Background: The HR domain is essential for the function of the T2SS.
Results: HR domain has similar structure to PilP of the T4PS and interacts with secretin.
Conclusion: The evolutionary link between T2SS and T4PS extends to proteins of the inner membrane subcomplex.
Significance: This work is crucial to understanding how this machine works and how it may be stopped.
Keywords: Bacterial Pathogenesis, Membrane Proteins, Membrane Transport, Molecular Evolution, Molecular Motors, Nuclear Magnetic Resonance, HR Domain, Secretin Interactions, Type II Secretion System
Abstract
The type II secretion system of Gram-negative bacteria is important for bacterial pathogenesis and survival; it is composed of 12 mostly multimeric core proteins, which build a sophisticated secretion machine spanning both bacterial membranes. OutC is the core component of the inner membrane subcomplex thought to be involved in both recognition of substrate and interaction with the outer membrane secretin OutD. Here, we report the solution structure of the HR domain of OutC and explore its interaction with the secretin. The HR domain adopts a β-sandwich-like fold consisting of two β-sheets each composed of three anti-parallel β-strands. This structure is strikingly similar to the periplasmic region of PilP, an inner membrane lipoprotein from the type IV pilus system highlighting the common evolutionary origin of these two systems and showing that all the core components of the type II secretion system have a structural or sequence ortholog within the type IV pili system. The HR domain is shown to interact with the N0 domain of the secretin. The importance of this interaction is explored in the context of the functional secretion system.
Introduction
Gram-negative bacteria have evolved multiple sophisticated machines to transport selected proteins across the two bacterial membranes. The type II secretion system (T2SS)3 is specifically used to secrete folded proteins such as lytic enzymes and toxins from the periplasm to the external medium or to host tissue (1, 2). This system is shared by many bacteria pathogenic for human, animals, and plants where it plays a pivotal role in pathogenesis. The T2S machinery is composed of 12 to 15 components with generic names GspA to GspO. The T2SS components of the phytopatogen bacteria Dickeya dadantii (formerly Erwinia chrysanthemi) are named Out. Most of these proteins are multimeric and build together a sophisticated machine spanning both bacterial membranes. The machine can be divided into three subassemblies. The secretin GspD (Gsp is the nomenclature for the enterotoxigenic Escherichia coli and Out for D. dadantii proteins) forms large gated channels in the outer membrane through which the proteins are secreted (3, 4). Five pseudopilins (GspG to GspK) are processed by the prepilin peptidase/methylase GspO and form a short pilus not exceeding the periplasm, which could push proteins to be secreted through the pore formed by the secretin. GspC, GspL, GspM, and GspF are thought to form a subcomplex in the inner membrane (5). An ATPase, GspE is associated with this subcomplex and provides energy to the secretion process probably by assuring assembly of the pseudopilus (6). However, the exact role of individual components and the molecular organization of the T2S machinery have remained elusive.
Phylogenetic analyses suggested that the T2SS is ancestrally related to the type IV pili system (T4PS) and share several homologous core elements (7). The current models of T2S also suggest its functional similarity with T4P system, notably, that short pseudopilus assembled in the periplasm push the protein across the pore formed by the secretin. Because the inner membrane components of T2SS and T4P, GspL/M/C, and PilM/N/O/P, respectively, do not share any sequence similarity, they have been assumed to execute functions specific for each of these systems (1, 8). However, recent structural analysis revealed that the periplasmic domains of GspL and PilN on one side and GspM and PilO on the other adopt the same ferredoxin-like fold and hence could play similar functions within T2S and T4P systems, respectively (9–11). Moreover, the cytoplasmic region of GspL adopts the actin-like fold of PilN (12). Therefore, GspC is yet the sole T2SS core component that has not a sequence or structural ortholog within the T4PS. GspC is a bitopic inner membrane protein. The single trans-membrane segment drives dimerization of the protein (13). The periplasmic region possesses a so-called homology region (HR) and, in most known GspCs, including OutC, a PDZ domain (see Fig. 1A). The PDZ domain is involved in secretion specificity but is not an essential module because in certain GspCs, it is absent or replaced by a coiled-coil domain (14). The exact function of HR is unknown; however, it was shown to interact in vitro with the N-terminal region of the secretin GspD and especially with the N0 domain (15, 16).
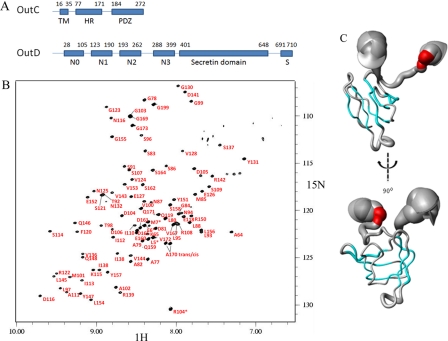
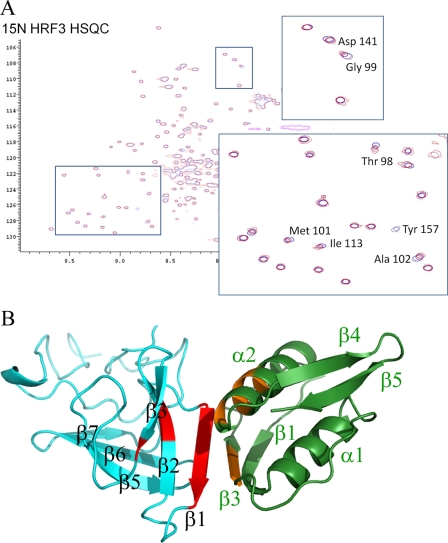
FIGURE 1.
