Background: Agonists can affect the subcellular localization of G protein-coupled receptors (GPCRs).
Results: Subcellular localization of AT1 angiotensin receptor rapidly changes in response to ligand binding.
Conclusion: Agonists have diverse effects on the subcellular dynamics of wild type and mutant AT1-Rs.
Significance: This study demonstrates that the effects of ligands on subcellular localization of GPCRs in living cells can be investigated by BRET.
Keywords: Angiotensin II, Bioluminescence Resonance Energy Transfer (BRET), Biosensors, Epidermal Growth Factor (EGF), G Protein-coupled Receptors (GPCR), Membrane Trafficking
Abstract
Initiation and termination of signaling of the type I angiotensin receptor (AT1-R) can lead to dynamic changes in its localization in plasma membrane microdomains. Several markers were recently developed to investigate membrane microdomains. Here, we used several YFP-labeled fusion constructs (i.e. raft or non-raft plasma membrane markers) to analyze the agonist-induced changes in compartmentalization of AT1-R, including internalization or lateral movement between plasma membrane compartments in response to stimulation using bioluminescence resonance energy transfer measurements. Our data demonstrate that angiotensin II (AngII) stimulus changes the microdomain localization of wild type or mutated (DRY → AAY or TSTS → AAAA) AT1-Rs co-expressed with the fluorescent probes in HEK293 cells. The comparison of the trafficking of AT1-R upon AngII stimulus with those of [Sar1,Ile8]AngII or [Sar1,Ile4,Ile8]AngII stimulus revealed different types of changes, depending on the nature of the ligand. The observed changes in receptor compartmentalization of the AT1-R are strikingly different from those of 5HT-2C and EGF receptors, which demonstrate the usefulness of the bioluminescence resonance energy transfer-based measurements in the investigation of receptor trafficking in the plasma membrane in living cell experiments.
Introduction
Studies during recent decades have revealed that the plasma membrane is not a uniform structure but rather a mosaic of microdomains, and this is a key concept of our current understanding of compartmentalized signaling. Membrane rafts are specific microdomains of the plasma membrane, which differ in their compositions from the rest of the plasma membrane (1). These cholesterol- and sphingolipid-rich plasma membrane microdomains play important roles in compartmentalization of cellular functions. Membrane rafts are frequently referred to as lipid rafts, whereas the rest of the plasma membrane can be called non-raft lipid domains or disordered membranes. The cholesterol- and sphingolipid-rich lipid rafts comprise up to 50% of the plasma membrane (2), their sizes are 10–200 nm, and they contain proteins clustered within them. The detergent-insoluble lipid rafts contain proteins that are modified posttranslationally by acylation or glycosylphosphatidylinositol modification but not by prenylation. Formation of rafts requires cholesterol; thus, cholesterol depletion by β-methyl-cyclodextrin treatment is widely used for disruption of lipid rafts.
Membrane microdomains are located in both leaflets of the plasma membrane. The Ras small G proteins are associated with inner leaflet clusters, as revealed by various methods, such as transmission electron microscopy and fluorescence recovery after photobleaching (3, 4). H-Ras and K-Ras are homologous proteins, but their C-terminal tails are significantly different. These C-terminal tails anchor the Ras proteins to the plasma membrane, but the different C termini of the Ras isoforms cause different interactions with membranes that can lead to distinct signaling outcomes. It was demonstrated that the GDP-bound H-Ras is located in lipid rafts, whereas the K-Ras is found in cholesterol-insensitive, non-raft lipid domains (4), and the different CAAX domains of the Ras proteins are targeted to distinct plasma membrane microdomains (5). It was also demonstrated by a fluorescence resonance energy transfer (FRET) approach that a lipid anchor on a fluorescent protein is sufficient to sequester the protein to different microdomains within the plasma membrane (6). Zacharias et al. (6) constructed several probes, in which the yellow fluorescent protein (YFP) was fused with short peptides containing consensus sequences for acylation, such as myristoylation and palmitoylation (MP-YFP).3 FRET measurements have revealed that the MP-YFP probe is clustered to membrane rafts, and β-methyl-cyclodextrin treatment interfered with its localization (6).
AngII is an octapeptide hormone, which is the main effector of the renin-angiotensin system and can bind and activate type 1 (AT1-R) and type 2 angiotensin receptors. AT1-R is a typical heptahelical, G protein-coupled receptor (GPCR), and the G protein-mediated “classical” signaling mechanisms are responsible for the majority of AngII-evoked cellular responses. These signaling mechanisms have a wide spectrum, including generation of second messengers (Ca2+ signal via inositol 1,4,5-trisphosphate, diacylglycerol), activation of small G proteins and cytoplasmic tyrosine kinases, regulation of ion channels, and transactivation of growth factor receptors. After binding of AngII to AT1-R, activation of heterotrimeric G proteins, Gq/11, mediates the hydrolysis of PtdIns(4,5)P2 by phosphoinositide-specific phospholipase Cβ, which leads to generation of second messengers (7). The down-regulation of this signaling includes several consecutive or parallel processes, such as desensitization, internalization into intracellular vesicles, and degradation of the receptors. Internalization of AT1-R is regulated by phosphorylation by GPCR kinases (GRKs), which promotes β-arrestin binding (7). Changes in the plasma membrane localization of AT1-R occur during the initiation and termination of signaling and also provide the possibility of resensitization that allows response to new extracellular stimuli.
