infections in a recently emerged, hypoendemic Peruvian Amazon community. Malar J. 2005;4:27. doi: 10.1186/1475-2875-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branquinho MS, Lagos CB, Rocha RM, Natal D, Barata JM, Cochrane AH, Nardin E, Nussenzweig RS, Kloetzel JK. Anophelines in the state of Acre, Brazil, infected with Plasmodium falciparum, P. vivax, the variant P. vivax VK247 and P. malariae. Trans R Soc Trop Med Hyg. 1993;87:391–394. doi: 10.1016/0035-9203(93)90008-e. [DOI] [PubMed] [Google Scholar]
- Camargo EP, Alves F, Pereira da Silva LH. Symptomless Plasmodium vivax infections in native Amazonians. Lancet. 1999;353:1415–1416. doi: 10.1016/s0140-6736(99)00941-1. [DOI] [PubMed] [Google Scholar]
- Camargo LM, dal Colletto GM, Ferreira MU, Gurgel Sde M, Escobar AL, Marques A, Krieger H, Camargo EP, da Silva LH. Hypoendemic malaria in Rondonia (Brazil, western Amazon region): seasonal variation and risk groups in an urban locality. Am J Trop Med Hyg. 1996;55:32–38. doi: 10.4269/ajtmh.1996.55.32. [DOI] [PubMed] [Google Scholar]
- Casimiro S, Coleman M, Hemingway J, Sharp B. Insecticide resistance in Anopheles arabiensis and Anopheles gambiae from Mozambique. J Med Entomol. 2006;43:276–282. doi: 10.1603/0022-2585(2006)043[0276:iriaaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Chareonviriyaphap T, Rongnoparut P, Chantarumporn P, Bangs MJ. Biochemical detection of pyrethroid resistance mechanisms in Anopheles minimus in Thailand. J Vector Ecol. 2003;28:108–116. [PubMed] [Google Scholar]
- Charlwood JD. Biological variation in Anopheles darlingi Root. Mem Inst Oswaldo Cruz. 1996;91:391–398. doi: 10.1590/s0074-02761996000400001. [DOI] [PubMed] [Google Scholar]
- Charlwood JD, Alcantara J, Pinto J, Sousa CA, Rompao H, Gil V, Rosario VE. Do bednets reduce malaria transmission by exophagic mosquitoes? Trans R Soc Trop Med Hyg. 2005;99:901–904. doi: 10.1016/j.trstmh.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Chouaibou M, Simard F, Chandre F, Etang J, Darriet F, Hougard JM. Efficacy of bifenthrin-impregnated bednets against Anopheles funestus and pyrethroid-resistant Anopheles gambiae in North Cameroon. Malar J. 2006;5:77. doi: 10.1186/1475-2875-5-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cienfuegos AV, Rosero DA, Naranjo N, Luckhart S, Conn JE, Correa MM. Evaluation of a PCR-RFLP-ITS2 assay for discrimination of Anopheles species in northern and western Colombia. Acta Trop. 2011;118:128–135. doi: 10.1016/j.actatropica.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn JE, Vineis JH, Bollback JP, Onyabe DY, Wilkerson RC, Povoa MM. Population structure of the malaria vector Anopheles darlingi in a malaria-endemic region of eastern Amazonian Brazil. Am J Trop Med Hyg. 2006;74:798–806. [PubMed] [Google Scholar]
- Coura JR, Suarez-Mutis M, Ladeia-Andrade S. A new challenge for malaria control in Brazil: asymptomatic Plasmodium infection - a review. Mem Inst Oswaldo Cruz. 2006;101:229–237. doi: 10.1590/s0074-02762006000300001. [DOI] [PubMed] [Google Scholar]
- Cruz Marques A. Human migration and the spread of malaria in Brazil. Parasitol Today. 1987;3:166–170. doi: 10.1016/0169-4758(87)90170-0. [DOI] [PubMed] [Google Scholar]
- Curtis CF, Maxwell CA, Magesa SM, Rwegoshora RT, Wilkes TJ. Insecticide-treated bed-nets for malaria mosquito control. J Am Mosq Control Assoc. 2006;22:501–506. doi: 10.2987/8756-971X(2006)22[501:IBFMMC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- da Silva NS, da Silva-Nunes M, Malafronte RS, Menezes MJ, D’Arcadia RR, Komatsu NT, Scopel KK, Braga EM, Cavasini CE, Cordeiro JA, Ferreira MU. Epidemiology and control of frontier malaria in Brazil: lessons from community-based studies in rural Amazonia. Trans R Soc Trop Med Hyg. 2010;104:343–350. doi: 10.1016/j.trstmh.2009.12.010. [DOI] [PubMed] [Google Scholar]
- da Silva-Nunes M, Codeco CT, Malafronte RS, da Silva NS, Juncansen C, Muniz PT, Ferreira MU. Malaria on the Amazonian frontier: transmission dynamics, risk factors, spatial distribution, and prospects for control. Am J Trop Med Hyg. 2008;79:624–635. [PubMed] [Google Scholar]
