Background: Ligand-dependent corepressor (LCoR) was identified as a repressor of nuclear hormone receptors.
Results: A screen for new interacting factors revealed that LCoR acts with Krüppel-like factor 6 (KLF6) to repress CDKN1A gene transcription.
Conclusion: KLF6 inhibits gene transcription by tethering a complex of proteins including LCoR to DNA.
Significance: Identification of a novel mechanism of action of LCoR, independent of nuclear receptors, with potential implications for prostate cancer.
Keywords: Gene Regulation, Nuclear Receptors, Prostate Cancer, Transcription Factors, Transcription Repressor, CDKN1A, KLF6, LCoR
Abstract
The widely expressed transcriptional coregulator, ligand-dependent corepressor (LCoR), initially characterized as a regulator of nuclear receptor-mediated transactivation, functions through recruitment of C-terminal binding proteins (CtBPs) and histone deacetylases (HDACs) to its N-terminal and central domains, respectively. We performed a yeast two-hybrid screen for novel cofactors, and identified an interaction between the C-terminal domain of LCoR and the transcription factor Krüppel-like factor 6 (KLF6), a putative tumor suppressor in prostate cancer. Subsequent experiments revealed LCoR regulation of several KLF6 target genes notably p21WAF1/CIP1 (CDKN1A) and to a lesser extent E-cadherin (CDH1), indicating that LCoR regulates gene transcription through multiple classes of transcription factors. In multiple cancer cells, LCoR and KLF6 bind together on the promoters of the genes encoding CDKN1A and CDH1. LCoR contributes to KLF6-mediated transcriptional repression in a promoter- and cell type-dependent manner. Its inhibition of reporter constructs driven by the CDKN1A and CDH1 promoters in PC-3 prostate carcinoma cells is sensitive to treatment with the HDAC inhibitor trichostatin A. Additionally, the LCoR cofactor CtBP1 bound the same promoters and augmented the LCoR-dependent repression in PC-3 cells. Consistent with their inferred roles in transcriptional repression, siRNA-mediated knockdown of KLF6, LCoR, or CtBP1 in PC-3 cells induced expression of CDKN1A and CDH1 and additional KLF6 target genes. We propose a novel model of LCoR function in which promoter-bound KLF6 inhibits transcription of the CDKN1A gene and other genes as well by tethering a transcriptional corepressor complex containing LCoR, with specific contributions by CtBP1 and HDACs.
Introduction
Aberrant gene regulation by transcription factors and associated regulatory proteins can disrupt the proper function of biological processes such as proliferative signaling, cell death, and energy metabolism, and result in oncogenic transformation (1, 2). The transcription factor, KLF6, which contains a highly conserved C2H2 zinc finger DNA-binding motif that interacts with “GC boxes” within responsive promoters (3), is frequently inactivated by loss of heterozygosity, somatic mutation, and/or decreased expression in human cancers (3). Evidence in prostate cancer (PCa),3 suggests that KLF6 acts as a tumor suppressor through transcriptional control of specific genes (4–7). For example, KLF6 regulates expression of CDKN1A, the gene encoding the cell cycle regulator p21WAF1/CIP1, providing a potential mechanistic explanation for its tumor suppressor function and its control of cellular proliferation (4, 8, 9). Indeed, numerous studies exist that demonstrate the growth suppressive capabilities of p21WAF1/CIP1 in cancer (10–14). However, overexpression of this gene has been demonstrated in an androgen-independent prostate carcinoma model derived from LNCaP cells (15), and was shown to be associated with a worse clinical outcome in PCa before and after androgen deprivation therapy (16–18). Thus, it would appear that p21WAF1/CIP1 has both tumor suppressive and oncogenic properties depending on the cellular contexts in which it is expressed (19). In addition, in an ovarian cancer cell line, KLF6 regulates expression of E-cadherin (CDH1) (7), a cell adhesion molecule. E-cadherin has putative tumor suppressor functions in multiple cancers, and CDH1 silencing has been shown to promote epithelial-to-mesenchymal transition, which culminates in metastasis (20).
Ligand-dependent corepressor (LCoR), initially identified as a factor that interacts with class I and II agonist-bound nuclear receptors (NR), is expressed in a wide variety of fetal and adult human tissues (21). LCoR is recruited to ligand-bound NRs through a single LXXLL motif/NR box, and suppresses target gene transcription by recruiting corepressors carboxyl (C)-terminal-binding protein (CtBP) 1 and 2 by tandem N-terminal extended PXDLS motifs (21, 22), and histone deacetylases (HDAC) 3 and 6 (21, 23) through central domains. A recent study has revealed that LCoR binds to and suppresses transactivation by the androgen receptor and inhibits PCa growth in vivo (24). LCoR is also present in a CtBP corepressor complex (25), and interacts with lysine-specific demethylase 1 (LSD1), a pivotal member of the ZEB1-CtBP-CoREST-LSD1 repressive complex (26). Surprisingly, in MCF-7 breast adenocarcinoma cells, knockdown of LCoR had either no effect or decreased expression of estrogen receptor α-regulated genes (22, 23, 27). Hence, LCoR may have promoter-specific effects on transcriptional regulation, similar to another member of the ligand-dependent repressor family, RIP140/NRIP1 (28, 29).
Here, using a yeast two-hybrid screen with the open reading frame of LCoR as bait, we identified Krüppel-like factor 6 (KLF6) as a novel LCoR-interacting transcription factor. We find that the two proteins interact directly in vitro, and on the CDKN1A and CDH1 promoters in human prostate adenocarcinoma PC-3 cells. LCoR functions as a corepressor of CDKN1A and other KLF6 target genes in PCa and breast cancer cells. Recruitment of CtBP1 and HDACs, previously identified LCoR cofactors, was required for LCoR corepression in PC-3 cells. LCoR recruitment to the proximal CDKN1A promoter, and corepression of reporter gene transcription driven by a CDKN1A promoter fragment, were dependent on endogenous KLF6, consistent with elevated expression of CDKN1A and other target genes in KLF6-deficient cells. These data suggest that KLF6 acts as a repressor of CDKN1A transcription and other genes as well through recruitment of LCoR and associated cofactors, and identify a novel function for LCoR independent of NRs.
EXPERIMENTAL PROCEDURES
Isolation of KLF6 cDNA Sequence
A yeast two-hybrid screen (9.8 × 105 transformants; BD Biosciences human fetal brain MATCHMAKER cDNA library HL4028AH; BD Biosciences) with an ORF of LCoR yielded 20 His+/LacZ+ colonies. Two colonies contained ∼1.0-kb inserts corresponding to KLF6. Homologies to human genomic sequences were found using BLASTN, and multiple alignments of the different cDNA clones were performed using ClustalW employing standard parameters.
Antibodies and Reagents
A rabbit polyclonal antibody was raised against LCoR amino acids 20–36 (QDPSQPNSTKNQSLPKA) fused to keyhole limpet hemocyanin and purified on a peptide affinity column (Bethyl Laboratories, Montgomery, TX). An additional LCoR (sc-134674) antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) as well as antibodies for KLF6 (sc-7158) and β-actin (sc-47778). An additional anti-KLF6 antibody was purchased from Invitrogen (37-8400). Antibody for FLAG (F1804) was purchased from Sigma. The antibody for CtBP1 (07-306) was purchased from Millipore (Billerica, MA).
Recombinant Plasmids
pSG5-LCoR-433, pcDNA3.1LCoR-M1M2, pcDNA3.1-FLAG-LCoR-433/M1M2, and pcDNA3-CtBP1 have been previously described (21). pCMV-LCoR-406 and pCMV-KLF6 were kind gifts from Dr. Y. Barak (Magee-Women's Institute, Pittsburgh, PA). CDKN1A-225bp-luc and CDH1-230bp-have been previously described (4, 7) as well as CDKN1A-2.5kbp-luc (30).
Cell Culture and Transfections
PC-3 and MCF-7 cells were obtained from the American Type Culture Collection (ATCC), and cultured in DMEM (319-005-CL, Multicell) supplemented with 10% FBS. CaCo-2 cells were obtained from ATCC, and cultured in α-MEM (12571-063, Invitrogen) supplemented with 10% FBS. All experiments were carried out between passage numbers 5 and 25.
