Background: Invariant natural killer T (iNKT) cells play a beneficial role during experimental influenza A virus (IAV) infection.
Results: iNKT cells produce IL-22 during infection, and IL-22 prevents the IAV-triggered cell death of pulmonary epithelium.
Conclusion: IL-22 produced by iNKT cells might be important during IAV infection.
Significance: Understanding how iNKT cells function during IAV infection might be instrumental to control IAV-associated pathogenesis.
Keywords: Dendritic Cells, Infectious Diseases, Influenza Virus, Innate Immunity, Interleukin, IL-22, Innate Sensors, Natural Killer T Cells, Dendritic Cells, Influenza A Virus
Abstract
Invariant natural killer T (iNKT) cells are non-conventional lipid-reactive αβ T lymphocytes that play a key role in host responses during viral infections, in particular through the swift production of cytokines. Their beneficial role during experimental influenza A virus (IAV) infection has recently been proposed, although the mechanisms involved remain elusive. Here we show that during in vivo IAV infection, mouse pulmonary iNKT cells produce IFN-γ and IL-22, a Th17-related cytokine critical in mucosal immunity. Although permissive to viral replication, IL-22 production by iNKT cells is not due to IAV infection per se of these cells but is indirectly mediated by IAV-infected dendritic cells (DCs). We show that activation of the viral RNA sensors TLR7 and RIG-I in DCs is important for triggering IL-22 secretion by iNKT cells, whereas the NOD-like receptors NOD2 and NLRP3 are dispensable. Invariant NKT cells respond to IL-1β and IL-23 provided by infected DCs independently of the CD1d molecule to release IL-22. In vitro, IL-22 protects IAV-infected airway epithelial cells against mortality but has no role on viral replication. Finally, during early IAV infection, IL-22 plays a positive role in the control of lung epithelial damages. Overall, IAV infection of DCs activates iNKT cells, providing a rapid source of IL-22 that might be beneficial to preserve the lung epithelium integrity.
Introduction
Invariant natural killer T (iNKT)8 cells represent a unique subpopulation of αβ T lymphocytes expressing markers associated with the NK lineage and recognizing lipid antigens (Ag) (for reviews, see Refs. 1 and 2). These cells express an invariant TCRα chain that pairs with a limited number of Vβ chains. Unlike conventional T lymphocytes that recognize peptide-MHC complexes, the TCR of iNKT cells detects self and foreign (i.e. microbial-derived) (glyco)lipids presented by the nonpolymorphic MHC class I-like protein CD1d expressed by Ag-presenting cells (APCs), including dendritic cells (DCs) (3, 4). Exposure of iNKT cells with the synthetic, non-physiological, glycolipid α-galactosylceramide (5) promptly induces the production of large amounts of both Th1-, Th2-, and Th17-associated cytokines that lead to downstream activation of DCs, NK cells, B cells, and conventional T cells (6, 7). This enables iNKT cells to influence the outcome of developing or ongoing immune reactions with, in general, beneficial effects on infection and cancer (1, 2). Activation of iNKT cells can also occur during stressful conditions, including infection (8, 9). Several in vivo models demonstrated that upon natural activation, iNKT cells are flexible in nature and can either suppress or enhance the outcome of infectious diseases (1, 8, 10, 11). Although the contribution of iNKT cells in different immune responses as regulators has been acknowledged, the exact mechanisms polarizing their effector functions are only poorly understood.
Invariant NKT cells have, in general, a positive role in anti-viral immunity, in particular through their ability to rapidly release IFN-γ (12–18). However, they can also display a detrimental role in virus-induced immunopathogenesis. For instance, using an experimental mouse model of chronic lung disease triggered by infection with Sendai virus, Kim et al. (19) showed that IL-13 production by iNKT cells is important in the pulmonary pathology. More recently, Stout-Delgado et al. (20) demonstrated that IL-17 production by iNKT cells from aged mice infected with herpes simplex virus 2 is sufficient to promote liver damage and death. Finally, we recently reported that iNKT cells produce IFN-γ during dengue virus infection and participate in the cytokine storm and vascular leakage, two major pathological features associated with severe dengue virus infection (21). Thus, the nature of the iNKT cell response is variable according to the virus and possibly the organ targeted. To control iNKT cell functions in the future for therapeutic purposes, it is important to better understand how these cells become activated. It is likely that the recruitment of innate sensors by viruses in sentinel cells, such as DCs, is important for the indirect activation of iNKT cells (8, 9). In this setting, activation of iNKT cells might be triggered by cytokines produced from DCs, by TCR ligation with self lipids, or both (8, 9). In light of recent demonstrations that iNKT cells play a natural role during experimental influenza A virus (IAV) infection (22–24), we herein attempted to study the nature of the iNKT cell cytokine response during IAV infection. Using a mouse-adapted H3N2 IAV strain, we show for the first time that iNKT cells produce IL-22 during infection, a critical cytokine in mucosal immunity. We further present detailed mechanisms by which iNKT cells release IL-22 in the context of IAV infection. Finally, we show that IL-22 protects the pulmonary epithelium against damages during IAV infection.
EXPERIMENTAL PROCEDURES
Mice and Viruses
The IAV strain used in this study (Scotland/20/74, H3N2) was grown in 10-day embryonated hens eggs by standard procedures and titrated on Madin-Darby canine kidney cells as previously described (25). Six- to eight-week-old male wild type (WT) C57BL/6 mice were purchased from Janvier (Le Genest-St-Isle, France). Mice were bred in the SPF facilities of Pasteur Institute, Lille and Transgenose Institute, CNRS, Orleans. Cd1d1−/− C57BL/6 mice were a gift from Dr. L. Van Kaer (Vanderbilt Institute, Nashville, TN) (26). Myeloid differentiation 88−/− (Myd88−/−), Toll-like receptor 7−/− (Tlr7−/−), IFN-β promoter stimulator 1−/− (Ips-1−/−), retinoic-acid-inducible gene-I (Rig-I−/−), nucleotide binding oligomerization domain 2−/− (Nod2−/−), and NOD-like receptor, pyrin domain containing 3−/− (Nlrp3−/−) C57BL/6 mice were from Drs. S. Akira and O. Takeuchi (Osaka University, Osaka, Japan), Dr. R. Flavell (Yale University, New Haven, CT), and the late Dr. J. Tschopp (University of Lausanne, Switzerland), respectively (27–32). Interleukin-22−/− C57BL/6 mice (33) were bred in the Ludwig Institute (Brussels, Belgium). For IAV infection, mice were maintained in a biosafety level 2 facility in the Animal Resource Center at the Pasteur Institute, Lille. All animal work conformed to the Pasteur Institute, Lille animal care and use committee guidelines (agreement no. AF 16/20090 from the Comité d'Ethique en Expérimentation Animale Nord Pas-De-Calais).
Reagents and Abs
Monoclonal Abs against mouse CD5 (APC-conjugated), NK1.1 (PerCP-Cy5.5-conjugated), TCRβ (FITC-conjugated), and isotype controls were purchased from BD Pharmingen. APC-conjugated and phosphatidylethanolamine-conjugated PBS-57-loaded CD1d tetramers were, respectively, obtained from ProImmune (Oxford, UK) and the NIAID (National Institutes of Health) Tetramer Facility (Emory University, Atlanta, GA). The monoclonal Ab against mouse IL-22 (clone 3F11), a kind gift from Dr. W. Ouyang (Genentech, San Francisco, CA), was labeled with the APC conjugation kit from AbD Serotec (Colmar, France). APC-conjugated control mouse IgG2a mAb was from eBioscience. The neutralizing goat IgGs directed against mouse IL1-β, IL-23, TNF-α, and IL-6 were from R&D Systems. Recombinant mouse IL-23 was from Clinisciences, and recombinant mouse IL-1β and IL-22 were from Peprotech (Neuilly-sur-Seine, France).
IAV Infection and Assessment of Gene Expression by Quantitative RT-PCR
Mice were anesthetized and administered intranasally with 50 μl of PBS containing 600 plaque forming units of virus (Scotland/20/74, H3N2) or PBS alone (mock). Total RNA from cell-sorted pulmonary iNKT cells of mock-treated or IAV-infected mice was extracted, and cDNA was synthesized by classical procedures. Quantitative RT-PCR was carried out in an ABI 7500 Thermocycler (Applied Biosystems, Foster City, CA) using 0.5 μm concentrations of specific primers and QuantiTect SYBR Green PCR Master Mix (Qiagen). Primers specific for gapdh (5′-TGCCCAGAACATCATCCCTG-3′) and (5′-TCAGATCCACGACGGACACA-3′), Ifng (5′-GTCTGAATAACTATTTTAACTCAAG-3′) and (5′-GTGGGTTGTTGACCTCAAACTTGGC-3′), Il-4 (5′-TGACGGCACAGAGCTATTGATGG-3′) and (5′-TCCCTTCTCCTGTGACCTCGTT-3′), Il-17A (5′-CCGCAATGAAGACCCTGATAGA-3′) and (5′-AGAATTCATGTGGTGGTCCAGC-3′), Il-17F (5′-TGTCCTCCCCTGGAGGATAAC-3′) and (5′-GAACTG GAGCGGTTCTGGAA-3′), Il-21 (5′-AAACTCAAGCCATCAAACCCTG-3′) and (5′-TGTTTCTTTCCTCCCCTCCTG-3′), and Il-22 (5′-TTCCAGCAGCCATACATCGTC-3′) and (5′-TCGGAACAGTTTCTCCCCG-3′) were designed by the Primer Express Program (Applied Biosystems) and used for amplification in triplicate assays. PCR amplification of gapdh was performed to control for sample loading and to allow normalization between samples. Quantitative comparison was obtained through the ΔΔCT method. For graphical representation, data are expressed as -fold mRNA level increase compared with the expression level in lung iNKT cells from mock-treated mice. Primers specific for IAV M2 gene 5′-AAGACCAATCCTGTCACCTCTGA-3′ and 5′-CAAAGCGTCTACGCTGCAGTCC-3′ and for mouse hprt 5′-CAGGCCAGACTTTGTTGGAT-3′ and 5′-TTGCGCTCATCTTAGGCTTT-3′ were used to quantify viral load in iNKT cells. IAV and hprt copies for each experimental sample were quantified in duplicate using the standard curves obtained by PCR amplification on serial dilutions of purified PCR products. Viral load is expressed as viral RNA normalized to hprt expression level.
