Background: Mitochondrial tRNA import in T. brucei is essential for mitochondrial biogenesis.
Results: Proteins necessary for mitochondrial tRNA import are identified.
Conclusion: Trypanosome tRNA import requires a novel mitochondrial membrane complex.
Significance: First report of the protein composition of a putative tRNA translocon in trypanosomes.
Keywords: Mitochondria, Protein Translocation, RNA Transport, RNA-binding Proteins, Transfer RNA (tRNA), Trypanosoma brucei, Kinetoplast, RNA Import, Tim17
Abstract
The mitochondrial genome of Trypanosoma brucei does not contain genes encoding tRNAs; instead this protozoan parasite must import nuclear-encoded tRNAs from the cytosol for mitochondrial translation. Previously, it has been shown that mitochondrial tRNA import requires ATP hydrolysis and a proteinaceous mitochondrial membrane component. However, little is known about the mitochondrial membrane proteins involved in tRNA binding and translocation into the mitochondrion. Here we report the purification of a mitochondrial membrane complex using tRNA affinity purification and have identified several protein components of the putative tRNA translocon by mass spectrometry. Using an in vivo tRNA import assay in combination with RNA interference, we have verified that two of these proteins, Tb11.01.4590 and Tb09.v1.0420, are involved in mitochondrial tRNA import. Using Protein C Epitope -Tobacco Etch Virus-Protein A Epitope (PTP)-tagged Tb11.01.4590, additional associated proteins were identified including Tim17 and other mitochondrial proteins necessary for mitochondrial protein import. Results presented here identify and validate two novel protein components of the putative tRNA translocon and provide additional evidence that mitochondrial tRNA and protein import have shared components in trypanosomes.
Introduction
The mitochondrial genomes of most eukaryotes contain genes encoding tRNAs, which function in maintaining active mitochondrial translation of proteins vital for mitochondrial function. However, some mitochondrial genomes do not encode complete sets of tRNA, and the cell must import tRNAs from the cytosol into the mitochondria (1, 2). Studies in mammals (3), fungi (4), plants (5), and protozoa (6–11) have examined the bioenergetic and sequence requirements for mitochondrial tRNA import. Although diverse mechanisms for translocation of tRNA have been identified, recent studies examining tRNA import in trypanosomes suggest that involvement of components of the mitochondrial protein translocation machinery may be a conserved feature of tRNA import in all organisms (12).
The mitochondrial genomes of the kinetoplastid protozoa Leishmania tropica and Trypanosoma brucei are completely devoid of mitochondrial tRNA genes. Instead, all of the mitochondrial tRNAs are encoded within the nucleus and show dual localization with ∼90% of each tRNA species in the cytosol and 10% in the mitochondrion, the exceptions being the selenocysteine and initiator methionyl-tRNAs, which are exclusively cytoplasmic (6, 13–15). The mechanism by which these tRNAs are imported into the trypanosome mitochondria is unknown; however, in vitro studies with isolated organelles have shown that translocation of tRNAs into the mitochondrion requires ATP hydrolysis and a proteinaceous membrane component (9, 16, 17). In L. tropica it has been reported that an outer mitochondrial membrane protein (8, 18) as well as an 11-subunit inner mitochondrial membrane RNA import complex (RIC)2 is required for tRNA import (19–22). Many of the essential subunits of this Leishmania inner mitochondrial membrane RIC complex are proposed to function both in tRNA import and as components of the mitochondrial respiratory complexes including the cytochrome c reductase (Complex III), cytochrome c oxidase (Complex IV), and the α-subunit of the F1F0-ATP synthase. However, the identification of several components of the RIC, including the Reiske iron sulfur protein and the α-subunit of the F1-ATP synthase, have recently come into question (12, 21, 23, 24). Furthermore, it is unlikely that the RIC of T. brucei has the same protein composition as Leishmania, as the bloodstream form (BF) of trypanosomes do not express the cytochrome c oxidase or cytochrome c reductase complexes, yet are able to import tRNAs into their mitochondria (25).
Although little is known about the mitochondrial tRNA import machinery of African trypanosomes, a cytosolic protein, eukaryotic elongation factor 1a (eEF1a), has recently been shown to play a role in mitochondrial tRNA import in T. brucei (17). In addition, the canonical translocase inner membrane 17 (Tim17) of the mitochondrial protein import complex has been shown to function in T. brucei protein and tRNA import (26). Here, we report the biochemical purification and characterization of a mitochondrial membrane complex in T. brucei involved in the translocation of tRNAs from the cytosol into the mitochondria. Two hypothetical proteins, Tb11.01.4590 and Tb09.v1.0420, were shown to function in mitochondrial tRNA import. The putative tRNA translocon also contains the Tim17 protein and other proteins proposed to function in mitochondrial protein import. These findings suggest that mitochondrial tRNA and protein import machinery in trypanosomes may either share protein components or that there is a common translocon for both proteins and tRNAs.
EXPERIMENTAL PROCEDURES
Trypanosome Cell Culture and Mitochondrial Purification
T. brucei 667 (TREU 667) and T. brucei 29−13 strains were used and maintained in a semi-defined media culture medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum (27). For tRNA and protein C-TEV-protein A (PTP) affinity purification experiments, mitochondria were isolated from procyclic form (PF) T. brucei TREU 667, and mitochondrial membrane and matrix fractions were prepared by differential detergent extraction of hypotonically isolated mitochondria (0.5% Triton X-100 for matrix followed by 2% n-dodecyl-β-d-maltoside for membrane) as previously described (28, 29). Mitochondrial matrix and membrane localization of PTP-tagged proteins were determined by probing for the protein A domain (PAP antibody, Abcam) Transmission electron microscopy of isolated mitochondria was conducted as previously described (30).
Native tRNA Binding Assay
Uniformly labeled T. brucei tRNALeu(CAA) (α-32P-UTP, 800 Ci/mmol) was generated by in vitro transcription by T7 polymerase using a DNA template with a T7 promoter according to the manufacturer's recommended procedure (Ambion, MEGAshortscript High Yield Transcription kit). To identify the tRNA import complex, solubilized total mitochondrial (2% n-dodecyl-β-d-maltoside) extracts were prepared from 4 × 109 cells. Each sample was mixed with an equal volume of a protein binding buffer (160 mm MOPS, 310 mm sucrose, 6.25 mm MgCl2, 100 mm KCl, 9 mm DTT, 2 units/300 μl of SUPERase-in (Ambion), 1 mg/ml BSA) and incubated for 30 min at 4 °C with uniformly labeled tRNALeu(CAA). After incubation, mitochondrial protein (30 μg) and associated tRNA were run for 18 h at 4 °C on blue native gradient gels (4–13%) (31). After electrophoresis, gels were dried and exposed to x-ray film (Eastman Kodak Co., Biomax MS) to visualize tRNA-protein complexes.
tRNA Affinity Purification of Mitochondrial Membrane Complexes
For tRNA affinity purification, 500 μl of paramagnetic streptavidin resin (DYNAL Magnetic Beads, Invitrogen) containing 6.7 × 108 beads/ml was washed 3 times in 500 μl of 0.5 × SSC buffer (1 × SSC; 150 mm NaCl, 15 mm trisodium citrate dihydrate, pH 7.2). The washed resin was resuspended in 0.5 × SSC, synthetic 5′-biotinylated T. brucei tRNALeu(CAA) (Dharmacon) was added to a final concentration of 0.2 μm and incubated at 65 °C for 10 min to form the tRNA-streptavidin affinity resin. After incubation, the tRNA-bound resin was washed 3 times with 300 μl of 0.1 × SSC and equilibrated in 300 μl of protein binding buffer (see above). tRNA binding complexes were affinity-purified after binding to intact mitochondria or detergent extracts (1% Triton X-100) of total mitochondria or purified mitochondrial membranes. Each of the starting samples was adjusted to 6.0 × 109 cell equivalents, and volumes were adjusted to 300 μl with protein binding buffer. Samples were incubated with the tRNA affinity resin for 30 min at 4 °C, unbound protein was collected, and the tRNA affinity resin was washed 6 times in 500 μl of protein binding buffer before elution of tRNA-bound proteins using buffers containing increasing ionic concentrations of NaCl (0.1–1 m NaCl; 20 mm MOPS, pH 7.2). Protein samples from each elution were precipitated and fractionated on 8–12% SDS-PAGE. For LC-MS/MS analysis, tRNA-bound proteins were eluted with 20 mm MOPS, 1 m NaCl, pH 7.2, and analyzed directly. Mitochondrial proteins were also incubated with paramagnetic streptavidin resin without bound tRNA as a control to identify proteins that bound to the resin nonspecifically. To evaluate the specificity of binding, synthetic 5′-biotinylated T. brucei-specific tRNALeu(CAA), tRNAMet-i, tRNALeu(CAA), D-loop (nucleotides 9–27), and tRNALeu(CAA) variable Loop/T-stem (nucleotides 44–74) pulldown assays of PTP-tagged Tb11.01.4590 were conducted exactly the same, except SUPERase-in (Ambion) was removed from the binding procedure as it was found to cross-react with both protein A/PAP (Abcam) and Protein C (Delta Biolabs) polyclonal antibodies. Also, before a single NaCl elution (1 m NaCl, 20 mm MOPS, pH 7.2), the bound fraction was washed 6 times in 600 μl of binding buffer in which the concentration of KCl had been increased to 300 mm.