GST Pulldown Assays and Immunoprecipitation
Glutathione S-transferase pulldown assays were performed using the MagneGSTTM Pull-down System (Promega) as per the manufacturer's instructions. Pulldown eluates were subsequently analyzed by Western blot using standard protocols and specified antibodies. All the GST pulldown conditions were run on the same gel, however, the input control was loaded on the far left, and it was moved to the right purely for esthetic reasons. The GST pulldowns were repeated at least three times with similar results, and 1 representative experiment was chosen for figure production. For immunoprecipitation of endogenous proteins, CaCo-2/PC-3 cells in 100-mm dishes were lysed for 10 min on ice in 500 μl of lysis buffer (20 mm Tris, pH 7.5, 100 mm NaCl, 0.5% Nonidet P-40, 0.5 mm EDTA, protease inhibitor mixture; Roche Applied Science). Cell debris were pelleted by centrifugation (10,000 × g for 10 min at 4 °C), and 300 μg of protein were pre-cleared with α-IgG and protein A/G-agarose beads for 1 h at 4 °C. Samples were immunoprecipitated with α-IgG, α-KLF6 (37-8400, SC-7158), or α-FLAG (F1804), and protein A/G-agarose beads overnight at 4 °C. Beads were washed in lysis buffer (4 times), boiled in Laemmli buffer, and Western blotted as previously described (31).
Luciferase Assays
For analysis of the effects of specific proteins on transactivation of the CDKN1A/CDH1 promoters, PC-3/MCF-7 cells were grown (80–90% confluent) in six-well plates. Two hours prior to transfection, the medium was replaced with fresh medium. After this period, cells were transfected in Opti-MEM (Invitrogen) with 10 μl of Lipofectamine 2000 (Invitrogen), 100 ng of pCMV-β-gal, 250 ng of CDKN1A/CDH1-luc, and the corresponding amounts of specified plasmids for 6 h. Transfected DNA quantity was kept constant in all conditions by adding a corresponding amount of empty vector. Then, the transfection medium was replaced, and cells were allowed to recover for 24 h, after which cells were harvested in 200 μl of reporter lysis buffer (Promega). For trichostatin A (TSA) treatments the corresponding amount of TSA was added to the medium for 24 h, after which samples were collected. Three independent biological experiments were performed in triplicate, and 1 representative experiment was chosen for figure production. All experiments were normalized to β-gal activity. Values reported are mean ± S.D.
Chromatin Immunoprecipitation (ChIP) and ReChIP Assays
ChIP and reChIP assays were essentially performed as previously described in PC-3, CaCo-2, and MCF-7 cells (23). Immunoprecipitations were performed with corresponding antibodies (SC-7158, F1804, sc-134674), and 2 sets of specific and 1 set of nonspecific (NS) primer sequences were used to validate protein binding. For the CDKN1A promoter, the primer sequences used were 5′-GCTGGGCAGCCAGGAGCCTG-3′ (forward) and 5′-CTGCTCACACCTCAGCTGGC-3′ (reverse), 5′-ATGTGTCCAGCGCACCAAC-3′ (forward), and 5′-GCGGCCCTGATATACAACC-3′ (reverse); and NS 5′-TGGGGTTATCTCTGTGTTAGGG-3′ (forward), and 5′-TAGCCTCTACTGCCACCATCTT-3′ (reverse). For the CDH1 promoter, the primers sequences used were 5′-CAGCTACTAGAGAGGCTGGGGCCAG-3′ (forward), and 5′-CGTACCGCTGATTGGCTGAGGGTTC-3′ (reverse), and 5′-GTCTTAGTGAGCCACCGGCGG-3′ (forward), and 5′-GTTCACCTGCCGGCCACAGCC-3′ (reverse), and NS, 5′-TTCTCCCTTCTAAGCTCCTGTG-3′ (forward), and 5′-CCACCCTCCTAACGTTCTAGTG-3′ (reverse). Three independent biological experiments were performed with similar results, and 1 representative experiment was chosen for figure production. Values reported are mean ± S.D.
siRNA Knockdowns
siRNA for LCoR (HSC.RNAI.N001170765.12.1/3) and KLF6 (HSC.RNAI.N001300.12.1/2) were purchased from IDT Integrated DNA Technologies. siRNA for CtBP1 (ON-TARGETplus, J-008609-08) was purchased from Thermo Scientific Dharmacon (Lafayette, CO). Briefly, PC-3 or MCF-7 cells were plated in 6-well plates, and grown to about 50–60% confluence. Two hours prior to transfection, the medium was replaced with fresh medium, and then the cells were transfected in Opti-MEM (Invitrogen) with corresponding siRNA for 5 (MCF-7) or 6 h (PC-3), and 10 μl of Lipofectamine 2000 (Invitrogen, for PC-3 cells) or Lipofectamine RNAI Max (Invitrogen, for MCF-7 cells). After this incubation, medium was added, and cells were allowed to recover. For protein knockdown and mRNA target gene analysis, cells were collected 48 and 72 h after transfection with siCtBP1 and siLCoR/siKLF6, respectively. mRNA expression analysis was carried out by quantitative PCR (qPCR). Three independent biological experiments were performed in triplicate, and 1 representative experiment was chosen for figure production. Values reported are mean ± S.D.
siRNA Knockdown and ChIP
PC-3 cells were grown in 100-mm dishes and the knockdown and ChIP assays were essentially performed as described in the previous sections.
siRNA Knockdown and Reporter Gene Experiments
PC-3 cells were grown (50–60% confluent) in 6-well plates, and plasmid DNA and siRNA were co-transfected in PC-3 cells as described under “Luciferase Assays” section. After a 72-h incubation period, cells were harvested in 200 μl of reporter lysis buffer (Promega). Three independent biological experiments were performed in triplicate, and pooled for figure production. All experiments were normalized to β-gal activity. Values reported are mean ± S.E.
RNA Isolation, cDNA Synthesis, and Quantitative Real-time PCR
RNA isolation, cDNA synthesis and quantitative real time PCR (RT-qPCR) were performed as previously described (23), with the following modifications to qPCR: the reagent used was SsoFast EvaGreen (172-5211, Bio-Rad), and the machine used was an Illumina Eco. Results were normalized to β-actin mRNA expression. The primers used are detailed in supplemental Table S1.
KLF6 Overexpression Experiments
PC-3 cells were grown (80–90% confluent) in 6-well plates. Two hours prior to transfection, the medium was replaced with fresh medium. After this period, cells were transfected in Opti-MEM (Invitrogen) with 10 μl of Lipofectamine 2000 (Invitrogen), 500 ng of pCMV-KLF6 or empty vector for 6 h. Cells were allowed to recover for 48 h, and then collected for protein analysis or RNA extraction.
Statistical Analysis
Statistical analysis was carried out with the program SYSTAT10.1 by performing Student's two-sample t test or one-way analysis of variance (ANOVA) followed by the Tukey test for multiple comparisons as indicated. Probability values are as follows: *, ≤0.05; **, ≤0.01; and ***, ≤0.001.
RESULTS
LCoR Interacts with Transcription Factor KLF6
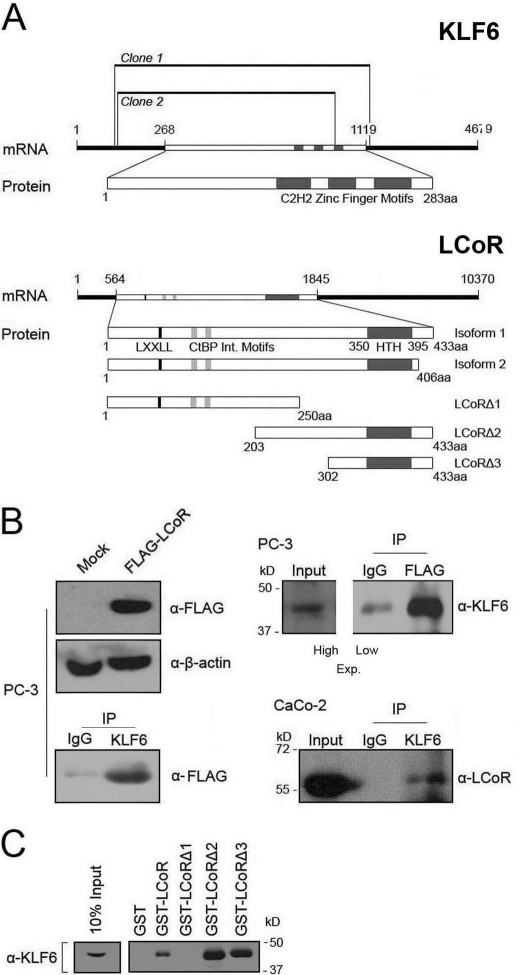
The early and widespread expression of LCoR (21) and its presence in multiprotein complexes (25) suggest that in addition to nuclear receptors, it may regulate other classes of transcription factors. Given the robust expression of LCoR in human fetal brain (21), we utilized a human fetal brain cDNA library (Clontech) to perform a yeast two-hybrid screen to identify new molecular partners. Two independent ∼1.0-kb long clones, encoding nearly the entire open reading frame of KLF6, were isolated from this screen (Fig. 1A). To confirm binding, KLF6- and FLAG-tagged LCoR were transfected into PC-3 cells, verified for FLAG-LCoR expression (Fig. 1B, upper left), immunoprecipitated with either anti-KLF6 or -FLAG antibody, and probed for FLAG or KLF6, respectively. Reciprocal arms of this experiment confirmed LCoR-KLF6 interaction in PC-3 cells (Fig. 1B). Immunoprecipitation of KLF6 from undifferentiated colorectal adenocarcinoma CaCo-2 cells also revealed an association between endogenous KLF6 and LCoR (Fig. 1B, lower right). To confirm the interaction between the two proteins and to delineate the KLF6-interacting domain of LCoR, we carried out GST pulldowns with full-length and truncated mutants of LCoR. These experiments localized KLF6 binding to the C-terminal domain encoded by amino acids 302 to 433 of LCoR (Fig. 1C).