Preparation of Pulmonary and Liver iNKT Cells
Pulmonary and hepatic mononuclear cells from mock-treated or IAV-infected mice were prepared by classical procedures. Briefly, lungs were perfused with PBS, excised, and finely minced followed by enzymatic digestion for 20 min at 37 °C in PBS containing 1 mg/ml collagenase type VIII and 1 μg/ml DNase type I (Sigma). After washing, lung and liver homogenates were resuspended in a 35% Percoll™ gradient, carefully layered onto 70% Percoll™, and centrifuged at 1000 × g without brake at 22 °C for 30 min. The layer at the interface between the two Percoll™ concentrations was carefully aspirated and washed in PBS 2% FCS. Red blood cells were removed with lysis buffer (Sigma). To purify iNKT cells, mononuclear cell s were labeled with phosphatidylethanolamine-conjugated PBS-57-loaded CD1d tetramer and FITC-conjugated anti-TCRβ Ab. After cell surface labeling, cells were sorted using a FACSAria (BD Biosciences). PBS57-loaded CD1d tetramer+ TCRβ+ cell purity after sorting was consistently >98%. In some cases iNKT cells were further purified according to the expression, or not, of the NK1.1 molecule using PerCP-Cy5.5-conjugated anti-NK1.1 Ab.
Collection of Bronchoalveolar Lavage Fluids
Bronchoalveolar lavage (BAL) fluids were collected 2 or 4 days post-infection (p.i.) by classical procedures in a 0.5-ml wash. Cytokines were quantified using kits from R&D Systems.
Intracellular FACS Staining
Lung mononuclear cells were cultured at 1 × 107 cells/ml in complete medium containing 10 ng/ml recombinant mouse IL-1β and IL-23 plus 10 μg/ml brefeldin A (Sigma) at 37 °C for 4 h. After activation, cells were washed and stained with LIVE/DEAD® Fixable Dead Cell Stain kit (Invitrogen) in PBS for 30 min. The cells were washed and incubated with appropriate dilutions of eFluor-conjugated CD45, phosphatidylethanolamine-conjugated PBS-57-loaded CD1d tetramer, V450-conjugated anti-TCRβ Ab, and PerCP-Cy5.5-conjugated anti-NK1.1 Ab for 30 min in PBS containing 2% FCS and 0.01% NaN3. Cells were washed and fixed using IC Fixation Buffer (eBioscience, CliniSciences, Montrouge, France). Fixed cells were then permeabilized in permeabilization buffer (eBioscience) according to the manufacturer's instructions. Cells were stained with APC-conjugated mAb against IL-22 or control mouse IgG2a mAb and analyzed on a LSR Fortessa (BD Biosciences).
Generation of Murine Bone Marrow-derived DCs, IAV Infection, and Co-culture
Bone marrow-derived DCs were generated exactly as described in Paget et al. (34). DCs (1 × 105 cells) were challenged with IAV at 1 multiplicity of infection (m.o.i.) for 1 h at 37 °C without FCS. After incubation, cells were extensively washed to remove unbound virus and incubated at 37 °C in medium containing FCS. Cell-free supernatants were harvested after 24 h for cytokine analysis. For co-culture experiments, treated DCs were cultured for 2 days with sorted iNKT cells (105 DCs + 105 iNKT cells/well) in round-bottom 96-well plates in RPMI supplemented with 5% FCS. In some cases neutralizing or control Abs were added during the co-culture. Co-culture supernatants were collected, and IFN-γ, IL-4, IL-17A/F, IL-21, and IL-22 concentrations were measured by ELISA (R&D systems, Abington, UK (IFN-γ, IL-2, IL-21, and IL-22)), eBiosciences, San Diego, CA (IL-17A and IL-17F), and BD Pharmingen (IL-4). Cytokines present in DC supernatants were quantified using commercial ELISA kits distributed by R&D systems (IL-12p40, IL-23, IL-1β, TNF-α), BD Pharmingen (IL-6), and PBL Biomedical Laboratories (Piscataway, NJ) (IFN-β).
Preparation and Infection of Murine Airway Epithelial Cells
Mouse primary airway epithelial cells (AEC) were prepared as follows. Briefly, lungs were digested for 25 min in dissociation buffer containing Pronase (1 mg/ml; Streptomyces griseus protease; Sigma). After macrophage depletion (adherence onto polypropylene plates), cells were resuspended on culture plates precoated with BD Matrigel (BD Biosciences) in airway epithelial cell growth medium (Promocell). After 2 weeks, more than 95% of the cells were cytokeratin positive (Abcam, Paris). Before use, cells were starved overnight in airway epithelial cell basal medium and preincubated with recombinant murine IL-22 (Peprotech) for 4 h. Cells were then infected with IAV at 1 m.o.i. for 1 h. Viral infection was stopped by intensive changes of culture medium and addition of serum. Expression of regenerating protein 3β (Reg3b) mRNA was performed by quantitative RT-PCR using 5′-CGCATTAGTTGCCCCAAGG-3′ and 5′-TCCAGGCCTCTTTTGGCAG-3′. Cell viability was evaluated 24 and 48 h after infection using Resorufin (CellTiter-Blue Reagent, Promega).
IAV Infection and Assessment of Pathology
Four days p.i. (100 plaque forming units), the BAL fluid and the whole lungs were collected from WT and Il-22−/− mice. Analysis of cell number in the BALs was performed as described (24). For histopathologic examination, fixed lung slices (5 μm sections) were subjected to hematoxylin and eosin staining (24). Evaluators who were blinded to genotype scored lung sections (0 (none)–3 (extreme) on the basis of hyperplasia of bronchial epithelium and intercellular cohesion.
Analysis of Virus Load by Plaque Assay
Cell or whole lung supernatants were diluted before being applied to 95% confluent Madin-Darby canine kidney cells. Virus was adsorbed onto the cells for 1 h at room temperature before being washed off four times with serum-free DMEM. Cells were then covered with serum-free DMEM containing trypsin and 0.8% agarose and incubated for 48 h at 37 °C with 5% carbon dioxide. Agarose was then removed, and the monolayer was stained with 1% gentian violet to allow for the visualization and counting of plaques.
Statistical Analysis
Results are expressed as the mean ± S.D. or ± S.E. The statistical significance of differences between experimental groups was calculated by one-way ANOVA with a Bonferroni post-test or an unpaired Student's t test (GraphPad Prism 4 Software, San Diego, CA). The possibility of using these parametric tests was assessed by checking if the population is Gaussian and the variance is equal (Bartlett's test). Results with a p value of less than 0.05 were considered significant. The percentages of inhibition are averages of at least three independent experiments indicated under “Results.”
RESULTS
Pulmonary iNKT Cells Produce IL-22 Early after IAV Infection
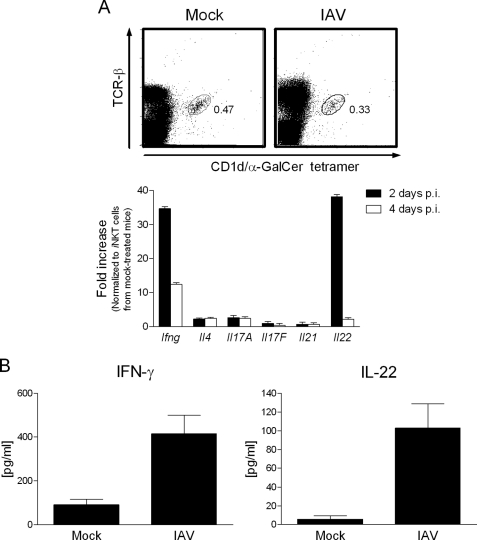
Ourselves and others have provided evidence that (i)NKT cells are important controllers of host pulmonary responses during experimental IAV infection by H1N1 (22, 23, 35) and H3N2 (24) strains in the mouse system. To determine the nature of the cytokines produced by iNKT cells, which may be important for their biological activities, the transcript levels of cytokines expressed by iNKT cells were monitored during H3N2 IAV infection by quantitative RT-PCR. As shown in Fig. 1A and relative to iNKT cells purified from mock-treated animals, iNKT cells from IAV-infected mice expressed a higher level of transcripts for IFN-γ, a cytokine routinely produced by activated iNKT cells (∼35- and ∼12-fold enhancement at days 2 and 4 p.i.). In contrast, IL-4 and IL-17A/F mRNAs were not up-regulated. Of interest was the finding that, relative to control cells, FACS-sorted iNKT cells expressed a higher level of IL-22 messenger (∼38-fold increase at day 2 p.i.), a cytokine produced mostly by terminally differentiated Th17 cells. This induction was transient as no enhanced IL-22 transcript level was detected at day 4 p.i. Notably, the expression level of IL-21 transcript, another member of the IL-17 family (for reviews, see Refs. 36 and 37)) remained near base line. To confirm these findings at the protein level, pulmonary iNKT cells were sorted from IAV-infected mice (60 h p.i.) and cultured without further restimulation. Compared with iNKT cells purified from mock-treated animals, pulmonary iNKT cells from IAV-infected mice produced higher levels of both IFN-γ and IL-22 proteins (Fig. 1B). In contrast, IL-4, IL17A/F, and IL-21 were not detected in the culture supernatant (data not shown). Collectively, upon IAV challenge, iNKT cells selectively produce IFN-γ and more, surprisingly, IL-22, a cytokine known to play a key role in mucosal defense (for reviews, see Refs.38–42). To our knowledge this is the first time that iNKT cells have been shown to produce IL-22 in the context of infection. For the rest of the study we focused on IL-22 production by iNKT cells.
FIGURE 1.