Subtractive Mass Spectrometry Analysis
Using the tRNA affinity purification strategy, mitochondrial proteins that specifically bound tRNA and those that nonspecifically interacted with the paramagnetic resin were prepared. Both samples were analyzed by LC-MS/MS (Thermo Fisher LTQ Linear Ion Trap), and peptides identified from the resin-only sample were subtracted from the indexed proteins specifically bound to the T. brucei tRNALeu(CAA) resin. Domain homology for each protein was determined by using the protein-protein BLAST (BLASTp) algorithm from the National Center for Biotechnology Information (NCBI) and the Conserved Domain Data base.
Inducible Expression of Variant tRNAMet-i
A tetracycline-inducible tRNA expression construct was previously generated and characterized (17). This tRNA is a variant of T. brucei tRNAMet-i (Var-tRNAMet-i) that contains an internal sequence tag within the D-loop and the T-stem from tRNAMet-e (supplemental Fig. 1). This construct has been shown to be aminoacylated and processed upon mitochondrial import by in vitro and in vivo assays (17, 32). The original construct containing the phleomycin resistance gene was replaced with the puromycin resistance gene by use of the MscI restriction sites flanking the phleomycin gene. Upon NotI digestion, linearized vector was electroporated and transfected into T. brucei 29−13 cell lines.
RNAi to Validate Candidate tRNA Import Complex Proteins
All RNAi constructs were prepared in the doxycycline-inducible pZJM vector containing opposing T7 promoters (33). RNAi inserts for eIF2 and eEF1a were generated as previously described (17). Inserts for Tb09.v1.0420 (nucleotides 1–597), Tb927.10.4280 (nucleotides 1–606), Tb927.10.15220 (nucleotides 1–438), Tb11.01.4590 (nucleotides 1–787), Tb11.02.0445 (nucleotides 1–282), gBP21 (nucleotides 1–621), and gBP25 (nucleotides 1–675) were also prepared. Constructs were linearized by cleavage with NotI and transfected into the clonal cell line containing the inducible Var-tRNAMet-i gene. Upon induction with doxycycline, each cell line simultaneously expressed the Var-tRNAMet-i and double-stranded RNA to knock down expression of targeted proteins.
Measurement of Mitochondrial Membrane Potential
RNAi cell lines for each protein were induced with doxycycline for 24, 48, or 72 h to determine the effect knockdown of each candidate protein had on mitochondrial membrane potential. Samples from untreated and doxycycline-induced (1 μg/ml) cells were incubated with the cationic fluorescent dye tetramethylrhodamine methyl ester (20 nm) for 10 min. Tetramethylrhodamine methyl ester rapidly accumulates in the mitochondria and has previously been used to determine the mitochondrial membrane potential by use of microfluorometry (34). After incubation with tetramethylrhodamine methyl ester, cells were centrifuged, and the pellet was resuspended in fresh SM media and allowed to equilibrate for 30 min followed by 2 washes with PBS. Washed cells were resuspended at a final concentration of 4 × 106 cells/ml in SM media. Mitochondrial membrane potential was then determined by analyzing 2 × 106 total cells in a microfluorometer with an excitation of 548 nm and emission of 573 nm (Luminescence Spectrometer LS 55, PerkinElmer Life Sciences).
RNA Isolation and Quantization of Imported tRNA
Mitochondrial and total cellular RNA was prepared from cells as previously described (17). Briefly, 4 × 108 cells were resuspended in 500 μl of 20 mm Tris-HCl, pH 7.5, 0.6 m sorbitol, 2 mm EDTA, pH 7.5, containing 0.05% digitonin and incubated for 5 min on ice. Cells were then centrifuged (8000 × g) at 4 °C for 5 min, and the supernatant was discarded. The pellet containing the crude mitochondrial fraction was then resuspended in 500 μl of micrococcal nuclease buffer (10 mm Tris, 1 mm CaCl2, pH 8.0, 10% glycerol) containing 300 units of micrococcal nuclease (U. S. Biochemical Corp.) and incubated at room temperature for 30 min to digest contaminating cytosolic RNAs. The nuclease reactions were stopped by the addition of 10 mm EDTA. The crude mitochondrial fraction was then centrifuged (8000 × g) at 4 °C for 5 min, and the supernatant was discarded. RNA was extracted from the mitochondrial and total cellular fractions with TriPure according to the manufacturer's recommendation (Roche Applied Science). Total cellular and mitochondrial RNAs from 2 × 107 cells were fractionated on 8 m urea, 8% polyacrylamide sequencing gels. Gels were stained with ethidium bromide to visualize RNAs before transfer to a positively charged nylon membrane (Amersham Biosciences Hybond N+; GE Healthcare) and subsequent probing with 32P end-labeled probe specific for the D-loop sequence tag of inducible Var-tRNAMet-i (CGCTCTTCCCCTGAGCCA) with a probe specific for the T-stem of the WT tRNAMet-i (GTTGGTTTCGATCCAACG) or with a probe specific for the mitochondrial-encoded 9 S rRNA (AATTACCGCAACGGCTGGCTGGCATCCATTTCTGAC). The amount of total cellular and mitochondrial tRNA was quantified by exposure of the probed membrane to a phosphorimaging plate (Storage Phosphor Screen, Molecular Dynamics) and then developed and analyzed by PhosphorImager (GE Healthcare model STORM-860).
Tandem Affinity of PTP Tb11.01.4590 and Associated Proteins
Constructs for PTP-Tb11.01.4590 (nucleotides 276–1047) were designed with Apa1 and NotI restriction sites to clone into the pC-PTP-Neo vector (35). PTP-tagged Tb11.01.4590 was linearized with BarI (SibEnzyme) and transfected into PF T. brucei TREU667 targeting a single allele for the endogenous expression of the PTP-tagged construct. Isolated mitochondria from 7.2 × 1010 cells were solubilized as previously described by resuspending (400 μg of mitochondria/40 μl) in solubilization buffer A (50 mm NaCl, 50 mm imidazole/HCl, 2 mm 6-aminohexanoic acid, 1 mm EDTA, pH 7.0) containing 2% dodecyl maltoside in a rotator for 30 min at 4 °C (31). PTP-tagged protein complexes were subsequently purified as described previously (36). Samples from the tandem affinity purification were examined by 8–10% polyacrylamide gel electrophoresis and immunoblot with anti-Protein C (Delta Biolabs). Eluted proteins were excised from gels and analyzed by LC-MS/MS (Thermo Fisher LTQ Linear Ion Trap). Raw tandem mass spectra were converted to mzXML files then into mascot generic files via the Trans-Proteomic Pipeline (Seattle Proteome Center, Seattle, WA). MGF files were searched using Mascot (Matrix Scientific, Boston, MA) against target and decoy NCBI databases for trypanosome proteins. ProteoIQ (Nusep, Bogart, GA) was used for data analysis, and a 5% protein false discovery rate was applied to confirm the presence of proteins.
