Background: The transcriptional coactivator YAP has a dual role, stimulating cell proliferation and promoting apoptosis.
Results: YAP is phosphorylated and activated in response to genotoxic stress.
Conclusion: The phosphorylation of YAP at multiple sites activates transcription and protects against apoptosis.
Significance: Hyperphosphorylation provides a mechanism by which YAP regulates transcription and apoptosis.
Keywords: Apoptosis, Caspase, Coactivator Transcription, DNA Damage Response, Phosphorylation
Abstract
The transcriptional coactivator Yes-associated protein (YAP) has been implicated in tumorigenesis by regulating cell proliferation and apoptosis. YAP interacts with the transcription factor TEAD and is essential in mediating TEAD-dependent gene expression. Here we show that YAP is hyperphosphorylated and activated in response to genotoxic stress such as UV irradiation and cisplatin treatment. Using high resolution mobility shift assay for phosphorylated proteins, we identified multiple sites of phosphorylation induced by UV irradiation. Pretreatment with p38 and JNK inhibitors completely suppressed the mobility retardation of phosphorylated YAP in UV-irradiated cells. Co-immunoprecipitation experiments showed that the physical interaction of YAP with TEAD was markedly enhanced by UV irradiation or CDDP treatment but suppressed by pretreatment with p38 and JNK inhibitors. Similarly, pretreatment with p38 and JNK inhibitors suppressed the expression of YAP/TEAD target genes, which were elevated on exposure to genotoxic stress. Using phosphomimetic and phosphorylation-deficient YAP mutants, we showed that the coactivator activity of YAP correlated with its state of phosphorylation and sensitivity to cisplatin-induced apoptosis. Our results demonstrate that multisite phosphorylation of YAP induces YAP/TEAD-dependent gene expression and provides a mechanism by which YAP regulates apoptosis differently depending on cellular context.
Introduction
The opposing actions of proliferation and apoptosis control cell numbers in particular tissues and organs and the coordination of cell fate is fundamental to animal development (1). The Hippo signaling pathway plays a key role in controlling both cell proliferation and apoptosis (2). Components of the Hippo pathway such as Merlin, Lats, and MST, are known to contribute to tumorigenesis (3–5). The MST-SAV complex phosphorylates and activates Lats, an NDR family kinase. Lats inhibits YAP,2 a transcriptional coactivator, via phosphorylation of HXRXX(S/T) motif. This mechanism of regulation is involved in cell contact inhibition and tissue growth control and epithelial-mesenchymal transition. YAP binds to and activates TEAD, a transcription factor essential to the biological functions of YAP (6–8). YAP is the candidate oncogene in the human chromosome 11q22 amplicon that is evident in several cancers (9, 10). Elevated YAP levels and increased nuclear localization have been reported in multiple cancerous tissues (11). Moreover, YAP overexpression in MCF10A induces epithelial-mesenchymal transition, a hallmark of tumorigenic transformation (10). YAP overexpression stimulates proliferation and increases saturation cell density in monolayer cultures of NIH-3T3 cells (11). In a conditional transgenic mouse model, overexpression of YAP causes a dramatic increase in liver size and eventually leads to tumorigenesis (12, 13). Conversely, YAP is a pro-apoptotic regulator in mammalian cells. Recent studies have established that p73 is a transcription factor that mediates YAP-dependent activation of pro-apoptotic genes (14, 15). p73, a p53 paralogue, has been shown to induce apoptosis in a variety of cell lines and supports transcription from promoters containing the p53 response element (16). YAP binds to p73, but not to p53, through its WW domain and a PPPY motif of p73. This interaction prevents p73 from proteasomal degradation by excluding the association of p73 with E3 ligase Itch, which also binds to p73 via WW domain (17). The binding of YAP to p73 activates the transcription of target genes. Upon exposure to DNA damage, YAP is tyrosine-phosphorylated by c-Abl and selectively coactivates p73 target genes such as BAX (18). In addition, p73/YAP is regulated by an autoregulatory feedback loop in which PML, a direct transcriptional target of p73/YAP, physically interacts with and stabilizes YAP (15). Additionally, YAP is phosphorylated on Ser-127 by Akt, and this modification attenuates p73-mediated apoptosis (19). Ser-127 phosphorylation, which is also generated by Lats, renders YAP transcriptionally inactive by binding to 14-3-3, a cytoplasmic anchor for phosphoproteins (11).
Here, we describe a novel regulatory mechanism for YAP. YAP was hyperphosphorylated and activated in response to genotoxic stress. We identified multiple sites of phosphorylation induced by UV irradiation, which was crucial to the coactivator function of YAP. Our results indicate that the multisite phosphorylation of YAP regulates the expression of YAP/TEAD target genes and provides a mechanism by which YAP regulates apoptosis.
EXPERIMENTAL PROCEDURES
Plasmids
YAP cDNA sequence was cloned from Image clones 5747370 and 5106309. The coding sequence was amplified using KOD polymerase (Toyobo) and cloned in-frame with a Myc tag into pcDNA3 and lentiviral vector. The cDNA encodes a YAP isoform of 488 residues with two WW domains. The numbering of the polypeptide sequence followed that of the encoded protein from GenBankTM accession number NM_001195044. YAP deletion constructs were amplified from wild-type cDNA with specific primers and cloned into pcDNA3 with an N-terminal Myc tag. Mutation was generated with the QuikChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions. The lentiviral pCSII vector encoding cDNA or short hairpin RNA (shRNA) was described previously (20, 21). To silence YAP gene in an inducible manner, a tetracycline-inducible (Tet-on) expression system of short hairpin RNA was utilized (21). DNA oligonucleotides encoding shRNA for YAP (shYAP) and TAZ (shTAZ) were annealed and subcloned into the lentiviral vector. The oligonucleotide sequences are (hairpin sequences shown in uppercase): shYAP, 5′-CCAGAGAATCAGTCAGAGTttcaagagaACTCTGACTGATTCTCTGGtttttt-3′; shTAZ, 5′-AGGTACTTCCTCAATCACAttcaagagaTGTGATTGAGGAAGTACCTcttttttt-3′. The lentiviral shRNA expression vector for LacZ was described previously (20). All constructs were verified by automated DNA sequencing (Applied Biosystems).
MATERIALS AND METHODS
YAP and Hsp90 antibodies were purchased from Santa Cruz, FLAG M2 was from Sigma, and TEAD and PP2A were from BD Transduction Laboratories. p-p38, p-JNK, PARP, cleaved caspase 9, and cleaved caspase 3 were from Cell Signaling Technology. Myc 9E10 monoclonal antibody was described previously (22). SB203580 and SP600125, anisomycin, and etoposide were purchased from Calbiochem. Cisplatin was from Sigma. Doxycycline was obtained from Nacalai Tesque, and Phos-tag ligand from Phos-tag.com.