Although membrane rafts are hot topics in the literature, their existence remains challenged (8). It is well established that some structural motifs of plasma membrane proteins are responsible for targeting into membrane microdomains, and several membrane markers were recently developed to investigate these microdomains (6). Although the concept of membrane rafts is based on biochemical experiments utilizing various detergent extraction methods, we decided to use another approach to investigate the relation of AT1-R to membrane microdomains in living cells. Earlier studies have demonstrated that AngII stimulation of AT1-R promotes association and trafficking of the receptors into caveolin-enriched/lipid rafts in vascular smooth muscle cells (9–11). Instead of focusing on the characterization of the biophysical and biochemical nature of the plasma membrane microdomains during AT1-R action, we followed the distribution of the AT1-Rs after ligand stimulus. FRET- and bioluminescence resonance energy transfer (BRET)-based methods are widely used to study oligomerization and protein-protein interactions of GPCRs (12, 13). The main advantage of these methods is that the measurements can be performed in living cells. For instance, it was shown by using FRET imaging in living cells that the neurokinin-1 receptors reside in very small (∼10-nm) membrane microdomains that are cholesterol-sensitive (14). We have constructed and used several YFP-labeled fusion constructs (i.e. raft or non-raft plasma membrane markers) to analyze the details of AT1-R trafficking, such as internalization or lateral movement between plasma membrane compartments upon stimulus in BRET measurements. Recently, a very similar targeting strategy was used to label different membrane microdomains (raft-targeted and non-raft-targeted reporters) to investigate the spatial compartmentalization in PI3K/Akt signaling (15). The BRET probes report energy transfer from Renilla luciferase to YFP, hence the molecular proximity of two fusion proteins. The BRET method is very sensitive due to the lack of excitation light, which results in low background (13, 16).
Using BRET experiments, we demonstrated that AT1-R changes its distribution between membrane microdomains in response to AngII stimulus in HEK293 cells. We also examined mutated AT1-Rs (either DRY/AAY mutation or TSTS/A mutation) co-expressed with fluorescent probes of different microdomains in order to determine the G protein- and phosphorylation-dependent steps in AT1-R trafficking. It was also revealed that the dynamics of AT1-R is different using diverse ligands of the receptor. The stimulus of AT1-R with peptide angiotensin analogues, such as [Sar1,Ile8]AngII or [Sar1,Ile4,Ile8]AngII, caused different changes in localization of AT1-R compared with AngII, whereas the non-peptide angiotensin antagonist candesartan led to complete inhibition of AT1-R trafficking.
EXPERIMENTAL PROCEDURES
Materials
Molecular biology enzymes were obtained from Fermentas (Burlington, Canada), Stratagene ( La Jolla, CA), and Invitrogen. Cell culture dishes and plates for BRET measurements were purchased from Greiner (Kremsmunster, Austria). Lipofectamine 2000 and coelenterazine h were from Invitrogen. The candesartan was obtained from Toronto Research Chemicals. The [Sar1,Ile8]AngII (SI-AngII) and [Sar1,Ile4,Ile8]AngII (SII-AngII) were purchased from Bachem AG (Bubendorf, Switzerland). Unless otherwise stated, all other chemicals and reagents were purchased from Sigma. The human embryonic kidney (HEK293) cells were from ATCC (American Type Culture Collection, Manassas, VA).
Molecular Biology
For the construction of YFP-labeled constructs, the plasmid backbones of eYFP-C1 or eYFP-N1 (Clontech, Mountain View, CA) were used. The cDNA of the eYFP-tagged β-arrestin2 (β-arrestin2-YFP) and eYFP-tagged AT1-R (AT1-R-YFP) were constructed as described previously (17). AT1-R-Rluc was constructed by replacing the eYFP coding region in AT1-R-YFP with Renilla luciferase. The luciferase-tagged 5-hydroxytryptamine receptor-2C receptor (5HT-2C-R) was constructed by subcloning the receptor cDNA into the codon humanized Renilla luciferase pRluc-N1 vector (PerkinElmer Life Sciences). The luciferase-tagged epidermal growth factor receptor (EGF-R) was constructed by replacing the eYFP coding region in EGF-R-YFP with Renilla luciferase. The MP targeting to plasma membrane rafts was based on the design of Zacharias et al. (6), where the MP tag was the N-terminal MGCIKSKRKDNLNDDE amino acid sequence from Lyn kinase (accession number NM_002350). In order to target the eYFP to the disordered plasma membrane microdomains, we have fused C-terminally the CAAX motif from the K-Ras small G protein, KMSKDGKKKKKKSKTKCVIM, consisting of the membrane targeting CAAX motif and hypervariable regions of K-Ras (accession number NM_004985). The eYFP-tagged phospholipase Cδ1 PH domain (PLCδ1-PH-YFP) was constructed as described previously (18). The eYFP-labeled full-length Rab5 was constructed by replacing the eGFP coding region with eYFP in Rab5-GFP, as described previously (19). The GRK2-YFP construct was a generous gift from Dr. Marc G. Caron (Duke University Medical Center).