RESULTS
Identification and Characterization of tRNA Binding Mitochondrial Membrane Protein Complexes
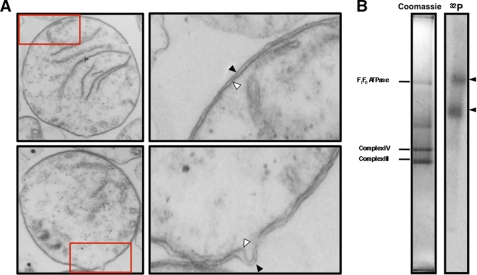
Previous studies on T. brucei suggested that mitochondrial membrane proteins were necessary for tRNA import (9). As a first step in the identification of the protein components necessary for tRNA import into mitochondria, we asked whether mild nonionic detergent (1% Triton X-100) extraction of mitochondrial membranes released protein complexes that could bind tRNAs. Mitochondria were isolated from PF T. brucei after hypotonic lysis and differential centrifugation. Transmission electron microscopy revealed that 87% (n = 100) of the mitochondria in these preparations contained both outer and inner mitochondrial membranes (Fig. 1A). A small fraction of mitochondria appeared to have a single membrane, consistent with the formation of mitoplasts lacking the outer membrane that is formed by hypotonic lysis and shearing. Detergent-extracted mitochondrial protein complexes were incubated with radiolabeled T. brucei tRNALeu(CAA) followed by separation of protein complexes using blue native gel electrophoresis (31). Under these conditions, tRNAs bound two high molecular weight protein complexes. The sizes of the tRNA binding complexes were estimated based on the known molecular weights of the T. brucei mitochondrial cytochrome c oxidase, cytochrome c reductase, and the ATP synthase, which were identified by LC-MS/MS in these preparations3 and by previous analysis of the ATP synthase (37). The larger complex was >820 kDa, whereas the lower tRNA binding complex was ∼500 kDa (Fig. 1B).
FIGURE 1.
Identification of tRNA binding complexes in extracts from T. brucei mitochondria. A, isolated mitochondria were characterized by transmission electron microscopy. Most mitochondria had intact outer (black arrowhead) and inner membranes (white arrowheads). A small fraction of mitochondria appear to have lost the outer membrane due to the hypotonic swelling and shearing protocol used in cell lysis (not shown). The areas designated by the red boxes in the lower magnification images were enlarged to better visualize the mitochondrial membranes (right panels). Total magnification: left panels, 27,272 ×; right panels, 89,089×. B, radiolabeled tRNALeu(CAA) binds two high molecular weight mitochondrial protein complexes (black arrowheads). Detergent extracts of T. brucei mitochondrial membrane were incubated with 32P-tRNALeu(CAA) and fractionated by blue native gel electrophoresis. The major mitochondrial membrane complexes visualized on stained blue native gel (left panel) were identified by LC-MS/MS as F1F0 ATP synthase (F1F0ATPase), cytochrome c oxidase (Complex IV), and cytochrome c reductase (Complex III) and were used as internal size standards.3 The tRNA binding complexes were detected by autoradiography (right panel).
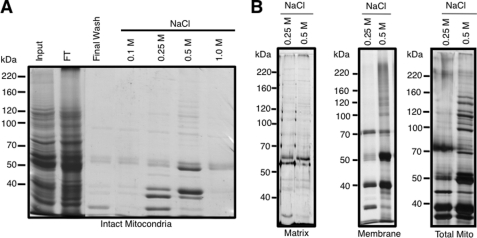
To purify these mitochondrial membrane complexes, we developed a tRNA affinity purification method using a synthetic T. brucei tRNALeu(CAA) biotinylated at the 5′ end and then anchored to a streptavidin paramagnetic resin. Using this as a ligand, we were able to pull down a specific subset of proteins from intact mitochondria and detergent extracts (1% Triton X-100) containing mitochondrial membranes (Fig. 2). Because the native tRNA translocon must be able to recognize and bind tRNAs presented on the cytosolic face of the mitochondrion, we reasoned that tRNA substrates must be able to bind mitochondrial membrane proteins on the purified, intact organelle. To test this, purified mitochondria were incubated with the tRNA affinity resin followed by treatment with non-ionic detergent (1% Triton X-100) then washed extensively with a low ionic strength buffer to remove non-specifically bound proteins. tRNA-binding proteins were released by stepwise salt elution. A subfraction of mitochondrial proteins was reproducibly recovered after treatment with 0.25–0.50 m NaCl (Fig. 2A). A similar set of proteins was seen when mitochondrial membrane proteins were fractionated using the biotinylated tRNA affinity method (Fig. 2B, middle and right panels) but not when using proteins extracted from the mitochondrial matrix (Fig. 2B, left panel). Protein profiles from the intact mitochondria and solubilized mitochondrial membranes were similar. This suggests that the biotinylated tRNA ligand interacts with a mitochondrial membrane complex that contains a tRNA binding domain exposed to the trypanosome cytoplasm. These results are consistent with a potential role for this complex as a tRNA translocon.
FIGURE 2.
Affinity purification of tRNA binding complexes from T. brucei mitochondria. A, isolated mitochondria were incubated with tRNALeu(CAA) affinity resin and treated with non-ionic detergent, and unbound proteins were collected in the flow-through (FT). Bound proteins were washed extensively with low salt buffer, and a final wash was collected (Final Wash) before stepwise elution with buffer containing increasing concentrations of NaCl. Samples were analyzed on 10% SDS-PAGE and stained with Coomassie Blue to visualize protein. Contaminating proteins found in all fractions were bovine serum albumin (66.8 kDa) from culture media and SUPERase-in (Ambion) from the affinity resin. B, purified mitochondria (left panel) and fractionated mitochondrial matrix (right panel) and membranes (right panel) were detergent-solubilized and incubated with tRNALeu(CAA) affinity resin, and associated proteins were eluted by treatment with increasing concentrations of NaCl. Proteins eluting with 0.25 and 0.5 m NaCl were fractionated on 10% SDS-PAGE and visualized by Coomassie Blue staining.
To identify components of the complex that interacted with the biotinylated tRNALeu(CAA), we used a subtractive mass spectrometry approach. Purified, intact mitochondria were incubated with resin lacking biotinylated tRNALeu(CAA) or resin containing biotinylated tRNALeu(CAA) followed by solubilization of the bound mitochondria and washing away unbound protein. Both samples were treated identically and were analyzed by LC-MS/MS. Proteins found to non-specifically bind to the affinity resin were subtracted from the analysis, whereas proteins that specifically bound to the biotinylated tRNALeu(CAA) resin were evaluated further. Proteins of interest were selected based on the MS score (>95% confidence), number of unique peptides (minimum of 2 peptides), predicted domain homology, predicted mitochondrial localization signals (MLS), or transmembrane helices. In addition, because tRNAs are imported into both BF and PF trypanosome mitochondria, only proteins encoded by constitutively expressed genes (44) in both developmental stages were analyzed further. From this subtractive LC-MS/MS analysis, 44 proteins fitting these criteria were identified that specifically interacted with the biotinylated tRNALeu(CAA). For each of these proteins a minimum of two unique peptides was identified with a probability score of greater than 95% (supplemental Table 1). Of these proteins, 29 are currently annotated as hypothetical in the GeneDB and TriTrypDB databases with nine having both predicted MLS and transmembrane helices domains. It is important to note that only two of the proteins identified in this analysis (Tb09.v1.0420 and Tb927.10.4280) are homologous to components of the 11-subunit Leishmania RIC. Based on the criteria described above, seven candidate proteins were selected for further analysis to directly test their role in mitochondrial tRNA import (Table 1).
TABLE 1.
Mitochondrial membrane proteins purified by tRNA binding
TM, transmembrane.
| tRNA-specific proteinsa | Protein ID | Predicted MLS and TMb | Conserved domainsc | PF/BF mRNA expressiond |
|---|---|---|---|---|
| Tb09.v1.0420e | Hypothetical | Both | None | Yes |
| Tb11.01.4860 | MRP2/gBP25 | MLS | RNA Binding | Yes |
| Tb11.55.0009 | MRP1/gBP21 | MLS | RNA Binding | Yes |
| Tb927.10.15220 | Hypothetical | Both | None | Yes |
| Tb11.01.4590 | Hypothetical | Both | None | Yes |
| Tb11.02.0445 | Hypothetical | Both | None | Yes |
| Tb927.10.4280e | Hypothetical | Both | UCR_14kD | Yes |
a >95% confidence/minimum 2 unique peptides.
b Predictions based on sequence analysis using Mito Prot/TMH MM/TMPred software and Panigrahi et al. (40).
c Domain homology by blasting protein sequences through NCBI.
d Life stage expression data from TriTryp DB/Nilsson et al. (44).
e Leishmania RIC homologue.