Tertiary structure of the HR domain of OutC. A, schematic representation of the domain organization of inner membrane subcomplex component OutC and secretin OutD. B, assignment of the 1H-15N HSQC spectra for the HR domain. C, sausage representation of the best 20 calculated OutC-HRF3 structures. The secondary structure is shown in color: α-helical residues are in red, and the β-sheet is in cyan. The diameter of the sausage reflects the dynamics of the protein in solution, which is plotted according to the residue α-carbon T2 relaxation profile.
Secretins constitute the outer membrane pore necessary for translocation of macromolecules. Beyond the T2SS system, secretins are also involved in T4P, T3SS and filamentous phage release (17). Secretins consist of two main regions, a variable N-terminal periplasmic region and a conserved pore-forming C-domain, also named the secretin domain. The N-terminal region has a modular structure and in secretins from different systems possesses a variable number of N0, N1/N2, and N3 domains (18, 19). A schematic of the domain organization of OutD the cognate secretin for OutC is also shown in Fig. 1A. This N-terminal region is thought to assure specific functions within each of the export pathways. Notably, it interacts with the inner membrane components of secretion systems and with the proteins to be exported (4, 19, 20).
In this work, we report determination of the solution structure of the homology region domain of OutC and investigate its interaction with the secretin OutD from the plant pathogenic bacteria Dickeya dadantii both in vitro and in vivo. This study reveals a striking structural similarity between the HR domain and the periplasmic domain of PilP from the T4PS, revealing that these important components of the inner membrane subcomplexes evolved from a common ancestor; this strengthens the view that these molecular machines have a common evolutionary origin. We also show that β1 of the HR domain interacts with the N0 domain in vitro and explore the importance of the interactions involved in vivo.
EXPERIMENTAL PROCEDURES
Protein Expression, Purification, and Analysis
The HRF3 fragment was discovered using limited proteolysis of the protein produced using the pGX-oC-HR construct (supplemental Table S1). Sequencing grade trypsin was used. The protein sample was bound to a C5 reverse-phase HPLC column in the presence of 1% formic acid solution and eluted using an acetonitrile gradient. Three fractions from the HPLC column were characterized by mass spectroscopy, HRF1, -2, and -3; HRF3 was the shortest of these nested fragments and was therefore selected for further study. N0, N1, and N1-N2 domains of OutD were produced in E. coli and purified by nickel-affinity chromatography as described previously (13).
The HRF3 DNA was amplified from HR construct (pGX-oC-HR) and cloned into the pET14b expression vector (strains and primers used are detailed in supplemental Tables S1 and S2). The expression vector was transformed into competent BL21 (DE3) E. coli cells. Following bacterial growth and induction the protein was loaded onto a 5-ml His Trap HP column. After washing, Thrombin (GE Healthcare) was used to release the His tag cleaved HRF3 from the column. HRF3 was then passed through a Superdex75 column (GE Healthcare) column. The fragment eluted at a volume consistent with HRF3 being a subunit in solution. This was confirmed by dynamic light scattering. For NMR spectroscopy, uniformly 15N- and 13C-labeled proteins were produced by growing cell cultures in M9 minimal medium that contained 1 g/liter 15N-ammonium chloride and 2 g/liter 13C-d-glucose (Cambridge Isotope Laboratories, Inc.) as the sources of nitrogen and carbon, respectively.
NMR Spectroscopy
NMR spectra were acquired at 15 °C using Bruker Avance 700 and 600 MHz spectrometers equipped with CryoProbes. NMR samples had a typical concentration of 0.3–0.7 mm in 20 mm Tris, pH7.0, and 10% D2O. 150 mm NaCl was included in the cross-titration studies. Fast chemical shift-based structure calculations were used initially employing CS-Rosetta (21, 22). All spectra were processed using NMRPipe/NMRDraw (23) and analyzed using XEASY (24). HNCA, HN(CO)CA, HNCO, HNCACB, and CBCA(CO)NH experiments were employed to obtain sequence-specific 1HN, 15N, 13Cα, 13Cβ, and 13C′ backbone assignments. Side chain aliphatic proton and carbon assignments were achieved by a combination of three-dimensional 15N-edited total correlation spectroscopy- and NOESY-heteronuclear single-quantum correlation spectroscopy (25). Secondary structure elements were determined from a combination of 1HN, 15N, 13Cα, 13Cβ, and 13C′ secondary chemical shifts using TALOS+ (26, 27) as well as from identification of interstrand NOE contacts. Hydrogen bonds were measured by direct trans-hydrogen bond (N-H–O=C) scalar coupling (28). Structure calculation used ARIA (version 1.2) (29) and input NOE data from three-dimensional 15N-edited NOESY-HSQC, 13C-edited NOESY-HSQC (all acquired with a mixing time of 100 ms), 76 ϕ and ψ torsion angles were derived from TALOS and residual dipolar coupling measurements. The standard ARIA protocol was used with nine iterations and 100 structures generated per iteration. In the final iteration, 30 structures with the lowest energy were used for water refinement and 20 structures with the lowest energy were selected as representatives of OutC-HRF3 structure.