Cell Culture, Transfection, and Treatments
Human pharyngeal carcinoma-derived KB, human embryonic kidney 293T, and human osteosarcoma U2OS and SAOS-2 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum (22). U2OS cells were plated on 6-well plates and transfected with Lipofectamine (Invitrogen) as described previously (23). For UV irradiation UV Linker (Funakoshi) was used. Cells on 6-well plates were irradiated with 100 J/m2 for the phosphorylation analysis or 30 J/m2 for other experiments and then incubated for the periods indicated.
Lentiviral Infection
For lentiviral production, 293T cells were transfected with pCSII vector and the accessory plasmids, VSV-G and REV for 12 h using calcium phosphate. After 36 h, the culture medium was filter-sterilized, concentrated by centrifuge for 16 h, and suspended in fresh DMEM with 10% FCS. KB cells were incubated with serial dilutions of viral suspension and selected for a week in the presence of antibiotics. To knock down YAP gene, KB cells infected with the lentiviral vector encoding Tet-on shYAP were incubated with 1 μg/ml doxycycline for at least 72 h.
Detection of Shift in Mobility of Phosphorylated Proteins
Phosphate affinity SDS-PAGE was performed according to the manufacturer's instructions with slight modifications (Phos-tag.com). 7.5% polyacrylamide gel was polymerized with 25 μm of Phos-tag ligand, and conventional SDS-PAGE was performed extensively. The gel was soaked in general transfer buffer containing 1 mm EDTA for 10 min and then buffer without EDTA for 10 min. After transfer to PVDF membrane, Western blotting was conducted as below.
Western Blotting and Immunoprecipitation
Cells were lysed in lysis buffer (50 mm Tris·Cl, pH 7.5, 150 mm NaCl, and 0.5% Nonidet P-40) supplemented with protease inhibitors (Complete Mini, Roche Applied Science). The lysates were resolved by SDS-PAGE and immunoblotted with the indicated antibodies. For immunoprecipitation, the cell lysates were incubated with the appropriate antibody (1∼2 μg) for 3 h or overnight at 4 °C followed by 1 h with protein G-Sepharose beads (GE Healthcare). For phosphatase treatment, immunoprecipitates were washed with lysis buffer and incubated with calf intestinal alkaline phosphatase (Toyobo) for 30 min at 37 °C. Immune complexes were washed at least three times with the lysis buffer before being resolved by SDS-PAGE and immunoblotted with the indicated antibodies. Quantification of the immunoblot bands was performed with MultiGauge software (Fuji).
Reverse Transcription PCR
RNA was isolated using Sepasol (Nacalai Tesque), and a reverse transcription (RT) reaction was performed using ReverTra AceRT kit (Toyobo). After mixing 10 ng of template and SYBR Green PCR Master mix (Toyobo), real-time RT PCR was performed using the StepOne real-time PCR system (Applied Biosystems) with the following primer sets: human YAP forward (5′-CCCGACAGGCCAGTACTGAT-3′) and reverse (5′-CAGAGAAGCTGGAGAGGAATGAG-3′); human TAZ forward (5′-CCAGCCAAATCTCGTGATGAA-3′ and reverse (5′-CGCATTGGGCATACTCATGA-3′); human CTGF forward (5′-TGCACCGCCAAAGATGGT-3′) and reverse (5′-GACTCTCCGCTGCGGTACAC-3′); human ANKRD1 forward (5′-GAACTGGTCACTGGAAAGAAGAATG-3′) and reverse (5′-GGTGGGCTAGAAGTGTCTTCAGA-3′); human p21 forward (5′-TGACAGATTTCTACCACTCCAAACG-3′) and reverse (5′-ATGTAGAGCGGGCCTTTGAG-3′); human BAX forward (5′-CCAAGGTGCCGGAACTGA-3′) and reverse (5′-CCCGGAGGAAGTCCAATGT-3′; human HDM2 forward (5′-CTACAGGGACGCCATCGAA-3′) and reverse (5′-CCAATCACCTGAATGTTCACTTACA-3′); human 14-3-3σ forward (5′-GCAGGCCGAACGCTATGA-3′) and reverse (5′-TCCACGGCGCCTTTCA-3′); human GAPDH forward (5′-GAGTCAACGGATTTGGTCGT-3′) and reverse (5′-GACAAGCTTCCCGTTCTCAG-3′). All primer pairs generated a single product as determined by a melt curve analysis and gel electrophoresis. For analysis of p73 isoform expression, RT PCR was performed using ThermoScript RT PCR system (Invitrogen) according to the manufacturer's instructions. Isoform-specific primer sets used were as follows: TAp73α/β and ΔNp73α/β, forward (5′-AGTTCGGCAGCTACACCCAA-3′) and reverse (5′-GTTGCTAGAGCGGAGCAGCT-3′); TAp73α/β forward (5′-TCTGGAACCAGACAGCACCT-3′) and reverse (5′-GTGCTGGACTGCTGGAAAGT-3′). The cycling parameters consisted of 1 cycle of 94 °C for 3 min then 35 cycles of 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 45 s followed by a single 10-min cycle at 72 °C. RT PCR products were electrophoresed on a 2% agarose gel.
Luciferase Reporter Assay
SAOS-2 cells were seeded in 12-well plates and cotransfected with 100 ng of luciferase reporter construct, 10 ng of Renilla luciferase, and 200 ng of Myc-YAP construct. After 24 h, cells were washed with PBS and lysed, and dual luciferase reporter assay (Promega) was performed according to the manufacturer's instructions. TEAD luciferase reporter was described previously (24). p53 luciferase reporter was from Promega.
Quantification of Apoptosis
KB cells were treated with CDDP (15 μm) for 18 h, and media and PBS wash as well as trypsinized cells were harvested and fixed in 70% ethanol. Cells were stained with 50 μg/ml propidium iodide, and the DNA content was determined by FACS analysis of using a FACSCanto II system (BD Biosciences) and Flowjo software, and the extent of apoptotic cell death was quantified by the percentage of cells showing subdiploid DNA content.