Production of IFN-γ and IL-22 by lung iNKT cells during in vivo IAV infection. A, shown is analysis of cytokine mRNA levels in sorted iNKT cells during IAV infection. Upper panel, iNKT cells were gated on lymphocytes expressing TCR-β and positive for the PBS57-loaded CD1d tetramer (shown are mock-treated and IAV-infected mice, 2 days p.i.). Despite a slight and not significant decrease in iNKT cell frequency, the number of detectable iNKT cells in the lung tissue remained stable 2 and 4 days p.i. Lower panel, invariant NKT cells were purified from the lungs of mock-treated and IAV-infected mice days 2 and 4 p.i. Sorted iNKT cells were analyzed for cytokine mRNA levels (lower panel). RNAs were prepared and IFN-γ (Ifng), IL-4 (Il4), IL-17A (Il17A), IL-17F (Il17F), IL-21 (Il21), and IL-22 (Il22) mRNA copy numbers were measured by quantitative RT-PCR. Data are normalized to expression of Gapdh and are expressed as -fold increase over average gene expression in iNKT cells isolated from mock-treated mice. Genes varying at least 2-fold were considered as significantly modulated. B, production of IFN-γ and IL-22 by iNKT cells sorted from IAV-infected mice is shown. Lung iNKT (PBS57-loaded CD1d tetramer+ TCRβ+) cells were purified from mock-treated or IAV-infected mice (60 h p.i.) and cultured for 2 days without restimulation (1 × 105 cells/well). Cytokines present in the supernatant were quantified by ELISA. A and B, data represent the mean ± S.D. (triplicates) of an experiment of two performed (pool of 10 mice/group).
IAV Entry into iNKT Cells Does Not lead to IL-22 Production
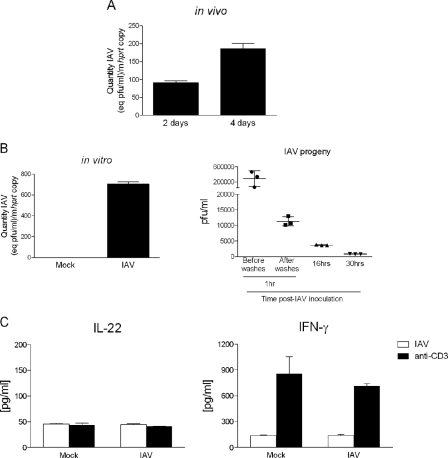
We next explored the mechanisms underlying the activation of iNKT cells in response to IAV. Invariant NKT cells have recently been shown to be targeted by herpes simplex type 2 virus to produce IL-17 (20). We thus hypothesized that IAV entry in iNKT cells could also result in cytokine release. Before addressing this issue in vitro, we first investigated whether iNKT cells are permissive to IAV. To this end, viral RNA (M2 transcript) was quantified in pulmonary iNKT cells purified from IAV-infected mice. As revealed in Fig. 2A, IAV M2 transcript was detected in iNKT cells 2 and 4 days p.i. Similarly, the transcript of the IAV M2 gene was detected in iNKT cells previously exposed to IAV in vitro (Fig. 2B, left panel). Thus, iNKT cells are susceptible to IAV infection. However, analysis of infectious progeny present in the culture supernatant by plaque assay indicated that IAV does not replicate productively in iNKT cells (Fig. 2B, right panel). Having established viral entry into iNKT cells, we next proceeded to test our hypothesis. For this, iNKT cells were exposed to IAV, and after 24 h, cytokines present in the supernatant were quantified by ELISA. As shown in Fig. 2C, IAV-infected iNKT cells did not produce IL-22 or IFN-γ. Of note, anti-CD3 re-stimulation, used here as a positive control, led to IFN-γ, but not IL-22, release by iNKT cells. To conclude, although permissive to IAV, iNKT cells are not directly activated by IAV to produce IL-22.
FIGURE 2.
Analysis of iNKT cell activation in response to IAV infection. A, shown is analysis of IAV mRNA in sorted iNKT cells during IAV infection (panel A) or in iNKT cells exposed in vitro with IAV (m.o.i. = 1) (panel B, left panel). M2 IAV mRNA levels were measured by quantitative RT-PCR. Data are normalized to expression of hprt. Shown are IAV M2/hprt mRNA expression ratios from iNKT (PBS57-loaded CD1d tetramer+ TCRβ+) cells sorted from the lungs of infected mice at days 2 and 4 p.i. (panel A) and from iNKT cells sorted from the liver of naive animals and exposed for 24 h with IAV (panel B, left panel). B, right panel, the viral titer in iNKT cell supernatants was determined by plaque assay at different time points after IAV exposure. pfu, plaque-forming units. C, purified iNKT cells were exposed to IAV (m.o.i. = 1), and 48 h later, cytokines present in the supernatant were quantified by ELISA. A–C, data represent the mean ± S.D. (triplicates) of an experiment of two (panel A) or three (panels B and C) performed.
IAV-infected DCs Activate NK1.1neg iNKT Cells to Produce IL-22
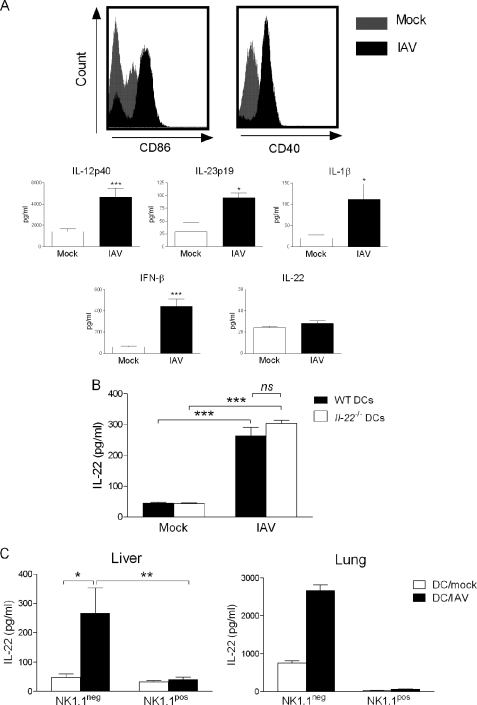
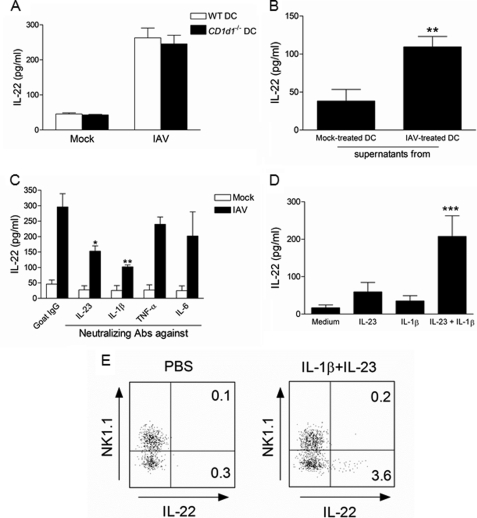
Next, we wanted to determine whether iNKT cells could be indirectly activated by IAV-infected DCs, a cell population known to stimulate iNKT cells during stressful conditions (for reviews, see Refs. 1, 8, and 9). As depicted in Fig. 3A, mouse bone marrow-derived DCs exposed to IAV (m.o.i. = 1) matured in terms of co-stimulatory molecule (CD40, CD86) expression (upper panel) and cytokine (IL-12p40, IL-23, IL-1β, TNF-α, IL-6, and IFN-β) production (lower panel and not shown). On the other hand, IAV-infected DCs failed to release IL-22 (Fig. 3A).
FIGURE 3.
Analysis of iNKT activation in response to IAV-infected DCs. A, effects of IAV infection on the surface expression of co-stimulatory molecules and on the release of cytokines on/by DCs are shown. Upper panel, bone marrow-derived DCs (CD11c+ MHC class+, ∼97% pure) were exposed or not to IAV (m.o.i. = 1) for 16 h. Afterward DCs were stained with the anti-CD86 or CD40 Abs and analyzed by flow cytometry. Lower panel, 24 h after stimulation, cytokine concentrations in culture supernatants were determined by ELISA. Data represent the mean ± S.E. of at least five independent experiments performed in triplicates. B, shown is the effect of IAV-infected DCs on cytokine release by iNKT cells. WT or Il-22−/− DCs were exposed or not to IAV (m.o.i. = 1) for 16 h and after extensive washing were co-cultured with sorted liver PBS57-loaded CD1d tetramer+ TCRβ+ cells. n.s., not significant. C, the same experiment was performed, but in this case, liver (left panel) or lung (right panel) iNKT cells were discriminated on the basis of NK1.1 expression. B and C, left panel, cytokine release was quantified 48 h later. Data represent the mean ± S.E. of at least three independent experiments performed in triplicate. A one-way ANOVA has been used to analyze the variance followed by a Bonferroni multiple comparison test to compare all groups. *, p < 0.05; **, p < 0.01; ***, p < 0.001. C, right panel, one representative experiment of two is shown (mean ± S.D., duplicate).
We next investigated whether DC maturation in response to IAV could lead to IL-22 release by iNKT cells. For this, iNKT cells were purified from the liver of naïve mice to obtain sufficient numbers of cells for in vitro studies. As seen in Fig. 3B, co-culture of IAV-infected DCs with iNKT cells resulted in IL-22 secretion. Dendritic cells have been shown to secrete IL-22 under certain circumstances (43, 44), but the use of DCs derived from Il-22−/− mice indicated that iNKT cells are the source of IL-22 in our setting. Invariant NKT cells can be subdivided into two main subsets based on the expression of NK1.1; the NK1.1pos subset being described to preferentially produce IFN-γ and IL-4 and the NK1.1neg subset, more prone to IL-17 release (45–49). As depicted in Fig. 3C, left panel, NK1.1neg iNKT cells produced IL-22 in response to IAV-infected DCs, whereas NK1.1pos iNKT cells failed to do so. Similar data were obtained when iNKT cells were sorted from the lung tissue (Fig. 3C, right panel). Collectively, these data show that, in response to IAV, DCs activate NK1.1neg iNKT cells to produce IL-22 in vitro.