In Vivo Analysis of Mutant OutC and OutD Variants
To test the functionality of OutC and OutD variants carrying site-specific substitutions, complementation assays were used with D. dadantii A3556 ΔoutC and A3558 ΔoutD strains. Bacteria carrying an appropriate pTdB plasmid with a mutant allele of either outC, or outD, or outC-outD were grown in Luria-Bertani medium supplemented with sodium galacturonate at 1 g·liter−1 at 30 °C aerobically at 150 rpm to steady-state; then, culture supernatant and cells were separated by centrifugation and analyzed by immunoblotting with PelD and PelI antibodies as described previously (13). Abundance of the OutC and OutD mutant variants in the cells was assessed by immunoblotting with OutC and OutD antibodies. For in vivo disulfide bonding analysis, D. dadantii cells carrying appropriate plasmids with mutant outC and/or outD genes were grown as above. Cells were washed in TBS (50 mm Tris-HCl, pH 7.5, 100 mm NaCl), and remaining free thiol groups were blocked with 20 mm iodoacetamide in TBS at 25 °C for 30 min. The cells were then pelleted, resuspended in Laemmli sample buffer without 2-mercaptoethanol and boiled for 10 min, and the extent of disulfide bonding was assessed by SDS-PAGE, followed by immunoblotting with anti-OutC and anti-OutD antibodies.
RESULTS
Identification of Folded Homology Region Domain (OutC-HR)
OutC comprises a short cytoplasmic sequence, a transmembrane helix, and two periplasmic regions: the homology region and a PDZ domain (Fig. 1A). The HR region is so named because a similar sequence is found in all GspC proteins. Examination of the 1H-15N HSQC spectrum of the periplasmic region of OutC (residues 60 to 272) reveals that in addition to the PDZ domain, there is also another folded domain. Limited proteolysis of this OutC derivative gave three bands (HRF1, HRF2, and HRF3) on both SDS-PAGE and native gel (supplemental Fig. S1). The native gel was used to assess the solubility of the proteolytic fragments as insoluble protein tends to aggregate and not enter the gel. The precise identities of the fragments were established by mass spectroscopy (supplemental Fig. S2) and confirmed by N-terminal sequencing. The smallest fragment (HRF3), corresponding to residues 77 to 172, was produced recombinantly by sub-cloning the appropriate part of the outC gene. The HRF3 protein produced in this way was both soluble and stable and was used for these studies. The 1H-15N HSQC spectrum of HRF3 was well dispersed and overlaid well with the previously measured spectrum for the entire periplasmic region of OutC, confirming that HRF3 comprises an independently folded domain (supplemental Fig. S3).
Solution Structure of Homology Region Domain of OutC
NMR relaxation measurements revealed that the structured region of HRF3 comprised 67 residues (residues 91 to 157), and we refer to this domain as the homology region (HR) domain. A nearly complete sequence-specific NMR signal assignment was achieved using a combination of three-dimensional triple resonance experiments using 15N,13C-labeled OutC-HR, and three-dimensional NOSEY-HSQC, TOCSY-HSQC using 15N-labeled protein (Fig. 1B). The 1954 experimental NOE distance restraints were obtained using isotope-edited NOESY spectra, and 76 ϕ and ψ torsion angle restraints were derived from secondary chemical shift analysis using the TALOS algorithm. Twenty eight hydrogen bonds were measured experimentally. The structure of the folded domain was determined with high precision as judged from the overall root mean square deviation (r.m.s.d.) of 0.46 and 1.26 Å for the backbone and heavy atoms, respectively (Table 1) for the pairwise r.m.s.d. for the family of 20 representative structures. It is significant that even the loop regions connecting the anti-parallel β-strands are well determined in this structure (Fig. 1C). It is interesting to note that the structure predicted by CS-ROSETTA, using only the chemical shift data, was very close to that determined using conventional methods.
TABLE 1.
NMR refinement statistics for the HR domain (OutC-HRF3)
| NMR distance and dihedral constraints | |
| Distance constraints | |
| Total NOE | 1954 |
| Intra-residue | 652 |
| Inter-residue | |
| Sequential (|i − j| = 1) | 381 |
| Medium range (|i − j| ≥ 2 ≤ 4) | 170 |
| Long range (|i − j| ≥ 5) | 751 |
| Hydrogen bonds (experimental measured) | 28 |
| Hydrogen bonds(observed in >50% of structures) | 64 |
| Total dihedral angle restraints (TALOS) | 76 |
| ϕ | 38 |
| ψ | 38 |
| Structure statistics | |
| Violations (mean ± S.D.) | |
| Distance constraints (>0.5) (Å) | 0 |
| Dihedral angle constraints (>5) (°) | 0 |
| Deviations from idealized geometry | |
| Bond lengths (Å) | 0.005 ± 0.0001 |
| Bond angles (°) | 0.671 ± 0.011 |
| Impropers (°) | 0.748 ± 0.024 |
| Average pairwise r.m.s.d. (Å)a | |
| Backbone | 0.46 ± 0.11 |
| Heavy | 1.26 ± 0.20 |
| R.m.s.d. from the mean structure (Å)a | 0.33 ± 0.07 |
| Residues in allowed regionsb | 98.3% |
| Residues in disallowed regions | 1.7% |
a Pairwise r.m.s.d. and r.m.s.d. from the mean structure were calculated among the 20 lowest energy structures from 50 refined structures (amino acid residues 91 to 157).
b Structure quality was analyzed with MolProbability over structured regions (amino acid residues 91–157). One residue in a tight β-turn between β5 and β6 (Pro140) is in a disallowed region.