In Vivo Validation of Candidate Protein Components of Trypanosome tRNA Import Complex
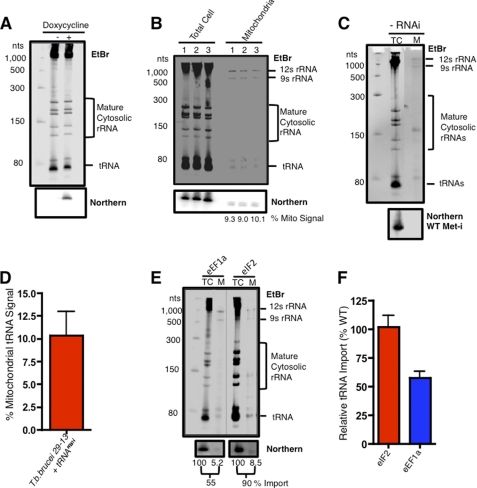
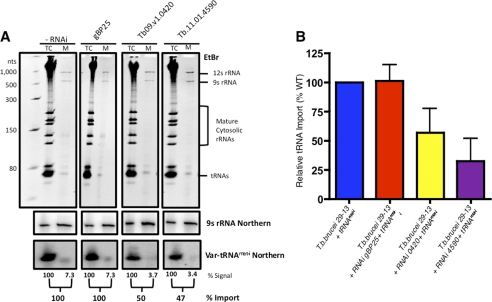
To determine the role of the candidate proteins in mitochondrial tRNA import, we utilized an in vivo import assay that combines the RNAi knockdown of a candidate protein with the induction of a newly synthesized Var-tRNAMet-i that is imported into the mitochondria (17). Using the Var-tRNAMet-i construct, we were able to show regulated expression of the Var-tRNAMet-i in the presence of doxycycline (Fig. 3A). To evaluate import of the Var-tRNAMet-i, purified mitochondria were treated with micrococcal nuclease to remove contaminating cytosolic RNAs, and mitochondrial RNA were isolated (Fig. 3B). By ethidium bromide staining, the mitochondrial RNA samples were enriched in the mitochondrial 9 S and 12 S rRNA and deficient in both large and small cytosolic rRNAs. To verify that the mitochondrial RNA samples were devoid of cytosolic tRNA contaminates, blots were hybridized with a probe for the initiator methionyl tRNA (WT Met-i) (Fig. 3C; supplemental Fig. 3). The absence of both ethidium bromide-stained cytosolic rRNAs and the cytosolic initiator methionyl tRNA indicated that our mitochondrial fractions were free of contaminating cytosolic RNAs. Using this fractionation method we found that the amount of tRNA imported into the mitochondria was highly reproducible over multiple experiments, with the average amount of Var-tRNAMet-i imported being 10.3% (Fig. 3, B and D).
FIGURE 3.
An in vivo assay for tRNA import in trypanosomes. A, shown is doxycycline-regulated expression of Var-tRNAMet-i in T. brucei. Total cell RNA from 2 × 107 trypanosomes were grown with (+) or without (−) the addition of doxycycline (1 μg/ml) for 24 h. RNA was fractionated by non-denaturing polyacrylamide gel electrophoresis (6%) (top panel), and abundant rRNA and tRNA were visualized by ethidium bromide (EtBr) staining. The Var-tRNAMet-i was detected by Northern blot hybridization and autoradiography (bottom panel). B, shown is mitochondrial import of Var-tRNAMet-i after doxycycline induction. Total cellular and mitochondrial RNA was isolated 24 h post-induction with doxycycline in three independent experiments. Samples were fractionated on 6% polyacrylamide gel, stained with EtBr to visualize the mitochondrial 9 S and 12 S rRNAs, small mature cytosolic rRNAs, and tRNAs (upper panel), and hybridized with a probe for Var-tRNAMet-i (lower panel). C, total cellular (TC) and mitochondrial (M) RNA from control (PC 29−13) was isolated and fractionated by non-denaturing polyacrylamide gel electrophoresis (8%) (top panel), and abundant rRNAs and tRNAs were visualized by ethidium bromide staining. The cytosolic-specific WT tRNAMet-i was detected by Northern blot hybridization and autoradiography. D, shown is quantitative analysis of Var-tRNAMet-i import at 24 h post induction with doxycycline (n = 8). The percentage of tRNA import into the mitochondrion was calculated based on the hybridization of the Var-tRNAMet-i probe to total cellular RNA and RNA extracted from purified mitochondria. E, shown is analysis of tRNA import after RNAi knockdown of eEF1a or eIF2. Samples were fractionated on 6% polyacrylamide gels, stained with EtBr (upper panel) total cellular (TC), and mitochondrial (M) RNA were analyzed by Northern blot (lower panel) for amount of Var-tRNAMet-i imported into mitochondria. F, quantitative analysis of tRNA import for eEF1a (n = 6) and eIF2 (n = 3) was expressed relative to the average level of Var-tRNAMet-i import in wild type cells (10.3%, n = 8).
Previously it was reported that RNAi knockdown of a cytosolic protein, eEF1a, reduced the amount of tRNA imported into the mitochondria, whereas RNAi knockdown of eIF2, also an essential cytosolic protein, had no effect on tRNA import (17). We have verified these results by simultaneous doxycycline induction of expression of the Var-tRNAMet-i and either eEF1a or eIF2 RNAi in our cell lines (Fig. 3, E and F). The import of Var-tRNAMet-i was reduced 50–60% after 24 h of RNAi induction in the eEF1a cells, whereas Var-tRNAMet-i import was unaffected after induction of eIF2 RNAi. Based on the corroboration of previous results (17), we can utilize this in vivo tRNA import assay to evaluate the role of candidate proteins in tRNA import.
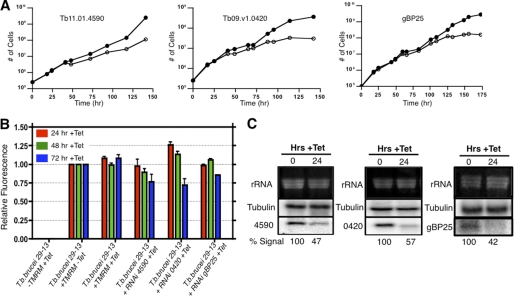
Identification of the tRNA import machinery by RNAi knockdown of candidate proteins is complicated by the fact that fully energized mitochondria are necessary for cell viability. Therefore, the loss of proteins affecting electron transport or oxidative phosphorylation in these experiments may give false positive results due to downstream effects on overall cellular viability. To ensure that we were monitoring tRNA import in viable cells, cell growth and the mitochondrial membrane potential were measured for each cell line after RNAi induction (supplemental Fig. 2; Fig. 4). Cell lines that displayed an RNAi-induced reduction in membrane potential at our 24-h assay time point were not further characterized, as any reduction in tRNA import could represent a downstream effect due to the overall health of the cell.
FIGURE 4.
RNAi analysis of candidate tRNA import complex proteins. A, shown is growth of Tb11.01.4590, Tb09.v1.0420, and gBP25 RNAi cell lines in the presence (open circles) or absence of doxycycline (1 μg/ml) (closed circles). B, shown is mitochondrial membrane potential analysis in wild type T. brucei 29−13 and RNAi cell lines Tb11.01.4590, Tb09.v1.0420, and gBP25. Cell cultures were treated with doxycycline (1 μg/ml), and the mitochondrial membrane potential was determined by relative fluorescence intensity of 2 × 106 total cells after the addition of tetramethylrhodamine methyl ester (TMRM; 20 nm). Samples were tested in triplicate 24, 48, and 72 h post induction. Tet, tetracycline. C, shown is a Northern blot analysis of the effects of RNAi on mRNA level for Tb11.01.4590, Tb09.v1.0420, and gBP25. Total cellular RNA was isolated 0 and 24 h post induction with 1 μg/ml doxycycline, and the percentage mRNA signal for each candidate was normalized to β-tubulin.