TLR7/MyD88 and RIG-I/IPS-1 Signaling in IAV-infected DCs Are Important to Activate IL-22 Release by iNKT Cells
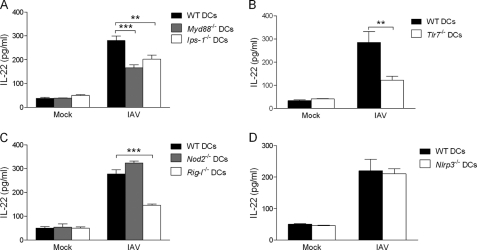
During infection, the activation of certain TLR members in DCs plays a role in the indirect activation of iNKT cells (34, 50–54). The potential role of other innate sensors has not yet been investigated. Influenza viral infection is recognized by distinct cellular sensors expressed in the endosome (particularly TLR7) or the cytosol (55–64). For the latter, the RNA helicase RIG-I and the NOD-like receptors (NLR) NOD2 and NLRP3 (also known as Nalp3) play pivotal functions in DC maturation in response to IAV. Recruitment of TLR7, RIG-I, and NOD2 triggers type I IFN and proinflammatory cytokine production through the adaptor proteins MyD88 (for TLR7) and IPS-1 (for RIG-I and NOD2) (59, 62). To study the respective role of TLR7 and NOD2/RIG-I signaling in the indirect activation of iNKT cells, IAV-infected Myd88−/− and Ips-1−/− DCs were co-cultured with sorted iNKT cells. Interestingly, Myd88 and Ips-1 deficiencies reduced by ∼50 and 40%, respectively, the release of IL-22 by iNKT cells (Fig. 4A). Relative to WT cells, Tlr7−/− DCs induced less IL-22 release by iNKT cells (∼65% reduction) (Fig. 4B). The impact of IPS-1 deficiency seen in Fig. 4A suggested a function for RIG-I and/or NOD2. As depicted in Fig. 4C, Nod2 deficiency in DCs had no effect on IL-22 production by iNKT cells, whereas Rig-I deficiency decreased it by ∼60%. As mentioned above, the NLRP3 inflammasome was shown to signal DCs upon IAV infection (55, 58, 63). However, Nlrp3 deficiency in DCs had no effect on IL-22 release by iNKT cells (Fig. 4D). In combination, these data indicate that, among innate sensors recruited by IAV in DCs, the RNA sensors TLR7 and RIG-I strongly contribute to the production of IL-22 by iNKT cells.
FIGURE 4.
Role of innate sensors in IL-22 release by iNKT cells. WT, Myd88−/− and Ips-1−/− (panel A), WT and Tlr7−/− (panel B), WT, Nod2−/−, and Rig-I−/− (panel C), or WT and Nlrp3−/− (panel D) DCs were exposed or not (mock) to IAV (m.o.i. = 1) for 16 h, extensively washed, and co-cultured for 48 h with purified liver iNKT cells. Cytokine production was quantified by ELISA. Data represent the mean ± S.E. of at least three independent experiments performed in triplicate. A–C, a one-way ANOVA has been used to analyze the variance followed by a Bonferroni multiple comparison test to compare all groups. **, p < 0.01; ***, p < 0.001. B–D, differences in mean were analyzed using the two-tailed Student's t tests. **, p < 0.01.
IAV-infected DCs Induce IL-22 Release by iNKT Cells via IL-23 and IL-1β
We next studied the mechanisms by which iNKT cells release IL-22 in response to IAV-infected DCs. In this setting inflammatory cytokines, in concert or not with CD1d-restricted lipids expressed by DCs, may be involved (8, 9). We first investigated whether IL-22 production was dependent on the CD1d molecule expressed by DCs. As shown in Fig. 5A, CD1d deficiency in DCs did not affect IL-22 production by iNKT cells. On the other hand, supernatant from IAV-infected DCs induced IL-22 release by iNKT cells (Fig. 5B). The potential role of DC-derived cytokines was next studied, with a special focus on IL-1β, TNF-α, IL-6, and IL-23, which are known to play a part in IL-22 synthesis by T cells (65). As revealed in Fig. 5C, neutralizing Abs against IL-1β and IL-23 reduced the synthesis of IL-22 by iNKT cells (by ∼65 and 55%, respectively), whereas the other tested Abs were without effect. Thus, IL-1β and IL-23 secretion by IAV-infected DCs is necessary and sufficient to activate the release of IL-22 by iNKT cells. To further confirm this, purified iNKT cells were treated with IL-1β and/or IL-23 in a DC-free culture system. As shown in Fig. 5D, whereas IL-1β or IL-23 individually failed to promote IL-22 synthesis, their combined addition induced IL-22 production by liver iNKT cells. Importantly, IL-22 production in response to IL-1β/IL-23 was not restricted to liver iNKT cells as pulmonary iNKT cells also produced it, as analyzed by intracellular FACS staining (Fig. 5E). Of note, only the NK1.1neg iNKT subset produced IL-22.
FIGURE 5.
Mechanisms involved in IAV-induced IL-22 release by NK1.1neg iNKT cells. A, IAV-infected WT or CD1d1−/− DCs were cultured with sorted liver iNKT cells. Of note, in response to the canonical iNKT cell agonist α-galactosylceramide, CD1d deficiency in DC fully abrogated IFN-γ production by iNKT cells (data not shown). B, after 16 h, undiluted supernatant from mock-treated or IAV-infected DCs was added to iNKT cells. C, IAV-infected DCs were cultured with iNKT cells in the presence of neutralizing anti-IL-23, anti-IL-1β, anti-TNF-α, anti-IL-6, or isotype control mAbs (5 μg/ml). D, purified iNKT cells were incubated with recombinant IL-23 and/or IL1-β protein(s) (1 ng/ml). Of note, TNF-α and IL-6 were without effect on IL-22 release (data not shown). A–D, two days later, IL-22 production was measured by ELISA. Data represent the mean ± S.D. of at least three independent experiments performed in triplicate. B, differences in mean were analyzed using the two-tailed Student's t test. **, p < 0.01. C and D, a one-way ANOVA was used to analyze the variance followed by a Bonferroni multiple comparison test to compare all groups. *, p < 0.05; **, p < 0.01; ***, p < 0.001. E, shown is intracellular staining of IL-22 in lung iNKT cells. Lung mononuclear cells treated with IL-1β and IL-23 (10 ng/ml) for 4 h in the presence of brefeldin A and gated iNKT cells expressing or not the NK1.1 marker were analyzed for intracellular IL-22 production. Gates were set based on the isotype control. The percentage of iNKT cells expressing IL-22 is shown. One representative experiment of two is depicted.
IL-22 Protects Lung Epithelial Cells against Death after in Vitro IAV Infection
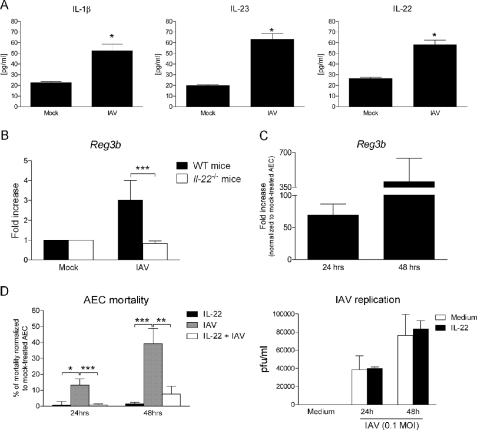
We next asked whether IL-1β and IL-23, which are able to promote IL-22 synthesis by iNKT cells in vitro, are produced in the lungs during IAV infection. As seen in Fig. 6A, IL-1β, IL-23, and IL-22 proteins were detected in the lungs of infected mice 2 days p.i. Moreover, the transcript level of REG3β, a known target of IL-22 (44), was enhanced during infection (Fig. 6B). This induction was IL-22-dependent as the level of REG3β mRNA remained at the base-line level in IAV-infected Il-22−/− mice. Thus, IL-22 is functional in the lungs of IAV-infected mice.
FIGURE 6.
Effect of IL-22 on the mortality of IAV-infected airway epithelial cells. A, BAL fluids from mock-treated or IAV-infected (2 days p.i.) mice were harvested, and IL1-β, IL-23, and IL-22 concentrations were quantified by ELISA. Data represent the mean ± S.E. of a representative experiment (4 mice/group) of four performed. Differences in mean were analyzed using the two-tailed Student's t test. *, p < 0.05. B, RNAs from whole lungs recovered from mock-treated or IAV-infected (4 days p.i.) mice were harvested, and REG3β (Reg3b) mRNA copy numbers were measured by quantitative RT-PCR. Data are normalized to expression of Gapdh and are expressed as -fold increase over average gene expression in lung tissue from mock-treated mice. Data represent the mean ± S.D. of a representative experiment (four mice/group) of two performed. A one-way ANOVA was used to analyze the variance followed by a Bonferroni multiple comparison test to compare all groups. ***, p < 0.001. C, AEC were cultured for 16 h with recombinant IL-22 (100 ng/ml), and cells were then analyzed for REG3β mRNA level. Data are normalized to expression of Gapdh and are expressed as -fold increase over average gene expression in untreated AEC. Data represent the mean ± S.D. of three independent experiments (triplicate). D, AEC, treated or not with IL-22, were exposed or not to IAV (m.o.i. = 1), and 24 and 48 h later, cell mortality was quantified using Resorufin (left panel). The viral titer in AEC supernatants was determined by plaque assay (right panel). Data represent the mean ± S.D. of four independent experiments (left panel) or represent an experiment of four performed (right panel). A one-way ANOVA was used to analyze the variance followed by a Bonferroni multiple comparison test to compare all groups. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
During infection, IAV initially targets the lung epithelium to replicate, a process leading to epithelial cell death and pathology. Although IL-22 was described to protect epithelial cells in response to a variety of environmental insults (65, 66), its potential role in the context of virus infection has not yet been evaluated so far. Before addressing this question, we first investigated the effect of IL-22 on primary AECs. As seen in Fig. 6C, IL-22 induced an increased transcript level of REG3β, which is known to play a role in epithelium fitness and survival (67). Having validated the biological activity of IL-22 on AEC, we next studied its potential role in the control of cell mortality triggered by IAV infection/replication. As represented in Fig. 6D, left panel, IAV induced cell mortality 24 h and, particularly, 48 h p.i. Strikingly, IL-22 strongly prevented the IAV-triggered cell death. In contrast, although IL-22 shares a structural similarity with the antiviral cytokine IFN-λ (68), it failed to reduce IAV replication as assessed by plaque assay (Fig. 6D, right panel). Thus, IL-22 may play a protective role on epithelial cells during IAV infection.