RESULTS
YAP Is Activated by Genotoxic Stress
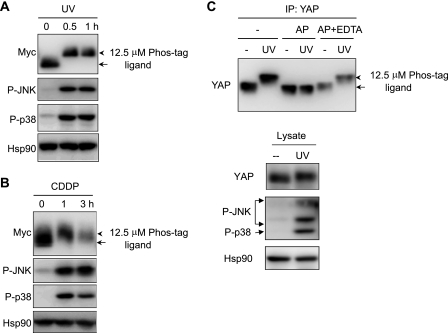
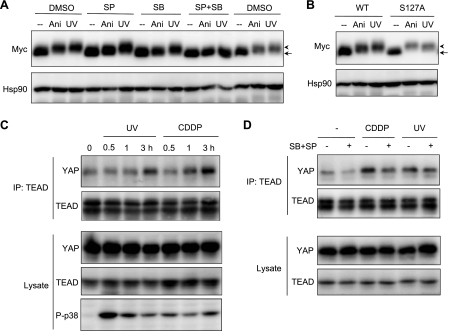
We sought to investigate whether YAP regulates apoptosis and proliferation in the same cellular context. We found that YAP protein mobility shifted on a gel containing Phos-tag ligand, which provides the affinity for phosphorylated proteins on SDS-PAGE in response to genotoxic stress. YAP shifted to a slowly migrating band after UV irradiation and treatment with cisplatin (CDDP) in 293T cells (Fig. 1A). This mobility shift was a rapid event occurring at 0.5 h after UV irradiation and 1 h after CDDP treatment. The time course of the mobility shift was similar to that of p38 and JNK activation, a well known event induced by genotoxic stress, as judged by the appearance of activating phosphorylation signals of these kinases. To further investigate the mobility shift, endogenous YAP was immunoprecipitated and incubated with alkaline phosphatase. The slow migration of endogenous YAP was detected in immunoprecipitates from UV-irradiated cells and, importantly, disappeared in immunoprecipitates incubated with alkaline phosphatase but not by the coaddition of EDTA, an inhibitor of alkaline phosphatase, indicating that the mobility shift was generated by the phosphorylation of YAP (Fig. 1C). Considering the time course of p38/JNK activation and mobility shift of YAP, these kinases were likely to be involved in YAP phosphorylation. Indeed, a mobility shift of Myc-YAP was induced again by anisomycin, a strong chemical inducer of p38/JNK activation, but disappeared partially when p38 or JNK inhibitor was pretreated and, importantly, disappeared entirely when the two inhibitors were added together (Fig. 2A). To examine whether the Hippo pathway is involved in the mobility shift of YAP, Myc-YAP 127A, which is not phosphorylated by Lats, was expressed in U2OS cells. Upon exposure to anisomycin or UV irradiation, Myc-YAP 127A still showed slow mobility compared with Myc-YAP wild-type (WT) (Fig. 2B), suggesting that YAP phosphorylation by the Hippo pathway is independent of the stress-induced shift in mobility of YAP.
FIGURE 1.
UV irradiation or CDDP-induced mobility shift of YAP. A and B, 293T cells were transfected with Myc-YAP construct, and cells were UV-irradiated (100 J/m2) (A) or treated with CDDP (100 μm) (B) for the indicated times. Cell lysates were analyzed by immunoblotting with the antibodies indicated. C, YAP was immunoprecipitated with anti-YAP antibody from UV-irradiated KB cell lysates. Immunoprecipitates (IP) were incubated in the absence or presence of calf intestinal alkaline phosphatase (AP) together with or without EDTA (50 mm) and immunoblotted with anti-YAP antibody. The arrows indicate the position of YAP in untreated sample, and arrowheads indicate YAP with slow mobility. Hsp90 was blotted as a loading control. For detection of mobility shift of YAP, cell lysates were resolved in polyacrylamide gels containing 12.5 μm Phos-tag ligand when indicated.
FIGURE 2.
p38 and JNK inhibitors suppress UV irradiation- or CDDP-induced interaction of YAP with TEAD. A, U2OS cells were transfected with Myc-YAP and preincubated for 2 h without (DMSO) or with JNK inhibitor, SP600125 (SP) (10 μm) and/or with p38 inhibitor, SB202190 (SB) (10 μm). Then cells were treated with anisomycin (Ani) (50 μg/ml) or UV-irradiated (100 J/m2). Cell lysates were analyzed by immunoblotting. B, U2OS cells expressing wild-type YAP (WT) or YAP 127A (127A) were treated and analyzed as in A. Immunoblot with anti-Myc antibody was performed on a gel containing 12.5 μm of Phos-tag ligand (A and B). C, KB cells were UV-irradiated or treated with CDDP (100 μm) for 3 h. Cell lysates were immunoprecipitated with anti-TEAD antibody, and coimmunoprecipitates (IP) were analyzed by immunoblotting with indicated antibodies. D, KB cells were preincubated in the absence or presence of p38/JNK inhibitors (SB+SP) for 1 h and then treated and analyzed as in C.
We then examined whether the function of YAP is regulated by genotoxic stress. Recent studies have shown that TEAD family of transcription factors physically interact with YAP (25, 26). Using coimmunoprecipitation assay, the interaction of endogenous YAP with TEAD was confirmed in untreated KB cells (supplemental Fig. S1). Significantly, the interaction of YAP with TEAD was increased at 3 h after UV irradiation and CDDP treatment (Fig. 2C), which was distinct from the rapid modification of YAP within 0.5 h after UV irradiation and 1 h after (Fig. 1A). Endogenous YAP and TEAD protein levels remained unaffected upon UV irradiation and CDDP treatment for up to 3 h. This result suggested that the modification of YAP is required to enhance formation of YAP-TEAD complex in response to genotoxic stress. As expected, the augmented interaction in UV-irradiated or CDDP-treated cells was decreased by pretreatment with p38/JNK inhibitors (Fig. 2D), which suppressed YAP modification (Fig. 2A). YAP-TEAD complex in untreated KB cells also slightly decreased on pretreatment with p38/JNK inhibitors, suggesting that the basal activity of p38/JNK contributed to the complex. The involvement of JNK and p38 in YAP regulation was further investigated using dominant-negative form of these kinases. Myc-YAP and FLAG-TEAD1 were cotransfected with or without dominant-negative JNK or/and p38 and stimulated by UV irradiation or CDDP treatment. FLAG-TEAD1 was immunoprecipitated, and cell lysates and coimmunoprecipitates were analyzed by immunoblot analysis (supplemental Fig. S2). Consistent with the results of inhibitor treatment (Fig. 2), the augmented interaction of YAP with TEAD1 in UV-irradiated or CDDP-treated cells was decreased by the expression of dominant negative JNK and p38. The mobility shift of YAP in UV-irradiated or CDDP-treated cells also disappeared when dominant-negative JNK and p38 were coexpressed.