FIGURE 1.
Identification and confirmation of interaction between LCoR and KLF6. A, top, schematic representation of the two KLF6 cDNA clones identified with the yeast two-hybrid. Bottom, LCoR truncation mutants used in this study. B, upper left panel, PC-3 cells were transfected with appropriate plasmids (pcDNA3.1FLAG-LCoR and pCMV-KLF6), and a Western blot was performed to confirm expression of transiently transfected FLAG-LCoR. Lower left panel, KLF6 was immunoprecipitated from PC-3 cells (SC-7158) and immunoprecipitates were probed for FLAG (F1804). Upper right panel, coimmunoprecipitation of KLF6 and FLAG-LCoR in PC-3 cells. FLAG was immunoprecipitated with specific antibody (F1804) followed by a Western blot for KLF6 (SC-7158). Due to the intensity of the signal from the IP, two different exposures had to be performed for the input (high) and immunoprecipitation (IP) (low). Lower right panel, coimmunoprecipitation of endogenous LCoR and KLF6 in CaCo-2 cells. KLF6 was immunoprecipitated with specific antibody (37–8400) followed by a Western blot for LCoR (Bethyl). C, results of a KLF6 (SC-7158) Western blot of pull downs with GST control or fusions with full-length LCoR or truncated mutants and in vitro bacterially translated KLF6.
KLF6 and LCoR Bind CDKN1A and CDH1 Promoters
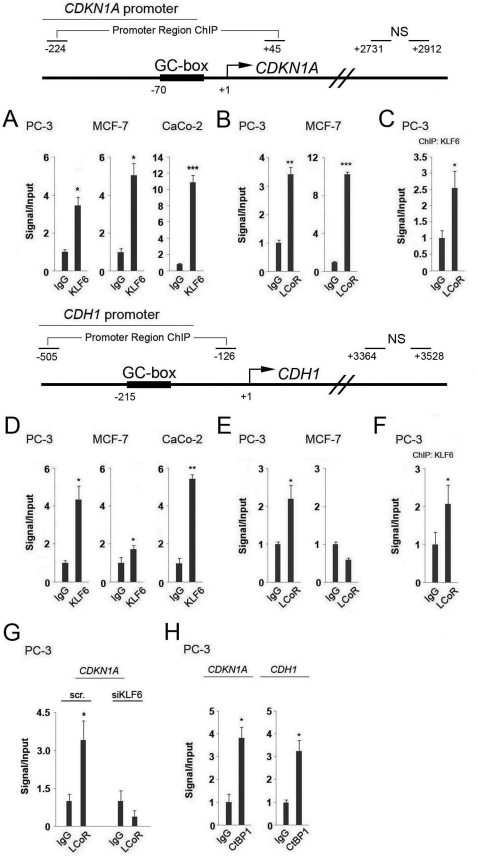
Chromatin immunoprecipitation (ChIP) assays, followed by semi-quantitative PCR or qPCR in PC-3, MCF-7, and CaCo-2 cells, confirmed that KLF6 binds to GC boxes situated within the CDKN1A and CDH1 promoters, as previously reported (Fig. 2, A and D, and data not shown) (7, 9). As a control for the binding specificity of the proteins studied, qPCR with primers targeting a NS region within the body of the gene were carried out. This control was performed for all subsequent ChIP assays, and revealed that no binding was detected within this nonspecific area studied (data not shown).
FIGURE 2.
Analysis of KLF6, LCoR, and CtBP1 binding to the CDKN1A and CDH1 promoters by ChIP assay. Top and middle, schematic representations of the CDKN1A and CDH1 promoter sections analyzed by ChIP. Regions amplified encompassing the GC boxes and NS control sequences are marked. A and D, ChIP assays were performed in PC-3, MCF-7, and CaCo-2 cells with KLF6 (SC-7158) antibody followed by qPCR with specific primers for the CDKN1A (A) or CDH1 (D) promoter. Two-sample t tests were performed to ascertain statistical significance on all assays. B and E, ChIP assays were performed in PC-3 and MCF-7 cells with anti-LCoR (sc-134674) antibody followed by qPCR with specific primers for the CDKN1A (B) or CDH1 (E) promoters. C and F, results of reChIP assays are shown, in which a first round of ChIP for KLF6 (SC-7158) followed by a second round of immunoprecipitation for LCoR (sc-134674) was performed in PC-3 cells, followed by qPCR with specific primers for the CDKN1A (C) or CDH1 (F) promoters. G, results of qPCR with specific primers for the CDKN1A promoter from ChIP assays for LCoR (sc-134674) following knockdown with a nonspecific control (scr) or KLF6-specific siRNA. H, ChIP assays were performed in PC-3 cells with an anti-CtBP1 (07-306) antibody followed by qPCR with specific primers for the CDKN1A/CDH1 promoters.
In the absence of suitable anti-LCoR antibodies capable of immunoprecipitation, initial ChIP assays were performed in cells transfected with FLAG-tagged LCoR, as described previously (22, 23), revealing an association of FLAG-LCoR with regions of the CDKN1A and CDH1 promoters bound by KLF6 in CaCo-2 cells (supplemental Fig. S1A, and data not shown). The subsequent availability of an IP-compatible anti-LCoR antibody facilitated ChIP analyses, which confirmed the presence of endogenous LCoR on the same KLF6 binding regions in PC-3 cells (Fig. 2, B and E). In contrast, whereas association of endogenous LCoR with the CDKN1A promoter was readily detected by ChIP in MCF-7 cells, no binding to the CDH1 promoter was observed (Fig. 2E), consistent with deficient KLF6 binding to this promoter (Fig. 2D). This data suggests that the CDH1 proximal promoter region in MCF-7 cells is neither bound by KLF6 nor LCoR. Furthermore, a ChIP for KLF6 followed by re-ChIP for endogenous LCoR or FLAG-LCoR showed that the two proteins are co-recruited to the CDKN1A promoter in PC-3 or CaCo-2 cells (Fig. 2C and supplemental Fig. S1B), and the CDH1 promoter in PC-3 cells (Fig. 2F).
To further verify that LCoR is recruited to the promoter region of the CDKN1A gene by endogenous KLF6, we knocked down its expression (see Fig. 7, below, for controls for knockdowns), and performed a ChIP for LCoR. Results of this assay revealed that recruitment of LCoR to the proximal promoter region of the CDKN1A gene was abolished in KLF6-deficient cells (Fig. 2G), strongly suggesting that KLF6 recruits LCoR to the GC box located near the transcription start site.
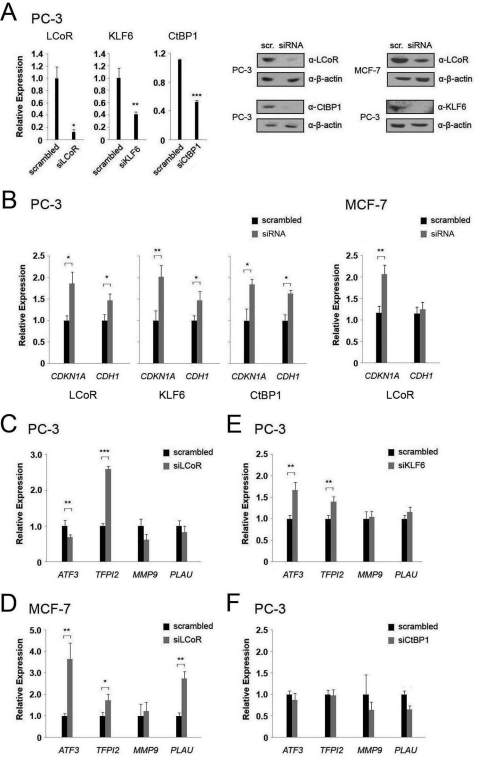
FIGURE 7.