Mutagenesis was performed using standard site-directed mutagenesis techniques. After verifying the mutations with dideoxy sequencing, the mutated fragment was exchanged between the wild type and mutated portion with suitable restriction sites to avoid the generation of unwanted mutations outside the sequenced regions. The DRY/AAY mutation of the AT1A-R (the highly conserved D125R126Y127 was mutated to A125A126Y127) and the TSTS/A mutant of the AT1A-R (T332S335T336S338 was substituted with alanine residues) were described earlier (20, 21).
Cell Culture and Transfection
The experiments were performed on the HEK293 cell line. The cells were cultured in DMEM with penicillin/streptomycin (Invitrogen) and 10% heat-inactivated fetal bovine serum (FBS) in 5% CO2 at 37 °C. The cells were cultured in plastic dishes, trypsinized prior to transfection, transiently transfected by using Lipofectamine 2000 (Invitrogen), and plated on polylysine-pretreated white 96-well plates at a density of 1 × 105 cells/well for BRET measurements. The DNA amounts were 0.25 μg of Rluc-containing construct/well and 0.0625 μg of YFP-containing construct/well; the amount of Lipofectamine 2000 was 0.5 μl/well.
BRET Measurement
We used a Renilla luciferase-fused receptor as the energy donor and an eYFP-tagged protein as the acceptor. The BRET measurements were performed after 24 h of the transfection on white 96-well plates. The medium of the cells was changed prior to measurements to a modified Krebs-Ringer buffer containing 120 mm NaCl, 4.7 mm KCl, 1.8 mm CaCl2, 0.7 mm MgSO4, 10 mm glucose, and 10 mm Na-HEPES, pH 7.4, and the BRET measurements were performed at 37 °C. The BRET measurements were started after the addition of the cell-permeable substrate, coelenterazine h (Invitrogen), at a final concentration of 5 μm, and the counts were recorded by using a Berthold Mithras LB 940 multilabel reader using filters at 485- and 530-nm wavelengths; the detection time was 0.25–0.5 s. The BRET ratios were calculated as the 530 nm/485 nm ratio. Measurements were done in triplicate. The BRET records are averages of at least three independent experiments. BRET ratios were base line-corrected to the vehicle curve using GraphPad Prism software.
Confocal Microscopy
The localization and distribution of the targeted probes were analyzed using a Zeiss LSM 510 confocal laser-scanning microscope in living cells plated on polylysine-pretreated glass coverslips (3 × 105 cells/35-mm dish).
RESULTS
BRET Assay for Detection of Changes in Compartmental Localization of AT1-R
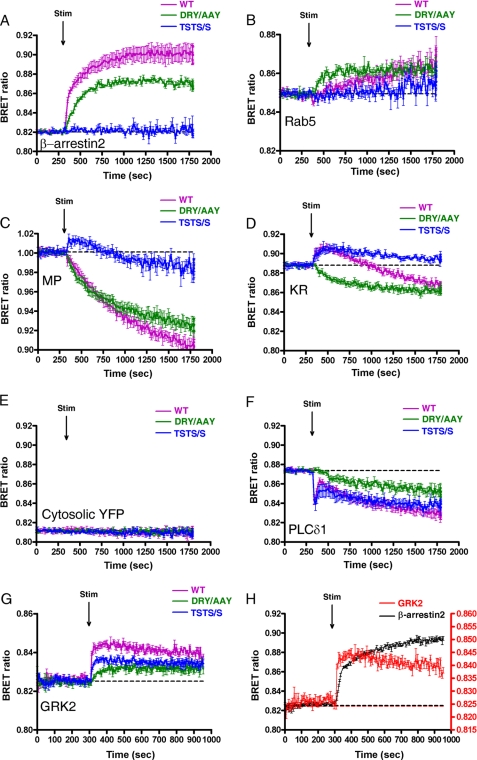
To detect the agonist-induced trafficking of AT1-R, our strategy was to use Renilla luciferase-labeled AT1-R, and we followed the BRET ratio of its interaction with a YFP-labeled protein counterpart. When the HEK293 cells co-expressing wild type AT1-R-luciferase and β-arrestin2-YFP were exposed to 100 nm AngII, the BRET ratio (Fig. 1A, magenta trace) elevated between the β-arrestin2-YFP and wild type AT1-R-luciferase, showing that the β-arrestin binds to the activated AT1-R. The internalized AT1-R then appears in the endocytic route, which could be detected with the energy transfer between the Rab5-YFP (as early endosome marker) and the wild type AT1-R-luciferase (Fig. 1B, magenta trace). To further analyze the compartmentalization of the receptors, we also used plasma membrane-targeted biosensors to investigate the possibility of the compartmentalized signaling of AT1-R. We used MP-YFP to label the lipid rafts (6), whereas the YFP-labeled CAAX domain of K-Ras (KR-YFP) was used as the marker of the non-raft lipid domains (disordered plasma membrane) (4, 5, 15). When MP-YFP was used, the BRET ratio between this construct and wild type AT1-R-luciferase dropped in response to AngII stimulation (Fig. 1C, magenta trace). However, when KR-YFP was used, we measured a significant BRET signal elevation prior to the drop in BRET ratio (Fig. 1D, magenta trace). The observed changes in the BRET ratio upon stimulus of AT1-R-luciferase using either MP-YFP or KR-YFP are not a consequence of