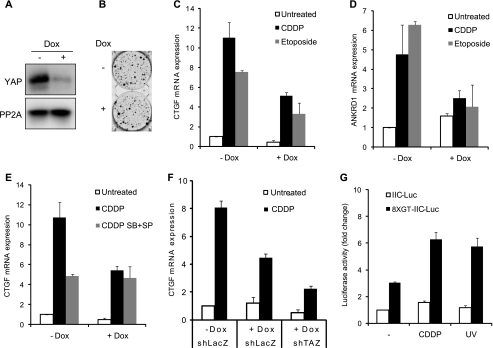
We next investigated the coactivator activity of YAP in response to genotoxic stress. To silence the YAP gene, we utilized an inducible Tet-on shRNA expression system using lentiviral expression vectors (21). YAP expression was efficiently down-regulated on treatment with doxycycline (Dox) for 72 h in KB cells expressing Tet-on shYAP (Fig. 3A). Cell proliferation was observed 3 weeks after plating in the presence or absence of Dox. As shown in Fig. 3B, knockdown of YAP did not significantly alter cell proliferation and did not induce cell death. The coactivator activity of YAP was monitored by real-time RT PCR analysis of two endogenous target genes, CTGF and ANKRD1 (27). Transcription of these genes was significantly induced by genotoxic stress from CDDP and etoposide (Fig. 3, C and D). As expected, the induction of CTGF and ANKDR1 expression was efficiently suppressed when YAP was depleted by Dox treatment. Co-treatment with p38/JNK inhibitors suppressed the induction of CTGF and ANKRD1 expression in CDDP or etoposide-treated cells but not in Dox-treated cells (Fig. 3E), indicating that p38 and JNK were involved in YAP-dependent expression of CTGF and ANKRD1. This result was consistent with the observation that the interaction of YAP with TEAD was enhanced by genotoxic stress and suppressed by p38/JNK inhibitors (Fig. 2, C and D). However, the suppression of CTGF and ANKRD1 expression was incomplete, although YAP was efficiently depleted in Dox-treated cells (Fig. 3E). We tested whether TAZ is involved in CTGF and ANKRD1 expression, as TAZ is similar in structure and function to YAP (28, 29). When TAZ was knocked down in KB cells expressing Tet-on shYAP and treated with CDDP, the expression of CTGF and ANKRD1 was more suppressed by incubation with Dox than in cells expressing control shLacZ, indicating that YAP and TAZ collectively regulated genotoxic stress-induced transcription. As our finding strongly suggested that the induced formation of YAP-TEAD complex activated the transcription of target genes in response genotoxic stress, we directly monitored the transcriptional activity of endogenous TEAD with luciferase reporter assay (Fig. 3G). TEAD reporter activity was significantly increased by CDDP treatment and UV irradiation, but the activity of a reporter without the TEAD binding element remained unaffected. Therefore, YAP is transcriptionally activated in response to genotoxic stress.
FIGURE 3.
Transcriptional activation of YAP in genotoxin-treated cells. A and B, KB cells expressing Tet-on shYAP were incubated with or without Dox (10 μg/ml) for 72 h (A) or 3 weeks (B). Cell lysates were analyzed by immunoblotting (A), or cells were stained with crystal violet and photographed (B). PP2A was monitored as a loading control (A). C–E, KB cells expressing Tet-on shYAP were incubated with or without Dox and treated with CDDP (15 μm) or etoposide (30 μm) for 20 h (C and D) in the absence or presence of p38 and JNK inhibitors (SB+SP) (E). Real-time RT PCR was performed with primers for CTGF (C) or ANKRD1 (D). F, KB cells expressing Tet-on shYAP were further infected with shLacZ or shTAZ constructs, treated, and analyzed as in C. G, KB cells were transfected with the control (IIC-Luc) or TEAD reporter construct (8XGT-IIC-Luc). After 24 h cells were treated with CDDP or UV-irradiated for 16 h and analyzed by dual luciferase reporter assay.
Multisite Phosphorylation of YAP in Response to Genotoxic Stress
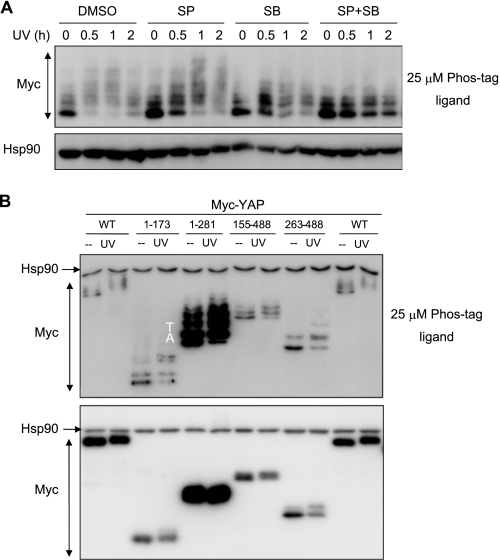
To further analyze the mobility shift of YAP induced by phosphorylation, we performed a high resolution mobility shift assay. U2OS cells were transfected with Myc-YAP, and cell lysates were resolved on the gel with Phos-tag ligand. Myc-YAP in untreated cells was detected as multiple bands (Fig. 4A). The fast-migrating band was most intense, and the slow-migrating bands faded in proportion to apparent molecular weight on SDS-PAGE, indicating that YAP was modified by phosphorylation in untreated cells. UV irradiation rapidly induced an overall retardation of the mobility of YAP with multiple bands and markedly decreased the fast migrating band. The retardation of the mobility was partially blocked by pretreatment with p38 or JNK inhibitor and completely blocked by both inhibitors. However, the retardation of the mobility remained unchanged when ERK inhibitor was treated (supplemental Fig. S3). Thus, YAP is hyperphosphorylated in response to UV irradiation, which is mediated by p38/JNK activation.
FIGURE 4.
Multisite phosphorylation of YAP revealed by high resolution mobility shift assay. A, U2OS cells were transfected with Myc-YAP, preincubated with p38 (SB) and/or JNK inhibitor (SP), and then UV-irradiated. Cell lysates were resolved in 7.5% polyacrylamide gel containing 25 μm of Phos-tag ligand and blotted with Myc antibody. Hsp90 was blotted as a control on gel without Phos-tag ligand. B, U2OS cells were transfected with Myc-YAP deletion constructs, and cell lysates were resolved on the gel with 25 μm of Phos-tag ligand (upper panel) or without Phos-tag (lower panel).
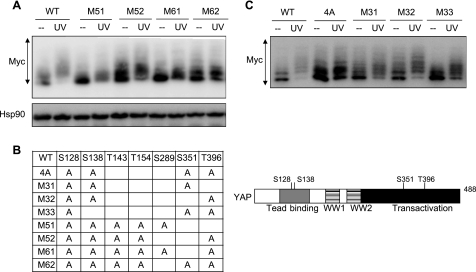
To analyze the region of YAP responsible for the retarded mobility, U2OS cells were transfected with YAP deletion constructs lacking functional domains of YAP (Fig. 4B). In untreated cells, all deletion constructs were expressed as multiple bands on phosphate affinity gel, indicating that there were several phosphorylation sites on YAP proteins. In UV-irradiated cells, YAP 1–281 including the N-terminal TEAD binding domain and central two WW domains, migrated with retarded mobility. YAP 1–173 deleted of WW domains still showed reduced mobility, indicating that TEAD binding domain was one target for phosphorylation. YAP 263–488 also migrated with retarded mobility in response to UV irradiation, suggesting that the C-terminal coactivator domain included sites of phosphorylation. To further investigate the multisite phosphorylation, we reasoned that p38/JNK phosphorylated YAP and focused on proline-directed phosphorylation sites of these kinases. Particularly, we focused on Pro-Ser or Pro-Thr residues in the N-terminal TEAD binding domain and C-terminal coactivator domain, as our results indicated that YAP activation in response to genotoxic stress was involved with the function of these domains. Ser or Thr residues in Pro-Ser or Pro-Thr were mutated to Ala residues, and the YAP mutants were analyzed on phosphate affinity gel. YAP with six mutations of Ser/Thr residues (YAP M62) showed no UV irradiation-induced retardation of mobility, although it migrated as multiple bands like YAP WT in untreated cells (Fig. 5, A and B). Then Ala residues in YAP M62 reverted to wild-type residues, and we generated a series of YAP mutants (Fig. 5B). We ultimately defined four sites of mutation (YAP 4A) showing no apparent mobility retardation by UV irradiation (Fig. 5, B and C). YAP 4A had mutations of S128A and S138A in the TEAD binding domain, which was consistent with our observation that p38 and JNK enhanced YAP-TEAD interaction, and S351A and T396A in the coactivator domain, which implied that UV irradiation-induced phosphorylation regulated the coactivator activity of YAP. We conclude that the four residues are major sites of phosphorylation induced by UV irradiation.