- da Silva-Nunes M, Ferreira MU. Clinical spectrum of uncomplicated malaria in semi-immune Amazonians: beyond the "symptomatic" vs "asymptomatic" dichotomy. Mem Inst Oswaldo Cruz. 2007;102:341–347. doi: 10.1590/s0074-02762007005000051. [DOI] [PubMed] [Google Scholar]
- de Arruda M, Carvalho MB, Nussenzweig RS, Maracic M, Ferreira AW, Cochrane AH. Potential vectors of malaria and their different susceptibility to Plasmodium falciparum and Plasmodium vivax in northern Brazil identified by immunoassay. Am J Trop Med Hyg. 1986;35:873–881. doi: 10.4269/ajtmh.1986.35.873. [DOI] [PubMed] [Google Scholar]
- de Castro MC, Monte-Mor RL, Sawyer DO, Singer BH. Malaria risk on the Amazon frontier. Proc Natl Acad Sci U S A. 2006;103:2452–2457. doi: 10.1073/pnas.0510576103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro MC, Sawyer DO, Singer BH. Spatial patterns of malaria in the Amazon: implications for surveillance and targeted interventions. Health Place. 2007;13:368–380. doi: 10.1016/j.healthplace.2006.03.006. [DOI] [PubMed] [Google Scholar]
- de Oliveira-Ferreira J, Lourenco-de-Oliveira R, Teva A, Deane LM, Daniel-Ribeiro CT. Natural malaria infections in anophelines in Rondonia State, Brazilian Amazon. Am J Trop Med Hyg. 1990;43:6–10. [PubMed] [Google Scholar]
- de Santana Filho FS, Arcanjo AR, Chehuan YM, Costa MR, Martinez-Espinosa FE, Vieira JL, Barbosa MG, Alecrim WD, Alecrim MG. Chloroquine-resistant Plasmodium vivax, Brazilian Amazon. Emerg Infect Dis. 2007;13:1125–1126. doi: 10.3201/eid1307.061386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte EC, Pang LW, Ribeiro LC, Fontes CJ. Association of subtherapeutic dosages of a standard drug regimen with failures in preventing relapses of vivax malaria. Am J Trop Med Hyg. 2001;65:471–476. doi: 10.4269/ajtmh.2001.65.471. [DOI] [PubMed] [Google Scholar]
- Eisle TP, Larson D, Steketee RW. Protective efficacy of interventions for preventing malaria mortality in children in Plasmodium falciparum endemic areas. Int J Epidemiol. 2010;39(Suppl 1):i88–101. doi: 10.1093/ije/dyq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson C. Learning and knowledge-production for public health: a review of approaches to evidence-based public health. Scand J Public Health. 2000;28:298–308. [PubMed] [Google Scholar]
- Feachem R, Sabot O. A new global malaria eradication strategy. Lancet. 2008;371:1633–1635. doi: 10.1016/S0140-6736(08)60424-9. [DOI] [PubMed] [Google Scholar]
- Fernández R, Carbajal F, Quintana J, Chauca H, Watts DM. Presencia del A. (N) darlingi (Diptera: Culicidae), en alrededores de la cuidad de Iquitos, Loreto-Peru. Bol Soc Peruana Enferm Inf Trop. 1996;5:10–20. [Google Scholar]
- Ferreira MU, da Silva-Nunes M. Evidence-based public health and prospects for malaria control in Brazil. J Infect Dev Ctries. 2010;4:533–545. doi: 10.3855/jidc.760. [DOI] [PubMed] [Google Scholar]
- Ferreira MU, Karunaweera ND, da Silva-Nunes M, da Silva NS, Wirth DF, Hartl DL. Population structure and transmission dynamics of Plasmodium vivax in rural Amazonia. J Infect Dis. 2007;195:1218–1226. doi: 10.1086/512685. [DOI] [PubMed] [Google Scholar]
- Flores-Mendoza C, Fernandez R, Escobedo-Vargas KS, Vela-Perez Q, Schoeler GB. Natural Plasmodium infections in Anopheles darlingi and Anopheles benarrochi (Diptera: Culicidae) from eastern Peru. J Med Entomol. 2004;41:489–494. doi: 10.1603/0022-2585-41.3.489. [DOI] [PubMed] [Google Scholar]
- Fonseca-Gonzalez I, Cardenas R, Quinones ML, McAllister J, Brogdon WG. Pyrethroid and organophosphates resistance in Anopheles (N) nuneztovari Gabaldon populations from malaria endemic areas in Colombia. Parasitol Res. 2009;105:1399–1409. doi: 10.1007/s00436-009-1570-2. [DOI] [PubMed] [Google Scholar]
- Freeman J, Laserson KF, Petralanda I, Spielman A. Effect of chemotherapy on malaria transmission among Yanomami Amerindians: simulated consequences of placebo treatment. Am J Trop Med Hyg. 1999;60:774–780. doi: 10.4269/ajtmh.1999.60.774. [DOI] [PubMed] [Google Scholar]
