SUMMARY
Little is known about how neutrophils and other cells establish a single zone of actin assembly during migration. A widespread assumption is that the leading edge prevents formation of additional fronts by generating long-range diffusible inhibitors or by sequestering essential polarity components. We use morphological perturbations, cell severing experiments, and computational simulations to show that diffusion-based mechanisms are not sufficient for long-range inhibition by the pseudopod. Instead, plasma membrane tension could serve as a long-range inhibitor in neutrophils. We find that membrane tension doubles during leading edge protrusion, and increasing tension is sufficient for long-range inhibition of actin assembly and Rac activation. Furthermore, reducing membrane tension causes uniform actin assembly. We suggest that tension, rather than diffusible molecules generated or sequestered at the leading edge, is the dominant source of long-range inhibition that constrains the spread of the existing front and prevents the formation of secondary fronts.
INTRODUCTION
The ability of cells to generate polarized distributions of signaling molecules enables numerous biological processes including asymmetric cell division, neurite specification, tissue formation, and cell motility. The Rac GTPase drives actin polymerization and protrusion at the leading edge in a wide range of migrating cells (Ridley et al., 1992; Sun et al., 2004). Efficient migration requires confining Rac activity to the leading edge: spatially uniform Rac activation abolishes movement (Allen et al., 1998; Inoue and Meyer, 2008; Srinivasan et al., 2003).
In neutrophils, Rac activity is highly polarized both in response to external gradients and in the presence of uniform chemoattractant (Gardiner et al., 2002; Weiner et al., 2007). Linked positive and negative feedback loops are thought to enable many cell types to polarize during chemotaxis or random migration (Jilkine and Edelstein-Keshet, 2011; Meinhardt, 1999; Neilson et al., 2011; Turing, 1952; Xiong et al., 2010). Positive feedback amplifies small, transient fluctuations into large, temporally persistent asymmetries. GTPase and/or phosphoinositide-based positive feedback loops have been implicated in the polarization of neutrophils (Inoue and Meyer, 2008; Weiner et al., 2002; Weiner et al., 2006), neurons (Fivaz et al., 2008), Dictyostelium (Sasaki et al., 2004), and budding yeast (Butty et al., 2002; Wedlich-Soldner et al., 2003). Positive feedback loops require a balancing mechanism of inhibition to prevent them from overtaking the entire cell. The positive feedback reaction can limit itself by generating long-range inhibition, which constrains the spread of the existing front and prevents the formation of secondary fronts. The inhibition is thought to arise either from the production of rapidly diffusing inhibitory molecules by the front (Figure 1A) or from the sequestration of limiting polarity components by the front (Figure 1B). These mechanisms of long-range inhibition depend on rapid diffusion of signaling components through the cytosol. In contrast to the components that participate in the positive feedback loops at the leading edge, the molecules responsible for long-range inhibition are unknown. It has not even been experimentally determined whether this inhibition is a diffusion-based process.
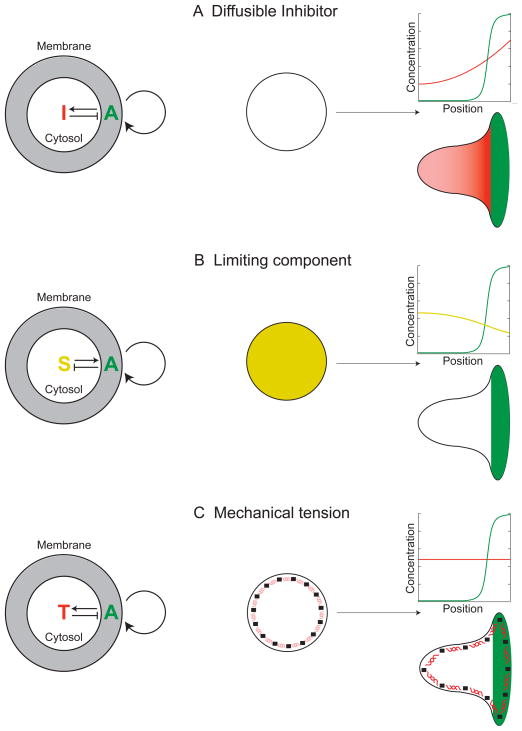
Figure 1. Conceptual mechanisms for long-range inhibition.
A) Diffusible Inhibitor. An autocatalytic activator (A, green) produces an inhibitory molecule (I, red) that diffuses throughout the cytoplasm to act as a long-range inhibitor of leading edge formation.
B) Limiting Component. An autocatalytic activator in the front inhibits activation elsewhere by consuming essential substrates (S, gold) of the positive feedback loop, rather than generating a diffusible inhibitor (as in (A)).
C) Mechanical Tension. Protrusion at the leading edge generates mechanical tension (T, depicted as red springs) in either the plasma membrane or the underlying cytoskeleton. This tension acts as a long-range inhibitor of protrusion.
Signaling-centered positive and negative feedback loops are not the only potential mechanisms of polarization. A model consisting entirely of mechanical interactions between the actin cytoskeleton, myosin, and plasma membrane accurately predicts the polarized morphologies of keratocytes (Kozlov and Mogilner, 2007) as well as the relation between cell shape and speed (Keren et al., 2008) without considering upstream signals. This model is insufficient to explain neutrophil polarity because cytoskeletal polarization and migration require Rac to be polarized (Inoue and Meyer, 2008; Srinivasan et al., 2003). Thus, for force to play a dominant role in neutrophil polarity, it must participate in the spatial patterning of signaling cascades, for example by acting as a long-range inhibitor of Rac activation (Figure 1C).
A significant challenge in discriminating between the many classes of models for cell polarity is that many of the underlying positive and negative feedback components have not been identified, and even for the known components, the key biophysical parameters are unknown. We performed experiments that discriminate between models of long-range inhibition without requiring detailed knowledge of the underlying molecular components. We used morphological perturbations and cell severing to push neutrophils into situations where existing diffusion-based long-range inhibition models break down, as verified by computational simulations. Mechanical tension is one mode of long-range inhibition that could explain our observations on stretched and severed cells. Consistent with this hypothesis, we find that membrane tension nearly doubles during leading edge protrusion, tension increases suffice for long-range inhibition of Rac activation, and reducing membrane tension activates actin assembly throughout the cell. Our data suggest that long-range inhibition is not solely based on diffusible molecules generated or sequestered at the leading edge (as has been widely assumed) but rather requires protrusion-based increases in plasma membrane tension to constrain the spread of the existing front and prevent the formation of secondary fronts.
RESULTS
Distinguishing between neutrophil polarity mechanisms with cell stretching and severing
We sought to distinguish between long-range inhibition mechanisms without requiring a detailed knowledge of the molecular players involved in the process. Due to their widely proposed roles in developmental patterning (Gierer and Meinhardt, 1972; Nakamura et al., 2006; Sick et al., 2006; Turing, 1952), diffusion-based systems are the most popular of the numerous hypothetical inhibition mechanisms in polarizing cells (Jilkine and Edelstein-Keshet, 2011). These usually involve a positive feedback reaction that either produces inhibitory molecules (Figure 1A) or depletes essential polarity components (Figure 1B). Importantly, to generate cell polarity, the inhibitory molecules or limiting components must diffuse more rapidly than the products of the positive feedback reactions. These models were typically developed for cells with spherical morphologies or assumed a 1D spatial geometry to denote the “front-back” axis. To test whether existing mathematical models are consistent with cell behavior, we devised novel experimental settings in which cellular morphologies attenuate diffusion.