protein overexpression because gradual reduction of the DNA amounts used for the transfection, resulting in very low counts in the BRET measurements, did not alter the shapes and extents of the BRET ratio changes (supplemental Fig. 1). Because the cytosolic YFP and AT1-R are not in the same compartment, the stimulus of the AT1-R-luciferase by 100 nm AngII did not change the BRET ratio (Fig. 1E, magenta trace). We also have investigated the binding of GRK2 to AT1-R upon stimulus because this GRK isoform is mostly responsible for AT1-R phosphorylation and β-arrestin recruitment in HEK293 cells (22). When the cells were exposed to 100 nm AngII, the BRET ratio immediately elevated between the GRK2-YFP and wild type AT1-R-luciferase (Fig. 1G, magenta trace). The time resolution of the BRET measurement allowed us to demonstrate that the agonist-induced binding of GRK2 to the AT1-R precedes the binding of β-arrestin2 to the receptor. Stimulation of the wild type AT1-R-luciferase evokes the binding of both the GRK2-YFP and β-arrestin2-YFP, but it is observable that the red trace of GRK2 precedes the black trace of β-arrestin2 (Fig. 1G), a phenomenon that was shown earlier by Hasbi et al. (23), using the oxytocin receptor, another GPCR.
FIGURE 1.
BRET assay between AT1-R and different proteins upon AngII stimulation in HEK293 cells. HEK293 cells were transfected with the plasmids of the indicated AT1-R-luciferase (magenta trace, wild type; green trace, DRY/AAY mutant; blue trace, TSTS/A mutant) and with the indicated YFP-fused proteins, and after 24 h, the cells were exposed to 100 nm AngII or vehicle (dashed line) at the indicated time points. BRET pairs were as follows: β-arrestin2-YFP and the indicated AT1-R-luciferase (A), Rab5-YFP and the indicated AT1-R-luciferase (B), MP-YFP and the indicated AT1-R-luciferase (C), KR-YFP and the indicated AT1-R-luciferase (D), untargeted YFP and the indicated AT1-R-luciferase (E), PLCδ1-PH-YFP and the indicated AT1-R-luciferase (F), and GRK2-YFP and the indicated AT1-R-luciferase (G). H, HEK293 cells were transfected with the plasmids of the wild type AT1-R-luciferase and either β-arrestin2-YFP (black trace) or GRK2-YFP (red trace), and after 24 h, the cells were exposed to 100 nm AngII or vehicle (dashed line). The left y axis is for β-arrestin2, and the right y axis is for GRK2. The BRET records are averages of at least three independent experiments. Mean values ± S.E. (error bars) are shown (n = 3).
Compartmentalization of Mutant AT1-Rs
The dissimilar BRET curves of MP-YFP or KR-YFP with the AT1-R-luciferase raised the possibility that we could monitor the compartmentalization of AT1-R between different plasma membrane compartments in living cells immediately in response to agonist stimulus. We further investigated whether the trafficking of the receptor is altered using mutant AT1-Rs (Fig. 1, DRY/AAY AT1-R (green traces) and TSTS/A AT1-R (blue traces)). It was revealed that the compartmentalization of DRY/AAY AT1-R is dramatically changed compared with wild type AT1-R. When the DRY/AAY AT1-R-luciferase was exposed to 100 nm AngII, the β-arrestin binding of the AngII-bound DRY/AAY mutant receptor was slightly decreased (Fig. 1A, green trace). The AngII-stimulated DRY/AAY mutant receptor was also able to translocate to Rab5 endocytic compartments (Fig. 1B, green trace). The BRET ratio also dropped immediately with MP-YFP using DRY/AAY AT1R-luciferase (Fig. 1C, green trace), similar to the wild type AT1-R-luciferase (Fig. 1C, magenta trace). The BRET ratio decreased after the stimulation of HEK293 cells with 100 nm AngII when we studied the interaction of DRY/AAY AT1-R-luciferase and KR-YFP (Fig. 1D, green trace). In this case, the decrease in BRET ratio was immediate and did not show the initial elevation we observed using the wild type AT1-R and the KR-YFP (Fig. 1D, magenta trace). We also determined the compartmentalization of another AT1-R mutant, TSTS/A. In agreement with earlier findings (21, 24), this receptor was not able to bind β-arrestin, and its AngII-induced internalization was significantly