Gene knockdowns confirm regulation of CDKN1A and CDH1 and additional target genes by KLF6 and LCoR. A, left panel, mRNA levels of the indicated genes were measured by qPCR after siRNA-mediated gene knockdown in PC-3 cells. Right panel, Western blots of PC-3/MCF-7 cell extracts were performed for the specified proteins after siRNA-mediated gene knockdowns. B, mRNA levels of the CDKN1A or CDH1 genes were measured by RT-qPCR after siRNA-mediated knockdown of LCoR, KLF6, and CtBP1 in PC-3 cells and LCoR in MCF-7 cells. C and D, mRNA levels of the indicated KLF6 target genes were measured by RT-qPCR after siRNA-mediated LCoR knockdown in PC-3 (C) and MCF-7 (D) cells. E, mRNA levels of the indicated KLF6 target genes were measured by RT-qPCR after siRNA-mediated KLF6 knockdown in PC-3 cells. F, mRNA levels of the indicated KLF6 target genes were measured by RT-qPCR after siRNA-mediated CtBP1 knockdown in PC-3 cells. Two-sample t tests were performed to determine statistical significance in all cases. PLAU, urokinase plasminogen activator.
The potential regulation of CDKN1A and CDH1 expression by LCoR is intriguing, as its cofactor CtBP1 has also been shown to regulate both promoters (25, 32, 33). As CtBP1 augments corepression by LCoR in transient expression experiments (22), we probed CtBP1 binding to KLF6 binding regions of both promoters by ChIP assays of extracts of PC-3 cells. These assays revealed that CtBP1 is present on both promoters (Fig. 2H), suggesting that LCoR may regulate KLF6 transcription of target genes through recruitment of CtBP1.
KLF6 and LCoR Regulate Transcription of CDKN1A or CDH1 Promoter-driven Reporter Gene
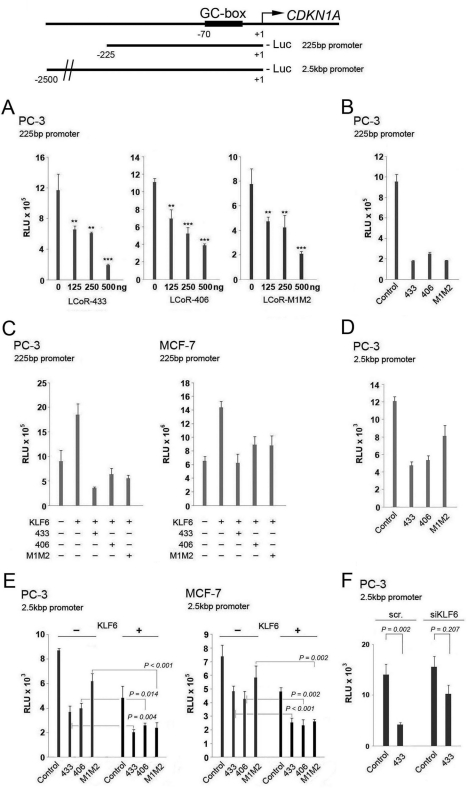
The National Center for Biotechnology Information (NCBI) curated genomic databases (ncbi.nlm.nih.gov/gene/84458) describe the existence of two LCoR isoforms: isoform 1 (LCoR-433) contains 433 amino acids, and isoform 2 (LCoR-406) differs in its 5′ UTR, 3′ UTR, and coding region relative to isoform 1, but maintains the reading frame, which results in a similar protein that lacks 27 C-terminal amino acids. KLF6 mediates transactivation of the CDKN1A promoter (4), and so we performed reporter gene assays with different CDKN1A promoter constructs to determine the effects of the different LCoR isoforms as well as a previously described LCoR deletion mutant that lacks CtBP1 binding sites (LCoR-M1M2) (22). Previous reports found that KLF6 binds to and transactivates the CDKN1A promoter through two GC boxes located between −70 bp and the transcriptional start site (4, 8). We tested two fragments of the proximal promoter, one stretching from −225 nt to +1 (225 bp), and a longer fragment running from −2500 to +1 (2.5 kbp) of the transcription start site.
As shown previously (4, 9), KLF6 transactivates the 225-bp promoter fragment of the CDKN1A promoter in PC-3 cells (supplemental Fig. S2A). However, KLF6 consistently suppressed expression of a reporter gene driven by the 2.5-kbp fragment in both PC-3 and MCF-7 cells (supplemental Fig. S2B). Additionally, as a control to test for function of the 2.5-kbp CDKN1A fragment, we confirmed its transactivation by transiently expressing p53, as previously reported (34) (supplemental Fig. S2C).
Transient expression experiments revealed that all three variants of LCoR were strong repressors of either the 225-bp or 2.5-kbp CDKN1A promoters in PC-3 cells (Fig. 3, A, B, and D). Both LCoR isoforms also corepressed promoter activity in MCF-7 cells, whereas the LCoR-M1M2 mutant was ineffective (supplemental Fig. S2D). When co-transfected along with KLF6 into PC-3 and MCF-7 cells, all three LCoR variants reversed KLF6-mediated transactivation of the 225 bp, and augmented KLF6-mediated repression of the 2.5-kb CDKN1A promoter fragment (Fig. 3, C and E).
FIGURE 3.
KLF6 and LCoR co-regulate the expression of a reporter gene driven by the CDKN1A promoter. Top, schematic representation of the CDKN1A promoter and the promoter recombinants used. A, luciferase reporter gene assays with a short fragment (225 bp) of the CDKN1A promoter were performed in PC-3 cells with different LCoR variants. One-way ANOVAs followed by the Tukey test for multiple comparisons were performed to establish statistical significance on all assays. B, a luciferase reporter gene assay with a short fragment of the CDKN1A promoter was performed in PC-3 cells with 500 ng of the different LCoR variants. C, co-expression luciferase reporter gene assay with a short CDKN1A promoter was performed in PC-3 and MCF-7 cells with 250 ng of WT KLF6 and 500 ng of different LCoR variants. D, a luciferase reporter gene assay with a long fragment (2.5 kbp) of the CDKN1A promoter was performed in PC-3 cells with 500 ng of the different LCoR variants. E, a co-expression luciferase reporter gene assay with a long (2.5 kb) CDKN1A promoter was performed with 250 ng of WT KLF6 and 500 ng of different LCoR variants in PC-3 and MCF-7 cells. F, luciferase reporter gene assays of the CDKN1A promoter (2.5 kbp) were performed in control (scr) and KLF6-deficient PC-3 cells coupled with transient expression of LCoR (500 ng). RLU, relative light units.
Given the discrepancy between the responses of the two different promoter lengths used when transiently expressing KLF6 alone, we performed an additional transient expression of KLF6, followed by qRT-PCR to determine endogenous CDKN1A levels. Results from this experiment revealed that endogenous CDKN1A expression decreases when KLF6 is overexpressed (supplemental Fig. S2E). This suggests that the longer CDKN1A promoter fragment reflects endogenous regulation by KLF6, and that KLF6 and LCoR may act together to suppress expression of the endogenous CDKN1A gene. To ensure that the weaker repression by the M1M2 mutant was not due to differential expression, we performed an additional reporter gene experiment with FLAG-tagged vectors, whose products could be distinguished from endogenous protein. This revealed comparable expression of tagged LCoR-433 and LCoR-M1M2. Moreover, the relative corepression by the tagged −433 and M1M2 variants was essentially identical to that of their untagged counterparts (compare supplemental Fig. S3 and Fig. 3D).
To further substantiate that the repressive effect of LCoR on the CDKN1A promoter was dependent on the presence of endogenous KLF6 we knocked down its expression in PC-3 cells (Fig. 3F) to determine whether corepression was attenuated in deficient cells (see Fig. 7, below, for controls for knockdowns). In a reporter gene assay of the 2.5-kbp CDKN1A promoter, ablation of KLF6 attenuated LCoR-mediated repression, consistent with a functional interaction of LCoR and KLF6 on this promoter. However, the only partial dependence of LCoR corepression on KLF6 expression suggests that LCoR may interact directly or indirectly with other transcription factors bound to other regions of the promoter.