If long-range inhibition depends on diffusion, then we can interfere with it by creating a thin neck between the pseudopod and the rest of the cell. In contrast, tension-based inhibition should still function in this context. Following brief heat shock in the presence of uniform chemoattractant, neutrophils adopt a stretched, growth-cone like morphology (Malawista and De Boisfleury Chevance, 1982); (Figure 2A). Despite this altered morphology, a single zone of actin assembly (tethered pseudopod) is observed with the cell body remaining inactive in 91% of these cells during the 250 second period of observation (N = 31, Figure 2B, Movie S1). The inactivity of the cell body is remarkable given that its communication with the pseudopod is restricted by a tether that is typically one micron in diameter and 25 microns long.
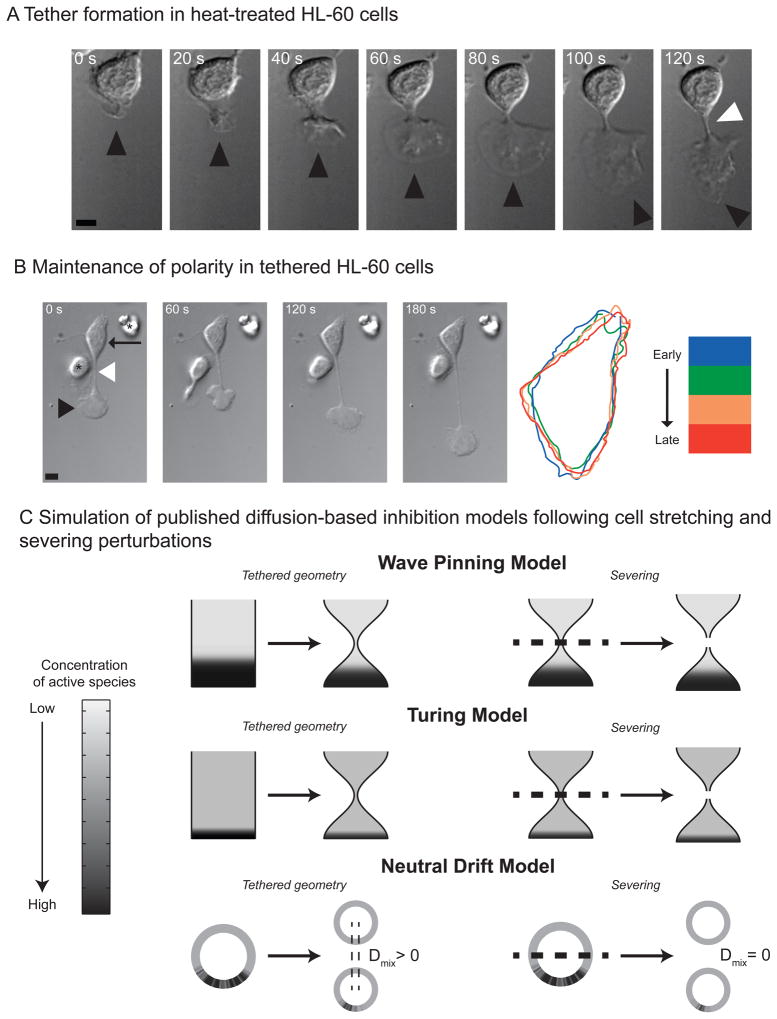
Figure 2. Maintenance of polarity in tethered cells.
A) Tether formation in heat-treated HL-60 cells. The cell initially forms a pseudopod (black arrowhead). The pseudopod crawls away from the fixed cell body, causing a tether (white arrowhead) to form between them. The scale bar is 5 microns.
B) Maintenance of polarity in tethered HL-60 cells. Left: The cell body (black arrow) remains completely fixed as the pseudopod (black arrowhead) migrates a significant distance. The asterisk in the first frame denotes a neighboring cell that lacks a tether. Right: cell outlines from successive time points are depicted in blue, green, orange, and red, respectively. The morphology of the cell body stays constant over the 250 second observation time in 91% of the uncut cells (N = 27). The scale bar is 5 microns.
C) Simulation of published diffusion-based inhibition models following cell stretching and severing perturbations. The top, middle and bottom panels depict simulation results of a wave pinning model (top), Turing model (middle), and neutral drift model (bottom) following cell stretching or severing. The concentration of the activator species (u) is represented as grayscale with black being highest concentration. In the left panels, spherical or cylindrical cells were allowed to develop polarized signals. We simulated the subsequent time evolution of this polarized signaling distribution in cells that were stretched into dumb-bell geometries, similar to our experimental tethers (Figure 2). In the right panels, the signals in spherical or cylindrical cells were polarized as before. We simulated the time evolution of the signals in cells that were severed into two equal halves. Steady state distributions of membrane-bound activators for all three models are shown.
We simulated how three classes of diffusion-based polarity models responded in a tethered morphology: Turing (Otsuji et al., 2007), wave pinning (Mori et al., 2008), and neutral drift (Altschuler et al., 2008). These models are conceptual, rolling many as-yet poorly characterized details of the polarity network into generic mechanisms of interaction and feedback. They share several common properties, namely: the total amount of molecules are assumed to be constant during the observed duration of polarization; molecules transition between two states, either at the membrane (active) or cytosol (inactive); and diffusion on the membrane is much slower than in the cytosol. In our simulations, recruitment of molecules to the membrane is autocatalytic and ultimately constrained by depletion of the cytoplasmic species. All three models predict that the cell body will remain inactive despite the tethered morphology (Figure 2C, left panels; Supplemental Figure 1). This could either reflect ongoing long-range inhibition of the cell body by the pseudopod or could be a consequence of depletion that occurred prior to tether formation and persisted indefinitely due to the lack of resynthesis of limiting components. To distinguish between these possibilities, we analyzed the behavior of the models when the cell body is severed from the pseudopod (Figure 2C, right panels; Supplemental Figure 1). All three models predict that the cell body remains inactive despite being disconnected from the pseudopod. Thus the inactivity of the cell body is not due to ongoing inhibition from the pseudopod. If it were, the cell body would have reanimated upon pseudopod removal.
All of the long-range inhibition mechanisms under consideration make strong predictions about the behavior of the cell body after it is severed from the pseudopod (Figure 3A). Diffusion-based competition for a fixed pool of molecules (limiting component) predicts that the cell body will remain inactive after pseudopod removal unless the limiting component is rapidly resynthesized. Similarly, if diffusible inhibitory molecules are responsible for long-range inhibition, then the cell body will also remain inactive unless the inhibitory molecules have a short lifetime. Both diffusion-based inhibition mechanisms predict that the cell body will remain inactive after severing unless the resynthesis/turnover rate is high. In contrast, a tension-based inhibition mechanism would allow the cell body to reanimate because the cell could relax into a reduced-tension morphology after severing. Thus, severing experiments discriminate between current long-range inhibition mechanisms.
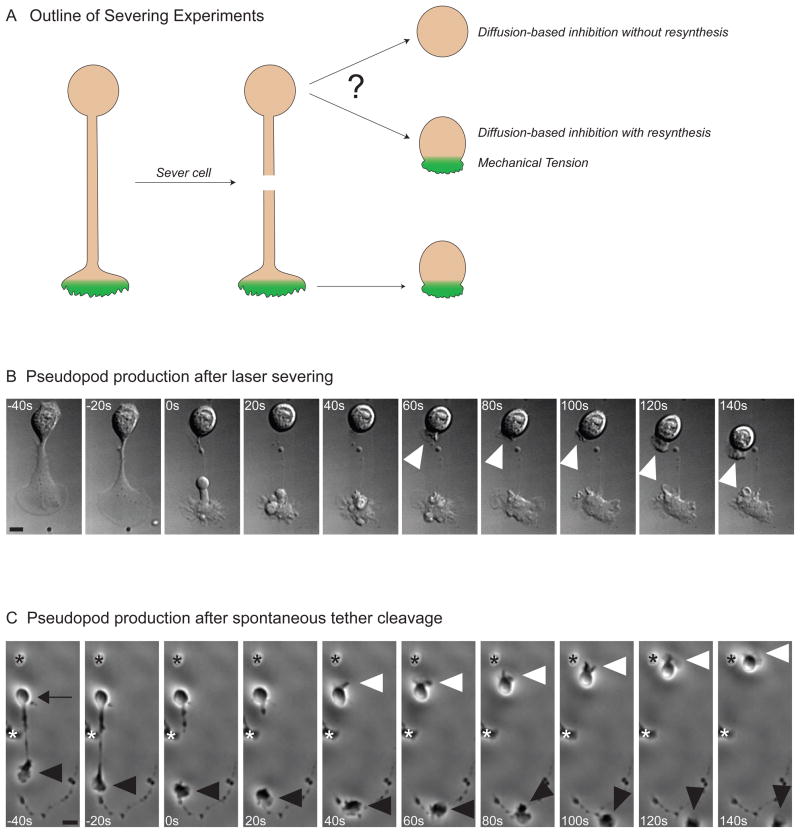
Figure 3. Cells generate a new pseudopod after severing.