- Galardo AK, Arruda M, D’Almeida Couto AA, Wirtz R, Lounibos LP, Zimmerman RH. Malaria vector incrimination in three rural riverine villages in the Brazilian Amazon. Am J Trop Med Hyg. 2007;76:461–469. [PubMed] [Google Scholar]
- Galardo AK, Zimmerman RH, Lounibos LP, Young LJ, Galardo CD, Arruda M, D’Almeida Couto AA. Seasonal abundance of anopheline mosquitoes and their association with rainfall and malaria along the Matapí River, Amapá, Brazil. Med Vet Entomol. 2009;23:335–349. doi: 10.1111/j.1365-2915.2009.00839.x. [DOI] [PubMed] [Google Scholar]
- Gamboa D, Ho MF, Bendezu J, Torres K, Chiodini PL, Barnwell JW, Incardona S, Perkins M, Bell D, McCarthy J, Cheng Q. A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3: implications for malaria rapid diagnostic tests. PLoS One. 2010;5:e8091. doi: 10.1371/journal.pone.0008091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil LH, Tada MS, Katsuragawa TH, Ribolla PE, da Silva LH. Urban and suburban malaria in Rondonia (Brazilian Western Amazon) II. Perennial transmissions with high anopheline densities are associated with human environmental changes. Mem Inst Oswaldo Cruz. 2007;102:271–276. doi: 10.1590/s0074-02762007005000013. [DOI] [PubMed] [Google Scholar]
- Girod R, Gaborit P, Carinci R, Issaly J, Fouque F. Anopheles darlingi bionomics and transmission of Plasmodium falciparum, Plasmodium vivax and Plasmodium malariae in Amerindian villages of the Upper-Maroni Amazonian forest, French Guiana. Mem Inst Oswaldo Cruz. 2008;103:702–710. doi: 10.1590/s0074-02762008000700013. [DOI] [PubMed] [Google Scholar]
- Gosling RD. Asymptomatic malaria associated with protection: not causal. Clin Infect Dis. 2008;47:147. doi: 10.1086/588849. author reply 147–148. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Hunt RH, Brooke BD, Mthembu J, Weeto MM, Awolola TS, Coetzee M. Anopheles arabiensis and An. quadriannulatus resistance to DDT in South Africa. Med Vet Entomol. 2003;17:417–422. doi: 10.1111/j.1365-2915.2003.00460.x. [DOI] [PubMed] [Google Scholar]
- Harvey SA, Olortegui MP, Leontsini E, Pezo CB, Pezantes LM, Winch PJ. The whole world will be able to see us: determining the characteristics of a culturally appropriate bed net among mestizo communities of the Peruvian Amazon. Am J Trop Med Hyg. 2008;79:834–838. [PubMed] [Google Scholar]
- Hill N, Lenglet A, Arnez AM, Carneiro I. Plant based insect repellent and insecticide treated bed nets to protect against malaria in areas of early evening biting vectors: double blind randomised placebo controlled clinical trial in the Bolivian Amazon. BMJ. 2007;335:1023. doi: 10.1136/bmj.39356.574641.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TA, Lima JB, Tada MS. Comparative susceptibility of anopheline mosquitoes to Plasmodium falciparum in Rondonia, Brazil. Am J Trop Med Hyg. 1991a;44:598–603. doi: 10.4269/ajtmh.1991.44.598. [DOI] [PubMed] [Google Scholar]
- Klein TA, Lima JB, Tada MS, Miller R. Comparative susceptibility of anopheline mosquitoes in Rondonia, Brazil to infection by Plasmodium vivax. Am J Trop Med Hyg. 1991b;45:463–470. doi: 10.4269/ajtmh.1991.45.463. [DOI] [PubMed] [Google Scholar]
- Klein TA, Lima JB, Tang AT. Biting behavior of Anopheles mosquitoes in Costa Marques, Rondonia, Brazil. Rev Soc Bras Med Trop. 1991c;24:13–20. doi: 10.1590/s0037-86821991000100003. [DOI] [PubMed] [Google Scholar]
- Kroeger A, Mancheno M, Alarcon J, Pesse K. Insecticide-impregnated bed nets for malaria control: varying experiences from Ecuador, Colombia, and Peru concerning acceptability and effectiveness. Am J Trop Med Hyg. 1995;53:313–323. doi: 10.4269/ajtmh.1995.53.313. [DOI] [PubMed] [Google Scholar]
- Kroeger A, Meyer R, Mancheno M, Gonzalez M, Pesse K. Operational aspects of bednet impregnation for community-based malaria control in Nicaragua, Ecuador, Peru and Colombia. Trop Med Int Health. 1997;2:589–602. doi: 10.1046/j.1365-3156.1997.d01-319.x. [DOI] [PubMed] [Google Scholar]
- Ladeia-Andrade S, Ferreira MU, de Carvalho ME, Curado I, Coura JR. Age-dependent acquisition of protective immunity to malaria in riverine populations of the Amazon Basin of Brazil. Am J Trop Med Hyg. 2009;80:452–459. [PubMed] [Google Scholar]