reduced (Fig. 1, A and B, blue traces). Because the internalization of the TSTS/A AT1-R is impaired compared with the wild type AT1-R, we observed a reduction in the late decrease in the BRET ratios (Fig. 1, C and D, blue traces). We think that the late component of the BRET signal is the consequence of the internalization of the AT1-R, resulting in depletion of the receptor in the plasma membrane. This assumption is confirmed using AT1-R-luciferase and PLCδ1-PH-YFP as BRET pairs. The PH domain of PLCδ1 binds to PtdIns(4,5)P2 in the plasma membrane (18). When the HEK293 cells co-expressing wild type AT1-R-luciferase and PLCδ1-PH-YFP were exposed to 100 nm AngII, the BRET ratio dropped very quickly, which was followed by an increase (Fig. 1F, magenta trace). The graph shows that the rapid initial dissociation (BRET ratio drop) of AT1-R-luciferase and PLCδ1-PH-YFP is followed by a transient association (a short BRET ratio increase) and a more prolonged dissociation (prolonged BRET ratio decrease) of these proteins. The initial drop in the BRET signal reflects that agonist-induced activation of AT1-R caused Gq/11 activation and PtdIns(4,5)P2 breakdown, resulting in the release of PLCδ1-PH-YFP from the plasma membrane. After a short period of time, the ratio began to increase because PtdIns(4,5)P2 resynthesis occurs very rapidly, and the majority of the PLCδ1-PH-YFP molecules rebind to the plasma membrane. This elevation in the BRET signal is followed by a slow decrease, which suggests that the AT1-R-luciferase and the PLCδ1-PH-YFP start to diverge from each other again, reflecting the internalization of the receptor. When HEK293 cells co-expressing DRY/AAY mutant AT1-R-luciferase and PLCδ1-PH-YFP were exposed to 100 nm AngII, the BRET ratio decreased slowly without the fast initial dissociation (Fig. 1F, green trace), which is consistent with the fact that the stimulus of the DRY/AAY AT1-R does not result in PtdIns(4,5)P2 breakdown and release of PLCδ1-PH-YFP from the plasma membrane (Fig. 1F), but the DRY/AAY AT1-R internalizes upon stimulus (Fig. 1B, green trace). We also investigated the effects of AngII on the binding of mutant AT1-Rs to GRK2. The TSTS/A mutant AT1-R mutant was able to bind to GRK2, although the binding was reduced, whereas the β-arrestin2 binding of this mutant was not detectable (Fig. 1, A and G, blue traces). Interestingly, the DRY/AAY AT1-R mutant is less capable of binding to GRK2, although the β-arrestin2 binding of this mutant is very similar to the wild type receptor (Fig. 1, A and G, green traces).
Effect of Agonist Stimulation on Distribution of Plasma Membrane Markers
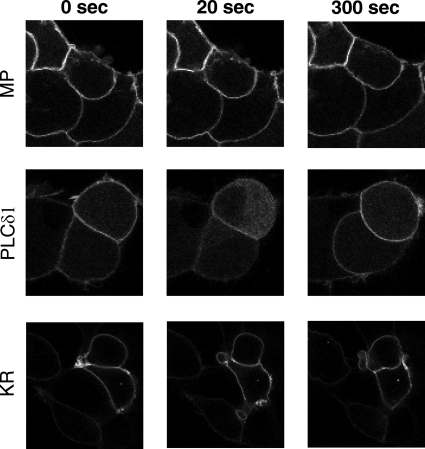
We examined the distribution of MP-YFP, PLCδ1-PH-YFP, and KR-YFP in HEK293 cells in response to AngII stimulation. As shown in Fig. 2, the AngII stimulus did not affect noticeably the distribution and amount of either MP-YFP or KR-YFP in the plasma membrane. In contrast, PLCδ1-PH-YFP is temporarily translocated to the cytoplasm and then returns to the plasma membrane, reflecting its PtdIns(4,5)P2 level (18). Taken together, we can conclude that the largest proportion of the observed changes in the BRET ratio using MP-YFP or KR-YFP along with AT1-R-luciferase probably reflect the alterations in the AT1-R distribution. Based on our data, we cannot rule out that the possibility that distributions of the MP-YFP or KR-YFP are not altered during the experiments, although this cannot be resolved by confocal microscopy.
FIGURE 2.
Effects of AngII stimulation on the distribution of MP-YFP, PLCδ1-PH-YFP, and KR-YFP in HEK293 cells. The cells were transfected with the AT1-R-luciferase and with the indicated YFP-fused proteins. After 24 h, the cells were exposed to 100 nm AngII. The probes were visualized by laser-scanning confocal microscopy (Zeiss LSM510). The representative confocal micrographs show the localization and cellular distribution of the indicated probes before (0 s) and 20 or 300 s after the AngII treatment. The YFP fluorescence was detected by Zeiss LSM510 confocal microscope. Bars, 10 μm.