A) Outline of severing experiments. Following cell polarization, the pseudopod is removed, and the behavior of the cell body is observed. If the pseudopod had sequestered a non-regenerating limiting component required for polarization, the cell body should not have the material to reanimate. Reanimation of the cell body following severing of the pseudopod would be consistent with short-lived inhibitor generated at the leading edge. This short-lived inhibitor could be due to mechanical tension, a rapidly synthesized limiting component, or a diffusible inhibitor with a short half-life.
B) Pseudopod production after laser severing. DIC images showing a tethered HL-60 that is severed with a laser beam just before 0 seconds. Following severing, the previously quiescent cell body generates a new pseudopod (white arrowhead). The cell body makes a pseudopod after severing in 47% of cells (N = 36). The scale bar is 2.5 microns.
C) Pseudopod production after spontaneous tether cleavage. Phase images of a cell whose pseudopod (black arrowhead) spontaneously breaks free from the cell body (black arrow) at 0 seconds. The cell body makes a new pseudopod within 50 seconds of severing (white arrowhead) and begins to migrate. The asterisks denote neighboring cells. There is significant reanimation of the cell body following spontaneous tether cleavage in 26% of cells (N = 62). The scale bar is 10 microns.
The tethered morphology enabled us to cut the tether with a focused laser beam without destroying either the cell body or the pseudopod. We find that the cell body becomes highly protrusive within 70 seconds of pseudopod removal in 47% of the cells (N = 36, Figure 3B, and Movie S2). Two sets of controls demonstrate that the reanimation of the cell body is not due to laser-induced generation of chemoattractant. Using cells containing a sensitive readout of actin assembly (SCAR/WAVE complex recruitment, Weiner et al., 2007), we observed activation of adjacent cells upon cell destruction with lasers but no detectable response following tether severing (Supplemental Figure 2). We also studied spontaneous cleavage of tethers, which occurs at a low frequency in the absence of laser cutting. Similar to laser-based severing, spontaneous cleavage of the tether also resulted in reanimation of the cell body within one minute of severing in 26% of the cells (N = 64, Figure 3C, Movie S2). Rapid reanimation of the cell body after severing is inconsistent with an inhibition mechanism involving slowly resynthesized limiting components. Our experiments suggest that the pseudopod continuously inhibits the cell body either by sequestering rapidly synthesized limiting components, producing short-lived diffusible inhibitory molecules, or generating mechanical tension.
Tethered morphologies dramatically attenuate diffusion-based exchange between pseudopod and cell body
We reasoned that the tether should severely attenuate diffusion-based communication between the pseudopod and cell body. We experimentally determined the mixing rate between the pseudopod and the cell body using FRAP (Figure 4). We first measured the rate of recovery following GFP photobleaching in the cell body of tethered cells (Figure 4B, Movie S3). To control for reversible bleaching and new GFP synthesis, we also performed photobleaching experiments in cells that lacked a tethered pseudopod (Supplemental Figure 3). We subtracted the recovery rate due to reversible bleaching from the overall recovery rate for tethered cells to determine the amount of recovery that was due to diffusion of GFP from the pseudopod through the tether (Figure 4B and Supplemental Figure 3). Our data demonstrate that the tethered morphology slows down the exchange of GFP between pseudopod and cell body by approximately 840-fold (N = 24, Figure 4C) compared to untethered cells. The experimentally determined mixing rates correlate very well with our computationally predicted mixing rates for measured cell tether lengths and diameters (R2 = 0.8, Figure 4D; Supplemental Information).
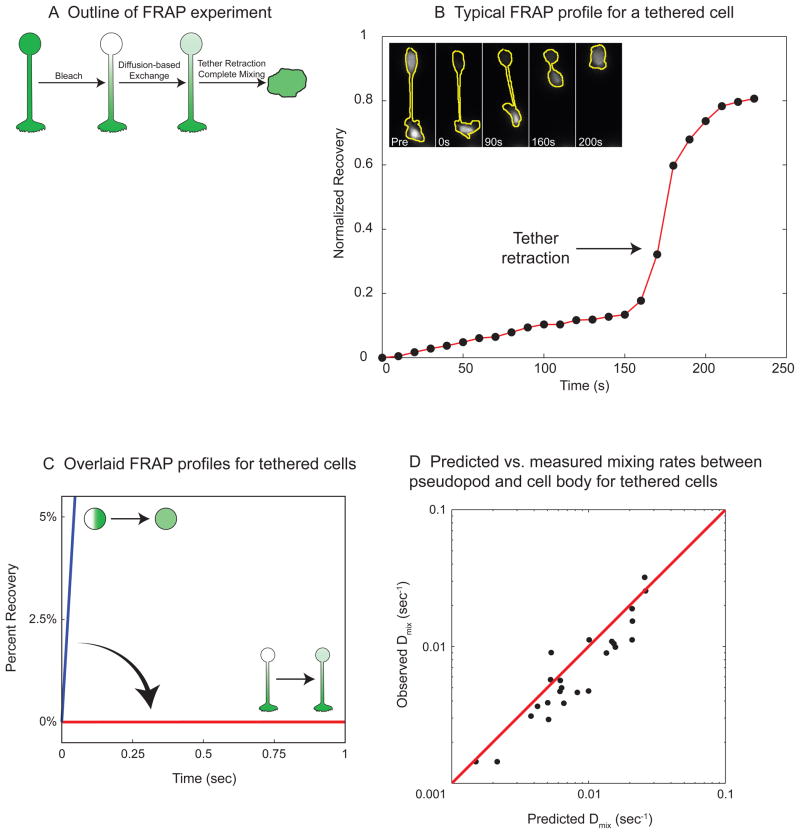
Figure 4. The tethered morphology dramatically attenuates diffusion.
A) Outline of FRAP (Fluorescence Recovery After Photobleaching) experiment. A GFP-expressing HL-60 cell is heated to generate a tethered pseudopod. We bleached the GFP in the cell body, and the recovery in the cell body was measured to monitor diffusion-based mixing through the tether. Retraction of the pseudopod causes the contents of the cell body and the pseudopod to mix completely.
B) Typical FRAP profile for a tethered cell. The graph shows the normalized fluorescence recovery due to diffusion for the cell whose GFP fluorescence is shown in the inset images (with cell outlines in yellow). There is slow linear recovery until 160 sec, when the tethered pseudopod retracts, and the GFP from the pseudopod rapidly mixes with the cell body.
C) Overlaid FRAP profiles for tethered cells. The measured fluorescence recoveries for all of the tethered cells during the first second after bleaching are overlaid in red. The expected initial fluorescence recovery for a non-tethered spherical cell (mixing rate constant = 1.2/sec) is shown in blue.