IL-22 Deficiency Leads to Morphological Alterations of Airway Epithelium during IAV Infection
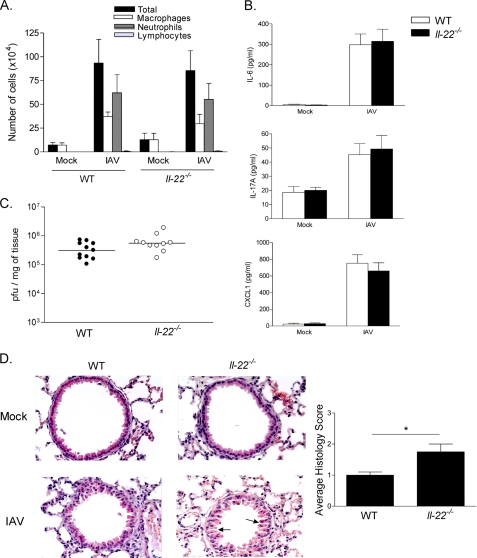
We then addressed the in vivo role of IL-22 during IAV infection, with a special focus on airway epithelium. The lack of IL-22 did not significantly modulate the recruitment of neutrophils and mononuclear cells (monocytes/macrophages and lymphocytes) in the BAL fluids 4 days p.i. (Fig. 7A). The IAV-induced production of inflammatory cytokines and chemokines, including IL-6, IL-17A, and CXCL1, was not modulated in the absence of IL-22 (Fig. 7B). Moreover, IL-22 deficiency had no effect on viral replication in the lung tissue (Fig. 7C). Interestingly, the lack of IL-22 led to a more marked morphological alteration of the airway epithelium relative to controls (Fig. 7D). Indeed, compared with WT animals, IAV-infected Il-22−/− mice developed an enhanced bronchial hyperplasia, a consequence of epithelial cell damages. This effect was associated with an augmented loss of the intercellular cohesion of the epithelium. Together, this suggests that IL-22 plays a part in the control of epithelial damages caused by IAV infection.
FIGURE 7.
Effect of IL-22 deficiency on IAV-associated epithelial damage in vivo. Age-matched WT or Il-22−/− mice were infected with 100 plaque-forming units of IAV Scotland/20/74/H3N2 strain and then sacrificed 4 days p.i. A, total cells, neutrophils, macrophages, and lymphocytes in the BALs were counted. B, IL-6, IL-17A, and CXCL1 concentrations in BAL fluids were quantified by ELISA. C, the viral loads, expressed as plaque forming units (pfu)/mg of lung tissue, were determined by plaque assay. D, representative hematoxylin and eosin-stained tissue sections (magnification × 400) are shown. Sections were scored blindly for levels of epithelial hyperplasia and loss of intercellular cohesion (arrows). Results are representative of three repeated experiments. Data represent the mean ± S.D. (n = 5–11 mice/group). Significant differences are designated by an asterisk (*, p < 0.05 (two-tailed Student's t test).
DISCUSSION
In some, but not all, experimental models, iNKT cells play a key role in antiviral immunity and associated immunopathogenesis (for reviews, see Refs. 11, 69, and 70). However, the mechanisms by which these cells become activated during viral infection as well as their precise functions have not been fully elucidated. Our data show for the first time that iNKT cells produce IL-22 in vivo during infection. We also present detailed mechanisms by which these cells release IL-22 in the context of IAV infection, and we propose that the innate production of IL-22 might protect the pulmonary epithelium against damage caused by viral replication.
The paradoxical role of iNKT cells during viral infection can be attributed to the nature of the cytokines they produce. For instance, production of IFN-γ by iNKT cells enhances the innate and Th1-dependent immune responses of NK cells and CD8+ T cells, ultimately leading to the elimination of virus-infected cells (14, 17, 18). On the other hand, iNKT cells can play a detrimental role in specific models of virus-induced immunopathogenesis by producing IL-17A or IL-13 (19, 20). Others (22, 23) (H1N1) and ourselves (24) (H3N2) have shown that iNKT cells play a positive role in the control of the IAV-associated pulmonary inflammation. To better understand how iNKT cells function in this system, we first monitored the nature of the cytokines they produce during the early course of IAV infection. Our data show that, relative to controls, iNKT cells from IAV-infected mice produce higher levels of transcripts for IFN-γ (but not IL-4) and IL-22. On the other hand, mRNA levels of genes associated with the Th17 lineage, such as IL-17A/F and IL-21, were unchanged. Importantly, the preferential synthesis of IFN-γ and IL-22 by iNKT cells in the context of IAV infection was confirmed at the protein level by ELISA.
Interleukin-22 is intensively studied at the moment due to its role in mucosal host defense and immunopathology, and it is of importance to better characterize IL-22-producing cell types as well as mechanisms involved in IL-22 expression. We thus attempted to identify the mechanisms by which iNKT cells release IL-22 in the context of IAV infection. Some viruses, including HIV, lymphocytic choriomeningitis virus, or herpes simplex viruses can selectively target iNKT cells (20, 71, 72). We first investigated whether iNKT cells could be directly activated by IAV to release IL-22. Our data show that H3N2 IAV can infect iNKT cells in vivo and in vitro. Of note, iNKT cells appear to support viral replication in vitro but, in clear contrast to epithelial cells, in a non-productive manner as revealed by plaque assay. In parallel, iNKT cells do not produce IL-22 in response to IAV, indicating that the mechanism of activation is indirect and probably requires APCs, such as DCs. Myeloid DCs can be infected by IAV in vitro and in vivo (73–75) and are particularly well equipped to directly activate iNKT cells. Our data show that IAV-infected DCs can promote IL-22 production by iNKT cells. Thus, our co-culture system mimics the in vivo situation, and we took advantage of this to identify the mechanisms implicated in IL-22 production by iNKT cells. Our data clearly show that the NK1.1neg subset, but not the NK1.1pos subset, produced IL-22 in response to IAV-exposed DCs.
During infection, the activation of innate sensors in DCs is important to activate iNKT cells (34, 50–53). Recognition of IAV by the host is elicited by various classes of pattern-recognition receptors including members of the TLR (TLR7, TLR3), NLR (NOD2 and NLRP3) and RIG-I-like receptors families (55–64, 76). So far, with the exception of TLR members, no studies have been devoted to investigate the role of cellular sensors expressed by DCs in iNKT cell activation. Our data show that TLR7/MyD88-dependent signaling pathways in DCs are important to promote IL-22 release by iNKT cells, whereas those from TLR3/TRIF are not (data not shown). In parallel, our data indicated a role for IPS-1/RIG-I signaling in IL-22 secretion by iNKT cells. On the other hand, the NLR NOD2 and NLRP3 are dispensable. This latter finding appeared surprising as stimulation of the NLRP3-dependent inflammasome activates caspase 1 to generate mature IL-1β, a cytokine involved in IL-22 production by iNKT cells (Fig. 6). However, the recent demonstration that RIG-I also triggers caspase-1-dependent inflammasome activation and, thus, IL-1β secretion by a mechanism independent of NLRP3 (77) likely explains our results. Together, our data strongly suggest that, in the context of IAV infection, recognition of virus genomic RNA in the endosome by TLR7 as well as recognition of the 5′ triphosphate end of viral RNA in the cytosol by RIG-I is critical to trigger, via DCs, IL-22 release by iNKT cells.
Stressed DCs have been shown to produce and present self lipid(s) to activate iNKT cells with the need for co-factors including IL-12, IL-18, and/or type I IFNs (34, 50, 52–54, 78, 79). Thus, we investigated whether the CD1d molecule is necessary to activate iNKT cells or whether cytokines released by IAV-infected DCs are sufficient to do so. As is the case after murine cytomegalovirus infection (54), our data indicate that CD1d expressed by DCs is dispensable for the activation of iNKT cells. We next hypothesized that activating cytokines produced by IAV-infected DCs may compensate the lack of CD1d function. Using neutralizing Abs, we showed that IL-1β and IL-23 are necessary to induce IL-22 synthesis by iNKT cells. Furthermore, using recombinant cytokines (DC-free system), we showed that the combination of IL-1β and IL-23 induced IL-22 release by iNKT cells. Thus, along with the inflammatory cytokines IL-12 and IL18 (IFN-γ) (52, 54, 79), the combination of IL-1β and IL-23 is sufficient to promote cytokine (IL-22) release by iNKT cells in the absence of a CD1d-restricted agonist. Although the potential role of self lipids presented by iNKT cells themselves cannot be completely ruled out (47, 54), our data suggest that cytokine-, rather than TCR-driven signals are crucial in iNKT cell activation (IL-22 production) during IAV challenge, at least in vitro.