FIGURE 5.
Identification of phosphorylated residues by phosphate affinity SDS-PAGE. A and C. U2OS cells were transfected with Myc-YAP mutant constructs depicted in B. Cell lysates were resolved on the gel containing 25 μm of Phos-tag ligand and blotted with antibodies indicated. B, YAP point mutants analyzed in this study are summarized. The residues at the indicated positions were changed to Ala. The mutated residues in YAP 4A are illustrated with the position of domains.
Multisite Phosphorylation Regulates Coactivator Activity of YAP
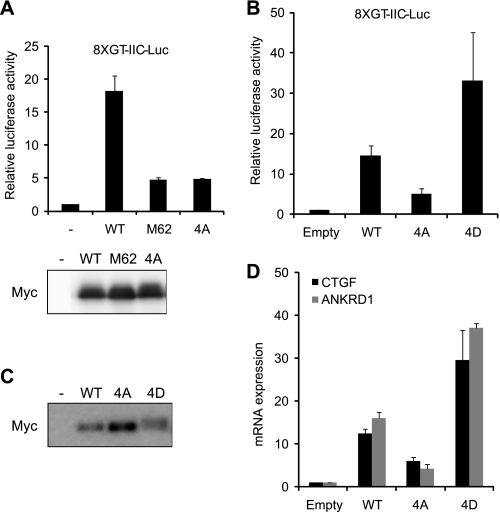
We then investigated whether UV irradiation-induced phosphorylation regulates the coactivator activity of YAP. We tested whether a TEAD reporter can be utilized to monitor the coactivator function of YAP. U2OS cells were cotransfected with YAP WT and TEAD reporter and analyzed by luciferase reporter assay (Fig. 6A). YAP WT strongly activated the TEAD reporter, indicating that the reporter was suitable for monitoring the YAP coactivator. We then compared the coactivator activity of YAP WT and YAP mutants. YAP M62 and YAP 4A, which showed little retarded mobility after UV irradiation (Fig. 5, A and B), had much less coactivator activity than YAP WT, suggesting that phosphorylation at the four sites was required for the coactivator function of YAP. We tested whether phosphorylation at the four sites is sufficient to activate YAP. For this purpose we generated a YAP construct by converting the four residues to Asp (YAP 4D) and utilized it as a phosphomimetic form mimicking YAP hyperphosphorylated by UV irradiation. YAP 4D migrated slower on SDS-PAGE (Fig. 6C), mimicking the retarded mobility induced by hyperphosphorylation (Figs. 1 and 2). Significantly, the phosphomimetic YAP 4D activated TEAD reporter more strongly and phosphorylation-deficient YAP 4A more weakly than YAP WT (Fig. 6B). Alternatively, the coactivator activity of YAP was monitored by real-time RT PCR (Fig. 6D). YAP 4D significantly augmented but YAP 4A did not induce the expression of CTGF and ANKRD1. Considering the expression level between YAP constructs (Fig. 6C), we conclude the coactivator activity of YAP 4A to be negligible. Taken together, hyperphosphorylation at the four sites is sufficient to activate YAP.
FIGURE 6.
Transcriptional activity of YAP phosphorylation mutants. A and B, U2OS cells were cotransfected with Myc-YAP constructs and TEAD reporter (8XGT-IIC-Luc). After 24 h cells were analyzed by dual luciferase reporter assay. Expression of Myc-YAP constructs was visualized by immunoblotting with Myc antibody. C and D, KB cells were infected with lentiviral vectors expressing Myc-YAP constructs indicated. Cell lysates were blotted with anti-Myc antibody (C) or analyzed by real-time RT PCR (D).
Multisite Phosphorylation of YAP Is Required to Protect against CDDP-induced Apoptosis
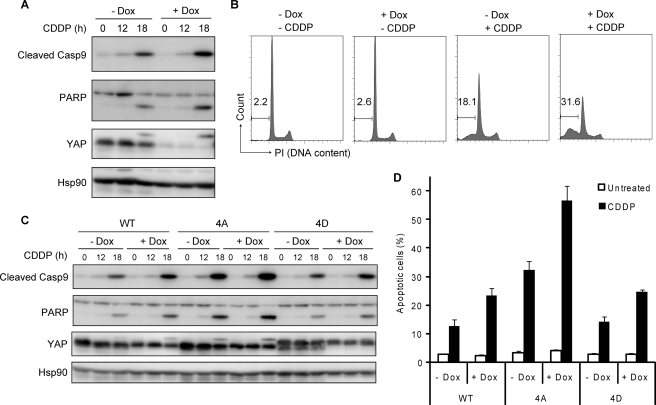
We then investigated whether YAP regulates genotoxic stress-induced apoptosis in KB cells. We observed the progression of apoptosis by monitoring the proteolytic activation of caspase 9 and cleavage of caspase 3 substrates. KB cells expressing Tet-on shYAP were cultured in the absence or presence of Dox, and apoptosis was triggered by treatment with CDDP. At 18 h of CDDP treatment, activation of caspase 9 and cleavage of PARP was observed in cells cultured without Dox (Fig. 7A). Importantly, in cells cultured with Dox, which efficiently depleted YAP proteins, cleavage of caspase 9 and PARP was more clearly observed, indicating that YAP functioned as an anti-apoptotic regulator. Apoptosis was monitored by measuring the percentage of cells showing subdiploid DNA content (Fig. 7B). KB cells cultured without Dox was moderately sensitive to CDDP-induced apoptosis, showing 18.1% of population to be subdiploid in DNA content. In contrast, KB cells expressing Tet-on shYAP and cultured in the presence of Dox were much more sensitive to apoptosis, showing 31.6% of population to be subdiploid. We then examined whether multisite phosphorylation of YAP is involved in the regulation of apoptosis. The expression of YAP was restored in KB cells expressing Tet-on shYAP by infection of lentiviral vectors encoding shYAP-resistant YAP WT and phospho-mimetic and phosphorylation-deficient YAP. The restored YAP protein level was comparable with endogenous YAP level (Fig. 7C). In YAP-restored cells, the endogenous YAP level was likely to be crucial in the regulation of apoptosis, as Dox treatment slightly accelerated the cleavage of caspase 9 and PARP in response to CDDP treatment. Significantly, phosphorylation-deficient YAP robustly accelerated cleavage of caspase 9 and PARP compared with WT and phospho-mimetic YAP. Apoptosis was again monitored by measuring the percentage of cells showing subdiploid DNA content (Fig. 7D). Consistent with the extent of cleavage of caspase 9 and PARP, apoptotic subdiploid population was increased in KB cells expressing phosphorylation-deficient YAP. Therefore, we concluded that phosphorylation-deficient YAP suppressed the function of endogenous YAP, and multisite phosphorylation was required to activate the anti-apoptotic program mediated by YAP.