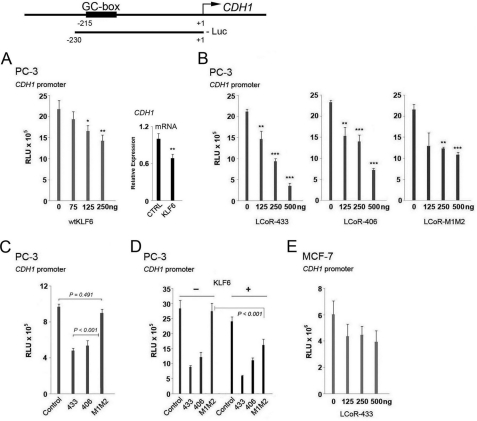
To extend these observations to additional KLF6 target genes, we analyzed luciferase expression driven by a −230 to +1 fragment of the CDH1 promoter containing a responsive GC box (7). Expression of transcription factor AP-2 transactivated the CDH1 promoter fragment, as previously described (35), confirming its functional integrity (supplemental Fig. S4). Whereas KLF6 was a strong dose-dependent inhibitor of the 2.5-kbp CDKN1A promoter (Fig. 3), it modestly inhibited reporter expression driven the CDH1 promoter in PC-3 cells (Fig. 4A). However, prior to proceeding with the LCoR reporter gene assays, we transiently overexpressed KLF6, and performed qRT-PCR to confirm that this construct accurately reflects endogenous CDH1 regulation by KLF6. KLF6 overexpression (see supplemental Fig. S2E for controls for overexpression) attenuated CDH1 mRNA levels confirming that this promoter construct reflects endogenous regulation by KLF6 (Fig. 4A).
FIGURE 4.
Analysis of KLF6 and LCoR regulation of the expression of a reporter gene driven by a CDH1 promoter fragment. Top, schematic representation of the CDH1 promoter and the promoter recombinant used. A, left, luciferase reporter gene assays with a short fragment (230 bp) of the CDH1 promoter were performed in PC-3 cells with wild type KLF6 (wtKLF6). One-way ANOVAs followed by the Tukey test for multiple comparisons were performed to examine statistical significance in all assays. Right, RT-qPCR with CDH1 primers of PC-3 extracts following transfection with a control or KLF6 expression vector. B, luciferase reporter gene assays with a short fragment (230 bp) of the CDH1 promoter were performed in PC-3 cells with different LCoR variants. C, a luciferase reporter gene assay with the same CDH1 promoter was performed in PC-3 cells with 500 ng of the different LCoR variants. D, a luciferase reporter gene assay with a short CDH1 promoter was performed in PC-3 cells cotransfected with 250 ng of WT KLF6 and 500 ng of different LCoR variants. E, a luciferase reporter gene assay with a short fragment of the CDH1 promoter and LCoR-433 was performed in MCF-7 cells. RLU, relative light units.
The LCoR isoform variants (433, 406) suppressed expression of the CDH1 promoter-driven reporter gene differently from the LCoR mutant variant (M1M2) in PC-3 cells (Fig. 4, B and C). In contrast, the LCoR-433 variant did not repress CDH1 expression in MCF-7 cells (Fig. 4E), consistent with the absence of interaction of endogenous LCoR with the promoter as assessed by the ChIP assay (Fig. 2). The weak corepression by LCoR-M1M2 in PC-3 cells is consistent with previous reports of inhibition of CDH1 expression by CtBP1 (25). Co-expression of KLF6 did not significantly enhance suppression of the CDH1 promoter by LCoR isoforms, with the exception of LCoR-M1M2 (Fig. 4D). Taken together, these results suggest that corepression of the CDH1 promoter by LCoR is cell-specific, and that, in PC-3 cells, its function is largely independent of KLF6 expression.
LCoR Corepresses KLF6 Target Genes through CtBP1- and HDAC-dependent Mechanisms
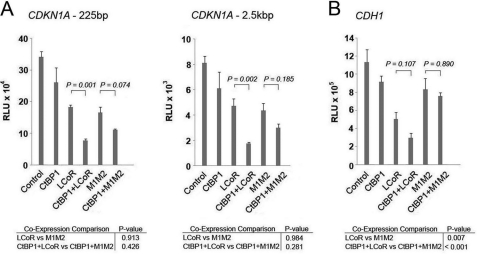
Our ChIP assays showing CtBP1 recruitment to the CDKN1A and CDH1 promoters (Fig. 2), and the attenuated corepression by LCoR-M1M2 mutants (Figs. 3 and 4), are consistent with CtBP1 functioning as an LCoR cofactor on KLF6 target genes. Additionally, CtBP1 moderately suppressed expression of a reporter gene driven by fragments of the CDKN1A and CDH1 promoters (supplemental Fig. S5, A and B). To determine whether the two proteins collaborate in repression of luciferase expression driven by either the 225-bp or 2.5-kb fragments of CDKN1A, we co-expressed LCoR-433 or its M1M2 mutant and CtBP1 (Fig. 5A). Although expression of CtBP1 on its own had little or no effect on reporter gene expression, it clearly augmented the repressive effect of LCoR, an effect that was attenuated but not eliminated in the presence of the LCoR-M1M2 mutant (Fig. 5A). A similar trend was obtained with the CDH1 promoter fragment, although the effect of CtBP1 on LCoR-mediated repression did not reach statistical significance (Fig. 5B). These results suggest that LCoR and CtBP1 collaborate to repress CDKN1A and CDH1 promoter activity. However, the observation that the combined effect of LCoR and CtBP1 on the CDKN1A promoter are only partially attenuated in the presence of the M1M2 mutant, suggests that CtBP1 can interact with other factors on the promoter in addition to LCoR.
FIGURE 5.
LCoR represses a reporter gene driven by the CDKN1A or CDH1 promoters through CtBP1-dependent mechanisms. Co-expression luciferase reporter gene assays with the 225-bp (left) or 2.5-kbp (right) fragment of the CDKN1A promoter was performed in PC-3 cells with 500 ng of CtBP1 and 500 ng of LCoR/M1M2. One-way ANOVAs followed by the Tukey test for multiple comparisons were performed to establish statistical significance in all assays. B, a co-expression luciferase reporter gene assay with a fragment of the CDH1 promoter was performed in PC-3 cells with 500 ng of CtBP1 and 500 ng of LCoR/M1M2. RLU, relative light units.
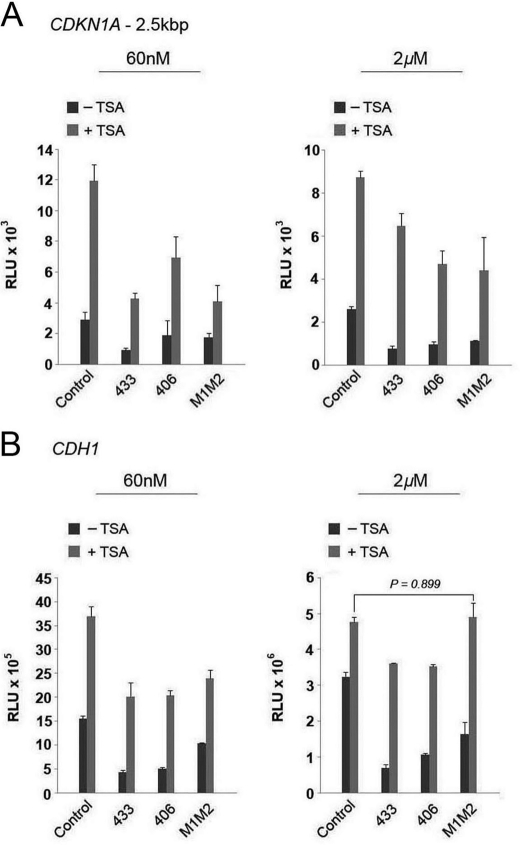
Previous reports have shown that HDAC inhibitors such as TSA increase both CDKN1A and CDH1 expression (36–38). Therefore, to ascertain if LCoR represses CDKN1A or CDH1-promoter-driven reporter expression through an HDAC-mediated mechanism, we treated transiently transfected cells with TSA at two concentrations. We decided to use a low (60 nm) and high dose (2 μm) of TSA as effective TSA concentrations reported vary from one study to the next (21, 25, 36). Both TSA concentrations attenuated LCoR-driven corepression (Fig. 6, A and B), with the effect being more pronounced at the higher concentration. This indicates that HDACs contribute substantially to repression of both promoters.
FIGURE 6.
LCoR represses a reporter gene driven by the CDKN1A or CDH1 promoters through HDAC-dependent mechanisms. A, luciferase reporter gene assays with a long fragment of the CDKN1A promoter were performed in PC-3 cells with 500 ng of the different LCoR variants and the indicated amounts of vehicle, Me2SO (−TSA) or TSA (+TSA). B, luciferase reporter gene assays with a fragment of the CDH1 promoter were performed in PC-3 cells with 500 ng of the different LCoR variants and the indicated amounts of vehicle, Me2SO (−TSA) or TSA (+TSA). One-way ANOVAs followed by the Tukey test for multiple comparisons were performed to establish statistical significance. RLU, relative light units.