Along with other lymphoid cell types, including NKp46+ NK1.1− mucosal cells (also termed NCR22), mucosa-associated lymphoid tissue inducer cells, conventional NK cells (for reviews, see Refs. 40, 80, and 81), and certain subsets of γδ T lymphocytes (82–84), the present study shows that NK1.1neg iNKT cells also have the potential to produce IL-22. Our finding is in line with that of Doisne and colleagues (47), who recently showed that peripheral lymph node-resident NK1.1neg iNKT cells produce IL-22 in response to stimuli that mimic (bacterial) infection. It is noteworthy that in this setting the transcription factor retinoic acid receptor-related orphan receptor-γt plays a crucial part in IL-22 synthesis (47). Our unpublished results indicate that 40–50% of pulmonary NK1.1neg iNKT cells express receptor-related orphan receptor-γt (15–20% of the total iNKT cell pool) and that this subset is the only producer of IL-22 upon IL-1β and IL-23 stimulation. Attempts are now in progress to determine the precise function of this iNKT cell subset in the context of IAV infection. In parallel, one can wonder whether the early production of IL-22 by iNKT cells is physiologically important in vivo. A recent study has provided the first evidence that this could be the case. Indeed, in response to ConA stimulation, hepatic iNKT cells lacking the herpesvirus entry mediator receptor produced IL-22 to protect against hepatitis (85). IL-22 has both proinflammatory and tissue-protective properties depending on the context in which it is expressed (38). In the lung tissue the beneficial role of IL-22 has recently been demonstrated in experimental models of lung fibrosis induced by repeated exposure to Bacillus subtilis (83), of pneumonia triggered by Klebsiella pneumoniae (86), and of ventilator-induced lung injury (87). On the other hand, IL-22 is deleterious in a model of acute lung inflammation induced by bleomycin (66). The role of IL-22 during (pulmonary) viral infection is still elusive. Interestingly, a recent study demonstrated that during IAV (H1N1) infection, NK also produce IL-22 in the lungs (88). Whether or not the early production of IL-22 by iNKT cells and/or by other cell types is important during IAV infection awaits further studies. Keeping in mind that direct or indirect interactions between iNKT cells and epithelial cells are viewed as important in many physiopathological situations, including infection (for reviews, see Refs. 89 and 90), it is possible that iNKT cell-derived IL-22 has a particular function during IAV infection. Our study shows that IL-22 is biologically active in the lung tissue during infection and that it can protect IAV-infected epithelial cells, the primary target of IAV, against mortality in vitro. Moreover, our finding indicates that IL-22 plays a positive role in the control of epithelial damage early after IAV infection. On the other hand, IL-22 lacks direct antiviral activity against IAV both in vitro and in vivo, as recently shown for hepatitis B and C viruses (91, 92). These data are in contrast with those from Guo and Topham (88), who suggested that IL-22 rather favors viral replication by sustaining epithelial cells. The protective role of IL-22 on epithelial cells is in line with our and other recent observations showing an enhanced damage of the airway epithelium in mice lacking iNKT cells (23, 24). It is possible that the cytoprotective effect of IL-22 is mediated through induction of anti-apoptotic molecules or REG3β (66). Thus, although this remains to be demonstrated in vivo, iNKT cell-derived IL-22 may be important to sustain the respiratory epithelium and preserve the epithelial barrier during IAV infection. In parallel, IL-22 may promote the synthesis of epithelial cytokines/chemokines as well as anti-microbial peptides, some of which are known to neutralize IAV (93–95). Through these mechanisms, IL-22 may be of particular significance during IAV pneumonia, a hypothesis that is currently under active investigation.
To conclude, our results show for the first time that iNKT cells produce IL-22 in the context of an infection. Our data also characterize RIG-I-like receptors as a new class of innate sensors important for iNKT cell activation during viral infection and also reinforce the concept (96) that iNKT cells are uniquely equipped for immediate, cytokine-driven activation during stressful conditions, including infection. Finally, we suggest that IL-22 is important to control epithelial damage caused by viral infection, a finding that may be relevant during IAV-associated pathogenesis.
Acknowledgments
We acknowledge the generous support from the NIAID (National Institutes of Health) Tetramer Facility (Emory University, Atlanta, GA) for supplying CD1d tetramers and Dr. W. Ouyang (Genentech, San Francisco, CA) for the anti-IL-22 Ab (clone 3F11). We gratefully acknowledge Dr. L. Van Kaer (Vanderbilt Institute, Nashville, TN) for the gift of Cd1d1−/− C57BL/6 mice. Drs. S. Akira and O. Takeuchi (Osaka University, Osaka, Japan) (Myd88−/−, Tlr7−/−, Ips-1−/−, Rig-I−/−), Dr. R. Flavell (Yale University, New Haven, CT) (NOD2−/−), and the late Dr. J. Tschopp (University of Lausanne, Switzerland) (Nlrp3−/−) are gratefully acknowledged for providing the knock-out mice used in this studies. We thank Dr. M. Chamaillard (CIIL, Institut Pasteur, Lille) for providing bone marrows from Nod2−/− C57BL/6 mice. C. Vendeville, L. Fauconnier, and B. Delaire are acknowledged for the technical assistance. We express gratitude to Dr. M. Leite de Moraes (Hôpital Necker, Paris), K. Benlagha (Hôpital Cochin St. Vincent de Paul, Paris), and R. Pierce (CIIL, Institut Pasteur de Lille) for helpful discussions. We thank the Plate-forme de Microscopie-Imagerie-Cytométrie du Campus Pasteur-Lille (MICPal) and the BioImaging Center Lille for the flow cytometry.
This work was supported by the Institut National de la Santé et de la Recherche Médicale, the CNRS, the University of Lille Nord de France, the Pasteur Institute of Lille, and the French National Research Agency (ANR) under reference ANR-08-MIEN-021-01 (to F. T.).
This paper is dedicated to the memory of Dr. J. Tschopp.
- iNKT cells
- invariant natural killer T cells
- Ag
- antigen
- APC
- Ag presenting cell
- DC
- dendritic cell
- IAV
- influenza A virus
- TLR
- Toll-like receptor
- RIG-I
- retinoic acid inducible gene-I
- Myd88
- myeloid differentiation 88
- IPS-1
- IFN-β promoter stimulator-1
- NOD
- nucleotide binding oligomerization domain
- NLRP3
- NOD-like receptor, pyrin domain containing 3
- p.i.
- post-infection
- m.o.i.
- multiplicity of infection
- AEC
- airway epithelial cells
- REG3β
- regenerating protein 3β
- NLR
- NOD-like receptor
- BAL
- bronchoalveolar lavage
- ANOVA
- analysis of variance
- TCR
- T cell receptor.
REFERENCES
- 1. Bendelac A., Savage P. B., Teyton L. (2007) The biology of NKT cells. Annu. Rev. Immunol. 25, 297–336 [DOI] [PubMed] [Google Scholar]
- 2. Kronenberg M. (2005) Toward an understanding of NKT cell biology. Progress and paradoxes. Annu. Rev. Immunol. 23, 877–900 [DOI] [PubMed] [Google Scholar]
- 3. Godfrey D. I., Kronenberg M. (2004) Going both ways. Immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 114, 1379–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Kaer L., Joyce S. (2005) Innate immunity. NKT cells in the spotlight. Curr. Biol. 15, R429–R431 [DOI] [PubMed] [Google Scholar]
- 5. Kawano T., Cui J., Koezuka Y., Toura I., Kaneko Y., Motoki K., Ueno H., Nakagawa R., Sato H., Kondo E., Koseki H., Taniguchi M. (1997) CD1d-restricted and TCR-mediated activation of vα14 NKT cells by glycosylceramides. Science 278, 1626–1629 [DOI] [PubMed] [Google Scholar]
- 6. Venkataswamy M. M., Porcelli S. A. (2010) Lipid and glycolipid antigens of CD1d-restricted natural killer T cells. Semin Immunol. 22, 68–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilson M. T., Singh A. K., Van Kaer L. (2002) Immunotherapy with ligands of natural killer T cells. Trends Mol. Med. 8, 225–231 [DOI] [PubMed] [Google Scholar]
- 8. Brigl M., Brenner M. B. (2010) How invariant natural killer T cells respond to infection by recognizing microbial or endogenous lipid antigens. Semin. Immunol. 22, 79–86 [DOI] [PubMed] [Google Scholar]
- 9. Tupin E., Kinjo Y., Kronenberg M. (2007) The unique role of natural killer T cells in the response to microorganisms. Nat. Rev. Microbiol. 5, 405–417 [DOI] [PubMed] [Google Scholar]
- 10. Sköld M., Behar S. M. (2003) Role of CD1d-restricted NKT cells in microbial immunity. Infect. Immun. 71, 5447–5455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tessmer M. S., Fatima A., Paget C., Trottein F., Brossay L. (2009) NKT cell immune responses to viral infection. Expert. Opin. Ther. Targets 13, 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ashkar A. A., Rosenthal K. L. (2003) Interleukin-15 and natural killer and NKT cells play a critical role in innate protection against genital herpes simplex virus type 2 infection. J. Virol. 77, 10168–10171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diana J., Griseri T., Lagaye S., Beaudoin L., Autrusseau E., Gautron A. S., Tomkiewicz C., Herbelin A., Barouki R., von Herrath M., Dalod M., Lehuen A. (2009) NKT cell-plasmacytoid dendritic cell cooperation via OX40 controls viral infection in a tissue-specific manner. Immunity 30, 289–299 [DOI] [PubMed] [Google Scholar]
- 14. Exley M. A., Bigley N. J., Cheng O., Shaulov A., Tahir S. M., Carter Q. L., Garcia J., Wang C., Patten K., Stills H. F., Alt F. W., Snapper S. B., Balk S. P. (2003) Innate immune response to encephalomyocarditis virus infection mediated by CD1d. Immunology 110, 519–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grubor-Bauk B., Arthur J. L., Mayrhofer G. (2008) Importance of NKT cells in resistance to herpes simplex virus, fate of virus-infected neurons, and level of latency in mice. J. Virol. 82, 11073–11083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grubor-Bauk B., Simmons A., Mayrhofer G., Speck P. G. (2003) Impaired clearance of herpes simplex virus type 1 from mice lacking CD1d or NKT cells expressing the semivariant Vα 14-Jα 281 TCR. J. Immunol. 170, 1430–1434 [DOI] [PubMed] [Google Scholar]