RNAi knockdowns of Tb927.10.15220, Tb11.02.0445 and gBP21 had a minimal affect on cell growth and tRNA import (supplemental Fig. 2). RNAi knockdown of the Tb927.10.4280, a member of the putative Leishmania RIC, slowed cell growth and reduced both the mitochondrial membrane potential and RNA import after 24 h of induction (supplemental Fig. 2). Because Tb927.10.4280 displays homology to components of complex III of the electron transport chain, the reduction in cell growth and the decrease in mitochondrial membrane potential were expected. The loss of mitochondrial membrane potential made it impossible to evaluate the role of Tb927.10.4280 in tRNA import. In addition, this protein was not identified in subsequent PTP pulldown assays with other putative tRNA translocon proteins, suggesting it was a contaminant in the initial MS analysis.
RNAi knockdown of the other three candidate proteins, Tb11.01.4590, Tb09.v1.0420, and gBP25, had no affect on cell growth or mitochondrial membrane potential in the first 24 h of induction. However, prolonged induction resulted (48–72 h) in a significant loss of mitochondrial membrane potential and arrest of cell growth, suggesting that these proteins are essential for trypanosome viability (Fig. 4, A and B). For each cell line, RNAi induction resulted in at least a 2-fold reduction of mRNA for each candidate protein after 24 h of induction (Fig. 4C).
To determine the role of each of these proteins in mitochondrial tRNA import, we used RNAi and monitored the movement of newly synthesized Var-tRNAMet-i across the mitochondrial membrane (Fig. 5A). RNAi of gBP25 mRNA had no effect on the import of Var-tRNAMet-i (Fig. 5, A and B). Thus, although essential for mitochondrial biogenesis, gBP25 is not required for tRNA import and was likely identified by pulldown analysis because of the known RNA binding properties of gBP25 (38). We next examined the effect of RNAi of Tb11.01.4590 and Tb09.v1.0420 expression on Var-tRNAMet-i import and found that the amount of Var-tRNAMet-i imported into the mitochondria was significantly reduced after 24 h of induction when compared with uninduced controls (Fig. 5, A and B). RNAi knockdown of Tb11.01.4590 and Tb09.v1.0420 expression reduced the amount of Var-tRNAMet-i imported into the mitochondria to 33 and 56% that of wild type levels, respectively (Fig. 5B). Furthermore, to examine the purity of mitochondrial RNA in these preparations, it was noted that cytosolic-specific rRNAs were not detected by ethidium bromide staining (Fig. 5A and supplemental Fig. 3). Mitochondrial RNA purity was also examined by probing for the cytosolic specific WT tRNAMet-i. It has previously been shown that WT tRNAMet-i is not imported into the mitochondria of T. brucei (39). By using a probe-specific for WT tRNAMet-i, we were able to show that our mitochondrial RNA preparations are devoid of cytosolic tRNA contamination (supplemental Fig. 3). These studies suggest that both Tb09.v1.0420 and Tb11.01.4590 are components of the mitochondrial tRNA import complex of T. brucei. Interestingly, Tb09.v1.0420 is the first protein found in both the trypanosome and Leishmania tRNA import complexes.
FIGURE 5.
Identification of two T. brucei mitochondrial membrane proteins required for tRNA import. A, shown is analysis of tRNA import into gBP25, Tb09.v1.0420, and Tb11.01.4590 RNAi cell lines. Total cell (TC) RNA and mitochondrial (M) RNA was isolated 24 h post-induction with doxycycline (1 μg/ml) and was fractionated by non-denaturing polyacrylamide gel electrophoresis (8%) (top panel), and abundant rRNAs and tRNAs was visualized by ethidium bromide (EtBr) staining. The Var-tRNAMet-i was detected by Northern blot hybridization and autoradiography (bottom panel). Percent import was expressed as the relative hybridization of mitochondrial to total cell Var-tRNAMet-i. 9 S rRNA was used as a loading control (middle panel). B, shown is quantitative analysis of relative tRNA import into the mitochondria for gBP25 (n = 5), Tb09.v1.0420 (n = 6), and Tb11.01.4590 (n = 8). tRNA import was expressed relative to level of Var-tRNAMet-i import in wild type cells (10.3%, n = 8).
Identification of Associated Components tRNA Import Complex by Tandem Affinity Purification
To further characterize the tRNA translocon of T. brucei, we used tandem affinity purification to identify proteins that interact with Tb11.01.4590 and Tb09.v1.0420 (35). Each protein was C-terminally PTP-tagged to avoid interference with a N-terminal MLS signal. However, the single allele knock-in of PTP-tagged Tb09.v1.0420 resulted in a slow growth phenotype as well as a significant reduction in mitochondrial membrane potential. Subcellular fractionation analysis indicated that PTP-tagged Tb09.v1.0420 was localized to the cytosol (not shown), although this protein has previously been shown to be a component of the mitochondria (40). Because the PTP-tagged Tb09.v1.0420 affected growth and mitochondrial activities, it could not be analyzed further.
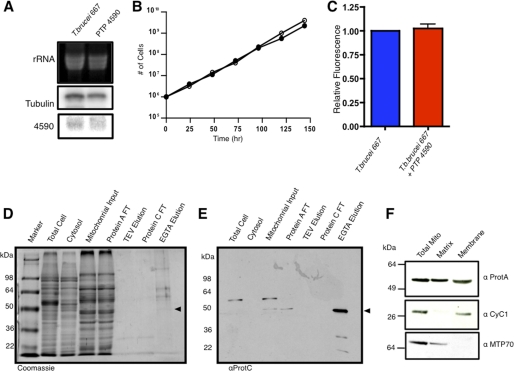
The PTP-tagged Tb11.01.4590 gene was expressed at levels comparable with the wild type Tb11.01.4590, and cells exhibited normal growth and mitochondrial membrane potential (Fig. 6, A, B, and C). Cell fractionation studies showed that the PTP-tagged protein of expected molecular weight (57.3 kDa) localized to the mitochondrion (Fig. 6E). Tandem affinity purification resulted in the elution of a highly purified complex composed of several polypeptides, one of which was the expected size (41.6 kDa) for the purified Tb11.01.4590 (Fig. 6D). The presence of Tb11.01.4590 in the tandem affinity-purified proteins was verified by Western blot (Fig. 6E). Furthermore, it was found that Tb11.01.4590 localizes to both the mitochondrial membrane and matrix fractions (Fig. 6F). Proteins from the PTP-tagged Tb11.01.4590 purification were fractionated on SDS-PAGE, and stained bands were excised and analyzed by LC-MS/MS. From this analysis, nine proteins associated with Tb11.01.4590 were identified (Table 2). Of these nine proteins, seven were specifically pulled down in our tRNA binding analysis (supplemental Table 1). Although some of these proteins did not satisfy all of the criteria we used for further analysis, the sequence scores were highly significant (minimum of two peptides with at least one peptide matched at >95% confidence). Four of the proteins identified (Tim17, mHsp70, mHsp60, and mHsp20) are known to function in mitochondrial protein import or as protein chaperones in other organisms and trypanosomes (26, 41–43). Of these proteins, mHsp70, mHsp60, and mHsp20 were also purified in the initial tRNA affinity purifications and were identified by mass spectrometry (supplemental Table 1). Four hypothetical proteins, Tb927.6.1680, Tb09.211.2530, Tb927.8.1740, and Tb11.03.0475 were also identified by PTP-tagged Tb11.01.4590 pulldown assays and by tRNA affinity purification. Based on BLASTp analysis, the hypothetical protein Tb927.6.1680 contains a C2H2 zinc finger motif; however, neither Tb927.8.1740 nor Tb11.03.0475 display domain homology by BLASTp. Consistent with the requirement for tRNA import throughout the life cycle of T. brucei, all proteins identified by the PTP pulldown of Tb11.01.4590 are constitutively expressed in both the BF and PF trypanosome life stages (44).