- Lehmann T, Licht M, Gimnig JE, Hightower A, Vulule JM, Hawley WA. Spatial and temporal variation in kinship among Anopheles gambiae (Diptera: Culicidae) mosquitoes. J Med Entomol. 2003;40:421–429. doi: 10.1603/0022-2585-40.4.421. [DOI] [PubMed] [Google Scholar]
- Lengeler C, Snow RW. From efficacy to effectiveness: insecticide-treated bednets in Africa. Bull World Health Organ. 1996;74:325–332. [PMC free article] [PubMed] [Google Scholar]
- Loaiza J, Scott M, Bermingham E, Rovira J, Sanjur O, Conn JE. Anopheles darlingi (Diptera: Culicidae) in Panama. Am J Trop Med Hyg. 2009;81:23–26. [PMC free article] [PubMed] [Google Scholar]
- Loaiza JR, Bermingham E, Scott ME, Rovira JR, Conn JE. Species composition and distribution of adult Anopheles (Diptera: Culicidae) in Panama. J Med Entomol. 2008;45:841–851. doi: 10.1603/0022-2585(2008)45[841:scadoa]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiola CC, da Silva CJ, Tauil PL. Malaria control in Brazil: 1965 to 2001. Rev Panam Salud Publica. 2002;11:235–244. doi: 10.1590/s1020-49892002000400005. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Conn JE. Malaria vector heterogeneity in South America. Amer Entomol. 2000;46:238–249. [Google Scholar]
- Lourenco-de-Oliveira R, Guimaraes AE, Arle M, da Silva TF, Castro MG, Motta MA, Deane LM. Anopheline species, some of their habits and relation to malaria in endemic areas of Rondonia State, Amazon region of Brazil. Mem Inst Oswaldo Cruz. 1989;84:501–514. doi: 10.1590/s0074-02761989000400008. [DOI] [PubMed] [Google Scholar]
- Macauley C. Aggressive active case detection: a malaria control strategy based on the Brazilian model. Soc Sci Med. 2005;60:563–573. doi: 10.1016/j.socscimed.2004.05.025. [DOI] [PubMed] [Google Scholar]
- MacDonald G. Epidemiological basis of malaria control. Bull WHO. 1956;15:613–626. [PMC free article] [PubMed] [Google Scholar]
- MacDonald G. The Epidemiology and Control of Malaria. Oxford University Press; Oxford: 1957. [Google Scholar]
- Magris M, Rubio-Palis Y, Menares C, Villegas L. Vector bionomics and malaria transmission in the Upper Orinoco River, Southern Venezuela. Mem Inst Oswaldo Cruz. 2007;102:303–311. doi: 10.1590/s0074-02762007005000049. [DOI] [PubMed] [Google Scholar]
- Magris M, Rubio-Palis Y, Alexander N, Ruiz B, Galván N, Frias D, Blanco M, Lines J. Community-randomized trial of lambdacyhalothrin-treated hammock nets for malaria control in Yanomami communities in the Amazon region of Venezuela. Trop Med Int Health. 2007;12:392–403. doi: 10.1111/j.1365-3156.2006.01801.x. [DOI] [PubMed] [Google Scholar]
- Males S, Gaye O, Garcia A. Long-term asymptomatic carriage of Plasmodium falciparum protects from malaria attacks: a prospective study among Senegalese children. Clin Infect Dis. 2008;46:516–522. doi: 10.1086/526529. [DOI] [PubMed] [Google Scholar]
- Marrelli MT, Malafronte RS, Flores-Mendoza C, Lourenco-de-Oliveira R, Kloetzel JK, Marinotti O. Sequence analysis of the second internal transcribed spacer of ribosomal DNA in Anopheles oswaldoi (Diptera: Culicidae) J Med Entomol. 1999;36:679–684. doi: 10.1093/jmedent/36.6.679. [DOI] [PubMed] [Google Scholar]
- Marrelli MT, Floeter-Winter LM, Malafronte RS, Tadei WP, Lourenço-de-Oliveira R, Flores-Mendoza C, Marinotti O. Amazonian malaria vector anopheline relationships interpreted from ITS2 rDNA sequences. Med Vet Entomol. 2005;19:208–218. doi: 10.1111/j.0269-283X.2005.00558.x. [DOI] [PubMed] [Google Scholar]
- Matson R, Rios CT, Chavez CB, Gilman RH, Florin D, Sifuentes VL, Greffa RC, Yori PP, Fernandez R, Portocarrero DV, Vinetz JM, Kosek M. Improved molecular technique for the differentiation of neotropical anopheline species. Am J Trop Med Hyg. 2008;78:492–498. [PMC free article] [PubMed] [Google Scholar]
- Mendis K, Rietveld A, Warsame M, Bosman A, Greenwood B, Wernsdorfer WH. From malaria control to eradication: The WHO perspective. Trop Med Int Health. 2009;14:802–809. doi: 10.1111/j.1365-3156.2009.02287.x. [DOI] [PubMed] [Google Scholar]