LCoR and CtBP1 Repress Endogenous KLF6 Target Gene Expression
To substantiate the results of reporter gene assays, we performed siRNA-mediated knockdown of KLF6, and CtBP1 in PC-3 cells, and LCoR in PC-3 and MCF-7 cells (Fig. 7A) and tested the effects on expression of several KLF6 target genes. Ablation of expression of LCoR, KLF6, or CtBP1 in PC-3 cells increased the expression of CDKN1A and CDH1 between 1.5- and 2-fold (Fig. 7B), substantiating findings above that these proteins are repressors of the CDKN1A and CDH1 promoters. Consistent with the apparent lack of association of LCoR with the CDH1 gene observed in ChIP assays above, and the weak or absent corepression of the promoter in reporter gene assays, ablation of LCoR in MCF-7 cells only increased the expression of CDKN1A, whereas the expression of CDH1 remained stable (Fig. 7B). In addition to CDKN1A and CDH1, KLF6 has been shown to regulate the expression of the growth/survival genes encoding activating transcription factor 3 (ATF3) and tissue factor pathway inhibitor 2 (TFPI2) (39, 40), and genes encoding proteins associated with tumor cell migration matrix metalloproteinase-9 (MMP9) and urokinase plasminogen activator (uPA/PLAU) (41, 42). LCoR ablation increased expression of the TFPI2 gene about 2-fold, whereas it slightly reduced ATF3 levels in PC-3 cells and had no significant effect on MMP9 or uPA expression (Fig. 7C). In MCF-7 cells, TFPI2 expression increased again around 2-fold after LCoR ablation, whereas ATF3 and uPA expression increased around 3-fold (Fig. 7D).
siRNA-mediated knockdown of KLF6 in PC-3 cells also increased mRNA expression of ATF3 and TFPI2, with effects around 1.5-fold (Fig. 7E). This is consistent with KLF6 being a transcriptional repressor of these genes in PC-3 cells. siRNA-mediated knockdown of CtBP1 in PC-3 cells had no significant effect on any of the other genes tested (Fig. 7F).
To further validate the siRNA knockdown study, ablation of LCoR and KLF6 was performed with a second independent siRNA, generating an essentially identical series of results confirming that LCoR is a corepressor of CDKN1A and TFPI2 (supplemental Fig. S6), whereas transcriptional regulation of ATF3 and CDH1 by LCoR is cell-specific. As LCoR ablation only moderately alters ATF3 mRNA expression in PC-3 cells, we performed a ChIP assay to determine whether LCoR was associated with the ATF3 promoter region bound by KLF6 (39), to rule out nonspecific effects. This revealed that LCoR binds a region that contains a KLF6 responsive GC box (supplemental Fig. S7), suggesting that LCoR can regulate ATF3 gene expression through recruitment by KLF6.
DISCUSSION
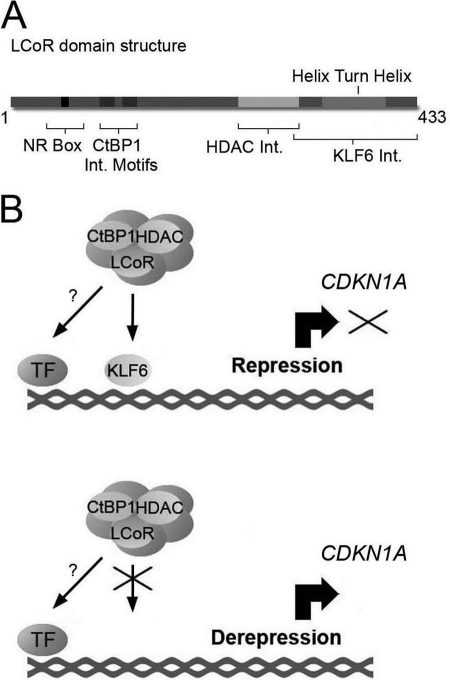
In this study, we identified a novel functional interaction between the transcription factor KLF6 and corepressor LCoR. These results reveal that the corepressor functions of LCoR are not specific to members of the nuclear receptor family of transcription factors. Moreover, we delineated the C-terminal region in LCoR between amino acids 302 and 433 that are required for the interaction with KLF6, which lies at the opposite end of the molecule from the NR box essential for nuclear receptor interactions, underlining the fact that LCoR is a multidomain protein (Fig. 8A). This region contains a helix-turn-helix motif, which previous studies have found to be important for LCoR-mediated corepression (22, 23). ChIP, reporter gene assays, and siRNA-mediated knockdowns revealed that KLF6, LCoR, and CtBP1 interact on the CDKN1A and CDH1 promoters, and corepress expression of CDKN1A, whereas repression of CDH1 is cell-specific. Indeed, CDH1 expression was not affected by LCoR ablation in MCF-7 cells. Moreover, knockdown of KLF6 did not completely abrogate LCoR repression of the long (2.5 kb) CDKN1A promoter suggesting that LCoR can interact either directly or indirectly with other transcription factors on other regions of this promoter.
FIGURE 8.
Model of LCoR-mediated corepression of the CDKN1A promoter. A, schematic representation of the domain structure of LCoR. B, a corepressor complex containing LCoR and associated co-factors is recruited to the promoter of the CDKN1A gene through the action of DNA-binding protein KLF6. TF refers to other potential interactions of the corepressor complex with transcription factors bound to other regions of the promoter.
Our reporter gene experiments supported previous reports of KLF6-mediated induction of CDKN1A expression in assays with a short 225-bp promoter fragment (4, 8, 9). However, we observed inhibition by KLF6 of expression driven by a 2.5-kb fragment of the CDKN1A promoter in two different cell lines, as well as the CDH1 promoter in transiently transfected PC-3 cells, which is consistent with the results of either the siRNA-mediated knockdowns with two independent KLF6 siRNAs that led to elevated expression of endogenous CDKN1A and CDH1, or the overexpression of KLF6, which decreased endogenous mRNA levels of CDKN1A and CDH1 in PC-3 cells. This suggests that the short CDKN1A promoter lacks additional promoter interactions required to reflect endogenous KLF6 gene regulation in these cells.
LCoR-mediated repression of CDKN1A in PC-3 cells was only partially blocked by TSA, and was not reduced significantly by mutation of its CtBP1 interaction sites, suggesting that other recruited cofactors may contribute to repression. For example, LCoR has been shown to be a component of a complex containing LSD1, a histone demethylase (25, 26). LSD1 has been found to repress CDKN1A expression in other cell types (43, 44), and hence could act through KLF6-LCoR recruitment. Other studies have shown that CtBP1 regulates CDKN1A expression via interactions with PARP1 and BRCA1 (33, 45). Additionally, LSD1 has also been shown to function in a complex with CtBP1 (25, 26), suggesting that KLF6 may associate with a large multiprotein transcriptional regulator complex to regulate CDKN1A expression.
Based on these considerations, we propose a novel mechanism for LCoR function in which KLF6, alone or in concert with other transcription factors provides the tethering point on the proximal CDKN1A promoter for a repressive complex containing LCoR, along with specific contributions from CtBP1 and HDACs (Fig. 8B). Inactivation of KLF6 through loss of heterozygozity, somatic mutation, and/or decreased expression in PCa, as described in many studies (for a review, see Ref. 3), would result in deficient recruitment of the corepressor complex containing LCoR on the proximal CDKN1A promoter, and the subsequent derepression and accumulation of CDKN1A (Fig. 8B). This mechanism would bridge the observations that exist between different expression studies that show KLF6 absence/inactivation in PCa (3), yet find p21WAF1/CIP1 overexpression in PCa (16–18). This model of transcriptional repression could also be applied to other genes. For example, ATF3 overexpression has also been found in PCa (46), and therefore a similar mechanism could be at play. Regulation of TFPI2 expression could also occur through this mechanism with promoter-specific contributions by CtBP1.
Given that transcription factors and coregulators can play opposing transcriptional roles in different physiological contexts (26, 28, 29, 47), the opposing results concerning KLF6 or LCoR regulation of target genes in different cell lines is not surprising as the original studies that identified these genes as KLF6 targets were carried out in various cell lines from liver to vascular endothelial cells (40, 41). Furthermore, the exact role of a transcriptional regulator depends on the additional interactions that occur on the promoter, which might differ in different tissues as was found for LSD1 whose role in transcriptional regulation is complex constituent-specific, and cell type-dependent (26). For instance, the KLF6-mediated repression of CDH1 expression, albeit modest, is in apparent contrast to studies in SKOV-3 ovarian cancer cells, in which targeted reduction of KLF6 led to a decrease in CDH1 expression and transient expression of KLF6 stimulated expression of the same CDH1 promoter-reporter recombinant used above (7). This is consistent with KLF6 regulating promoters in a cell-specific manner. Additionally, our studies suggest that KLF6 plays only a secondary role in LCoR-mediated repression of the CDH1 promoter. LCoR may regulate transcription of this gene through the action of other Krüppel-like transcription factors. For example, the Krüppel-like transcription factor ZEB1, a well established repressor of CDH1 (48, 49), has been identified in a complex containing LCoR (26). Therefore, LCoR could potentially regulate transcription of CDH1 through ZEB1.