Compartmentalization of AT1-Rs Using Various Ligands
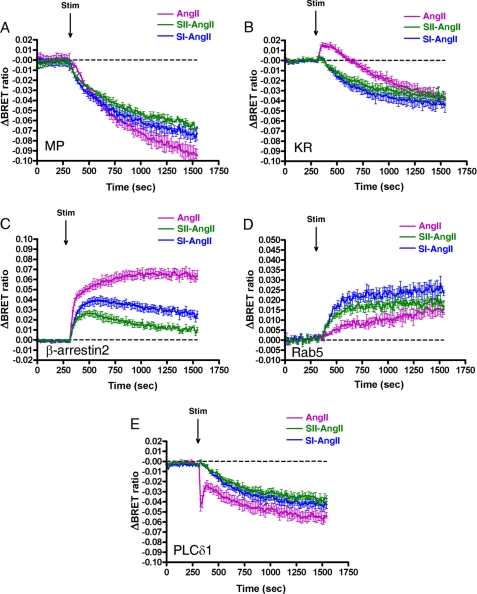
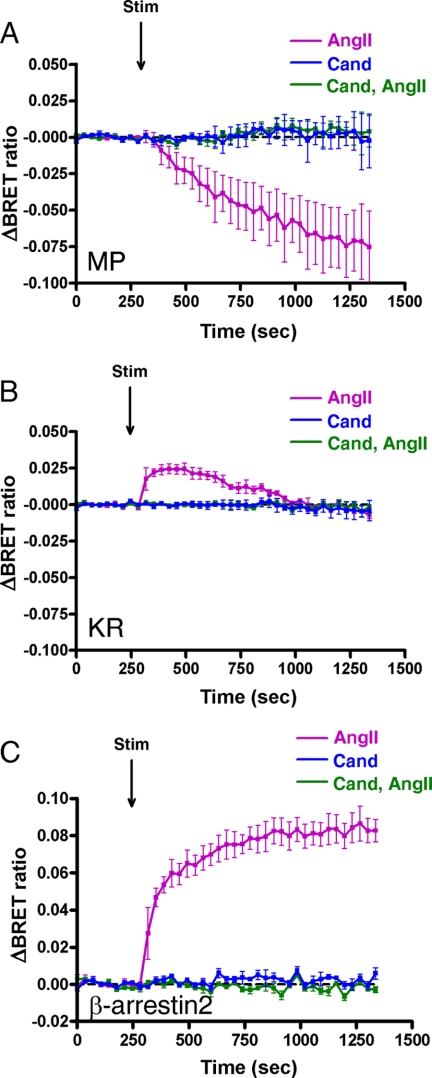
We further investigated whether the binding of different ligands to the AT1-R leads to dissimilar changes upon stimulus. SII-AngII is a biased peptide agonist of the AT1-R, which is not able to activate G proteins but can stimulate G protein-independent mechanisms, such as β-arrestin binding and consequent ERK activation (25, 26). Stimulation of AT1-R with this peptide caused different compartmentalization of AT1-R compared with the effects of AngII (Fig. 3). It is shown in Fig. 3, A and B, that motion of the AT1-R is impaired using SII-AngII, but the receptor is able to internalize, which is consistent with previous reports that SII-AngII can induce AT1-R internalization (27). The BRET ratio drops with the MP-YFP immediately after SII-AngII stimulation (Fig. 3A, green trace), and the kinetics of this process seems to be more rapid compared with AngII stimulus (Fig. 3A, magenta trace). It is remarkable that the BRET ratio between KR-YFP and AT1-R-luciferase upon SII-AngII treatment decreases immediately, without the initial elevation compared with AngII stimulation (Fig. 3B). This observation is very reminiscent of the AngII stimulus of DRY/AAY AT1-R with KR-YFP (Fig. 1D, green trace). The SII-AngII-induced binding of AT1-R-luciferase to β-arrestin2 is impaired compared with the effect of AngII stimulation (Fig. 3C). In addition, a higher increase in the BRET interaction between Rab5-YFP and AT1-R-luciferase was observed after SII-AngII treatment compared with AngII stimulation (Fig. 3D), which is similar to the results obtained in the studies for AngII-induced association of wild type and DRY/AAY mutant AT1-R-luciferase with Rab5-YFP (Fig. 1B). Because the SII-AngII stimulus of AT1-R does not activate Gq-mediated PtdIns(4,5)P2 breakdown, the BRET ratio with the PLCδ1-PH-YFP decreases immediately and continually without an initial peak in the drop (Fig. 3E, green trace). The SI-AngII is an octapeptide angiotensin analog, which is an antagonist of AT1-R (28), but it was shown earlier that it is able to initiate receptor internalization (29). Stimulation of AT1-R-luciferase with SI-AngII also caused altered receptor compartmentalization compared with AngII stimulation (Fig. 3). Because the SI-AngII is an AT1-R antagonist, stimulation with this analog does not result in PtdIns(4,5)P2 hydrolysis (Fig. 3B, blue trace). In addition, treatment of AT1-R with a non-peptide AT1-R antagonist, candesartan, did not change the distribution of AT1-R, and the pretreatment of HEK293 cells with candesartan prior to BRET measurement completely inhibited the trafficking of AT1-R in response to AngII stimulation (Fig. 4).
FIGURE 3.