D) Predicted vs. measured mixing rates between pseudopod and cell body for tethered cells. Each black dot represents the diffusion-based mixing rate constant for an individual photobleached cell. The y coordinate for each cell is the experimentally measured mixing rate constant (Dmix, obs). The x coordinate for each cell is the predicted mixing rate constant using the formula: ; where Dmix,pred is the predicted mixing rate constant; DGFP is the known diffusion coefficient of GFP in cytoplasm (27μm2/s, (Swaminathan et al., 1997)); L is the tether length; and Vcell and Vtether are the volumes of the cell and the tether, respectively. The values L, Vcell and Vtether were measured for each cell from brightfield images. The predicted mixing rates correlate with the measured values (R2 = 0.8, N=24, red line is y = x). The tethered geometry reduces mixing rate by 134 - 4472 fold for all of the cells in the experiment.
We investigated whether inclusion of synthesis and degradation of the limiting component could enable this mechanism to replicate our experimental observations in tethered and severed cells. We chose to analyze the limiting component in the context of the neutral drift model because it is the most analytically tractable. For the cell body to reanimate within 35 seconds of severing, the limiting component must be resynthesized at a rate of at least 6 particles/sec (Supplemental Information). For the cell body to remain quiescent in the tethered state in the absence of severing, resynthesis must be balanced by loss through the tether. Since the tethered morphology dramatically reduces the rate at which molecules diffuse from the cell body to the pseudopod, the limiting component would require a diffusion coefficient of 330 μm2/sec to maintain quiescence of the cell body (Supplemental Information). This diffusion rate is an order of magnitude larger than those reported for cytosolic proteins (Swaminathan et al., 1997) and approximately two-fold greater than that for metabolites in cytoplasm (Garcia-Perez et al., 1999). Similar constraints make a diffusible inhibitor emanating from the pseudopod unlikely, because the inhibitor requires a short lifetime to enable rapid reanimation from the cell body following severing, but such a short-lived inhibitor cannot survive the slow journey through the tether to prevent cell body activity in the tethered morphology.
In summary, the reanimation of the cell body after severing demonstrates that the pseudopod actively inhibits protrusions elsewhere. However, our FRAP experiments on tethered cells indicate that diffusion is too highly attenuated for efficient diffusion-based long-range inhibition. Even with resynthesis, the neutral drift model requires the limiting component to diffuse at a rate that is over an order of magnitude larger than cytoplasmic proteins. In fact, the necessary diffusion coefficient is comparable to that of water itself. Our data are inconsistent with long-range inhibition mechanisms based solely on diffusion. The leading edge must inhibit activation of the cell body through more rapid modes of communication such as active transport, propagating waves, or mechanical forces.
Membrane tension increases during cellular protrusion
Because mechanical propagation of information (such as tension) and diffusion-based communication do not share the same geometrical limitations, we hypothesized that pseudopod protrusion could generate tension in the plasma membrane that rapidly propagates to inhibit protrusive activity in the rest of the cell. This hypothesis requires tension to increase during polarization. Consistent with this hypothesis, aspiration experiments on suspended neutrophils have shown that the cellular cortical tension increases during polarization (Zhelev et al., 1996). However, it is unknown whether the cortical tension represents tension in the cytoskeleton, plasma membrane, or both (Hochmuth, 2000). We used optical traps (Dai and Sheetz, 1999) to measure plasma membrane tension during chemoattractant-stimulated polarization (Figure 5, Movie S4).
Figure 5. Membrane tension increases during protrusion.
A) Schematic outline of membrane tension measurement experiment. The tension in the plasma membrane can be measured by pulling a thin tube of membrane from the cell surface with an adhesive polystyrene bead in an optical trap. Increases in membrane tension result in higher pulling forces on the bead. We hypothesized that cell spreading, induced by uniform fMLP addition, should cause the membrane tension to increase. As a control, we flow in buffer, which does not induce spreading and should not increase membrane tension.
B) Pulling force over time for a representative cell. For primary human neutrophils, the tube was first pulled to a length of ~2 microns (pull 1, arrow, light green bar) and held there briefly (hold 1, light blue bar). The tube was then extended to a length of ~10 microns (pull 2, arrow, dark green bar) and held there (hold 2, dark blue bar) before fMLP (arrow) was flowed in. The colored bars denote the time period over which the forces were averaged for the graph in D; these regions were selected to avoid sudden force jumps. Addition of fMLP caused the cell to spread and the pulling force to increase dramatically (red bar). The inset graph shows the increase in spread area (green) and the increase in tether force (blue), both of which were normalized to the total area or force increase that occurred during the response. Brightfield images of the cell, with the outline superimposed in yellow, are shown below. The tether position, determined with a fluorescent membrane dye (DiI), is also superimposed in yellow.
C) Pulling force over time for individual cells following buffer addition or fMLP stimulation. The left panel shows the force traces of tubes held at constant length as buffer is flowed through the chamber to control for the effects of flow on the force measurements. The right panel shows the force traces of tubes held at constant length as the cells were stimulated by flowing fMLP through the chamber. In both panels, flow begins at the beginning of each trace.
D) Pulling force at different stages of the experiment. The graph shows the forces at different times during the experiment (denoted by the colored bars in B) for the eight fMLP-stimulated cells depicted in C. Each black dot represents the force measurement of an individual cell. The large and small maroon bars indicate mean force values and standard errors, respectively. After fMLP addition, the cell spreads and the force increases dramatically (p = 0.0006) and briefly plateaus (post-spread, red bar in B) before the tube detaches from the bead.
Addition of chemoattractant caused a significant increase in cell protrusion accompanied by a significant increase in membrane tension (N=8, Figure 5B, C, p=0.0006, Movie S4). On average, the pulling force nearly doubled (from 8.5 to 16.6pN) during protrusion (Figure 5D) in a manner that closely correlated with cell spreading (Figure 5B, inset). We suggest that cellular protrusion is responsible for the significant (roughly 4-fold) increase in plasma membrane tension. The pulling force was not a response to the mechanical forces associated with fluid exchange (N=8, Figure 5C). Pretreatment with blebbistatin, which causes a highly elongated morphology, also causes basal membrane tension to increase (Supplemental Figure 4).