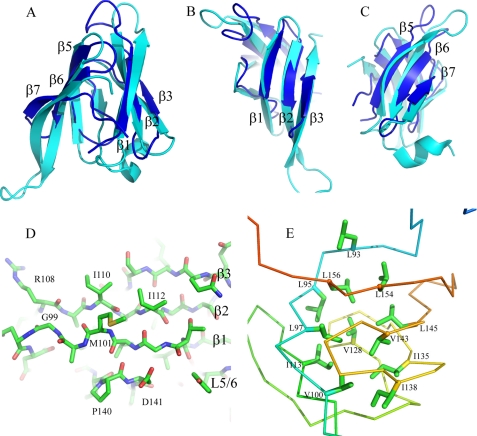
The architecture of the central 67 residues is β-sandwich-like comprising two three-stranded (up-down-up) anti-parallel β-sheets with short hairpin loops connecting adjacent β-strands within the two sheets and a longer loop between the two sheets (Fig. 2, A–C). The angle between the strands in the two β-sheets is ∼45°. In several members of the family of structures, there is a fourth β-strand in the second β-sheet. This strand is more dynamic in solution than the other six β-strands, which are present in each of the 20 structures. The first β-strand is involved in interaction with the N0 domain of OutD and is illustrated in Fig. 2D (see also results below). Aliphatic residues, mostly leucines, from the internal surface of the β-sheets form the hydrophobic core of the HR domain (Fig. 2E). To both the N- and C- terminal ends of the β-sandwich, there are ∼20 residues that are less regular in structure.
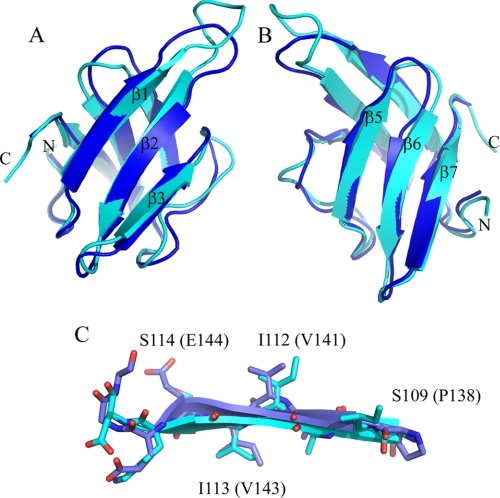
FIGURE 2.
HR domain structure compared with PilP. A, schematic representation of the structure of the HR domain (blue) superimposed on PilP (cyan). The β-strands are labeled according to PilP numbering. B and C look down onto the first and second sheet of the HR domain, respectively. D, the first β-sheet in stick representation, highlighting β1, which is important in the interaction of HR with OutD-N0 (see Fig. 5). E, hydrophobic residues forming the core of the HR domain in stick representation with backbone displayed as a ribbon.
HR Domain Is Structurally Similar to PilP
A Dali database search revealed that the HR domain is similar to the periplasmic domain of PilP, an inner membrane core component of the T4PS of Neisseria meningitidis (Protein Data Bank code 2IVW) (30). The Z score is 3.7, and root mean square deviation is 6.8 Å for 64 equivalent α-carbon atoms. The HR domain has the same β-strand topology and common β-sandwich type fold as PilP (Fig. 2, A–C). In some of the HR family of structures, the conserved β4 strand of PilP is frequently not formed revealing greater structural flexibility in this part of the HR domain. To aid comparison with PilP, and in light of this flexible “β4” region, we use the same β-strand numbering for HR as used previously for PilP. Sequence alignment of PilP homologs from various T4PS indicates that strand β4 together with short neighboring loops L4 and L5 constitute a more conserved region than the corresponding region of the HR sequence (supplemental Fig. S4).
Several Conserved Residues Form Hydrophobic Core of HR
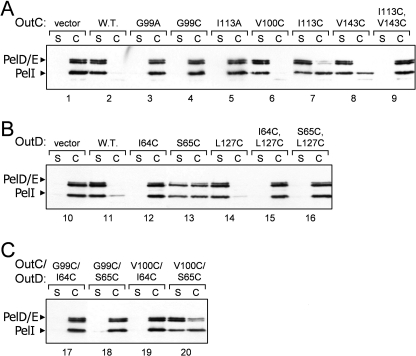
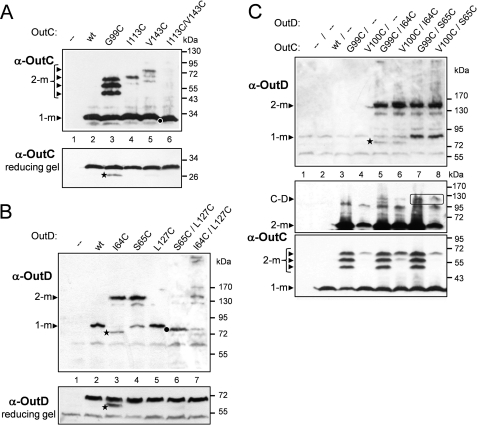
The two β-sheets composing the HR domain seem largely independent and are connected together through several hydrophobic interactions, including the side chains of well conserved Leu93, Leu97, Ile113, Val143, Leu154, and Leu156 and semiconserved Leu/Ile145, Ile/Leu/Val135, and Ile/Val138 (Fig. 2E and supplemental Fig. S4). Most of these residues are also conserved in the PilP periplasmic domain highlighting their structural importance. Ile113 and Val143 contribute to the hydrophobic packing between the two sheets. The proximity of Ile113 and Val143 was assessed in vivo by site-directed mutagenesis and cysteine mediated cross-linking analysis. Substitution of one of these residues with cysteine did not affect notably the secretion in D. dadantii, whereas substitution with alanine impaired it (Fig. 3A, lanes 5, 7, and 8), indicating that in contrast to cysteine, alanine is not able to replace these hydrophobic side chains. A non-reducing gel revealed that OutCI113C and OutCV143C remained mostly as a non-cross-linked subunit (Fig. 4A, lanes 4 and 5), implying that the corresponding side chains are poorly accessible for intermolecular disulfide formation. Double I113C/V143C substitution fully prevented secretion (Fig. 3, lane 9) and provoked an efficient intramolecular disulfide bond within OutC (Fig. 4A, lane 6). Indeed, on non-reducing gel, OutCI113C/V143C migrated slightly faster indicative of its more compact shape. These mutational analyses are consistent with the solution structure determined for the HR domain.