- 17. Ilyinskii P. O., Wang R., Balk S. P., Exley M. A. (2006) CD1d mediates T-cell-dependent resistance to secondary infection with encephalomyocarditis virus (EMCV) in vitro and immune response to EMCV infection in vivo. J. Virol. 80, 7146–7158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson T. R., Hong S., Van Kaer L., Koezuka Y., Graham B. S. (2002) NK T cells contribute to expansion of CD8(+) T cells and amplification of antiviral immune responses to respiratory syncytial virus. J. Virol. 76, 4294–4303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim E. Y., Battaile J. T., Patel A. C., You Y., Agapov E., Grayson M. H., Benoit L. A., Byers D. E., Alevy Y., Tucker J., Swanson S., Tidwell R., Tyner J. W., Morton J. D., Castro M., Polineni D., Patterson G. A., Schwendener R. A., Allard J. D., Peltz G., Holtzman M. J. (2008) Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat. Med. 14, 633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stout-Delgado H. W., Du W., Shirali A. C., Booth C. J., Goldstein D. R. (2009) Aging promotes neutrophil-induced mortality by augmenting IL-17 production during viral infection. Cell Host Microbe 6, 446–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Renneson J., Guabiraba R., Maillet I., Marques R. E., Ivanov S., Fontaine J., Paget C., Quesniaux V., Faveeuw C., Ryffel B., Teixeira M. M., Trottein F. (2011) A detrimental role for invariant natural killer T cells in the pathogenesis of experimental dengue virus infection. Am. J. Pathol. 179, 1872–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Santo C., Salio M., Masri S. H., Lee L. Y., Dong T., Speak A. O., Porubsky S., Booth S., Veerapen N., Besra G. S., Gröne H. J., Platt F. M., Zambon M., Cerundolo V. (2008) Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J. Clin. Invest. 118, 4036–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kok W. L., Denney L., Benam K., Cole S., Clelland C., McMichael A. J., Ho L. P. (2011) Pivotal advance: invariant NKT cells reduce accumulation of inflammatory monocytes in the lungs and decrease immune-pathology during severe influenza A virus infection. J. Leukoc. Biol. doi: 10.1189/jlb.0411184 [DOI] [PubMed] [Google Scholar]
- 24. Paget C., Ivanov S., Fontaine J., Blanc F., Pichavant M., Renneson J., Bialecki E., Pothlichet J., Vendeville C., Barba-Spaeth G., Barba-Speath G., Huerre M. R., Faveeuw C., Si-Tahar M., Trottein F. (2011) Potential role of invariant NKT cells in the control of pulmonary inflammation and CD8+ T cell response during acute influenza A virus H3N2 pneumonia. J. Immunol. 186, 5590–5602 [DOI] [PubMed] [Google Scholar]
- 25. Guillot L., Le Goffic R., Bloch S., Escriou N., Akira S., Chignard M., Si-Tahar M. (2005) Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 280, 5571–5580 [DOI] [PubMed] [Google Scholar]
- 26. Mendiratta S. K., Martin W. D., Hong S., Boesteanu A., Joyce S., Van Kaer L. (1997) CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity 6, 469–477 [DOI] [PubMed] [Google Scholar]
- 27. Adachi O., Kawai T., Takeda K., Matsumoto M., Tsutsui H., Sakagami M., Nakanishi K., Akira S. (1998) Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9, 143–150 [DOI] [PubMed] [Google Scholar]
- 28. Hemmi H., Kaisho T., Takeuchi O., Sato S., Sanjo H., Hoshino K., Horiuchi T., Tomizawa H., Takeda K., Akira S. (2002) Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3, 196–200 [DOI] [PubMed] [Google Scholar]
- 29. Kato H., Takeuchi O., Sato S., Yoneyama M., Yamamoto M., Matsui K., Uematsu S., Jung A., Kawai T., Ishii K. J., Yamaguchi O., Otsu K., Tsujimura T., Koh C. S., Reis e Sousa C., Matsuura Y., Fujita T., Akira S. (2006) Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 [DOI] [PubMed] [Google Scholar]
- 30. Kobayashi K. S., Chamaillard M., Ogura Y., Henegariu O., Inohara N., Nuñez G., Flavell R. A. (2005) Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307, 731–734 [DOI] [PubMed] [Google Scholar]
- 31. Kumar H., Kawai T., Kato H., Sato S., Takahashi K., Coban C., Yamamoto M., Uematsu S., Ishii K. J., Takeuchi O., Akira S. (2006) Essential role of IPS-1 in innate immune responses against RNA viruses. J. Exp. Med. 203, 1795–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martinon F., Pétrilli V., Mayor A., Tardivel A., Tschopp J. (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 [DOI] [PubMed] [Google Scholar]
- 33. Kreymborg K., Etzensperger R., Dumoutier L., Haak S., Rebollo A., Buch T., Heppner F. L., Renauld J. C., Becher B. (2007) IL-22 is expressed by Th17 cells in an IL-23-dependent fashion but not required for the development of autoimmune encephalomyelitis. J. Immunol. 179, 8098–8104 [DOI] [PubMed] [Google Scholar]
- 34. Paget C., Mallevaey T., Speak A. O., Torres D., Fontaine J., Sheehan K. C., Capron M., Ryffel B., Faveeuw C., Leite de Moraes M., Platt F., Trottein F. (2007) Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity 27, 597–609 [DOI] [PubMed] [Google Scholar]
- 35. Ishikawa H., Tanaka K., Kutsukake E., Fukui T., Sasaki H., Hata A., Noda S., Matsumoto T. (2010) IFN-γ production downstream of NKT cell activation in mice infected with influenza virus enhances the cytolytic activities of both NK cells and viral antigen-specific CD8+ T cells. Virology 407, 325–332 [DOI] [PubMed] [Google Scholar]
- 36. Kolls J. K., Khader S. A. (2010) The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 21, 443–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ouyang W., Kolls J. K., Zheng Y. (2008) The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 28, 454–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eyerich S., Eyerich K., Cavani A., Schmidt-Weber C. (2010) IL-17 and IL-22. Siblings, not twins. Trends Immunol. 31, 354–361 [DOI] [PubMed] [Google Scholar]
- 39. Sonnenberg G. F., Fouser L. A., Artis D. (2011) Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 12, 383–390 [DOI] [PubMed] [Google Scholar]
- 40. Vivier E., Spits H., Cupedo T. (2009) Interleukin-22-producing innate immune cells. New players in mucosal immunity and tissue repair? Nat. Rev. Immunol. 9, 229–234 [DOI] [PubMed] [Google Scholar]
- 41. Wolk K., Kunz S., Witte E., Friedrich M., Asadullah K., Sabat R. (2004) IL-22 increases the innate immunity of tissues. Immunity 21, 241–254 [DOI] [PubMed] [Google Scholar]
- 42. Zenewicz L. A., Flavell R. A. (2008) IL-22 and inflammation. Leukin' through a glass onion. Eur. J. Immunol. 38, 3265–3268 [DOI] [PubMed] [Google Scholar]
- 43. Pickert G., Neufert C., Leppkes M., Zheng Y., Wittkopf N., Warntjen M., Lehr H. A., Hirth S., Weigmann B., Wirtz S., Ouyang W., Neurath M. F., Becker C. (2009) STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 206, 1465–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zheng Y., Valdez P. A., Danilenko D. M., Hu Y., Sa S. M., Gong Q., Abbas A. R., Modrusan Z., Ghilardi N., de Sauvage F. J., Ouyang W. (2008) Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14, 282–289 [DOI] [PubMed] [Google Scholar]
- 45. Coquet J. M., Chakravarti S., Kyparissoudis K., McNab F. W., Pitt L. A., McKenzie B. S., Berzins S. P., Smyth M. J., Godfrey D. I. (2008) Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc. Natl. Acad. Sci. U.S.A. 105, 11287–11292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Doisne J. M., Becourt C., Amniai L., Duarte N., Le Luduec J. B., Eberl G., Benlagha K. (2009) Skin and peripheral lymph node invariant NKT cells are mainly retinoic acid receptor-related orphan receptor (γ)t+ and respond preferentially under inflammatory conditions. J. Immunol. 183, 2142–2149 [DOI] [PubMed] [Google Scholar]
- 47. Doisne J. M., Soulard V., Bécourt C., Amniai L., Henrot P., Havenar-Daughton C., Blanchet C., Zitvogel L., Ryffel B., Cavaillon J. M., Marie J. C., Couillin I., Benlagha K. (2011) Cutting edge. Crucial role of IL-1 and IL-23 in the innate IL-17 response of peripheral lymph node NK1.1- invariant NKT cells to bacteria. J. Immunol. 186, 662–666 [DOI] [PubMed] [Google Scholar]
- 48. Michel M. L., Keller A. C., Paget C., Fujio M., Trottein F., Savage P. B., Wong C. H., Schneider E., Dy M., Leite-de-Moraes M. C. (2007) Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J. Exp. Med. 204, 995–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rachitskaya A. V., Hansen A. M., Horai R., Li Z., Villasmil R., Luger D., Nussenblatt R. B., Caspi R. R. (2008) Cutting edge. NKT cells constitutively express IL-23 receptor and RORγt and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J. Immunol. 180, 5167–5171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brigl M., Bry L., Kent S. C., Gumperz J. E., Brenner M. B. (2003) Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat. Immunol. 4, 1230–1237 [DOI] [PubMed] [Google Scholar]
- 51. Mattner J., Debord K. L., Ismail N., Goff R. D., Cantu C., 3rd, Zhou D., Saint-Mezard P., Wang V., Gao Y., Yin N., Hoebe K., Schneewind O., Walker D., Beutler B., Teyton L., Savage P. B., Bendelac A. (2005) Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434, 525–529 [DOI] [PubMed] [Google Scholar]
- 52. Nagarajan N. A., Kronenberg M. (2007) Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J. Immunol. 178, 2706–2713 [DOI] [PubMed] [Google Scholar]
- 53. Salio M., Speak A. O., Shepherd D., Polzella P., Illarionov P. A., Veerapen N., Besra G. S., Platt F. M., Cerundolo V. (2007) Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc. Natl. Acad. Sci. U.S.A. 104, 20490–20495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tyznik A. J., Tupin E., Nagarajan N. A., Her M. J., Benedict C. A., Kronenberg M. (2008) Cutting edge. The mechanism of invariant NKT cell responses to viral danger signals. J. Immunol. 181, 4452–4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Allen I. C., Scull M. A., Moore C. B., Holl E. K., McElvania-TeKippe E., Taxman D. J., Guthrie E. H., Pickles R. J., Ting J. P. (2009) The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30, 556–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Diebold S. S., Kaisho T., Hemmi H., Akira S., Reis e Sousa C. (2004) Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531 [DOI] [PubMed] [Google Scholar]