Cellular deformation induces long-range inhibition of protrusion signals
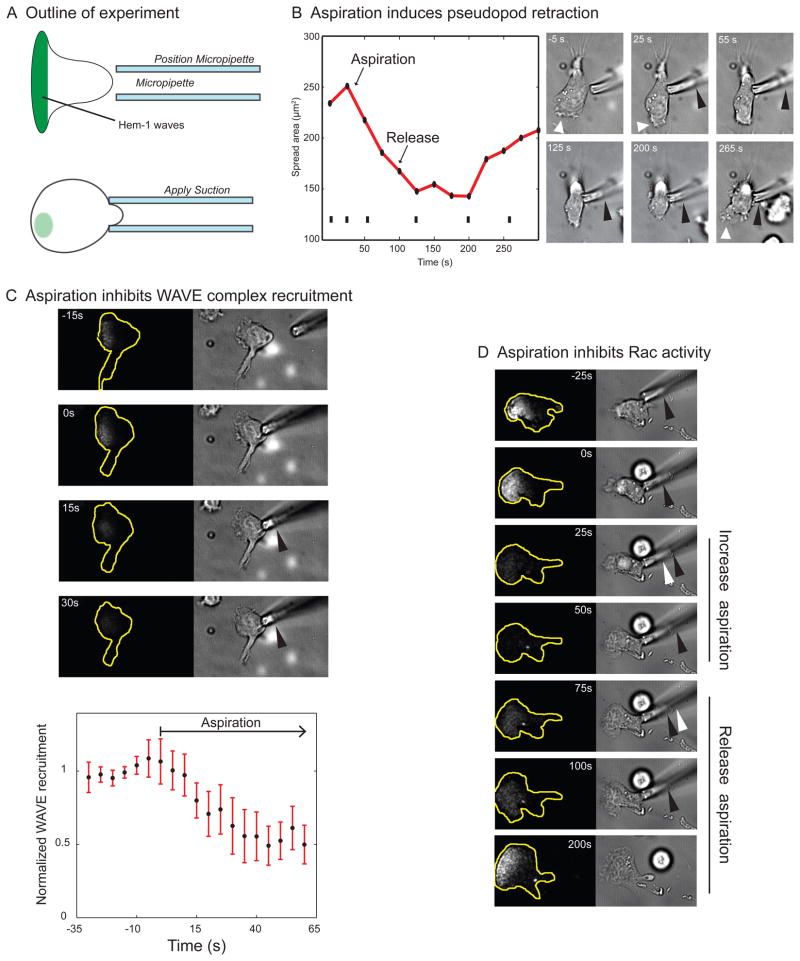
If tension is a long-range inhibitor of protrusion and leading edge signaling, then increases in cell tension (even when they are not a result of pseudopod generation) should inhibit protrusion and leading edge signals throughout the cell. To test this hypothesis, we used micropipette aspiration to mimic the mechanical increases in tension that accompany protrusion (Herant et al., 2005). This perturbation likely increases tension in both the cytoskeleton and plasma membrane when performed on migrating neutrophils. We assayed the effects of tension increases on cell morphology as well as leading edge signals using TIRF-based imaging readouts of Rac activity and SCAR/WAVE complex recruitment. Rac and the SCAR/WAVE complex localize to the leading edge of neutrophils and are essential for actin assembly in these cells (Sun et al., 2004; Weiner et al., 2006).
We brought a micropipette into contact with the cell surface and applied suction to cause the cell to bulge into the micropipette, thereby increasing cell tension (Figure 6A). Within seconds, this mechanical deformation inhibited leading edge protrusion. Following aspiration, the pseudopod retracts into the cell body, leading to a significant reduction in spread area (Figure 6B, Movie S5). Aspiration also inhibited SCAR/WAVE complex recruitment (Figure 6C, Movie S5) and Rac activation (Figure 6D, Movie S5).
Figure 6. Increasing tension with aspiration reversibly inhibits leading edge protrusion and signaling.
A) Outline of experiment. Schematic showing the predicted results of aspiration experiments for a long-range inhibitor based on cell tension. The deformation of the cell due to aspiration increases tension, which would be predicted to inhibit protrusion and reduce SCAR/WAVE complex recruitment.
B) Aspiration induces pseudopod retraction. Aspiration of the trailing edge acts as a long-range inhibitor of protrusion. Left: a graph of the spread area over time during aspiration. The spread area decreases dramatically upon aspiration and then eventually rebounds after release. Tick marks indicate bright field frames shown at right. Right: brightfield images of the same cell. The tip of aspirated cytoplasm is shown with a black arrowhead. The pseudopod (white arrowhead) dies and retracts shortly after aspiration. When the aspirated cytoplasm is released, a new pseudopod forms with a delay of about 100 seconds.
C) Aspiration inhibits SCAR/WAVE complex recruitment. Top: A crawling neutrophil expressing the SCAR/WAVE complex reporter Hem-1-YFP is shown before (−15s and 0s) and during micropipette aspiration. The black arrowhead in the brightfield image denotes the portion of the cell aspirated into the pipette. Increasing tension via aspiration inhibits SCAR/WAVE complex recruitment throughout the cell. Bottom: Quantitation of Hem-1-YFP recruitment during aspiration experiments (N = 10); aspiration begins at frame seven (arrow).
D) Aspiration inhibits Rac activity. A crawling neutrophil expressing the Rac activation reporter PAK-PBD-YFP is shown. The fluorescence channel shows PAK-PBD-YFP visualized in TIRF mode. Each brightfield frame shows the portion of the cell aspirated into the pipette for the current (black arrowhead) and previous (white arrowhead) frames. Aspiration-mediated increases in cell tension result in a dramatic decrease in Rac activation in 85% of cells (N =27). Rac activation returns upon the release of aspiration pressure 65% of the time.
Aspiration-induced inhibition of protrusion and leading edge signals was reversible (Figure 6B,D late time points, Movie S5). Thus, inhibition was not due to trivial reasons such as irreversible cell damage following aspiration. We also monitored plasma membrane integrity during aspiration with cytoplasmic dyes. We did not detect leakage of cytosolic GFP or the entrance of extracellular rhodamine phalloidin, indicating that our aspirations leave the plasma membrane intact (Supplemental Figure 5). Thus, mechanical tension suffices to act as a long-range inhibitor of protrusion and leading edge signals in migrating neutrophils.
Membrane tension restricts signals to the front of migrating neutrophils
Our aspiration data show that tension increases are sufficient for long-range inhibition of protrusion, but two important questions remain. First, does the cell require tension to spatially confine signals to the front? Second, which structures transmit the inhibitory tension: cytoskeleton, plasma membrane or both? To answer these questions we monitored leading edge signaling while reducing membrane tension with hypertonic buffers (Keren, 2011) or cytoskeletal tension with myosin inhibition (Lee et al., 2011; Pasternak et al., 1989). Treating neutrophils with the myosin inhibitor blebbistatin causes cytoskeletal tension to decrease (Lee et al., 2011) but plasma membrane tension to increase (Supplemental Figure 4). Blebbistatin treatment caused cell elongation due to a defect in tail retraction but did not cause an expansion of signals beyond the leading edge (Figure 7A,C). Elongation often led to reduced SCAR/WAVE complex recruitment at later time points (Figure 7A,C), possibly because of increased membrane tension. After several minutes of blebbistatin treatment, the cells often developed a stellate morphology with multiple arm-like projections that appeared to be leading edges. However, close inspection revealed that only one of these projections actively protrudes at a time (Supplemental Figure 6); the others are inanimate husks of previous leading edges that were not retracted after they died. The addition of hypertonic buffer (150mM sucrose) to blebbistatin-treated cells caused an immediate and spatially uniform accumulation of SCAR/WAVE complex at the plasma membrane and resulted in a loss of cell polarity (Figure 7B,C), whereas hypotonic buffer resulted in cell rounding and a disappearance of SCAR/WAVE complex recruitment (Supplemental Figure 6). Hypertonic buffer in the absence of blebbistatin also caused SCAR/WAVE complex accumulation although the effect was smaller (Figure 7C; Supplemental Figure 6). At later time points, neutrophils in hypertonic buffer often spread uniformly with wider leading edges and exhibited multiple pseudopodia for long periods of time (Supplemental Figure 6). These behaviors are consistent with ectopic leading edge signaling throughout the cell. Based on the significant expansion of SCAR/WAVE complex recruitment in cells treated with hypertonic buffer but minimal effect of blebbistatin alone, we conclude that membrane tension plays the dominant role in restricting signals to the leading edge.
Figure 7. Membrane Tension Reduction Causes Expansion of Leading Edge Signaling.
A) Blebbistatin treatment causes cellular elongation but no enhancement of leading edge signaling. Top: A crawling neutrophil expressing the SCAR/WAVE complex reporter Hem-1-YFP (visualized in TIRF mode, shown as a heat map) is shown before (−10s) and during (5s, 25s, 55s) application of blebbistatin, which reduces cytoskeletal tension but increases membrane tension. The cells become elongated, but SCAR/WAVE complex recruitment does not expand beyond the leading edge. SCAR/WAVE complex recruitment decreases at later time points, likely due to elongation-induced increases in membrane tension. Bottom: Brightfield images of the same cell to visualize morphology.