FIGURE 6.
Purification of tRNA translocon by PTP-tagged Tb11. 01.4590. A, shown is a Northern blot analysis of total cellular RNA in PTP-tagged Tb11.01.4590 cell lines. The level of expression of the PTP-tagged Tb11.01.4590 mRNA was compared with the level of Tb11.01.4590 in wild type T. brucei (667). B, the growth rates of the PTP-tagged Tb11.01.4590 (open circles) and wild type T. brucei (closed circles) were determined. C, shown is measurement of mitochondrial membrane potential for wild type T. brucei (blue bar) and PTP-tagged Tb11.01.4590 (red bar). D, shown is subcellular fractionation and affinity purification of PTP-tagged Tb11.01.4590 and associated proteins. Samples from total cell protein, mitochondrial, and cytosolic fractions and from tandem affinity purification were stained with Coomassie Blue to visualize proteins. The flow-through (FT) and TEV protease eluate (TEV elution) from the protein A affinity purification and the flow-through (Protein C FT) and EGTA eluate (EGTA Elution) from the protein C affinity purification are shown. E, shown is a Western blot analysis of the gel in panel E probed with anti-protein C. The PTP-tagged Tb11.01.4590 from the mitochondrial fraction is 57.3 kDa, whereas the final purified protein after TEV cleavage is 41.6 kDa. F, mitochondrial fractionation and Western blots of PTP-tagged Tb11.01.4590 localizes to both the matrix and membrane of the mitochondria. Cytochrome C1 (CyC1) and mitochondrial HSP70 (MTP70) were used as membrane and matrix markers, respectively.
TABLE 2.
LC-MS/MS identification of proteins purified by Tb11.01.4590 PTP pulldown assays
TM, transmembrane.
| Gene ID | Protein ID | Pulled down by tRNA? | Predicted MLS or TMa | Domain homologyb | PF/BF mRNA expressionc |
|---|---|---|---|---|---|
| Tb927.8.1740 | Hypothetical | Yesd | Mito | None | Yes |
| Tb11.01.4870 | Tim17 | No | Both | Protein import | Yes |
| Tb927.3.3330 | HSP20 | Yesd | Mito | Chaperone | Yes |
| Tb927.6.1680 | Hypothetical | Yesd | Mito | C2H2-zinc finger | Yes |
| Tb927.10.6400 | HSP60 | Yes | Mito | Protein import | Yes |
| Tb11.03.0475 | Hypothetical | Yesd | Mito | None | Yes |
| Tb927.6.3740 | HSP70 | Yes | Mito | Protein import | Yes |
| Tb11.02.3310 | Hypothetical | Yesd | Both | None | Yes |
| Tb09.211.2530 | Hypothetical | No | Both | None | Yes |
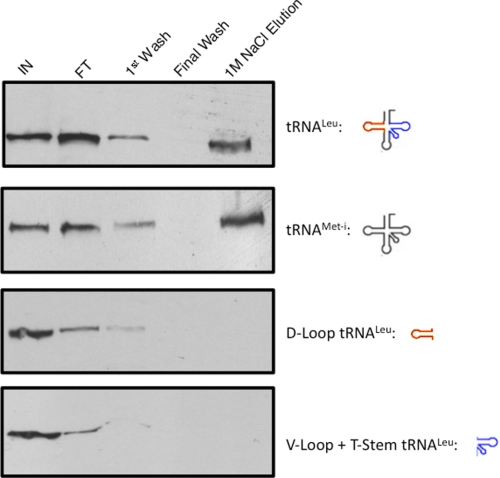
Finally, the identification of Tb11.01.4590 as potential component of the tRNA translocon allowed us to address the specificity of this complex for imported tRNAs. Biotinylated tRNALeu(CAA) and cytosol-specific tRNAMet-i were coupled to resin and used as affinity ligands for mitochondrial protein preparations (Fig. 7). Binding to the putative tRNA translocon was evaluated using our standard purification assay monitoring the elution of PTP-tagged Tb11.01.4590 under stringent wash conditions by Western blot with anti-protein A. Both full-length tRNAs bound the PTP-tagged Tb11.01.4590-containing complex. However, binding was not nonspecific. We found that intact tRNAs were needed for high affinity binding as truncated products, either the D-loop of tRNALeu(CAA) or the V-loop/T-stem of tRNALeu(CAA), were unable to bind the PTP-tagged Tb11.01.4590-containing complex (Fig. 7).
FIGURE 7.
Binding of PTP-tagged Tb11.01.4590 containing complexes to tRNAs. Isolated, intact mitochondria from cells constitutively expressing the PTP-tagged Tb11.01.4590 protein were incubated with a resin that either contained full-length tRNALeu(CAA), the D-loop from tRNALeu(CAA) (red), the variable loop/T-stem from tRNALeu(CAA) (blue), or the cytosolic-specific WT tRNAMet-i. Unbound mitochondria were washed away (IN), and the bound mitochondrial fraction was solubilized with 1% Triton (FT). After solubilization, bound protein was washed extensively using a high salt wash buffer (1st and Final Wash). Bound protein was eluted using a single 1 m NaCl salt elution. PTP-tagged Tb11.01.4590 was detected by Western blot analysis of the gels with anti-protein A.
DISCUSSION
Here we report the identification of two mitochondrial membrane proteins (Tb11.01.4590 and Tb09.v1.0420) that are functionally involved in tRNA import. Tb09.v1.0420 is the only shared component of the RNA import machinery identified in trypanosomes and Leishmania. Using Tb11.01.4590 in tandem affinity purification, nine associated proteins were identified including multiple subunits of the mitochondrial protein import machinery. Seven of these proteins were also identified in LC-MS/MS analysis of tRNA binding complexes (supplemental Table 1), further supporting the role for components of the protein import machinery in tRNA import.
Previous characterization of L. tropica led to the identification of an 11-subunit protein complex, called the RIC, involved in the translocation of tRNAs into the mitochondrion. However, multiple protein components of the Leishmania RIC are subunits of mitochondrial oxidative phosphorylation complexes, including subunits IV and VI of cytochrome c oxidase and the Reiske iron sulfur protein from cytochrome bc1 reductase (22). If these proteins have dual functions in mitochondrial ATP production and tRNA import in Leishmania, it is likely that African trypanosomes use a distinctly different tRNA translocon. Also, because of their proposed dual function in mitochondrial bioenergetics and tRNA import, care must be taken to distinguish a primary role in tRNA translocation and simply compromising mitochondrial metabolic function.
African trypanosomes have complex life cycles with extensive metabolic changes associated with transition of the parasites from the digestive tract of the insect vector to the bloodstream of the mammalian host. Whereas the mitochondrion of the PF of the parasite is fully active, generating much of its ATP by electron transport coupled oxidative phosphorylation, the BF of T. brucei contains no detectable cytochromes, lacks oxidative phosphorylation, and produces ATP solely by glycolysis (45–47). Transcript and proteomic analysis have verified that many of the nuclear-encoded subunits of the cytochrome c oxidase and cytochrome c reductase are absent in BF T. brucei (46–49). Because tRNAs are imported into both PF and BF T. brucei mitochondria to the same extent, developmentally regulated subunits of the electron transport chain cannot be part of the constitutive tRNA translocon (25). In addition, recent studies have brought into question the role of the Reiske iron sulfur protein and the α-subunit of the F1F0-ATP synthase in Leishmania tRNA import complex (12, 23). In this study we were able to identify only two subunits of the Leishmania RIC (Tb09.v1.0420 and Tb927.10.4280) by tRNA affinity purification from T. brucei mitochondria (Table 1 and Fig. 2). These results suggest that whereas closely related organisms may share some components of the tRNA import machinery, differences may have evolved as a consequence of distinct metabolic differences between the two. Alternatively, the presence of subunits of the mitochondrial respiratory complexes may simply reflect contamination of the Leishmania RIC with abundant mitochondrial membrane proteins (23). Additional studies are needed to resolve this issue.