FIGURE 7.
Regulation of apoptosis by YAP phosphorylation mutants. A and B, KB cells expressing Tet-on shYAP were incubated in the presence or absence of Dox for 72 h and then treated with CDDP (15 μm) for the periods indicated. Cell lysates were analyzed by immunoblotting with indicated antibodies (A). At 18 h after CDDP treatment, the extent of apoptotic cell death was quantified by the percentage of cells showing subdiploid DNA content by FACS analysis (B). PI, propidium iodide. C and D, KB cells expressing Tet-on shYAP were restored with shRNA-resistant YAP constructs. Cells were treated and analyzed as in A, and the extent of apoptotic cell death was analyzed as in D.
Anti-apoptotic Function of YAP Is Not Associated with p73 in KB Cells
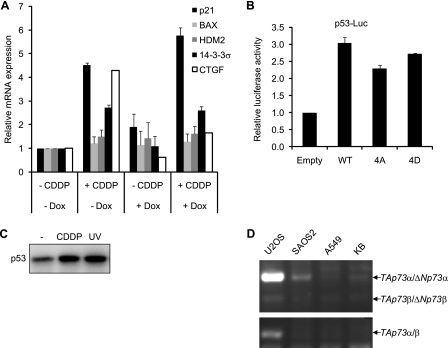
YAP has been reported to enhance p73-dependent apoptosis in response to DNA damage (14, 17). Therefore, we asked whether the anti-apoptotic function of YAP is associated with the regulation of p73 or p53. KB cells expressing shYAP were treated with CDDP, and the expression of representative target genes of p73/p53 was monitored by real-time RT PCR (Fig. 8A). The expression of p21 and 14-3-3σ was moderately increased by CDDP treatment, but that of BAX and HDM2 remained unaffected, indicating that target genes were selectively induced under our experimental conditions. We confirmed that p53 protein accumulated in KB cells treated with CDDP or UV irradiation as reported in other cell types (Fig. 8C). However, we failed to detect p73 protein even in cells treated with CDDP or UV irradiation (data not shown). The CDDP-induced expression of p21 and 14-3-3σ remained unaffected by treatment with Dox (Fig. 8A), whereas CDDP-induced expression of CTGF was efficiently blocked as in Fig. 3C. We concluded that the transcriptional activity of p73/p53 was not affected by the depletion of YAP in KB cells. It should be noted that the concentration of CDDP was sufficient to induce apoptosis (Fig. 7). We examined whether YAP phosphorylation regulates p73 transcriptional activity. As p73 shares common binding elements with p53, we utilized the p53 reporter to measure the transcriptional activity of p73. p53 reporter was cotransfected with p73 and YAP constructs in KB cells, and reporter activity was monitored by luciferase assay (Fig. 8B). YAP WT induced about a 3-fold increase in luciferase activity. Phospho-mimetic YAP as well as phosphorylation-deficient YAP similarly induced p53 reporter activity, indicating that multisite phosphorylation of YAP was not involved in the regulation of p73. This observation clearly contrasted to the role of YAP phosphorylation in the regulation of TEAD (Fig. 6). In KB cells, we failed to detect the expression of transcriptionally active TAp73α or TAp73β, whereas in U2OS cells we clearly detected TAp73α/β by RT PCR analysis (Fig. 8D). Finally, we examined whether restoration of p73α/β alters sensitivity to apoptosis. As shown in supplemental Fig. S4, activation of caspase 3 was more evident with lower concentrations of CDDP in KB cells expressing FLAG-p73, particularly the β isoform. Altogether, we conclude that YAP functions as an anti-apoptotic regulator in KB cells which are deficient in 73α/β expression.
FIGURE 8.
p73 is not regulated by YAP in KB cells. A, KB cells expressing Tet-on shYAP were incubated in the presence or absence of Dox for 72 h and then treated with CDDP (15 μm) for 14 h. Real-time RT PCR was performed with primers indicated. B, U2OS cells were cotransfected with YAP constructs and the p53-Luc reporter. Cell lysates were analyzed by dual luciferase reporter assay. C, KB cells were treated with CDDP (15 μm) or UV-irradiated (100 J/m2), and p53 levels were monitored by immunoblotting. D, RNA was extracted from the cell lines indicated and analyzed by RT PCR.
DISCUSSION
YAP has a dual role in stimulating proliferation and protecting against apoptosis but also promoting apoptosis. YAP has been implicated as an oncogene in human cancers and animal models (9, 10, 12, 13). On the contrary, the YAP gene is lost in breast cancer, and knockdown of YAP in breast cancer cell lines suppresses cell death and enhances tumor growth in nude mice, indicating YAP to be a tumor suppressor (30). Our results support that YAP functions as an anti-apoptotic regulator. Knockdown of YAP enhanced caspase 9 activation and cleavage of caspase substrate upon CDDP treatment (Fig. 7). The dual role of YAP may be explained by the selective regulation of different transcription factors (31). The dual role of YAP may be mediated mainly by TEAD and p73. The state of p73 expression is likely to be crucial in the pro-apoptotic function of YAP as the expression of p73, and its isoforms vary widely depending on tissue and cell types (16, 32). However, we show that in p73-deficent cells such as KB, a protective anti-apoptotic signal is relayed to TEAD via YAP phosphorylation on exposure to genotoxic stress such as CDDP. Our results indicate that multisite phosphorylation of YAP is crucial and required to protect against apoptosis.