- Mirabello L, Vineis JH, Yanoviak SP, Scarpassa VM, Povoa MM, Padilla N, Achee NL, Conn JE. Microsatellite data suggest significant population structure and differentiation within the malaria vector Anopheles darlingi in Central and South America. BMC Ecol. 2008;8:3. doi: 10.1186/1472-6785-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra SP, Webber R, Lines J, Jaffar S, Bradley DJ. Malaria control: bednets or spraying? Spray versus treated nets using deltamethrin—a community randomized trial in India. Trans R Soc Trop Med Hyg. 1999;93:456–457. doi: 10.1016/s0035-9203(99)90335-8. [DOI] [PubMed] [Google Scholar]
- Monteiro de Barros FS, Honório NA, Arruda ME. Temporal and spatial distribution of malaria within an agricultural settlement of the Brazilian Amazon. J Vector Ecol. 2011;36:159–169. doi: 10.1111/j.1948-7134.2011.00153.x. [DOI] [PubMed] [Google Scholar]
- Moody A. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev. 2002;15:66–78. doi: 10.1128/CMR.15.1.66-78.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JE, Rubio-Palis Y, Paez E, Perez E, Sanchez V. Abundance, biting behaviour and parous rate of anopheline mosquito species in relation to malaria incidence in gold-mining areas of southern Venezuela. Med Vet Entomol. 2007;21:339–349. doi: 10.1111/j.1365-2915.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- Moutinho PR, Gil LHS, Cruz RB, Ribolla PEM. Population dynamics, structure and behaviour of Anopheles darlingi in a rural settlement in the Amazonian rainforest of Acre, Brazil. Malar J. 2011;10:174. doi: 10.1186/1475-2875-10-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najera JA. Malaria control: achievements, problems and strategies. Parassitologia. 2001;43:1–89. [PubMed] [Google Scholar]
- Norris DE. Mosquito-borne diseases as a consequence of land use change. EcoHealth. 2004;1:19–24. [Google Scholar]
- Oliveira-Ferreira J, Lacerda MV, Brasil P, Ladislau JL, Tauil PL, Daniel-Ribeiro CT. Malaria in Brazil: an overview. Malar J. 2010;9:115. doi: 10.1186/1475-2875-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orjuela-Sánchez P, da Silva-Nunes M, da Silva NS, Scopel KKG, Gonçalves RM, Malafronte RS, Ferreira MU. Population dynamics of genetically diverse Plasmodium falciparum lineages: community-based prospective study in rural Amazonia. Parasitology. 2009a;136:1097–1105. doi: 10.1017/S0031182009990539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orjuela-Sánchez P, da Silva NS, da Silva-Nunes M, Ferreira MU. Recurrent parasitemias and population dynamics of Plasmodium vivax polymorphisms in rural Amazonia. Am J Trop Med Hyg. 2009b;81:961–968. doi: 10.4269/ajtmh.2009.09-0337. [DOI] [PubMed] [Google Scholar]
- Orjuela-Sánchez P, Karunaweera ND, da Silva-Nunes M, da Silva NS, Scopel KKG, Gonçalves RM, Amaratunga C, Sá JM, Socheat D, Fairhust RM, Gunawardena S, Thavakodirasah T, Galapaththy GL, Abeysinghe R, Kawamoto F, Wirth DF, Ferreira MU. Single-nucleotide polymorphism, linkage disequilibrium and geographic structure in the malaria parasite Plasmodium vivax: prospects for genome-wide association studies. BMC Genet. 2010;11:65. doi: 10.1186/1471-2156-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAHO. Report on the Situation of Malaria in the Americas, 2008. Pan American Health Organization; Washington, D.C.: 2009. [Google Scholar]
- Pampana E. A Textbook of Malaria Eradication. Oxford University Press; London: 1963. pp. 360–375. [Google Scholar]
- Parekh FK, Hernandez JN, Krogstad DJ, Casapia WM, Branch OH. Prevalence and risk of Plasmodium falciparum and P. vivax malaria among pregnant women living in the hypoendemic communities of the Peruvian Amazon. Am J Trop Med Hyg. 2007;77:451–457. [PMC free article] [PubMed] [Google Scholar]
- Pinedo-Cancino V, Sheen P, Tarazona-Santos E, Oswald WE, Jeri C, Vittor AY, Patz JA, Gilman RH. Limited diversity of Anopheles darlingi in the Peruvian Amazon region of Iquitos. Am J Trop Med Hyg. 2006;75:238–245. [PMC free article] [PubMed] [Google Scholar]