Effects of angiotensin peptides on the BRET interaction between AT1-R and different proteins in HEK293 cells. HEK293 cells were transfected with the plasmids of the AT1-R-luciferase and the indicated YFP-fused proteins, and after 24 h, the cells were exposed to either 100 nm AngII (magenta trace), 100 nm SI-AngII (blue trace), 10 μm SII-AngII (black trace), or vehicle (dashed line) at the indicated time points. BRET pairs were as follows: MP-YFP and wild type AT1-R-luciferase (A); KR-YFP and wild type AT1-R-luciferase (B); β-arrestin2-YFP and wild type AT1-R-luciferase (C); Rab5-YFP and wild type AT1-R-luciferase (D); PLCδ1-PH-YFP and wild type AT1-R-luciferase (E). The BRET records are averages of three independent experiments. Mean values ± S.E. (error bars) are shown (n = 3).
FIGURE 4.
Effects of AngII and candesartan on the BRET interaction between AT1-R and different proteins in HEK293 cells. HEK293 cells were transfected with the plasmids of the AT1-R-luciferase and the indicated YFP-fused proteins, and after 24 h, the cells were exposed to either 100 nm candesartan (blue trace) or 100 nm AngII without pretreatment (magenta trace) or with 100 nm candesartan pretreatment for 10 min (green trace). BRET pairs were as follows: MP-YFP and wild type AT1-R-luciferase (A), KR-YFP and wild type AT1-R-luciferase (B), and β-arrestin2-YFP and wild type AT1-R-luciferase (C). The BRET records are averages of three independent experiments. Mean values ± S.E. (error bars) are shown (n = 3).
BRET Assay for Detection of Compartmentalization of 5HT-2C-R and EGF-R
The preceding results suggest that the plasma membrane compartmentalization of a receptor could be mapped by the help of BRET measurements. Next, we tested the agonist-induced compartmentalization of other membrane receptors using our BRET approach to compare their kinetics with those of AT1-R. We analyzed the agonist-induced dynamics of 5HT-2C-R (supplemental Fig. 2) and EGF-R (supplemental Fig. 3). The 5HT-2C-R (30) also couples to Gq protein, similarly to the AT1-R. After serotonin (5-hydroxytryptamine, 5HT) binding, the receptor bound β-arrestin2 (Fig. 5C) and became internalized, as shown by the elevation of the BRET ratio between Rab5 and 5HT-2C-R (supplemental Fig. 2D). The internalization was also apparent using the plasma membrane-located MP-YFP and KR-YFP (supplemental Fig. 2, A and B). However, in contrast to AT1-R, we could not detect a strikingly different distribution change between using MP-YFP and KR-YFP. The EGF-R is a growth factor receptor with tyrosine kinase activity, which is widely used for the study of receptor internalization (31). In contrast to GPCRs, such as AT1-R or 5HT-2C-R, the EGF-R does not interact with β-arrestin (supplemental Fig. 3C) but internalizes upon EFG treatment (supplemental Fig. 3D). Similarly to 5HT-2C-R, we also did not observe different distribution change between using MP-YFP and KR-YFP with EGF-R-luciferase (supplemental Fig. 3, A and B).
FIGURE 5.
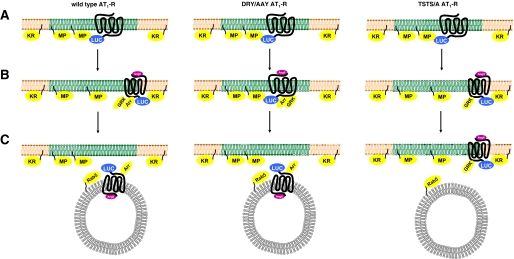
Compartmentalized AT1-R signaling in the plasma membrane. A, plasma membrane localization of the indicated AT1-Rs in unstimulated cells. B, changes immediately after the ligand treatment. C, localization of the receptors 5 min after the stimulus. The plasma membrane and endosome are shown.
DISCUSSION
In the present study, we have investigated the compartmentalization of the AT1-R in the plasma membrane in response to ligand binding. Instead of biochemical characterization of AT1-R localization in the plane of the plasma membrane, we used a BRET-based approach to investigate the distribution of the receptor in response to hormone stimulus. We used several YFP-labeled fusion constructs and Renilla luciferase fused receptors as intermolecular probe pairs in BRET measurements.
Interactions of YFP-labeled fusion constructs (plasma membrane targeted markers, β-arrestin, GRK2, and Rab5) with luciferase-tagged AT1-Rs were evaluated to follow the compartmentalization of the receptor. MP-YFP and YFP-labeled CAAX domain of K-Ras (KR-YFP) were used to label the lipid rafts (6) and non-raft lipid domains (disordered plasma membrane), respectively (4, 5, 15). Because our goal was to analyze the agonist-induced changes in receptor compartmentalization in live cells, we did not measure the actual distribution of the YFP-labeled markers in our HEK293 cells by biochemical preparation methods, such as detergent solubilization and ultracentrifugation. To achieve our goal, we used BRET-based probes, assuming that, although the MP-YFP and KR-YFP are apparently uniformly localized in the inner surface of the plasma membrane (see the confocal images of Fig. 2), they probably differently label submicroscopic microdomains (4–6, 15). The different properties of these microdomains also manifested when their interaction was studied with AT1-R-luciferase in BRET measurements in living cells (Fig. 1). These data demonstrate that AT1-R probably is not distributed uniformly in the native plasma membrane but is instead localized in specific microdomains. The changes in compartmentalization of AT1-Rs are different when followed by various plasma membrane markers in BRET measurements. Our BRET experiments indicate that the localization of the AT1-R rapidly changes after AngII stimulation in HEK293 cells (Figs. 1 and 3). However, we cannot exclude the possibility that the BRET ratio changes can be also the result of the lateral diffusion of YFP-labeled biosensors because the membrane microdomains are not static structures. It is noteworthy that the absolute level of BRET signal is higher when using MP-YFP compared with KR-YFP (∼1.0 versus ∼0.89; the absolute BRET ratio is ∼0.82 when using cytosolic YFP and the AT1-R-luciferase). The higher basal BRET signal could reflect that the unstimulated AT1-R-luciferase is preferably located in the plasma membrane microdomain marked by MP-YFP in resting conditions.