B) Combination of hypertonic buffer and blebbistatin causes uniform SCAR/WAVE complex recruitment. Top: A blebbistatin-treated (100 μM) neutrophil expressing the SCAR/WAVE complex reporter Hem-1-YFP (visualized in TIRF mode, shown as a heat map) is shown before (−20s and −10s) and during (5s, 15s and 475s) application of hypertonic buffer (150mM sucrose + 100 μM blebbistatin), which reduces membrane tension. Reduction in membrane tension causes SCAR/WAVE complex recruitment throughout the cell. Bottom: Brightfield images of the same cell to visualize morphology. Note the uniform spreading between the 15s and 475s time points.
C) Quantification of tension reduction effects on signaling. Quantitation of Hem-1-YFP recruitment during treatment with either hypertonic buffer + blebbistatin (red, N=24), hypertonic buffer alone (blue, N=28), or blebbistatin alone (green, N=12). The number of pixels containing Hem-1 signal were quantified at each time point (see Methods and Materials) and normalized to the pre-treatment signal.
DISCUSSION
We used microdissection and perturbations of cell morphology to push neutrophils into regimes where existing diffusion-based polarity models break down. Our results support a polarity mechanism in which membrane tension provides a long-range inhibitory signal that restricts signals to the leading edge. In contrast to diffusion-based inhibition, tension can effectively propagate through the cell even when the cellular cross-section is small. This ability could be physiologically important as leukocytes frequently have small cross-sections as they crawl through tight spaces in vivo, for example during transendothelial migration (Peters et al., 2008).
We suggest that tension acts as a long-range inhibitor in the following manner. First, pseudopod protrusion increases tension in the plasma membrane (as we observe with our optical trap measurements in Figure 5). This tension rapidly propagates throughout the cell to act as a long-range inhibitor of leading edge formation. In support of this hypothesis, increases in tension suffice for long-range inhibition of Rac activation and protrusion (Figure 6), and decreases in tension expand leading edge activities (Figure 7). During cell polarization, tension only becomes significant after the front has formed, by which point positive feedback enables the existing front to maintain itself. Furthermore, since the front is the source of tension, any fluctuations in front size are immediately balanced by compensatory changes in tension levels. The observation that tension spatially restricts signals such as Rac to the leading edge differentiates our model from purely mechanical (signaling-independent) models of polarity.
For tension to be an effective long-range inhibitor, it must remain high for the entire duration of neutrophil migration. If remodeling of the membrane (due to exocytosis) dissipates tension over time, then the inhibition should decay and polarization would eventually fail. Importantly, others have shown that neutrophil membrane tension remains high long after stimulation (Shao and Xu, 2002) and that persistent deformations cause persistent increases in membrane tension (Herant et al., 2005). Resting neutrophils, like many immune cells, have numerous small wrinkles in their plasma membrane which act as a reservoir that doles out plasma membrane as membrane tension increases during cellular deformation (Hallett and Dewitt, 2007; Herant et al., 2005). Because the unfolding of wrinkles is energetically unfavorable yet reversible, membrane tension remains high as the cell tries to restore its wrinkles (Herant et al., 2005). Thus, the membrane can transmit long-range inhibitory tension in the neutrophil as in other highly motile cells like keratocytes (Keren et al., 2008).
Tension could collaborate with diffusion-based systems to guide migration
We have shown that membrane tension restricts protrusion to the leading edge, thereby allowing the neutrophil to polarize and migrate. However, it is likely that other signaling systems align migration with external cues. Latrunculin-treated cells, which cannot polymerize actin and protrude, can still align internal signals such as PIP3 with external chemoattractant gradients (Janetopoulos et al., 2004; Servant et al., 2000). Thus, cells can interpret chemical gradients without protrusion-based increases in tension. However, neutrophils require F-actin to polarize leading edge signals such as PIP3 production in response to uniform chemoattractant (Wang et al., 2002). Furthermore, multiple patches of front signals coexist in latrunculin-treated Dictyostelium cells exposed to multiple sources of chemoattractant, whereas a single patch of activity dominates in untreated cells (Devreotes and Janetopoulos, 2003; Janetopoulos et al., 2004). Thus, while gradient alignment can occur without actin, important parts of the polarity program (polarity in response to uniform chemoattractant, single site of polarity in response to multiple cues) depend on the actin network, possibly reflecting the role of tension. Although diffusion-based inhibitors may collaborate with tension for polarity in response to uniform chemoattractant, we suggest that tension is the dominant inhibitor. Tension can operate under conditions where diffusive mechanisms fail (tethered cells), and decreasing tension interferes with the restriction of protrusive signals to the leading edge.
Tension antagonizes protrusion in many cell types
Both cytoskeletal and membrane tension are capable of transmitting forces over long range (Dai and Sheetz, 1999; Keren et al., 2008; Mayer et al., 2010) to inhibit cell protrusion. Membrane tension is the loading force which growing actin filaments fight in order to protrude the membrane (Keren et al., 2008). Tension in the cytoskeleton (arising from myosin contraction) can also antagonize actin-based protrusion by pulling actin filaments away from the membrane, thereby reducing the amount of protrusion generated by a given amount of actin assembly (Cai et al., 2010).
In fibroblasts, both cytoskeletal and membrane tension limit cell protrusion. Increasing membrane tension halts spreading while decreasing membrane tension enhances the rate of lamellipodial protrusion and transiently causes uniform spreading (Raucher and Sheetz, 2000). Decreasing cytoskeletal tension (through myosin inhibition) causes faster spreading and a larger final spread area (Cai et al., 2010). Furthermore, increasing tension with biaxial cellular stretching downregulates Rac activity (Katsumi et al., 2002).
For fish keratocytes, membrane tension acts as a long-range coupling mechanism for cell polarization (Keren et al., 2008; Kozlov and Mogilner, 2007). Protrusion in one location promotes retraction in other locations and vice versa due to changes in membrane tension. Actin polymerization in a membrane bag reproduces the wide range of morphologies observed by keratocytes and accurately predicts quantitative relationships between migration speed and morphology without requiring free parameter fitting (Keren et al., 2008). Decreases in cytoskeletal tension (through the myosin inhibitor blebbistatin) does not destroy keratocyte polarity and only slightly reduces their speed, suggesting that the plasma membrane (not the cytoskeleton) carries the important tension in this system. Although keratocyte motility has been primarily considered as a pure mechanical system (with no need for signaling inputs), a graded distribution of actin assembly is necessary for the existing models of keratocytes motility (Barnhart et al., 2011; Keren et al., 2008). In light of our findings, it will be interesting to examine leading edge signals in keratocytes and determine whether membrane tension restricts them to the front as well.
In Dictyostelium, cytoskeletal tension plays an important role in restricting signals to the leading edge. Traction force microscopy experiments have identified large myosin-based cytoskeletal tension increases during Dictyostelium migration (Meili et al., 2010). Genetic deletion of Myosin II reduces cytoskeletal tension dramatically (Pasternak et al., 1989) and increases lateral pseudopod production (Wessels et al., 1988) and Ras activation (Lee et al., 2010). These data strongly support a role for cytoskeletal tension in Dictyostelium polarity. Whether plasma membrane tension also plays a role in Dictyostelium polarity is unknown.
In neutrophils, membrane tension appears to be the dominant inhibitory mechanism for cell polarization. We find that membrane tension increases during neutrophil protrusion and that decreasing membrane tension results in the expansion of leading edge signals and a loss of polarity. In contrast, we find that decreasing cytoskeletal tension with myosin inhibition has no effect on leading edge signals, though myosin inhibition potentiates leading edge signaling increases observed in hypertonic buffer, suggesting that cytoskeletal tension may be partially redundant with membrane tension in restricting leading edge activities.