Phosphorylation is a fundamental post-translational modification that regulates the function, localization, and binding specificity of target proteins. YAP is phosphorylated at multiple sites including a well characterized phosphorylation at Ser-127. However, it is not known how other phosphorylation regulates the function of YAP in cell proliferation and apoptosis. Our findings indicate that YAP is regulated by hyperphosphorylation upon exposure to genotoxic stress. Supporting our results, Tomlinson et al. (33) recently reported that JNK phosphorylated YAP to regulate apoptosis, although the mechanism was different from our findings. They showed that YAP protected keratinocytes from UV irradiation-induced apoptosis by binding and stabilizing pro-proliferative ΔNp63α. However, it was not described whether YAP phosphorylation by JNK regulated the coactivator activity of YAP or YAP binding to ΔNp63α controlled the transcription of target genes. We clearly show that multisite phosphorylation is associated with the coactivator activity of YAP and required for protection from apoptosis. We conclude that the multisite phosphorylation of YAP activates TEAD-dependent transcription, although we cannot rule out that ΔNp63α is another regulator of anti-apoptotic transcription in KB cells. It is likely that the regulation of ΔNp63α by YAP is also cell type-specific and dependent on the expression of p73 as the expression of p63 and its isoforms is wildly various depending on cell types (16, 32).
Our results indicate that both p38 and JNK induce multisite phosphorylation of YAP on exposure to UV irradiation. However, it was reported that YAP was phosphorylated by JNK but not by p38 in vitro (33). This observation suggests YAP is activated by p38 through the phosphorylation by downstream kinases of p38 such as MAPKAPK, PRAK, and MSK or priming by JNK provides a site on YAP for subsequent phosphorylation by p38 activation, although the possibility is not excluded that the experimental conditions used were not suitable for monitoring the phosphorylation of YAP by p38 in vivo. Importantly, we show that multisite phosphorylation of YAP induced by p38/JNK is required for transcriptional coactivation of YAP. Activation of the Hippo pathway leads to the phosphorylation of YAP at Ser-127, which in turn inhibits the coactivator activity of YAP. Therefore, our findings clearly contrast with inactivation of YAP in the Hippo pathway and show the complexity of YAP regulation by phosphorylation. Phosphorylation-dependent regulation of YAP might be even more complex, as recent studies revealed that multiple Ser/Thr residues were phosphorylated including Ser-128 and Ser-138, which were also identified in our study (34–36). In addition, other sites of phosphorylation have been reported in the Drosophila homolog Yorkie (34). However, except for the multisite phosphorylation in our study it remains unknown whether the coactivator activity of YAP is regulated by the phosphorylation of Ser/Thr residues.
Regulation of YAP protein stability in response to DNA damage demonstrates another level of regulation mechanism. It was reported that YAP increased on CDDP treatment and was stabilized upon phosphorylation by c-Abl (17, 18). Inconsistent with these reports, we could not confirm an elevation in YAP protein by UV irradiation or CDDP treatment in KB cells (Fig. 2). Instead, the interaction of YAP with TEAD increased in response to genotoxic stress and decreased on pretreatment with p38/JNK inhibitors. Although the molecular mechanism is not clear, this result suggests that phosphorylation of Ser-128 and Ser-138 in the TEAD binding domain augments affinity for TEAD. The transcriptional activity of YAP-TEAD complex may be further amplified by phosphorylation of Ser-351 and Thr-396 in the coactivator domain of YAP. The role of each phosphorylation remains to be determined in detail.
The transcriptional coactivator TAZ is closely related to YAP (28, 29). The two proteins share high sequence homology with a similar topology containing two central WW domains and a C-terminal transactivation domain. We showed that YAP and TAZ cooperated to regulate gene expression on exposure to genotoxic stress. This result raises the possibility that TAZ is also regulated by multisite phosphorylation as three of four sites of phosphorylation in YAP are conserved in TAZ. It would be interesting to explore the phosphorylation of TAZ and the involvement of p38/JNK on exposure to CDDP or UV irradiation.
In addition to the regulation of apoptosis in genotoxic stress-induced cells, the multisite phosphorylation of YAP might be involved in other biological processes besides the Hippo pathway. YAP functions as a downstream element in how cells perceive their physical microenvironment, such as extracellular matrix rigidity, which requires Rho GTPase activity and tension of stress fibers (27). In cells under such circumstance or physiological stress, it is tempting to speculate that YAP is temporally phosphorylated by JNK and/or p38 and functions as a transcriptional regulator in various biological settings. In this respect, it is of note that recent studies reported that YAP/TAZ binds to and regulates a number of proteins including Runx, Smad, β-catenin, and Crumbs complex (37–40). It remains to be analyzed whether the functions of these proteins are associated with the multisite phosphorylation of YAP revealed in this study. Although some genes implicated in apoptosis are regulated by YAP (8, 13), it is not clear whether these genes are directly regulated by YAP phosphorylation. Our findings should help to define the genetic program of apoptosis regulated by YAP hyperphosphorylation.
Supplementary Material
Acknowledgments
We thank Hiroshi Sasaki for FLAG-TEAD constructs and TEAD luciferase reporter. We thank Eisuke Nishida for HA-JNK VPF and HA-p38 AGF.

This article contains supplemental Figs. S1–S4.
- YAP
- Yes-associated protein
- CDDP
- cisplatin
- Dox
- doxycycline
- PARP
- poly(ADP-ribose) polymerase
- TEAD
- TEA domain.
REFERENCES
- 1. Conlon I., Raff M. (1999) Size control in animal development. Cell 96, 235–244 [DOI] [PubMed] [Google Scholar]
- 2. Zhao B., Li L., Lei Q., Guan K. L. (2010) The Hippo-YAP pathway in organ size control and tumorigenesis. An updated version. Genes Dev. 24, 862–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McClatchey A. I., Giovannini M. (2005) Membrane organization and tumorigenesis. The NF2 tumor suppressor, Merlin. Genes Dev. 19, 2265–2277 [DOI] [PubMed] [Google Scholar]
- 4. St John M. A., Tao W., Fei X., Fukumoto R., Carcangiu M. L., Brownstein D. G., Parlow A. F., McGrath J., Xu T. (1999) Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumors, and pituitary dysfunction. Nat. Genet. 21, 182–186 [DOI] [PubMed] [Google Scholar]
- 5. Zhou D., Conrad C., Xia F., Park J. S., Payer B., Yin Y., Lauwers G. Y., Thasler W., Lee J. T., Avruch J., Bardeesy N. (2009) Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16, 425–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu S., Liu Y., Zheng Y., Dong J., Pan D. (2008) The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell 14, 388–398 [DOI] [PubMed] [Google Scholar]