- Pinto J, Donnelly MJ, Sousa CA, Gil V, Ferreira C, Elissa N, do Rosario VE, Charlwood JD. Genetic structure of Anopheles gambiae (Diptera: Culicidae) in Sao Tome and Principe (West Africa): implications for malaria control. Mol Ecol. 2002;11:2183–2187. doi: 10.1046/j.1365-294x.2002.01587.x. [DOI] [PubMed] [Google Scholar]
- Pinto J, Donnelly MJ, Sousa CA, Malta-Vacas J, Gil V, Ferreira C, Petrarca V, do Rosario VE, Charlwood JD. An island within an island: genetic differentiation of Anopheles gambiae in Sao Tome, West Africa, and its relevance to malaria vector control. Heredity. 2003;91:407–414. doi: 10.1038/sj.hdy.6800348. [DOI] [PubMed] [Google Scholar]
- Pluess B, Tanser FC, Lengeler C, Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database Syst Rev. 2010;14:CD006657. doi: 10.1002/14651858.CD006657.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal S, Carret C, Recker M, Armitage AE, Gonçalves LA, Epiphanio S, Sullivan D, Roy C, Newbold CI, Drakesmith H, Mota MM. Host-mediated regulation of superinfection in malaria. Nat Med. 2011;17:732–737. doi: 10.1038/nm.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povoa MM, Conn JE, Schlichting CD, Amaral JC, Segura MN, Da Silva AN, Dos Santos CC, Lacerda RN, De Souza RT, Galiza D, Santa Rosa EP, Wirtz RA. Malaria vectors, epidemiology, and the re-emergence of Anopheles darlingi in Belém, Pará, Brazil. J Med Entomol. 2003;40:379–386. doi: 10.1603/0022-2585-40.4.379. [DOI] [PubMed] [Google Scholar]
- Prata A, Urdaneta M, McGreevy PB, Tada MS. Infrequency of asymptomatic malaria in an endemic area in Amazonas, Brazil. Rev Soc Bras Med Trop. 1988;21:51–54. doi: 10.1590/s0037-86821988000200003. [DOI] [PubMed] [Google Scholar]
- Quinones ML, Ruiz F, Calle DA, Harbach RE, Erazo HF, Linton YM. Incrimination of Anopheles (Nyssorhynchus) rangeli and An(Nys.) oswaldoi as natural vectors of Plasmodium vivax in Southern Colombia. Mem Inst Oswaldo Cruz. 2006;101:617–623. doi: 10.1590/s0074-02762006000600007. [DOI] [PubMed] [Google Scholar]
- Rejmánková E, Rubio-Palis Y, Villegas L. Larval habitats of anopheline mosquitoes in the Upper Orinoco, Venezuela. J Vector Ecol. 1999;24:130–137. [PubMed] [Google Scholar]
- Roberts DR, Laughlin LL, Hsheih P, Legters LJ. DDT, global strategies, and a malaria control crisis in South America. Emerg Infect Dis. 1997;3:295–302. doi: 10.3201/eid0303.970305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DR, Manguin S, Mouchet J. DDT house spraying and re-emerging malaria. Lancet. 2000;356:330–332. doi: 10.1016/s0140-6736(00)02516-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Perez L, Caicedo JC, Prieto G, Arroyo JA, Kaur H, Suarez-Mutis M, de La Hoz F, Lines J, Alexander N. Composition and biting activity of Anopheles (Diptera: Culicidae) in the Amazon region of Colombia. J Med Entomol. 2009;46:307–315. doi: 10.1603/033.046.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper MH, Carrion Torres RS, Cava Goicochea CG, Andersen EM, Aramburu Guarda JS, Calampa C, Hightower AW, Magill AJ. The epidemiology of malaria in an epidemic area of the Peruvian Amazon. Am J Trop Med Hyg. 2000;62:247–256. doi: 10.4269/ajtmh.2000.62.247. [DOI] [PubMed] [Google Scholar]
- Rosa-Freitas MG. Anopheles (Nyssorhynchus) deaneorum: a new species in the Albitarsis complex (Diptera: Culicidae) Mem Inst Osw Cruz (Brazil) 1989;84:535–543. [Google Scholar]
- Rosa-Freitas MG, Broomfield G, Priestman A, Milligan PJ, Momen H, Molyneux DH. Cuticular hydrocarbons, isoenzymes and behavior of three populations of Anopheles darlingi from Brazil. J Am Mosq Control Assoc. 1992;8:357–366. [PubMed] [Google Scholar]
- Roshanravan B, Kari E, Gilman RH, Cabrera L, Lee E, Metcalfe J, Calderon M, Lescano AG, Montenegro-James S, Calampa C, Vinetz JM. Endemic malaria in the Peruvian Amazon region of Iquitos. Am J Trop Med Hyg. 2003;69:45–52. [PubMed] [Google Scholar]
- Rozendaal JA. Biting and resting behavior of Anopheles darlingi in the Suriname rainforest. J Am Mosq Control Assoc. 1989;5:351–358. [PubMed] [Google Scholar]