Possible mechanism of tension sensing
Our experiments suggest that membrane tension acts as a long-range inhibitor of protrusion in migrating neutrophils. How do cells sense and respond to tension? Tension-gated ion channels in the plasma membrane could transduce membrane tension into an inhibitory signal. Another potential mode of tension sensation relies on the properties of the actin nucleation machinery in neutrophils. The Scar/WAVE complex forms multiple propagating waves that organize the neutrophil leading edge (Weiner et al., 2007) and extinguish if mechanical barriers prevent them from protruding. Increases in membrane tension could similarly extinguish waves by antagonizing protrusion. Since the SCAR/WAVE complex is required for Rac activation in neutrophils (Weiner et al., 2006), the destruction of waves by membrane tension also inhibits Rac activation. Because cytoskeletal tension also antagonizes protrusion, it might inhibit signaling via SCAR/WAVE complex dynamics as well. Why don’t protrusions at the leading edge extinguish themselves through increases in tension? Perhaps waves at the leading edge preferentially survive because of their high density, which could enhance survival by activating Rac or by creating a strong actin network that can protrude against the load provided by membrane tension, similar to how membrane tension limits protrusion to areas of high actin density in keratocytes (Keren et al., 2008). Tension-based polarization mechanisms appear to operate in a wide range of migratory cells, although though the sources of the tension (protrusion vs. contraction) and structures bearing the tension (membrane vs. cytoskeleton) can vary from one cell type to the next.
EXPERIMENTAL PROCEDURES
Cell culture
HL-60 cells were generated, cultured, and differentiated as described in Weiner et al, 2007. Primary neutrophils were obtained by pinprick as described in Weiner et al, 2006.
Cell severing and FRAP
Tethered cells were generated by brief heat shock as in Malawista and De Boisfleury Chevance, 1982. Cell severing and FRAP was performed with a 435 nm dye cell laser (Manually Controlled Micropoint System, Photonic Instruments).
Cell aspiration
Cell aspiration was performed with a heat-polished microneedle (3 μm diameter) positioned with a Narishige MM-89 micromanipulator with fine hydraulic control. Suction pressure was controlled with a Narishige IM-300 microinjection system.
Microscopy
Brightfield, epifluorescence and TIRF experiments were performed on Nikon TE-2000 and Ti microscopes.
Membrane tension measurements
Membrane tubes were pulled with 2 μm ConA-coated beads positioned by a 1064 nm holographic optical trap. To calculate the tether force, we measured the distance between the bead and the center of the trap (see Supplemental Methods).
Cytoskeletal and membrane tension perturbations
For hypertonic treatment experiments, we added an equal volume of buffer + 300 mM sucrose. To inhibit myosin, we added blebbistatin to 66 μM final concentration. For combined treatment we pretreated with 100 μM blebbistatin for 10 minutes prior to adding hypertonic buffer. For hypotonic treatment experiments, we added an equal volume of hypotonic buffer (H2O + 1 mM MgCl2 + 1.2 mM CaCl2).
Computational simulations
Described in Supplemental Methods.
Statistical Analysis
Data is presented at mean ± SEM, and a students t test (two-tailed distribution, two-sample unequal variance) was used to calculate p values.
Supplementary Material
HIGHLIGHTS.
Neutrophil leading edge generates long-range inhibition of protrusion
Diffusive mechanisms do not suffice to account for long-range inhibition
Membrane tension doubles during cell polarization
Membrane tension is necessary to confine signals to the leading edge, and tension increases suffice to inhibit protrusion.
Acknowledgments
We thank our labs, Henry Bourne, John Clements, Robert Hochmuth, Dyche Mullins, and Michael Springer for helpful discussion and a critical reading of the manuscript. Support: AHA Predoctoral Fellowship (ARH), NSERC Postdoctoral Fellowship (AJ), DBI-0619674 (ERD), U54 RR022232 (ODW, ERD), NSF-DMS0705431 (SBA), GM081549 & Welch Foundation I-1644 (LFW), R01 GM071794 & Welch Foundation I-1619 (SJA), and Searle Scholars Award & GM084040 (ODW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen WE, Zicha D, Ridley AJ, Jones GE. A role for Cdc42 in macrophage chemotaxis. J Cell Biol. 1998;141:1147–1157. doi: 10.1083/jcb.141.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler SJ, Angenent SB, Wang Y, Wu LF. On the spontaneous emergence of cell polarity. Nature. 2008;454:886–889. doi: 10.1038/nature07119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart EL, Lee KC, Keren K, Mogilner A, Theriot JA. An adhesion-dependent switch between mechanisms that determine motile cell shape. PLoS Biol. 2011;9:e1001059. doi: 10.1371/journal.pbio.1001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butty AC, Perrinjaquet N, Petit A, Jaquenoud M, Segall JE, Hofmann K, Zwahlen C, Peter M. A positive feedback loop stabilizes the guanine-nucleotide exchange factor Cdc24 at sites of polarization. Embo J. 2002;21:1565–1576. doi: 10.1093/emboj/21.7.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Rossier O, Gauthier NC, Biais N, Fardin MA, Zhang X, Miller LW, Ladoux B, Cornish VW, Sheetz MP. Cytoskeletal coherence requires myosin-IIA contractility. J Cell Sci. 2010;123:413–423. doi: 10.1242/jcs.058297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Sheetz MP. Membrane tether formation from blebbing cells. Biophys J. 1999;77:3363–3370. doi: 10.1016/S0006-3495(99)77168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreotes P, Janetopoulos C. Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J Biol Chem. 2003;278:20445–20448. doi: 10.1074/jbc.R300010200. [DOI] [PubMed] [Google Scholar]
- Fivaz M, Bandara S, Inoue T, Meyer T. Robust neuronal symmetry breaking by Ras-triggered local positive feedback. Curr Biol. 2008;18:44–50. doi: 10.1016/j.cub.2007.11.051. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez AI, Lopez-Beltran EA, Kluner P, Luque J, Ballesteros P, Cerdan S. Molecular crowding and viscosity as determinants of translational diffusion of metabolites in subcellular organelles. Arch Biochem Biophys. 1999;362:329–338. doi: 10.1006/abbi.1998.1051. [DOI] [PubMed] [Google Scholar]
- Gardiner EM, Pestonjamasp KN, Bohl BP, Chamberlain C, Hahn KM, Bokoch GM. Spatial and temporal analysis of Rac activation during live neutrophil chemotaxis. Curr Biol. 2002;12:2029–2034. doi: 10.1016/s0960-9822(02)01334-9. [DOI] [PubMed] [Google Scholar]
- Gierer A, Meinhardt H. A theory of biological pattern formation. Kybernetik. 1972;12:30–39. doi: 10.1007/BF00289234. [DOI] [PubMed] [Google Scholar]
- Hallett MB, Dewitt S. Ironing out the wrinkles of neutrophil phagocytosis. Trends Cell Biol. 2007;17:209–214. doi: 10.1016/j.tcb.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Herant M, Heinrich V, Dembo M. Mechanics of neutrophil phagocytosis: behavior of the cortical tension. J Cell Sci. 2005;118:1789–1797. doi: 10.1242/jcs.02275. [DOI] [PubMed] [Google Scholar]
- Hochmuth RM. Micropipette aspiration of living cells. J Biomech. 2000;33:15–22. doi: 10.1016/s0021-9290(99)00175-x. [DOI] [PubMed] [Google Scholar]