FIGURE 3.
Functional analysis of OutC and OutD point mutants. The secretion activity of OutC and OutD mutants (indicated on top of gels) was estimated in a complementation assays with D. dadantii strains A3656 ΔoutC (A and C) and A3658 ΔoutD (B). Bacteria carrying a plasmid with mutant alleles of either outC (A), or outD (B), or outC and outD (C) were grown to steady-state; then, culture supernatant (S) and cells (C) were separated and analyzed by immunoblotting with PelD and PelI antibodies. The amount of secreted proteins (PelD, PelE, and PelI) in culture supernatant reflects secretion efficiency. The abundance of the corresponding OutC and OutD variants is shown on Fig. 4.
FIGURE 4.
Disulfide cross-linking analysis of OutC and OutD interactions within the functional T2SS. A, disulfide cross-linking analysis of the HR domain of OutC. The G99C substitution provokes efficient homodimerization of OutC, whereas I113C or V143C single substitutions do not. Double substitution I113C/V143C leads to an intramolecular disulfide within OutC. B, disulfide cross-linking analysis of N0-N1 interaction sites in OutD. I64C and S65C single substitutions provoke efficient homodimerization of OutD, whereas L127C substitution does not. Double substitution S65C/L127C causes an intramolecular disulfide between N0 and N1 domains of OutD, whereas I64C/L127C does not. C, disulfide bonding analysis of HR-N0 interacting site. Combination of G99C and V100C substitutions in OutC with I64C and S65C substitutions in OutD does not provoke an efficient cross-linking between OutC and OutD. Some amounts of putative OutC·OutD complex are surrounded. D. dadantii strains A3556 ΔoutC (A and C) and A3558 ΔoutD (B) carrying a plasmid with mutant alleles of either outC (A), or outD (B), or outC and outD (C) (cysteine substitutions indicated on top) were grown aerobically to steady-state. Remaining free thiol groups were blocked with iodoacetamide, and the extent of disulfide bonding was assessed by SDS-PAGE under non-reducing conditions followed by immunoblotting with anti-OutC and anti-OutD antibodies. The same samples but treated with 2-mercaptoethanol were used to estimate abundance of the corresponding OutC and OutD variants (reducing gel). In C, the middle panel was overexposed in comparison with the lower panel to show high molecular mass species. Positions of monomers (1-m) and dimers (2-m) and putative OutC·OutD complex (C and D) are indicated by arrowheads. Stars indicate degradative products of OutCG99C and OutDI64C variants. Dots indicate OutCI113C/V143C and OutDS65C/L127C variants that migrate slightly faster than the corresponding wild-type monomers, indicating formation of an intramolecular disulfide bond.
OutC-HR Domain Interacts with OutD-N0 Domain
Previous NMR studies had demonstrated that the periplasmic part of OutC (residues 60 to 172 corresponding to the periplasmic region of OutC with the PDZ domain deleted) interacted with the N-terminal domains (N0, N1, N2, N3) of the secretin OutD (16). When unlabeled N0 domain (residues 28 to 112) was titrated into 15N-labeled HR domain (residues 77 to 172), the peaks loosing intensity on the 1H-15N-HSQC spectra were those seen to lose intensity when the N-terminal domains of OutD were titrated into OutC60–272 (supplemental Fig. S3). This confirms that it is the HR domain that is interacting with the periplasmic domains of OutD. However, shifts were not observed when the domain pair N1-N2 (residues 116 to 285) was titrated into the labeled HR domain. These data reveal that the N0 domain and not the N1-N2 domains interact with the HR domain. The major peak shifts on the 1H-15N-HSQC spectra when N0 was added involved β1 of the HR domain (Fig. 5A).
FIGURE 5.
HADDOCK model of the OutC-HR·OutD-N0 complex in schematic representation. A, chemical shifts measured for the 15N-HR domain when unlabeled N0 is added. B, the HR domain is on the left in cyan, and the N0 domain on the right in green. Chemical shifts are mapped onto the structures of HR domain in red and onto the N0 domain in orange, respectively. β1 of the HR domain interacts with β3 and α2 of N0. The peaks shifted on HRF3 are Thr98, Gly99, Val200, Met101, Ala102, Ile112, Ile113, Phe120, Asp141, and Tyr88. On N0, the peaks shifted are Thr63, Ile64, Ser65, Phe79, Ser83, and Val84. This is a modeled structure not an experimentally derived structure.
Non-linear sampling methods were then used to collect data from 13C,15N-labeled N0 domain allowing the assignment of 65 of the 80 residues. The backbone chemical shifts confirmed that the secondary structure of D. dadantii N0 was the same as that of E. coli N0, and a reasonable model of the D. dadantii N0 domain could therefore be built using the E. coli domain as a template. The chemical shifts observed when unlabeled HR domain was added could now be mapped to the surface of the N0 domain. The chemical shifts observed reveal that residues on strands β1, β2, and β3 of the HR domain interact with N0 with residues on β1 most affected (Fig. 5). Residues on β1 and α2 of the N0 domain were the most affected, but the shifts were relatively small. Small shifts were seen for residues: Thr63, Ile64, Ser65, Phe79, Ser83, and Val84. A model of the HR·N0 complex was produced using HADDOCK (31) and is shown in Fig. 5B. The model brings β1 of HR and β3 of N0 together such that they could form a continuous anti-parallel β-sheet across both domains HR and N0. It is important to note that this is only a model of the interaction and the position and conformation of interfacial residues has not been defined experimentally. Met101 appears to be an important hydrophobic residue at the interface with the more remote Asp141 also showing large chemical shifts. The interaction between the D. dadantii HR and N0 domains is weak, but it does persist in the presence of 150 mm sodium chloride. The biological relevance of the interaction is assessed in the next section.