Although much is known about the physical properties of tRNA import into trypanosome mitochondria, only two mitochondrial proteins, Tim17 and mHsp70, have been shown to be directly involved in translocation (12). Thus far, the only other protein that has been identified as having a function in mitochondrial tRNA import is the cytoplasmic eEF1a (17). Our studies fully support a role for eEF1a in tRNA import (Fig. 3F). We also show two other trypanosome mitochondrial proteins (Tb11.01.4590 and Tb09.v1.0420) are necessary for tRNA import (Fig. 5). Furthermore, Tb11.01.4590 was pulled down by mitochondrial (tRNALeu(CAA))- and cytosolic (WT tRNAMet-i)-localized tRNAs (Fig. 7). However, when the variable loop/T-stem or the D-loop from tRNALeu(CAA) were used as bait, Tb11.01.4590 was not pulled down. This suggests that the import complex recognizes full-length tRNA substrates and that the specificity for delivery of tRNA into mitochondria may lie within the previously characterized chaperone protein eEF1a (17), which binds specifically to the T-stem of mitochondria-targeted tRNA and not to the cytosolic WT tRNAMet-i. In addition, we identified a nine-subunit mitochondrial protein complex that interacts with Tb11.01.4590 and is composed of five hypothetical proteins and three proteins (Tim17, mHsp70, and mHsp60) that function as protein chaperones in mitochondrial protein import (Table 2) (26, 41, 42). The other protein identified, mHsp20, has been shown to interact with mHsp70 to refold denatured protein after import into mitochondria (43). All of the proteins identified as interacting with Tb11.01.4590 by tandem affinity purification have previously been shown to localize to the mitochondria (40). Based on the known subcellular localization of many of the identified proteins in the purified tRNA import complex (Table 2), it is likely that this complex represents an inner mitochondrial membrane tRNA translocon in T. brucei. Furthermore, the identification of proteins known to function in mitochondrial protein import strongly suggests that there is either a shared mechanism for mitochondrial protein and tRNA import or shared components between translocons.
The identification of components of the mitochondrial protein import machinery, as components of the T. brucei tRNA translocon, is consistent with mitochondrial RNA import in other systems. In yeast it has been shown that a single nuclear-encoded mitochondrial tRNA is co-imported into the mitochondrion with its corresponding pre-tRNA synthetase and requires specific interactions with components of the TIM and TOM proteins import complexes (4). Similarly, in plants, tRNA import requires interactions with Tom20 and Tom40 before mitochondrial translocation (5). Although specific mitochondrial components have not been identified, characterization of the mechanism of 5 S rRNA import into mammalian mitochondria showed a dependence on protein import machinery and co-transport with the cytoplasmic chaperone protein rhodanese (50, 51). In each case a shared mechanism of import has been suggested for protein and RNA translocation into mitochondria.
Recent studies have shown that the T. brucei Tim17 and mHsp70 are necessary for both protein and tRNA import into trypanosome mitochondria (12). Here we show that Tim 17, mHsp70, and two other proteins implemented in protein import are physically associated with the tRNA translocon of T. brucei. These results further support the possibility of a membrane transporter capable of moving both RNA and protein into trypanosome mitochondria and suggest that yeast, plant, mammals, and trypanosomes may have functionally similar translocons. Further functional analysis of the components of the tRNA translocon will need to be conducted to determine whether there is truly a shared mechanism between mitochondrial protein and tRNA import in these organisms.
Supplementary Material
Acknowledgments
We thank Andre Schneider, Arthur Gunzl and David Engman for providing plasmids and antibodies used in this study. We thank Steven Sykes for aiding with native gel preparation and proteomic analysis. We also thank Melissa Lerch, Robert Sabatini, and current and past members of the Hajduk laboratory for discussion throughout the research presented here.
This work was supported, in whole or in part, by National Institutes of Health Grant R01AI061356.

This article contains supplemental Figs. 1–3.
S. Sykes and S. Hajduk, personal communication.
- RIC
- RNA import complex
- eEF1a
- eukaryotic elongation factor 1a
- Tim17
- translocase inner membrane 17
- BF
- bloodstream form
- PF
- procyclic form
- MLS
- mitochondrial localization signal
- PTP
- Protein C Epitope-Tobacco Etch Virus-Protein A Epitope.
REFERENCES
- 1. Schneider A., Maréchal-Drouard L. (2000) Mitochondrial tRNA import. Are there distinct mechanisms? Trends Cell Biol. 10, 509–513 [DOI] [PubMed] [Google Scholar]
- 2. Salinas T., Duchêne A. M., Maréchal-Drouard L. (2008) Recent advances in tRNA mitochondrial import. Trends Biochem. Sci. 33, 320–329 [DOI] [PubMed] [Google Scholar]
- 3. Rubio M. A., Rinehart J. J., Krett B., Duvezin-Caubet S., Reichert A. S., Söll D., Alfonzo J. D. (2008) Mammalian mitochondria have the innate ability to import tRNAs by a mechanism distinct from protein import. Proc. Natl. Acad. Sci. 105, 9186–9191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tarassov I., Entelis N., Martin R. P. (1995) An intact protein translocating machinery is required for mitochondrial import of a yeast cytoplasmic tRNA. J. Mol. Biol. 245, 315–323 [DOI] [PubMed] [Google Scholar]
- 5. Salinas T., Duchêne A. M., Delage L., Nilsson S., Glaser E., Zaepfel M., Maréchal-Drouard L. (2006) The voltage-dependent anion channel, a major component of the tRNA import machinery in plant mitochondria. Proc. Natl. Acad. Sci. U.S.A. 103, 18362–18367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schneider A., Martin J., Agabian N. (1994) A nuclear encoded tRNA of Trypanosoma brucei is imported into mitochondria. Mol. Cell. Biol. 14, 2317–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rusconi C. P., Cech T. R. (1996) The anticodon is the signal sequence for mitochondrial import of glutamine tRNA in Tetrahymena. Genes Dev. 10, 2870–2880 [DOI] [PubMed] [Google Scholar]
- 8. Adhya S., Ghosh T., Das A., Bera S. K., Mahapatra S. (1997) Role of an RNA-binding protein in import of tRNA into Leishmania mitochondria. J. Biol. Chem. 272, 21396–21402 [DOI] [PubMed] [Google Scholar]
- 9. Yermovsky-Kammerer A. E., Hajduk S. L. (1999) In vitro import of a nuclearly encoded tRNA into the mitochondrion of Trypanosoma brucei. Mol. Cell. Biol. 19, 6253–6259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lima B. D., Simpson L. (1996) Sequence-dependent in vivo importation of tRNAs into the mitochondrion of Leishmania tarentolae. RNA 2, 429–440 [PMC free article] [PubMed] [Google Scholar]
- 11. Sherrer R. L., Yermovsky-Kammerer A.E., Hajduk S. L. (2003) A sequence motif within trypanosome precursor tRNAs influences abundance and mitochondrial localization. Mol. Cell. Biol. 23, 9061–9072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tschopp F., Charrière F., Schneider A. (2011) In vivo study in Trypanosoma brucei links mitochondrial transfer RNA import to mitochondrial protein import. EMBO Rep. 12, 825–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simpson A. M., Suyama Y., Dewes H., Campbell D. A., Simpson L. (1989) Kinetoplastid mitochondria contain functional tRNAs which are encoded in nuclear DNA and also contain small minicircle and maxicircle transcripts of unknown function. Nucleic Acids Res. 17, 5427–5445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hancock K., Hajduk S. L. (1990) The mitochondrial tRNAs of Trypanosoma brucei are nuclear encoded. J. Biol. Chem. 265, 19208–19215 [PubMed] [Google Scholar]
- 15. Tan T. H., Pach R., Crausaz A., Ivens A., Schneider A. (2002) tRNAs in Trypanosoma brucei. Genomic organization, expression, and mitochondrial import. Mol. Cell. Biol. 22, 3707–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mukherjee S., Bhattacharyya S. N., Adhya S. (1999) Stepwise transfer of tRNA through the double membrane of Leishmania mitochondria. J. Biol. Chem. 274, 31249–31255 [DOI] [PubMed] [Google Scholar]