Considering the cell-specific effects of KLF6 on target gene expression observed here, and the literature that exists concerning differential tissue-specific transcriptional regulation, we cannot rule out the possibility that KLF6 or LCoR may function in either activation or repression of a given gene under different physiological or pathophysiological conditions. Our previous studies in LCoR-replete and -deficient MCF-7 cells have suggested that LCoR may contribute to estrogen-induced expression of some genes (23). Given the identification of a complex containing LCoR and LSD1 (26), this suggests a possible mechanism that would underlie tissue-specific activation/repression transcriptional roles. Remarkably, in addition to a transcriptional role being complex-constituent specific, a study identified a phosphorylation mark on a histone threonine that regulates methylation of different lysine residues by LSD1, thereby preventing LSD1 repression and promoting LSD1-androgen receptor-mediated gene expression in LNCaP cells (50). This suggests that LCoR may participate in both repression and activation with a histone mark-specific transcriptional role. Thus, the transcriptional outcome of the KLF6-LCoR interactions may not always result in repression, but may be dependent on the additional molecular constituents of the complex and the histone marks associated with it.
In conclusion, our results present evidence for a novel functional interaction between the transcription factor KLF6 and the transcriptional regulator LCoR, highlighting a new role in transcriptional regulation for LCoR independent of NRs. These proteins corepress several KLF6 target genes, but notably CDKN1A and CDH1 in a cell type-dependent manner, with promoter-specific contributions from CtBP1 and HDACs.
Supplementary Material
Acknowledgments
We thank Drs. Yaacov Barak and Manuella Bouttier for critical reading of the manuscript.
This work was supported in part by a Canadian Institutes of Health Research Operating grant (to J. H. W.).

This article contains supplemental Tables S1 and Figs. S1–S7.
- PCa
- prostate cancer
- LCoR
- ligand-dependent corepressor
- NR
- nuclear receptor
- CtBP
- carboxyl-terminal-binding protein
- HDAC
- histone deacetylase
- KLF6
- Krüppel-like factor 6
- NS
- nonspecific
- qPCR
- quantitative PCR
- ANOVA
- analysis of variance
- TSA
- trichostatin A
- ATF3
- activating transcription factor 3
- TFPI2
- tissue factor pathway inhibitor 2.
REFERENCES
- 1. Hanahan D., Weinberg R. A. (2011) Hallmarks of cancer. The next generation. Cell 144, 646–674 [DOI] [PubMed] [Google Scholar]
- 2. Shen M. M., Abate-Shen C. (2010) Molecular genetics of prostate cancer. New prospects for old challenges. Genes Dev. 24, 1967–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DiFeo A., Martignetti J. A., Narla G. (2009) The role of KLF6 and its splice variants in cancer therapy. Drug Resist. Updat. 12, 1–7 [DOI] [PubMed] [Google Scholar]
- 4. Narla G., Heath K. E., Reeves H. L., Li D., Giono L. E., Kimmelman A. C., Glucksman M. J., Narla J., Eng F. J., Chan A. M., Ferrari A. C., Martignetti J. A., Friedman S. L. (2001) KLF6, a candidate tumor suppressor gene mutated in prostate cancer. Science 294, 2563–2566 [DOI] [PubMed] [Google Scholar]
- 5. Benzeno S., Narla G., Allina J., Cheng G. Z., Reeves H. L., Banck M. S., Odin J. A., Diehl J. A., Germain D., Friedman S. L. (2004) Cyclin-dependent kinase inhibition by the KLF6 tumor suppressor protein through interaction with cyclin D1. Cancer Res. 64, 3885–3891 [DOI] [PubMed] [Google Scholar]
- 6. Ito G., Uchiyama M., Kondo M., Mori S., Usami N., Maeda O., Kawabe T., Hasegawa Y., Shimokata K., Sekido Y. (2004) Krüppel-like factor 6 is frequently down-regulated and induces apoptosis in non-small cell lung cancer cells. Cancer Res. 64, 3838–3843 [DOI] [PubMed] [Google Scholar]
- 7. DiFeo A., Narla G., Camacho-Vanegas O., Nishio H., Rose S. L., Buller R. E., Friedman S. L., Walsh M. J., Martignetti J. A. (2006) E-cadherin is a novel transcriptional target of the KLF6 tumor suppressor. Oncogene 25, 6026–6031 [DOI] [PubMed] [Google Scholar]
- 8. Narla G., Kremer-Tal S., Matsumoto N., Zhao X., Yao S., Kelley K., Tarocchi M., Friedman S. L. (2007) In vivo regulation of p21 by the Kruppel-like factor 6 tumor-suppressor gene in mouse liver and human hepatocellular carcinoma. Oncogene 26, 4428–4434 [DOI] [PubMed] [Google Scholar]
- 9. Li D., Yea S., Dolios G., Martignetti J. A., Narla G., Wang R., Walsh M. J., Friedman S. L. (2005) Regulation of Kruppel-like factor 6 tumor suppressor activity by acetylation. Cancer Res. 65, 9216–9225 [DOI] [PubMed] [Google Scholar]
- 10. Liu S., Bishop W. R., Liu M. (2003) Differential effects of cell cycle regulatory protein p21(WAF1/Cip1) on apoptosis and sensitivity to cancer chemotherapy. Drug Resist. Updat. 6, 183–195 [DOI] [PubMed] [Google Scholar]
- 11. Yang Z. Y., Perkins N. D., Ohno T., Nabel E. G., Nabel G. J. (1995) The p21 cyclin-dependent kinase inhibitor suppresses tumorigenicity in vivo. Nat Med 1, 1052–1056 [DOI] [PubMed] [Google Scholar]
- 12. Roy S., Singh R. P., Agarwal C., Siriwardana S., Sclafani R., Agarwal R. (2008) Down-regulation of both p21Cip1 and p27Kip1 produces a more aggressive prostate cancer phenotype. Cell Cycle 7, 1828–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sugibayashi R., Kiguchi Y., Shimizu T., Suzuki T., Hamada H., Takeda K. (2002) Up-regulation of p21(WAF1/CIP1) levels leads to growth suppression of prostate cancer cell lines. Anticancer Res. 22, 713–719 [PubMed] [Google Scholar]
- 14. Hall C. L., Zhang H., Baile S., Ljungman M., Kuhstoss S., Keller E. T. (2010) p21CIP-1/WAF-1 induction is required to inhibit prostate cancer growth elicited by deficient expression of the Wnt inhibitor Dickkopf-1. Cancer Res. 70, 9916–9926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu S., Tsai S. Y., Tsai M. J. (1999) Molecular mechanisms of androgen-independent growth of human prostate cancer LNCaP-AI cells. Endocrinology 140, 5054–5059 [DOI] [PubMed] [Google Scholar]
- 16. Baretton G. B., Klenk U., Diebold J., Schmeller N., Löhrs U. (1999) Proliferation- and apoptosis-associated factors in advanced prostatic carcinomas before and after androgen deprivation therapy. Prognostic significance of p21WAF1/CIP1 expression. Br. J. Cancer 80, 546–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aaltomaa S., Lipponen P., Eskelinen M., Ala-Opas M., Kosma V. M. (1999) Prognostic value and expression of p21waf1/cip1 protein in prostate cancer. Prostate 39, 8–15 [DOI] [PubMed] [Google Scholar]
- 18. Fizazi K., Martinez L. A., Sikes C. R., Johnston D. A., Stephens L. C., McDonnell T. J., Logothetis C. J., Trapman J., Pisters L. L., Ordoñez N. G., Troncoso P., Navone N. M. (2002) The association of p21((WAF-1/CIP1)) with progression to androgen-independent prostate cancer. Clin. Cancer Res. 8, 775–781 [PubMed] [Google Scholar]
- 19. Abbas T., Dutta A. (2009) p21 in cancer. Intricate networks and multiple activities. Nat. Rev. Cancer 9, 400–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thiery J. P. (2002) Epithelial-mesenchymal transitions in tumor progression. Nat. Rev. Cancer 2, 442–454 [DOI] [PubMed] [Google Scholar]
- 21. Fernandes I., Bastien Y., Wai T., Nygard K., Lin R., Cormier O., Lee H. S., Eng F., Bertos N. R., Pelletier N., Mader S., Han V. K., Yang X. J., White J. H. (2003) Ligand-dependent nuclear receptor corepressor LCoR functions by histone deacetylase-dependent and -independent mechanisms. Mol. Cell 11, 139–150 [DOI] [PubMed] [Google Scholar]