- 7. Zhang L., Ren F., Zhang Q., Chen Y., Wang B., Jiang J. (2008) The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev. Cell 14, 377–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao B., Ye X., Yu J., Li L., Li W., Li S., Yu J., Lin J. D., Wang C. Y., Chinnaiyan A. M., Lai Z. C., Guan K. L. (2008) TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22, 1962–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zender L., Spector M. S., Xue W., Flemming P., Cordon-Cardo C., Silke J., Fan S. T., Luk J. M., Wigler M., Hannon G. J., Mu D., Lucito R., Powers S., Lowe S. W. (2006) Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell 125, 1253–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Overholtzer M., Zhang J., Smolen G. A., Muir B., Li W., Sgroi D. C., Deng C. X., Brugge J. S., Haber D. A. (2006) Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc. Natl. Acad. Sci. U.S.A. 103, 12405–12410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao B., Wei X., Li W., Udan R. S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L., Zheng P., Ye K., Chinnaiyan A., Halder G., Lai Z. C., Guan K. L. (2007) Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Camargo F. D., Gokhale S., Johnnidis J. B., Fu D., Bell G. W., Jaenisch R., Brummelkamp T. R. (2007) YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 17, 2054–2060 [DOI] [PubMed] [Google Scholar]
- 13. Dong J., Feldmann G., Huang J., Wu S., Zhang N., Comerford S. A., Gayyed M. F., Anders R. A., Maitra A., Pan D. (2007) Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Strano S., Monti O., Pediconi N., Baccarini A., Fontemaggi G., Lapi E., Mantovani F., Damalas A., Citro G., Sacchi A., Del Sal G., Levrero M., Blandino G. (2005) The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA damage. Mol. Cell 18, 447–459 [DOI] [PubMed] [Google Scholar]
- 15. Lapi E., Di Agostino S., Donzelli S., Gal H., Domany E., Rechavi G., Pandolfi P. P., Givol D., Strano S., Lu X., Blandino G. (2008) PML, YAP, and p73 are components of a proapoptotic autoregulatory feedback loop. Mol. Cell 32, 803–814 [DOI] [PubMed] [Google Scholar]
- 16. Blandino G., Dobbelstein M. (2004) p73 and p63. Why do we still need them? Cell Cycle 3, 886–894 [PubMed] [Google Scholar]
- 17. Levy D., Adamovich Y., Reuven N., Shaul Y. (2007) The Yes-associated protein 1 stabilizes p73 by preventing Itch-mediated ubiquitination of p73. Cell Death Differ. 14, 743–751 [DOI] [PubMed] [Google Scholar]
- 18. Levy D., Adamovich Y., Reuven N., Shaul Y. (2008) Yap1 phosphorylation by c-Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Mol. Cell 29, 350–361 [DOI] [PubMed] [Google Scholar]
- 19. Basu S., Totty N. F., Irwin M. S., Sudol M., Downward J. (2003) Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3and attenuation of p73-mediated apoptosis. Mol. Cell 11, 11–23 [DOI] [PubMed] [Google Scholar]
- 20. Kiriyama M., Kobayashi Y., Saito M., Ishikawa F., Yonehara S. (2009) Interaction of FLASH with arsenite resistance protein 2 is involved in cell cycle progression at S phase. Mol. Cell. Biol. 29, 4729–4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kobayashi Y., Yonehara S. (2009) Novel cell death by down-regulation of eEF1A1 expression in tetraploids. Cell Death Differ 16, 139–150 [DOI] [PubMed] [Google Scholar]
- 22. Lee K. K., Yonehara S. (2002) Phosphorylation and dimerization regulate nucleocytoplasmic shuttling of mammalian STE20-like kinase (MST). J. Biol. Chem. 277, 12351–12358 [DOI] [PubMed] [Google Scholar]
- 23. Lee K. K., Ohyama T., Yajima N., Tsubuki S., Yonehara S. (2001) MST, a physiological caspase substrate, highly sensitizes apoptosis both upstream and downstream of caspase activation. J. Biol. Chem. 276, 19276–19285 [DOI] [PubMed] [Google Scholar]
- 24. Ota M., Sasaki H. (2008) Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development 135, 4059–4069 [DOI] [PubMed] [Google Scholar]
- 25. Cao X., Pfaff S. L., Gage F. H. (2008) YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev. 22, 3320–3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vassilev A., Kaneko K. J., Shu H., Zhao Y., DePamphilis M. L. (2001) TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 15, 1229–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., Elvassore N., Piccolo S. (2011) Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 [DOI] [PubMed] [Google Scholar]
- 28. Lei Q. Y., Zhang H., Zhao B., Zha Z. Y., Bai F., Pei X. H., Zhao S., Xiong Y., Guan K. L. (2008) TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol. Cell. Biol. 28, 2426–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kanai F., Marignani P. A., Sarbassova D., Yagi R., Hall R. A., Donowitz M., Hisaminato A., Fujiwara T., Ito Y., Cantley L. C., Yaffe M. B. (2000) TAZ. A novel transcriptional coactivator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 19, 6778–6791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yuan M., Tomlinson V., Lara R., Holliday D., Chelala C., Harada T., Gangeswaran R., Manson-Bishop C., Smith P., Danovi S. A., Pardo O., Crook T., Mein C. A., Lemoine N. R., Jones L. J., Basu S. (2008) Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ 15, 1752–1759 [DOI] [PubMed] [Google Scholar]
- 31. Zhao B., Lei Q. Y., Guan K. L. (2008) The Hippo-YAP pathway. New connections between regulation of organ size and cancer. Curr. Opin. Cell Biol. 20, 638–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang A., McKeon F. (2000) P63 and P73. P53 mimics, menaces and more. Nat. Rev. Mol. Cell Biol. 1, 199–207 [DOI] [PubMed] [Google Scholar]
- 33. Tomlinson V., Gudmundsdottir K., Luong P., Leung K. Y., Knebel A., Basu S. (2010) JNK phosphorylates Yes-associated protein (YAP) to regulate apoptosis. Cell Death Dis. 1, e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oh H., Irvine K. D. (2009) In vivo analysis of Yorkie phosphorylation sites. Oncogene 28, 1916–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olsen J. V., Blagoev B., Gnad F., Macek B., Kumar C., Mortensen P., Mann M. (2006) Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127, 635–648 [DOI] [PubMed] [Google Scholar]
- 36. Beausoleil S. A., Jedrychowski M., Schwartz D., Elias J. E., Villén J., Li J., Cohn M. A., Cantley L. C., Gygi S. P. (2004) Large scale characterization of HeLa cell nuclear phosphoproteins. Proc. Natl. Acad. Sci. U.S.A. 101, 12130–12135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Varelas X., Samavarchi-Tehrani P., Narimatsu M., Weiss A., Cockburn K., Larsen B. G., Rossant J., Wrana J. L. (2010) The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev Cell 19, 831–844 [DOI] [PubMed] [Google Scholar]
- 38. Varelas X., Miller B. W., Sopko R., Song S., Gregorieff A., Fellouse F. A., Sakuma R., Pawson T., Hunziker W., McNeill H., Wrana J. L., Attisano L. (2010) The Hippo pathway regulates Wnt/β-catenin signaling. Dev Cell 18, 579–591 [DOI] [PubMed] [Google Scholar]
- 39. Heallen T., Zhang M., Wang J., Bonilla-Claudio M., Klysik E., Johnson R. L., Martin J. F. (2011) Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 332, 458–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alarcón C., Zaromytidou A. I., Xi Q., Gao S., Yu J., Fujisawa S., Barlas A., Miller A. N., Manova-Todorova K., Macias M. J., Sapkota G., Pan D., Massagué J. (2009) Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-β pathways. Cell 139, 757–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.