Assessment of HR Residues Involved in HR-N0 Interaction in Vivo
NMR cross-titration studies indicated that the HR-N0 interface includes the first β-strand of HR, notably Gly99, Val100, and Met101 (Figs. 2D and 5). Moreover, the latter residue and Asp141 showed large chemical shifts on binding. To explore the functional importance of these residues, they were substituted with alanine and cysteine and assessed in secretion assays (Fig. 3A). The D141A substitution was fully functional, indicating that this conserved residue (supplemental Fig. S4) is not crucial for the interaction with N0 and therefore has some other functional significance. Similarly, substitution of Val100 with cysteine did not affect the secretion (Fig. 3A, lane 6), implying that cysteine can be accommodated in place of the valine without perturbation of the β-sandwich architecture. In contrast, Gly99 and Met101 could not be substituted without total loss of secretion function (Fig. 3A), indicating their crucial structural and functional roles, respectively. Substitution of Met101 with alanine would lead to a loss of hydrophobic surface affecting interactions involving the HR-β1 interface. Gly99 is the most conserved residue among HR and PilP (supplemental Fig. S4). It is located in the center of β1 strand and introduces a bend to facilitate formation of β-bulge. Contrary to the other single cysteine substitutions in HR, OutCG99C formed dimers very efficiently (Fig. 4A, compare lane 3 with lanes 4 and 5, Fig. 4C, lower panel, compare lane 3 with 4), suggesting that in the assembled secretion system, neighboring HR domains might interact via the first β-strand.
In Vivo Assessment of HR-N0 and N0-N1 Interactions
To probe in vivo proximity of β1 of HR with β3 of N0 domain, several residues located on these two β-strands were simultaneously substituted with cysteine in an attempt to isolate a disulfide bridged OutC and OutD pair. Single cysteine substitution of Ile64 and Ser65 on β3 of N0 provoked homodimerization of the mutant OutD (Fig. 4B, lanes 3 and 4), suggesting that in the functional T2SS, neighboring N0 domains could interact together through the β3 strand. When these mutations in OutD were combined pairwise with G99C and V100C substitutions in OutC, only low amounts of OutC·OutD complex was observed with OutCG99C/OutDS65C couple (Fig. 4C, middle panel, lane 7). Perhaps an optimal arrangement of residues or the distance adequate for disulfide formation was not achieved.
We further probed by cysteine cross-linking analysis the crystal interface observed previously between the β3 of N0 and β6 of N1. This interface includes several pairs of proximal residues, notably Ser65 and Leu127, on β3 and β6, respectively. Single L127C substitution behaved as the wild-type protein, was fully functional and was monomeric (Fig. 3B, lane 14, and Fig. 4B, lane 5). However, double S65C/L127C substitution became non functional (Fig. 3B, lane 16) and formed an intramolecular disulfide bond because it migrated faster than monomeric OutD (Fig. 4B, compare lanes 5 and 6). In contrast, L127C combined with I64C did not generate such an intramolecular cysteine bonding (Fig. 4B, lane 7). These data are compatible with mutual arrangement of the assessed residues within the N0/N1 crystal interface, where Ile64 is buried, whereas Ser65 and Leu127 are exposed to the interface. These results reveal that β3 of the N0 domain can interact as well with an adjacent N0 domain (via β3-β3 strand interaction) as with N1 domain (via β3-β6 strand interaction) (Fig. 4B, lanes 4 and 6), suggesting a dynamic arrangement of the secretin N-terminal domains in the functional secretion system.
DISCUSSION
The similarity in structure of the HR domain of the T2SS and PilP of the T4PS suggests their evolution from a common ancestral domain, which strengthens the evolutionary link between these export machines, a link that did not previously extend to these core proteins of the inner membrane subcomplex. As both domains are located in the periplasm and are associated with machines that assemble pilins either to form a pilus (T4PS) or to push proteins through the outer membrane secretin (T2SS), the structural similarity may extend to a similarity in function. The HR domain binds to the N0 domain of the T2SS secretin and so by analogy, PilP has been shown to interact with the T4PS secretin stabilizing the complex (32).
It is clear from the in vivo studies that the interactions within the secretion system are dynamic. Probing the interaction between N0 and N1 of OutD in the functional secretion system confirmed that the interaction surface seen in the crystal complex of the N-terminal periplasmic domains of GspD with nanobody (18), mainly involving strands β3 and β6, corresponds to one of the functional arrangements of these domains in vivo, but we also discovered that the N0 domain can interact with a neighboring N0 domain through the same β3 strand. Interestingly, the crystal structure of the N-terminal region of EscC, the secretin from the type III secretion system, showed another mode of mutual arrangement of N0 and N1 domains (19), suggesting the dynamic character of interactions involving N-terminal domains of secretin. The binding of the OutC-HR domain to OutD-N0 must also be dynamic as β3 strand of N0 is also detected in this interaction. When this paper was being published, the crystal structure of the enterotoxigenic E. coli GspC-HR·GspD-N0-N1 complex (Protein Data Bank code 3OSS) was described (33). The GspC-HR crystal structure is closely similar to our solution structure of OutC-HR (Fig. 6). The two HR domains have 35% sequence identity, and the 65 equivalent α-carbon atoms superimpose with an r.m.s.d. of 1.3 Å. In the crystal structure β1 of HR interacts with β1 of N0 and not β3 of N0. One explanation for this might be that the β3 region of N0 is particularly sticky and that it prefers to bind N1 but that in the absence of N1, it binds β1 of HR. There is clearly an argument that with both N0 and N1 domains present in the crystal structure, it will be more representative of the situation in the assembled secretion system, but both the solution structure and the crystal structure may be poor mimics of the organization present in the functional secretion system where the dodecameric arrangement of GspD/OutD subunits interacts with a likely hexameric or dodecameric arrangement of GspC/OutC subunits as well as with the other components of the T2SS and secreted proteins.