- 17. Bouzaidi-Tiali N., Aeby E., Charrière F., Pusnik M., Schneider A. (2007) Elongation factor 1a mediates the specificity of mitochondrial tRNA import in T. brucei. EMBO J. 26, 4302–4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mahapatra S., Ghosh S., Bera S. K., Ghosh T., Das A., Adhya S. (1998) The D arm of tRNATyr is necessary and sufficient for import into Leishmania mitochondria in vitro. Nucleic Acids Res. 26, 2037–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhattacharyya S. N., Chatterjee S., Goswami S., Tripathi G., Dey S. N., Adhya S. (2003) “Ping-pong” interactions between mitochondrial tRNA import receptors within a multiprotein complex. Mol. Cell. Biol. 23, 5217–5224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chatterjee S., Home P., Mukherjee S., Mahata B., Goswami S., Dhar G., Adhya S. (2006) An RNA binding respiratory component mediates import of type II tRNAs into Leishmania mitochondria. J. Biol. Chem. 281, 25270–25277 [DOI] [PubMed] [Google Scholar]
- 21. Goswami S., Dhar G., Mukherjee S., Mahata B., Chatterjee S., Home P., Adhya S. (2006) A bifunctional tRNA import receptor from Leishmania mitochondria. Proc. Natl. Acad. Sci. U.S.A. 103, 8354–8359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mukherjee S., Basu S., Home P., Dhar G., Adhya S. (2007) Necessary and sufficient factors for the import of transfer RNA into the kinetoplast mitochondrion. EMBO Rep. 8, 589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paris Z., Rubio M. A., Lukes J., Alfonzo J. D. (2009) Mitochondrial tRNA import in Trypanosoma brucei is independent of thiolation and the Rieske protein. RNA 15, 1398–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schekman R. (2010) Editorial expression of concern: a bifunctional tRNA import receptor from Leishmania mitochondria. Proc. Natl. Acad. Sci. U.S.A. 107, 9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cristodero M., Seebeck T., Schneider A. (2010) Mitochondrial translation is essential in bloodstream forms of Trypanosoma brucei. Mol. Microbiol. 78, 757–769 [DOI] [PubMed] [Google Scholar]
- 26. Singha U. K., Peprah E., Williams S., Walker R., Saha L., Chaudhuri M. (2008) Characterization of the mitochondrial inner membrane protein translocator Tim17 from Trypanosoma brucei. Mol. Biochem. Parasitol. 159, 30–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cunningham I. (1977) New culture medium for maintenance of tsetse tissues and growth of trypanosomatids. J. Protozool. 24, 325–329 [DOI] [PubMed] [Google Scholar]
- 28. Harris M. E., Moore D. R., Hajduk S. L. (1990) Addition of uridines to edited RNAs in trypanosome mitochondria occurs independently of transcription. J. Biol. Chem. 265, 11368–11376 [PubMed] [Google Scholar]
- 29. Ochsenreiter T., Hajduk S. L. (2006) Alternative editing of cytochrome c oxidase III mRNA in trypanosome mitochondria generates protein diversity. EMBO Rep. 7, 1128–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tetley L., Vickerman K. (1985) Differentiation in Trypanosoma brucei. Host-parasite cell junctions and their persistence during acquisition of the variable antigen coat. J. Cell Sci. 74, 1–19 [DOI] [PubMed] [Google Scholar]
- 31. Wittig I., Braun H. P., Schägger H. (2006) Blue native PAGE. Nat. Protoc. 1, 418–428 [DOI] [PubMed] [Google Scholar]
- 32. Crausaz Esseiva A., Maréchal-Drouard L., Cosset A., Schneider A. (2004) The T-stem determines the cytosolic or mitochondrial localization of trypanosomal tRNAs Met. Mol. Biol. Cell 15, 2750–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Z., Morris J. C., Drew M. E., Englund P. T. (2000) Inhibition of Trypanosoma brucei gene expression by RNA interference using an integratable vector with opposing T7 promoters. J. Biol. Chem. 275, 40174–40179 [DOI] [PubMed] [Google Scholar]
- 34. Scaduto R. C., Jr., Grotyohann L. W. (1999) Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys. J. 76, 469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schimanski B., Nguyen T. N., Günzl A. (2005) Highly efficient tandem affinity purification of trypanosome protein complexes based on a novel epitope combination. Eukaryot. Cell 4, 1942–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gunzl A., Schimanski B. (2009) Unit 19.19 Tandem affinity purification of proteins. Curr. Protoc. Protein Sci. 55, 19.19.11–19.19.16 [DOI] [PubMed] [Google Scholar]
- 37. Zíková A., Schnaufer A., Dalley R. A., Panigrahi A. K., Stuart K. D. (2009) The F0F1-ATP synthase complex contains novel subunits and is essential for procyclic Trypanosoma brucei. PLoS Pathog. 5, e1000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Blom D., Burg J. v., Breek C. K., Speijer D., Muijsers A. O., Benne R. (2001) Cloning and characterization of two guide RNA-binding proteins from mitochondria of Crithidia fasciculata. gBP27, a novel protein, and gBP29, the orthologue of Trypanosoma brucei gBP21. Nucleic Acids Res. 29, 2950–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tan T. H., Bochud-Allemann N., Horn E. K., Schneider A. (2002) Eukaryotic-type elongator tRNAMet of Trypanosoma brucei becomes formylated after import into mitochondria. Proc. Natl. Acad. Sci. U.S.A. 99, 1152–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Panigrahi A. K., Ogata Y., Zíková A., Anupama A., Dalley R. A., Acestor N., Myler P. J., Stuart K. D. (2009) A comprehensive analysis of Trypanosoma brucei mitochondrial proteome. Proteomics 9, 434–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Manning-Krieg U. C., Scherer P. E., Schatz G. (1991) Sequential action of mitochondrial chaperones in protein import into the matrix. EMBO J. 10, 3273–3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bömer U., Meijer M., Maarse A. C., Hönlinger A., Dekker P. J., Pfanner N., Rassow J. (1997) Multiple interactions of components mediating preprotein translocation across the inner mitochondrial membrane. EMBO J. 16, 2205–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee G. J., Vierling E. (2000) A small heat shock protein cooperates with heat shock protein 70 systems to reactivate a heat-denatured protein. Plant Physiol. 122, 189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nilsson D., Gunasekera K., Mani J., Osteras M., Farinelli L., Baerlocher L., Roditi I., Ochsenreiter T. (2010) Spliced leader trapping reveals widespread alternative splicing patterns in the highly dynamic transcriptome of Trypanosoma brucei. PLoS Pathog. 6, e1001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vickerman K. (1985) Developmental cycles and biology of pathogenic trypanosomes. Br. Med. Bull. 41, 105–114 [DOI] [PubMed] [Google Scholar]
- 46. Michelotti E. F., Hajduk S. L. (1987) Developmental regulation of trypanosome mitochondrial gene expression. J. Biol. Chem. 262, 927–932 [PubMed] [Google Scholar]
- 47. Priest J. W., Hajduk S. L. (1994) Developmental regulation of Trypanosoma brucei cytochrome c reductase during bloodstream to procyclic differentiation. Mol. Biochem. Parasitol. 65, 291–304 [DOI] [PubMed] [Google Scholar]
- 48. Tasker M., Timms M., Hendriks E., Matthews K. (2001) Cytochrome oxidase subunit VI of Trypanosoma brucei is imported without a cleaved presequence and is developmentally regulated at both RNA and protein levels. Mol. Microbiol. 39, 272–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mayho M., Fenn K., Craddy P., Crosthwaite S., Matthews K. (2006) Post-transcriptional control of nuclear-encoded cytochrome oxidase subunits in Trypanosoma brucei. Evidence for genome-wide conservation of life cycle stage-specific regulatory elements. Nucleic Acids Res. 34, 5312–5324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Entelis N. S., Kolesnikova O. A., Dogan S., Martin R. P., Tarassov I. A. (2001) 5 S rRNA and tRNA import into human mitochondria. Comparison of in vitro requirements. J. Biol. Chem. 276, 45642–45653 [DOI] [PubMed] [Google Scholar]
- 51. Smirnov A., Comte C., Mager-Heckel A. M., Addis V., Krasheninnikov I. A., Martin R. P., Entelis N., Tarassov I. (2010) Mitochondrial enzyme rhodanese is essential for 5 S ribosomal RNA import into human mitochondria. J. Biol. Chem. 285, 30792–30803 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.