- Ruiz F, Quinones ML, Erazo HF, Calle DA, Alzate JF, Linton YM. Molecular differentiation of Anopheles (Nyssorhynchus) benarrochi and An(N.) oswaldoi from southern Colombia. Mem Inst Oswaldo Cruz. 2005;100:155–160. doi: 10.1590/s0074-02762005000200008. [DOI] [PubMed] [Google Scholar]
- Sallum MA, Marrelli MT, Nagaki SS, Laporta GZ, Dos Santos CL. Insight into Anopheles (Nyssorhynchus) (Diptera: Culicidae) species from Brazil. J Med Entomol. 2008;45:970–981. doi: 10.1603/0022-2585(2008)45[970:iiandc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Santos RL, Fayal A da S, Aguiar AE, Vieira DB, Povoa MM. Evaluation of the residual effect of pyrethroids on Anopheles in the Brazilian Amazon. Rev Saude Publica. 2007;41:276–283. doi: 10.1590/s0034-89102007000200015. [DOI] [PubMed] [Google Scholar]
- Scarpassa VM, Conn JE. Population genetic structure of the major malaria vector Anopheles darlingi (Diptera: Culicidae) from the Brazilian Amazon, using microsatellite markers. Mem Inst Oswaldo Cruz. 2007;102:319–327. doi: 10.1590/s0074-02762007005000045. [DOI] [PubMed] [Google Scholar]
- Schoeler GB, Flores-Mendoza C, Fernandez R, Davila JR, Zyzak M. Geographical distribution of Anopheles darlingi in the Amazon Basin region of Peru. J Am Mosq Control Assoc. 2003;19:286–296. [PubMed] [Google Scholar]
- Scopel KKG, Fontes CJ, Nunes AC, Horta MF, Braga EM. High prevalence of Plamodium malariae infections in a Brazilian Amazon endemic area (Apiacás-Mato Grosso State) as detected by polymerase chain reaction. Acta Trop. 2005;90:61–64. doi: 10.1016/j.actatropica.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Shaukat AM, Breman JG, McKenzie FE. Using the entomological inoculation rate to assess the impact of vector control on malaria parasite transmission and elimination. Malar J. 2010;9:122. doi: 10.1186/1475-2875-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh OP, Dykes CL, Lather M, Agrawal OP, Adak T. Knockdown resistance (kdr)-like mutations in the voltage-gated sodium channel of a malaria vector Anopheles stephensi and PCR assays for their detection. Malar J. 2011;10:59. doi: 10.1186/1475-2875-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinka ME, Rubio-Palis Y, Manguin S, Patil AP, Temperley WH, Gething PW, Van Boeckel T, Kabaria CW, Harbach RE, Hay SI. The dominant Anopheles vectors of human malaria in the Americas: occurrence data, distribution maps and bionomic precis. Parasit Vectors. 2010;3:72. doi: 10.1186/1756-3305-3-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Silva FA, da Silva-Nunes M, Sanchez BA, Ceravolo IP, Malafronte RS, Brito CF, Ferreira MU, Carvalho LH. Naturally acquired antibodies to Plasmodium vivax Duffy binding protein (DBP) in Brazilian Amazon. Am J Trop Med Hyg. 2010;82:185–193. doi: 10.4269/ajtmh.2010.08-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez MF, Quinones ML, Palacios JD, Carrillo A. First record of DDT resistance in Anopheles darlingi. J Am Mosq Control Assoc. 1990;6:72–74. [PubMed] [Google Scholar]
- Suarez-Mutis MC, Cuervo P, Leoratti FM, Moraes-Avila SL, Ferreira AW, Fernandes O, Coura JR. Cross sectional study reveals a high percentage of asymptomatic Plasmodium vivax infection in the Amazon Rio Negro area, Brazil. Rev Inst Med Trop Sao Paulo. 2007;49:159–164. doi: 10.1590/s0036-46652007000300005. [DOI] [PubMed] [Google Scholar]
- Tadei WP, Thatcher BD, Santos JM, Scarpassa VM, Rodrigues IB, Rafael MS. Ecologic observations on anopheline vectors of malaria in the Brazilian Amazon. Am J Trop Med Hyg. 1998;59:325–335. doi: 10.4269/ajtmh.1998.59.325. [DOI] [PubMed] [Google Scholar]
- Tadei WP, Dutary-Thatcher B. Malaria vectors in the Brazilian Amazon Anopheles of the subgenus Nyssorhynchus. Rev Inst Med Trop S Paulo. 2000;42:87–94. doi: 10.1590/s0036-46652000000200005. [DOI] [PubMed] [Google Scholar]
- Taylor CE, Toure YT, Coluzzi M, Petrarca V. Effective population size and persistence of Anopheles arabiensis during the dry season in west Africa. Med Vet Entomol. 1993;7:351–357. doi: 10.1111/j.1365-2915.1993.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Tineo ET, Medina CA, Fallaque SC, Chavez CL, Quispe FS, Mercado AM, Zevallos GJ, Leon CW, Palomino SM. Distribucion geografica y comportamiento estacional de la picadura del Anopheles (Nyssorhynchus) darlingi Root 1926 en localidades de la frontera Peru-Bolivia, Madre de Dios, Peru. Rev Peru Med Exp Salud Publica. 2003;20:6. [Google Scholar]