FIGURE 6.
Comparison of the solution structure and the crystal structure of the HR domain. The solution structure of OutC-HR and crystal structure of GspD-HR are in cyan and blue, respectively. A and B are schematic representations looking down on to the first and second β-sheets. C illustrates the structural similarity between the structures for a central sheet strand, β2. Residue numbers are for OutC-HR, with those for GspD-HR in parentheses.
Our NMR study was initiated because the binding between the soluble regions of OutC and OutD was weak and so co-crystallization was not a possibility. The effectiveness of pulldown assays of OutC with OutD can be improved by co-expression or by partial unfolding in urea followed by refolding, but even then the complex is not stable in size exclusion chromatography even in conditions of low salt (16). The corresponding interaction for the enterotoxigenic E. coli GspC/GspD is clearly of higher affinity and can apparently be trapped more easily in the crystal. This apparently points to subtle differences between the secretion systems. Further research is clearly needed to establish the interactions between these and other domains in the context of the assembled secretion system and the way in which these interactions change during the secretion cycle.
In conclusion, this study completes the picture revealing that all core components of the T2SS have a structural ortholog within the T4PS. It also suggests the importance of β1 of the HR domain of the inner membrane subcomplex in interaction with β3 of the N0 domain of the secretin.
Supplementary Material
This work was supported by the Biology and Biotechnology Sciences Research Council (U.K), Higher Education Funding Council for England, and Queen Mary University of London.
The atomic coordinates and structure factors (code 2LNV) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
NMR spectra and assignments have been deposited in the BioMagResBank under accession no. 18181.

This article contains supplemental Tables S1 and S2 and Figs. S1–S4.
- T2SS
- type II secretion system
- T4PS
- type IV pili system
- HR
- homology region
- r.m.s.d.
- root mean square deviation.
REFERENCES
- 1. Filloux A. (2004) The underlying mechanisms of type II protein secretion. Biochim. Biophys. Acta 1694, 163–179 [DOI] [PubMed] [Google Scholar]
- 2. Cianciotto N. P. (2005) Type II secretion: A protein secretion system for all seasons. Trends Microbiol. 13, 581–588 [DOI] [PubMed] [Google Scholar]
- 3. Chami M., Guilvout I., Gregorini M., Rémigy H. W., Müller S. A., Valerio M., Engel A., Pugsley A. P., Bayan N. (2005) Structural insights into the secretin PulD and its trypsin-resistant core. J. Biol. Chem. 280, 37732–37741 [DOI] [PubMed] [Google Scholar]
- 4. Reichow S. L., Korotkov K. V., Hol W. G., Gonen T. (2010) Structure of the cholera toxin secretion channel in its closed state. Nat. Struct. Mol. Biol. 17, 1226–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Py B., Loiseau L., Barras F. (2001) An inner membrane platform in the type II secretion machinery of Gram-negative bacteria. EMBO Rep. 2, 244–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Camberg J. L., Johnson T. L., Patrick M., Abendroth J., Hol W. G., Sandkvist M. (2007) Synergistic stimulation of EpsE ATP hydrolysis by EpsL and acidic phospholipids. EMBO J. 26, 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peabody C. R., Chung Y. J., Yen M. R., Vidal-Ingigliardi D., Pugsley A. P., Saier M. H. (2003) Type II protein secretion and its relationship to bacterial type IV pili and archaeal flagella. Microbiology 149, 3051–3072 [DOI] [PubMed] [Google Scholar]
- 8. Hazes B., Frost L. (2008) Towards a systems biology approach to study type II/IV secretion systems. Biochim. Biophys. Acta 1778, 1839–1850 [DOI] [PubMed] [Google Scholar]
- 9. Sampaleanu L. M., Bonanno J. B., Ayers M., Koo J., Tammam S., Burley S. K., Almo S. C., Burrows L. L., Howell P. L. (2009) Periplasmic domains of Pseudomonas aeruginosa PilN and PilO form a stable heterodimeric complex. J. Mol. Biol. 394, 143–159 [DOI] [PubMed] [Google Scholar]
- 10. Abendroth J., Rice A. E., McLuskey K., Bagdasarian M., Hol W. G. (2004) The crystal structure of the periplasmic domain of the type II secretion system protein EpsM from Vibrio cholerae: The simplest version of the ferredoxin fold. J. Mol. Biol. 338, 585–596 [DOI] [PubMed] [Google Scholar]
- 11. Abendroth J., Kreger A. C., Hol W. G. (2009) The dimer formed by the periplasmic domain of EpsL from the type 2 secretion system of Vibrio parahaemolyticus. J. Struct. Biol. 168, 313–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karuppiah V., Derrick J. P. (2011) Structure of the PilM-PilN inner membrane type IV pilus biogenesis complex from Thermus thermophilus. J. Biol. Chem. 286, 24434–24442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Login F. H., Shevchik V. E. (2006) The single transmembrane segment drives self-assembly of OutC and the formation of a functional type II secretion system in Erwinia chrysanthemi. J. Biol. Chem. 281, 33152–33162 [DOI] [PubMed] [Google Scholar]