- 57. Hornung V., Ellegast J., Kim S., Brzózka K., Jung A., Kato H., Poeck H., Akira S., Conzelmann K. K., Schlee M., Endres S., Hartmann G. (2006) 5'-Triphosphate RNA is the ligand for RIG-I. Science 314, 994–997 [DOI] [PubMed] [Google Scholar]
- 58. Ichinohe T., Pang I. K., Iwasaki A. (2010) Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat. Immunol. 11, 404–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Koyama S., Ishii K. J., Kumar H., Tanimoto T., Coban C., Uematsu S., Kawai T., Akira S. (2007) Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J. Immunol. 179, 4711–4720 [DOI] [PubMed] [Google Scholar]
- 60. Lund J. M., Alexopoulou L., Sato A., Karow M., Adams N. C., Gale N. W., Iwasaki A., Flavell R. A. (2004) Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. U.S.A. 101, 5598–5603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pichlmair A., Schulz O., Tan C. P., Näslund T. I., Liljeström P., Weber F., Reis e Sousa C. (2006) RIG-I-mediated antiviral responses to single-stranded RNA bearing 5'-phosphates. Science 314, 997–1001 [DOI] [PubMed] [Google Scholar]
- 62. Sabbah A., Chang T. H., Harnack R., Frohlich V., Tominaga K., Dube P. H., Xiang Y., Bose S. (2009) Activation of innate immune antiviral responses by Nod2. Nat. Immunol. 10, 1073–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thomas P. G., Dash P., Aldridge J. R., Jr., Ellebedy A. H., Reynolds C., Funk A. J., Martin W. J., Lamkanfi M., Webby R. J., Boyd K. L., Doherty P. C., Kanneganti T. D. (2009) The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 30, 566–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yoneyama M., Kikuchi M., Natsukawa T., Shinobu N., Imaizumi T., Miyagishi M., Taira K., Akira S., Fujita T. (2004) The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, 730–737 [DOI] [PubMed] [Google Scholar]
- 65. Wolk K., Witte E., Witte K., Warszawska K., Sabat R. (2010) Biology of interleukin-22. Semin. Immunopathol. 32, 17–31 [DOI] [PubMed] [Google Scholar]
- 66. Sonnenberg G. F., Nair M. G., Kirn T. J., Zaph C., Fouser L. A., Artis D. (2010) Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J. Exp. Med. 207, 1293–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Graf R., Schiesser M., Reding T., Appenzeller P., Sun L. K., Fortunato F., Perren A., Bimmler D. (2006) Exocrine meets endocrine. Pancreatic stone protein and regenerating protein; two sides of the same coin. J. Surg. Res. 133, 113–120 [DOI] [PubMed] [Google Scholar]
- 68. Gad H. H., Dellgren C., Hamming O. J., Vends S., Paludan S. R., Hartmann R. (2009) Interferon-λ is functionally an interferon but structurally related to the interleukin-10 family. J. Biol. Chem. 284, 20869–20875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Diana J., Lehuen A. (2009) NKT cells. Friend or foe during viral infections? Eur. J. Immunol. 39, 3283–3291 [DOI] [PubMed] [Google Scholar]
- 70. Kulkarni R. R., Haeryfar S. M., Sharif S. (2010) The invariant NKT cell subset in anti-viral defenses. A dark horse in anti-influenza immunity? J. Leukoc. Biol. 88, 635–643 [DOI] [PubMed] [Google Scholar]
- 71. Hobbs J. A., Cho S., Roberts T. J., Sriram V., Zhang J., Xu M., Brutkiewicz R. R. (2001) Selective loss of natural killer T cells by apoptosis following infection with lymphocytic choriomeningitis virus. J. Virol. 75, 10746–10754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Motsinger A., Haas D. W., Stanic A. K., Van Kaer L., Joyce S., Unutmaz D. (2002) CD1d-restricted human natural killer T cells are highly susceptible to human immunodeficiency virus 1 infection. J. Exp. Med. 195, 869–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bender A., Albert M., Reddy A., Feldman M., Sauter B., Kaplan G., Hellman W., Bhardwaj N. (1998) The distinctive features of influenza virus infection of dendritic cells. Immunobiology 198, 552–567 [DOI] [PubMed] [Google Scholar]
- 74. Hao X., Kim T. S., Braciale T. J. (2008) Differential response of respiratory dendritic cell subsets to influenza virus infection. J. Virol. 82, 4908–4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Manicassamy B., Manicassamy S., Belicha-Villanueva A., Pisanelli G., Pulendran B., García-Sastre A. (2010) Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proc. Natl. Acad. Sci. U.S.A. 107, 11531–11536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Le Goffic R., Balloy V., Lagranderie M., Alexopoulou L., Escriou N., Flavell R., Chignard M., Si-Tahar M. (2006) Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2, e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Poeck H., Bscheider M., Gross O., Finger K., Roth S., Rebsamen M., Hannesschläger N., Schlee M., Rothenfusser S., Barchet W., Kato H., Akira S., Inoue S., Endres S., Peschel C., Hartmann G., Hornung V., Ruland J. (2010) Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1β production. Nat. Immunol. 11, 63–69 [DOI] [PubMed] [Google Scholar]
- 78. Leite-De-Moraes M. C., Hameg A., Arnould A., Machavoine F., Koezuka Y., Schneider E., Herbelin A., Dy M. (1999) A distinct IL-18-induced pathway to fully activate NK T lymphocytes independently from TCR engagement. J. Immunol. 163, 5871–5876 [PubMed] [Google Scholar]
- 79. Wesley J. D., Tessmer M. S., Chaukos D., Brossay L. (2008) NK cell-like behavior of Vα14i NK T cells during MCMV infection. PLoS Pathog. 4, e1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Colonna M. (2009) Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity 31, 15–23 [DOI] [PubMed] [Google Scholar]
- 81. Spits H., Di Santo J. P. (2011) The expanding family of innate lymphoid cells. Regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 12, 21–27 [DOI] [PubMed] [Google Scholar]
- 82. Martin B., Hirota K., Cua D. J., Stockinger B., Veldhoen M. (2009) Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity 31, 321–330 [DOI] [PubMed] [Google Scholar]
- 83. Simonian P. L., Wehrmann F., Roark C. L., Born W. K., O'Brien R. L., Fontenot A. P. (2010) γδ T cells protect against lung fibrosis via IL-22. J. Exp. Med. 207, 2239–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sutton C. E., Lalor S. J., Sweeney C. M., Brereton C. F., Lavelle E. C., Mills K. H. (2009) Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity 31, 331–341 [DOI] [PubMed] [Google Scholar]
- 85. Wahl C., Wegenka U. M., Leithäuser F., Schirmbeck R., Reimann J. (2009) IL-22-dependent attenuation of T cell-dependent (ConA) hepatitis in herpes virus entry mediator deficiency. J. Immunol. 182, 4521–4528 [DOI] [PubMed] [Google Scholar]
- 86. Aujla S. J., Chan Y. R., Zheng M., Fei M., Askew D. J., Pociask D. A., Reinhart T. A., McAllister F., Edeal J., Gaus K., Husain S., Kreindler J. L., Dubin P. J., Pilewski J. M., Myerburg M. M., Mason C. A., Iwakura Y., Kolls J. K. (2008) IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 14, 275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hoegl S., Bachmann M., Scheiermann P., Goren I., Hofstetter C., Pfeilschifter J., Zwissler B., Muhl H. (2011) Protective properties of inhaled IL-22 in a model of ventilator-induced lung injury. Am. J. Respir. Cell Mol. Biol. 44, 369–376 [DOI] [PubMed] [Google Scholar]
- 88. Guo H., Topham D. J. (2010) Interleukin-22 (IL-22) production by pulmonary Natural Killer cells and the potential role of IL-22 during primary influenza virus infection. J. Virol. 84, 7750–7759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nieuwenhuis E. E., Matsumoto T., Lindenbergh D., Willemsen R., Kaser A., Simons-Oosterhuis Y., Brugman S., Yamaguchi K., Ishikawa H., Aiba Y., Koga Y., Samsom J. N., Oshima K., Kikuchi M., Escher J. C., Hattori M., Onderdonk A. B., Blumberg R. S. (2009) Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J. Clin. Invest. 119, 1241–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wingender G., Kronenberg M. (2008) Role of NKT cells in the digestive system. IV. The role of canonical natural killer T cells in mucosal immunity and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G1–G8 [DOI] [PubMed] [Google Scholar]
- 91. Dambacher J., Beigel F., Zitzmann K., Heeg M. H., Göke B., Diepolder H. M., Auernhammer C. J., Brand S. (2008) The role of interleukin-22 in hepatitis C virus infection. Cytokine 41, 209–216 [DOI] [PubMed] [Google Scholar]
- 92. Pagliaccetti N. E., Chu E. N., Bolen C. R., Kleinstein S. H., Robek M. D. (2010) λ and α interferons inhibit hepatitis B virus replication through a common molecular mechanism but with different in vivo activities. Virology 401, 197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Doss M., White M. R., Tecle T., Gantz D., Crouch E. C., Jung G., Ruchala P., Waring A. J., Lehrer R. I., Hartshorn K. L. (2009) Interactions of α-, β-, and θ-defensins with influenza A virus and surfactant protein D. J. Immunol. 182, 7878–7887 [DOI] [PubMed] [Google Scholar]
- 94. Klotman M. E., Chang T. L. (2006) Defensins in innate antiviral immunity. Nat. Rev. Immunol. 6, 447–456 [DOI] [PubMed] [Google Scholar]
- 95. Leikina E., Delanoe-Ayari H., Melikov K., Cho M. S., Chen A., Waring A. J., Wang W., Xie Y., Loo J. A., Lehrer R. I., Chernomordik L. V. (2005) Carbohydrate binding molecules inhibit viral fusion and entry by cross-linking membrane glycoproteins. Nat. Immunol. 6, 995–1001 [DOI] [PubMed] [Google Scholar]
- 96. Brigl M., Tatituri R. V., Watts G. F., Bhowruth V., Leadbetter E. A., Barton N., Cohen N. R., Hsu F. F., Besra G. S., Brenner M. B. (2011) Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J. Exp. Med. 208, 1163–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]