- 22. Palijan A., Fernandes I., Verway M., Kourelis M., Bastien Y., Tavera-Mendoza L. E., Sacheli A., Bourdeau V., Mader S., White J. H. (2009) Ligand-dependent corepressor LCoR is an attenuator of progesterone-regulated gene expression. J. Biol. Chem. 284, 30275–30287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Palijan A., Fernandes I., Bastien Y., Tang L., Verway M., Kourelis M., Tavera-Mendoza L. E., Li Z., Bourdeau V., Mader S., Yang X. J., White J. H. (2009) Function of histone deacetylase 6 as a cofactor of nuclear receptor coregulator LCoR. J. Biol. Chem. 284, 30264–30274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Asim M., Hafeez B. B., Siddiqui I. A., Gerlach C., Patz M., Mukhtar H., Baniahmad A. (2011) Ligand-dependent corepressor acts as a novel androgen receptor corepressor, inhibits prostate cancer growth, and is functionally inactivated by the Src protein kinase. J. Biol. Chem. 286, 37108–37117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shi Y., Sawada J., Sui G., Affar el B., Whetstine J. R., Lan F., Ogawa H., Luke M. P., Nakatani Y. (2003) Coordinated histone modifications mediated by a CtBP co-repressor complex. Nature 422, 735–738 [DOI] [PubMed] [Google Scholar]
- 26. Wang J., Scully K., Zhu X., Cai L., Zhang J., Prefontaine G. G., Krones A., Ohgi K. A., Zhu P., Garcia-Bassets I., Liu F., Taylor H., Lozach J., Jayes F. L., Korach K. S., Glass C. K., Fu X. D., Rosenfeld M. G. (2007) Opposing LSD1 complexes function in developmental gene activation and repression programmes. Nature 446, 882–887 [DOI] [PubMed] [Google Scholar]
- 27. Kaipparettu B. A., Malik S., Konduri S. D., Liu W., Rokavec M., van der Kuip H., Hoppe R., Hammerich-Hille S., Fritz P., Schroth W., Abele I., Das G. M., Oesterreich S., Brauch H. (2008) Estrogen-mediated down-regulation of CD24 in breast cancer cells. Int J Cancer 123, 66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cavaillès V., Dauvois S., L'Horset F., Lopez G., Hoare S., Kushner P. J., Parker M. G. (1995) Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. EMBO J. 14, 3741–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Treuter E., Albrektsen T., Johansson L., Leers J., Gustafsson J. A. (1998) A regulatory role for RIP140 in nuclear receptor activation. Mol. Endocrinol. 12, 864–881 [DOI] [PubMed] [Google Scholar]
- 30. Lee Y. K., Thomas S. N., Yang A. J., Ann D. K. (2007) Doxorubicin down-regulates Kruppel-associated box domain-associated protein 1 sumoylation that relieves its transcription repression on p21WAF1/CIP1 in breast cancer MCF-7 cells. J. Biol. Chem. 282, 1595–1606 [DOI] [PubMed] [Google Scholar]
- 31. Tavera-Mendoza L. E., Quach T. D., Dabbas B., Hudon J., Liao X., Palijan A., Gleason J. L., White J. H. (2008) Incorporation of histone deacetylase inhibition into the structure of a nuclear receptor agonist. Proc. Natl. Acad. Sci. U.S.A. 105, 8250–8255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Devlin A. M., Bottiglieri T., Domann F. E., Lentz S. R. (2005) Tissue-specific changes in H19 methylation and expression in mice with hyperhomocysteinemia. J. Biol. Chem. 280, 25506–25511 [DOI] [PubMed] [Google Scholar]
- 33. Madison D. L., Lundblad J. R. (2010) C-terminal binding protein and poly(ADP)-ribose polymerase 1 contribute to repression of the p21waf1/cip1 promoter. Oncogene 29, 6027–6039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gartel A. L., Tyner A. L. (1999) Transcriptional regulation of the p21((WAF1/CIP1)) gene. Exp. Cell Res. 246, 280–289 [DOI] [PubMed] [Google Scholar]
- 35. Batsché E., Muchardt C., Behrens J., Hurst H. C., Crémisi C. (1998) RB and c-Myc activate expression of the E-cadherin gene in epithelial cells through interaction with transcription factor AP-2. Mol. Cell. Biol. 18, 3647–3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sowa Y., Orita T., Minamikawa S., Nakano K., Mizuno T., Nomura H., Sakai T. (1997) Histone deacetylase inhibitor activates the WAF1/Cip1 gene promoter through the Sp1 sites. Biochem. Biophys. Res. Commun. 241, 142–150 [DOI] [PubMed] [Google Scholar]
- 37. Richon V. M., Sandhoff T. W., Rifkind R. A., Marks P. A. (2000) Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc. Natl. Acad. Sci. U.S.A. 97, 10014–10019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peinado H., Ballestar E., Esteller M., Cano A. (2004) Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)-HDAC2 complex. Mol. Cell. Biol. 24, 306–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang X., Li X., Guo B. (2008) KLF6 induces apoptosis in prostate cancer cells through up-regulation of ATF3. J. Biol. Chem. 283, 29795–29801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guo H., Lin Y., Zhang H., Liu J., Zhang N., Li Y., Kong D., Tang Q., Ma D. (2007) Tissue factor pathway inhibitor-2 was repressed by CpG hypermethylation through inhibition of KLF6 binding in highly invasive breast cancer cells. BMC Mol. Biol. 8, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Das A., Fernandez-Zapico M. E., Cao S., Yao J., Fiorucci S., Hebbel R. P., Urrutia R., Shah V. H. (2006) Disruption of an SP2/KLF6 repression complex by SHP is required for farnesoid X receptor-induced endothelial cell migration. J. Biol. Chem. 281, 39105–39113 [DOI] [PubMed] [Google Scholar]
- 42. Kojima S., Hayashi S., Shimokado K., Suzuki Y., Shimada J., Crippa M. P., Friedman S. L. (2000) Transcriptional activation of urokinase by the Krüppel-like factor Zf9/COPEB activates latent TGF-β1 in vascular endothelial cells. Blood 95, 1309–1316 [PubMed] [Google Scholar]
- 43. Saramäki A., Diermeier S., Kellner R., Laitinen H., Vaïsänen S., Carlberg C. (2009) Cyclical chromatin looping and transcription factor association on the regulatory regions of the p21 (CDKN1A) gene in response to 1α,25-dihydroxyvitamin D3. J. Biol. Chem. 284, 8073–8082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Foster C. T., Dovey O. M., Lezina L., Luo J. L., Gant T. W., Barlev N., Bradley A., Cowley S. M. (2010) Lysine-specific demethylase 1 regulates the embryonic transcriptome and CoREST stability. Mol. Cell. Biol. 30, 4851–4863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li S., Chen P. L., Subramanian T., Chinnadurai G., Tomlinson G., Osborne C. K., Sharp Z. D., Lee W. H. (1999) Binding of CtIP to the BRCT repeats of BRCA1 involved in the transcription regulation of p21 is disrupted upon DNA damage. J. Biol. Chem. 274, 11334–11338 [DOI] [PubMed] [Google Scholar]
- 46. Pelzer A. E., Bektic J., Haag P., Berger A. P., Pycha A., Schäfer G., Rogatsch H., Horninger W., Bartsch G., Klocker H. (2006) The expression of transcription factor activating transcription factor 3 in the human prostate and its regulation by androgen in prostate cancer. J. Urol. 175, 1517–1522 [DOI] [PubMed] [Google Scholar]
- 47. Huff V. (2011) Wilms' tumors. About tumor suppressor genes, an oncogene and a chameleon gene. Nat. Rev. Cancer 11, 111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grooteclaes M. L., Frisch S. M. (2000) Evidence for a function of CtBP in epithelial gene regulation and anoikis. Oncogene 19, 3823–3828 [DOI] [PubMed] [Google Scholar]
- 49. Graham T. R., Zhau H. E., Odero-Marah V. A., Osunkoya A. O., Kimbro K. S., Tighiouart M., Liu T., Simons J. W., O'Regan R. M. (2008) Insulin-like growth factor-I-dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 68, 2479–2488 [DOI] [PubMed] [Google Scholar]
- 50. Metzger E., Imhof A., Patel D., Kahl P., Hoffmeyer K., Friedrichs N., Müller J. M., Greschik H., Kirfel J., Ji S., Kunowska N., Beisenherz-Huss C., Günther T., Buettner R., Schüle R. (2010) Phosphorylation of histone H3T6 by PKCbeta(I) controls demethylation at histone H3K4. Nature 464, 792–796 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.