- Turell MJ, Sardelis MR, Jones JW, Watts DM, Fernandez R, Carbajal F, Pecor JE, Klein TA. Seasonal distribution, biology, and human attraction patterns of mosquitoes (Diptera: Culicidae) in a rural village and adjacent forested site near Iquitos, Peru. J Med Entomol. 2008;45:1165–1172. doi: 10.1603/0022-2585(2008)45[1165:sdbaha]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Van den Eede P, Soto-Calle VE, Delgado C, Gamboa D, Grande T, Rodriguez H, Llanos-Cuentas A, Anné J, D’Alessandro U, Erhart A. Plasmodium vivax sub-patent infections after radical treatment are common in Peruvian patients: results of a 1-year prospective cohort study. PLoS One. 2011;6:e16257. doi: 10.1371/journal.pone.0016257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eede P, Van der Auwera G, Delgado C, Huyse T, Soto-Calle VE, Gamboa D, Grande T, Rodriguez H, Llanos A, Anné J, Erhart A, D’Alessandro U. Multilocus genotyping reveals high heterogeneity and strong local population structure of the Plasmodium vivax population in the Peruvian Amazon. Malar J. 2010;9:151. doi: 10.1186/1475-2875-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinetz JM, Gilman RH. Asymptomatic Plasmodium parasitemia and the ecology of malaria transmission. Am J Trop Med Hyg. 2002;66:639–640. doi: 10.4269/ajtmh.2002.66.639. [DOI] [PubMed] [Google Scholar]
- Vittor AY, Gilman RH, Tielsch J, Glass G, Shields T, Lozano WS, Pinedo-Cancino V, Patz JA. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg. 2006;74:3–11. [PubMed] [Google Scholar]
- Vittor AY, Pan W, Gilman RH, Tielsch J, Glass G, Shields T, Sanchez-Lozano W, Pinedo VV, Salas-Cobos E, Flores S, Patz JA. Linking deforestation to malaria in the Amazon: characterization of the breeding habitat of the principal malaria vector, Anopheles darlingi. Am J Trop Med Hyg. 2009;81:5–12. [PMC free article] [PubMed] [Google Scholar]
- Voorham J. Intra-population plasticity of Anopheles darlingi’s (Diptera, Culicidae) biting activity patterns in the state of Amapa, Brazil. Rev Saude Publica. 2002;36:75–80. doi: 10.1590/s0034-89102002000100012. [DOI] [PubMed] [Google Scholar]
- Wilkerson RC, Gaffigan TV, Bento Lima J. Identification of species related to Anopheles (Nyssorhynchus) albitarsis by random amplified polymorphic DNA-polymerase chain reaction (Diptera: Culicidae) Mem Inst Oswaldo Cruz. 1995a;90:721–732. doi: 10.1590/s0074-02761995000600013. [DOI] [PubMed] [Google Scholar]
- Wilkerson RC, Parsons TJ, Klein TA, Gaffigan TV, Bergo E, Consolim J. Diagnosis by random amplified polymorphic DNA polymerase chain reaction of four cryptic species related to Anopheles (Nyssorhynchus) albitarsis (Diptera: Culicidae) from Paraguay, Argentina, and Brazil. J Med Entomol. 1995b;32:697–704. doi: 10.1093/jmedent/32.5.697. [DOI] [PubMed] [Google Scholar]
- Wondji C, Simard F, Lehmann T, Fondjo E, Same-Ekobo A, Fontenille D. Impact of insecticide-treated bed nets implementation on the genetic structure of Anopheles arabiensis in an area of irrigated rice fields in the Sahelian region of Cameroon. Mol Ecol. 2005;14:3683–3693. doi: 10.1111/j.1365-294X.2005.02699.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. World Malaria Report 2008. World Health Organization; Geneva: 2008. [Google Scholar]
- Zimmerman RH, Voorham J. Use of insecticide-impregnated mosquito nets and other impregnated materials for malaria control in the Americas. Rev Panam Salud Publica. 1997;2:18–25. doi: 10.1590/s1020-49891997000700004. [DOI] [PubMed] [Google Scholar]
- Zimmerman RH, Galardo AK, Lounibos LP, Arruda M, Wirtz R. Bloodmeal hosts of Anopheles species (Diptera: Culicidae) in a malaria-endemic area of Brazilian Amazon. J Med Entomol. 2006;43:947–956. doi: 10.1603/0022-2585(2006)43[947:bhoasd]2.0.co;2. [DOI] [PubMed] [Google Scholar]