- 14. Bouley J., Condemine G., Shevchik V. E. (2001) The PDZ domain of OutC and the N-terminal region of OutD determine the secretion specificity of the type II out pathway of Erwinia chrysanthemi. J. Mol. Biol. 308, 205–219 [DOI] [PubMed] [Google Scholar]
- 15. Korotkov K. V., Krumm B., Bagdasarian M., Hol W. G. (2006) Structural and functional studies of EpsC, a crucial component of the type 2 secretion system from Vibrio cholerae. J. Mol. Biol. 363, 311–321 [DOI] [PubMed] [Google Scholar]
- 16. Login F. H., Fries M., Wang X., Pickersgill R. W., Shevchik V. E. (2010) A 20-residue peptide of the inner membrane protein OutC mediates interaction with two distinct sites of the outer membrane secretin OutD and is essential for the functional type II secretion system in Erwinia chrysanthemi. Mol. Microbiol. 76, 944–955 [DOI] [PubMed] [Google Scholar]
- 17. Korotkov K. V., Gonen T., Hol W. G. (2011) Secretins: Dynamic channels for protein transport across membranes. Trends Biochem. Sci. 36, 433–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Korotkov K. V., Pardon E., Steyaert J., Hol W. G. (2009) Crystal structure of the N-terminal domain of the secretin GspD from ETEC determined with the assistance of a nanobody. Structure 17, 255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spreter T., Yip C. K., Sanowar S., André I., Kimbrough T. G., Vuckovic M., Pfuetzner R. A., Deng W., Yu A. C., Finlay B. B., Baker D., Miller S. I., Strynadka N. C. (2009) A conserved structural motif mediates formation of the periplasmic rings in the type III secretion system. Nat. Struct. Mol. Biol. 16, 468–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shevchik V. E., Robert-Baudouy J., Condemine G. (1997) Specific interaction between OutD, an Erwinia chrysanthemi outer membrane protein of the general secretory pathway, and secreted proteins. EMBO J. 16, 3007–3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shen Y., Lange O., Delaglio F., Rossi P., Aramini J. M., Liu G., Eletsky A., Wu Y., Singarapu K. K., Lemak A., Ignatchenko A., Arrowsmith C. H., Szyperski T., Montelione G. T., Baker D., Bax A. (2008) Consistent blind protein structure generation from NMR chemical shift data. Proc. Natl. Acad. Sci. U.S.A. 105, 4685–4690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shen Y., Vernon R., Baker D., Bax A. (2009) De novo protein structure generation from incomplete chemical shift assignments. J. Biomol. NMR 43, 63–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 24. Bartels C., Xia T. H., Billeter M., Guntert P., Wuthrich K. (1995). The program XEASY for computer-supported NMR spectral-analysis of biological macromolecules. J. Biomol. NMR 6, 1–10 [DOI] [PubMed] [Google Scholar]
- 25. Fesik S. W., Zuiderweg E. R. (1988) Heteronuclear 3-dimensional NMR spectroscopy–A strategy for the simplification of homonuclear two-dimensional NMR-spectra. J. Magn. Reson. 78, 588–593 [Google Scholar]
- 26. Shen Y., Bax A. (2007) Protein backbone chemical shifts predicted from searching a database for torsion angle and sequence homology. J. Biomol. NMR 38, 289–302 [DOI] [PubMed] [Google Scholar]
- 27. Shen Y., Delaglio F., Cornilescu G., Bax A. (2009) TALOS+: A hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J. Biomol. NMR 44, 213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cordier F., Grzesiek S. (1999) Direct observation of hydrogen bonds in proteins by interresidue (3h)J(NC') scalar couplings. J. Am. Chem. Soc. 121, 1601–1602 [DOI] [PubMed] [Google Scholar]
- 29. Linge J. P., Habeck M., Rieping W., Nilges M. (2003) ARIA: Automated NOE assignment and NMR structure calculation. Bioinformatics 19, 315–316 [DOI] [PubMed] [Google Scholar]
- 30. Golovanov A. P., Balasingham S., Tzitzilonis C., Goult B. T., Lian L. Y., Homberset H., Tønjum T., Derrick J. P. (2006) The solution structure of a domain from the Neisseria meningitidis lipoprotein PilP reveals a new β-sandwich fold. J. Mol. Biol. 364, 186–195 [DOI] [PubMed] [Google Scholar]
- 31. Dominguez C., Boelens R., Bonvin A. (2003) HADDOCK: A protein-protein docking approach based on biochemical or biophysical information. J. Am. Chem. Soc. 125, 1731–1737 [DOI] [PubMed] [Google Scholar]
- 32. Balasingham S. V., Collins R. F., Assalkhou R., Homberset H., Frye S. A., Derrick J. P., Tønjum T. (2007) Interactions between the lipoprotein PilP and the secretin PilQ in Neisseria meningitidis. J. Bacteriol. 189, 5716–5727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Korotkov K. V., Johnson T. L., Jobling M. G., Pruneda J., Pardon E., Heroux A., Turley S., Steyaert J., Holmes R. K., Sandkvist M., Hol W. G. (2011) Structural and functional studies of the interaction of GspC and GspD in the type II secretion system. PloS Pathog. 7, e1002228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.