- Inoue T, Meyer T. Synthetic activation of endogenous PI3K and Rac identifies an AND-gate switch for cell polarization and migration. PLoS One. 2008;3:e3068. doi: 10.1371/journal.pone.0003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janetopoulos C, Ma L, Devreotes PN, Iglesias PA. Chemoattractant-induced phosphatidylinositol 3,4,5-trisphosphate accumulation is spatially amplified and adapts, independent of the actin cytoskeleton. Proc Natl Acad Sci U S A. 2004;101:8951–8956. doi: 10.1073/pnas.0402152101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilkine A, Edelstein-Keshet L. A comparison of mathematical models for polarization of single eukaryotic cells in response to guided cues. PLoS Comput Biol. 2011;7:e1001121. doi: 10.1371/journal.pcbi.1001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumi A, Milanini J, Kiosses WB, del Pozo MA, Kaunas R, Chien S, Hahn KM, Schwartz MA. Effects of cell tension on the small GTPase Rac. J Cell Biol. 2002;158:153–164. doi: 10.1083/jcb.200201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren K. Cell motility: the integrating role of the plasma membrane. Eur Biophys J. 2011;40:1013–1027. doi: 10.1007/s00249-011-0741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren K, Pincus Z, Allen GM, Barnhart EL, Marriott G, Mogilner A, Theriot JA. Mechanism of shape determination in motile cells. Nature. 2008;453:475–480. doi: 10.1038/nature06952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov MM, Mogilner A. Model of polarization and bistability of cell fragments. Biophys J. 2007;93:3811–3819. doi: 10.1529/biophysj.107.110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, Herant M, Heinrich V. Target-specific mechanics of phagocytosis: protrusive neutrophil response to zymosan differs from the uptake of antibody-tagged pathogens. J Cell Sci. 2011;124:1106–1114. doi: 10.1242/jcs.078592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Shen Z, Robinson DN, Briggs S, Firtel RA. Involvement of the cytoskeleton in controlling leading-edge function during chemotaxis. Mol Biol Cell. 2010;21:1810–1824. doi: 10.1091/mbc.E10-01-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malawista SE, De Boisfleury Chevance A. The cytokineplast: purified, stable, and functional motile machinery from human blood polymorphonuclear leukocytes. J Cell Biol. 1982;95:960–973. doi: 10.1083/jcb.95.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M, Depken M, Bois JS, Julicher F, Grill SW. Anisotropies in cortical tension reveal the physical basis of polarizing cortical flows. Nature. 2010;467:617–621. doi: 10.1038/nature09376. [DOI] [PubMed] [Google Scholar]
- Meili R, Alonso-Latorre B, del Alamo JC, Firtel RA, Lasheras JC. Myosin II is essential for the spatiotemporal organization of traction forces during cell motility. Mol Biol Cell. 2010;21:405–417. doi: 10.1091/mbc.E09-08-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt H. Orientation of chemotactic cells and growth cones: models and mechanisms. J Cell Sci. 1999;112:2867–2874. doi: 10.1242/jcs.112.17.2867. [DOI] [PubMed] [Google Scholar]
- Mori Y, Jilkine A, Edelstein-Keshet L. Wave-pinning and cell polarity from a bistable reaction-diffusion system. Biophys J. 2008;94:3684–3697. doi: 10.1529/biophysj.107.120824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Mine N, Nakaguchi E, Mochizuki A, Yamamoto M, Yashiro K, Meno C, Hamada H. Generation of robust left-right asymmetry in the mouse embryo requires a self-enhancement and lateral-inhibition system. Dev Cell. 2006;11:495–504. doi: 10.1016/j.devcel.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Neilson MP, Veltman DM, van Haastert PJ, Webb SD, Mackenzie JA, Insall RH. Chemotaxis: a feedback-based computational model robustly predicts multiple aspects of real cell behaviour. PLoS Biol. 2011;9:e1000618. doi: 10.1371/journal.pbio.1000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuji M, Ishihara S, Co C, Kaibuchi K, Mochizuki A, Kuroda S. A mass conserved reaction-diffusion system captures properties of cell polarity. PLoS Comput Biol. 2007;3:e108. doi: 10.1371/journal.pcbi.0030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak C, Spudich JA, Elson EL. Capping of surface receptors and concomitant cortical tension are generated by conventional myosin. Nature. 1989;341:549–551. doi: 10.1038/341549a0. [DOI] [PubMed] [Google Scholar]
- Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S, Lawyer P, Fay MP, Germain RN, Sacks D. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321:970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucher D, Sheetz MP. Cell spreading and lamellipodial extension rate is regulated by membrane tension. J Cell Biol. 2000;148:127–136. doi: 10.1083/jcb.148.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Sasaki AT, Chun C, Takeda K, Firtel RA. Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement. J Cell Biol. 2004;167:505–518. doi: 10.1083/jcb.200406177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287:1037–1040. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao JY, Xu J. A modified micropipette aspiration technique and its application to tether formation from human neutrophils. J Biomech Eng. 2002;124:388–396. doi: 10.1115/1.1486469. [DOI] [PubMed] [Google Scholar]
- Sick S, Reinker S, Timmer J, Schlake T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science. 2006;314:1447–1450. doi: 10.1126/science.1130088. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Wang F, Glavas S, Ott A, Hofmann F, Aktories K, Kalman D, Bourne HR. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J Cell Biol. 2003;160:375–385. doi: 10.1083/jcb.200208179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CX, Downey GP, Zhu F, Koh AL, Thang H, Glogauer M. Rac1 is the small GTPase responsible for regulating the neutrophil chemotaxis compass. Blood. 2004;104:3758–3765. doi: 10.1182/blood-2004-03-0781. [DOI] [PubMed] [Google Scholar]
- Swaminathan R, Hoang CP, Verkman AS. Photobleaching recovery and anisotropy decay of green fluorescent protein GFP-S65T in solution and cells: cytoplasmic viscosity probed by green fluorescent protein translational and rotational diffusion. Biophys J. 1997;72:1900–1907. doi: 10.1016/S0006-3495(97)78835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turing AM. The Chemical Basis of Morphogenesis. Philosophical Transactions of the Royal Society of London. 1952;237:37–72. doi: 10.1098/rstb.2014.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Herzmark P, Weiner OD, Srinivasan S, Servant G, Bourne HR. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat Cell Biol. 2002;4:513–518. doi: 10.1038/ncb810. [DOI] [PubMed] [Google Scholar]
- Wedlich-Soldner R, Altschuler S, Wu L, Li R. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science. 2003;299:1231–1235. doi: 10.1126/science.1080944. [DOI] [PubMed] [Google Scholar]
- Weiner OD, Marganski WA, Wu LF, Altschuler SJ, Kirschner MW. An actin-based wave generator organizes cell motility. PLoS Biol. 2007;5:e221. doi: 10.1371/journal.pbio.0050221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner OD, Neilsen PO, Prestwich GD, Kirschner MW, Cantley LC, Bourne HR. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat Cell Biol. 2002;4:509–513. doi: 10.1038/ncb811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner OD, Rentel MC, Ott A, Brown GE, Jedrychowski M, Yaffe MB, Gygi SP, Cantley LC, Bourne HR, Kirschner MW. Hem-1 complexes are essential for Rac activation, actin polymerization, and myosin regulation during neutrophil chemotaxis. PLoS Biol. 2006;4:e38. doi: 10.1371/journal.pbio.0040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels D, Soll DR, Knecht D, Loomis WF, De Lozanne A, Spudich J. Cell motility and chemotaxis in Dictyostelium amebae lacking myosin heavy chain. Dev Biol. 1988;128:164–177. doi: 10.1016/0012-1606(88)90279-5. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Huang CH, Iglesias PA, Devreotes PN. Cells navigate with a local-excitation, global-inhibition-biased excitable network. Proc Natl Acad Sci U S A. 2010;107:17079–17086. doi: 10.1073/pnas.1011271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhelev DV, Alteraifi AM, Hochmuth RM. F-actin network formation in tethers and in pseudopods stimulated by chemoattractant. Cell Motil Cytoskeleton. 1996;35:331–344. doi: 10.1002/(SICI)1097-0169(1996)35:4<331::AID-CM5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.