We also examined the agonist-induced changes in compartmentalization of mutated AT1-Rs (DRY/AAY or TSTS/A mutation) in order to determine the G protein- and phosphorylation-dependent steps in AT1-R trafficking. Comparing the data, which were obtained utilizing either wild type or mutated AT1-R co-expressed with the fluorescent probes in HEK293 cells, revealed changes in their distribution between membrane microdomains in response to AngII stimulation. It is also possible that the initial BRET ratio elevation between wild type AT1-R-luciferase and KR-YFP upon AngII stimulus is the consequence of the change of the distribution of the KR-YFP (the energy acceptor molecules are enriched during AT1-R action). The compartmentalization of DRY/AAY AT1-R, which is not able to activate Gq/11 protein (20), is dramatically changed compared with wild type AT1-R. Stimulation of DRY/AAY AT1-R did not result in PtdIns(4,5)P2 breakdown and concomitant release of PLCδ1-PH-YFP from the plasma membrane (Fig. 1F), but DRY/AAY AT1-R internalizes upon stimulus, in agreement with previous reports that DRY/AAY AT1-R undergoes internalization after AngII stimulation (20, 26). The β-arrestin binding of the AngII-bound DRY/AAY mutant receptor is G protein-independent (Fig. 1A) as was shown earlier (26, 32, 33). It seems that the rapid distribution change of AT1-R-luciferase in response to AngII stimulus between the MP-YFP and KR-YFP is G protein-dependent. The BRET ratio between DRY/AAY AT1-R-luciferase and the KR-YFP decreased after AngII stimulation without the initial elevation, contrary to the wild type AT1-R (Fig. 1D). In addition, the decline in the BRET ratio seemed more immediate when interaction of DRY/AAY AT1R-luciferase was studied with MP-YFP compared with the wild type AT1-R-luciferase (Fig. 1C) or when MP-YFP and AT1-R-luciferase were studied upon SII-AngII or SI-AngII stimuli (Fig. 3A). TSTS/A mutant AT1-R is not able to bind β-arrestin2, and its internalization is significantly reduced in response to AngII (21, 24). We also confirmed these properties of this mutant and also showed that the TSTS/A AT1-R still anchors GRK2 (Fig. 1, A, B, and G). Because the internalization of the TSTS/A AT1-R is reduced compared with wild type AT1-R, the late decrease component is similarly slower, which is consistent with our conclusion that this component is caused by the internalization of the receptor (Fig. 1, C, D, and F).
Our studies also revealed that treatment of AT1-R with either [Sar1,Ile8]AngII or [Sar1,Ile4,Ile8]AngII caused different changes in the compartmentalization of AT1-R compared with the effect of AngII (Fig. 3), whereas candesartan did not induce changes in microdomain localization or trafficking of AT1-R (Fig. 4). The biased agonist, SII-AngII, is not able to activate G proteins but can stimulate G protein-independent mechanisms, such as β-arrestin binding and ERK activation (25, 26). According to our data, the rapid distribution change of AT1-R-luciferase upon stimulus between plasma membrane markers is G protein-dependent or reflects the different conformational change of the receptor using distinct ligands. The binding of an antagonist (SI-AngII) to AT1-R also caused altered compartmentalization of the receptor compared with AngII stimulus (Fig. 3), which supports the idea that the agonist-induced compartmentalization of the AT1-Rs in the plasma membrane is G protein-dependent and/or requires the physiological conformational change.
Based on our findings, we propose that the AT1-R preferably localized in raft domains in resting cells. According to the model, the stimulation of wild type AT1-R with AngII causes conformational change and redistribution of the receptor in the plasma membrane as well as binding to GRK2 and β-arrestin2, which leads to internalization of the receptor (Fig. 5, left column). In the case of stimulation of wild type AT1-R with SII-AngII or stimulation of DRY/AAY mutant AT1-R with AngII, cause another conformational change and the redistribution of the receptor is bypassed (Fig. 5, middle column). This leads to accelerated association with Rab5. In contrast, stimulation of TSTS/AAY mutant AT1-R with AngII causes similar conformational change and redistribution of the receptor as in the case of the wild type receptor. This mutant is not able to bind β-arrestin2, which results in impaired internalization, leading to the sustained “mislocalization” of the receptor among the membrane microdomains (Fig. 5, right column). Taken together, it is likely that the AT1-R is not uniformly located in the plasma membrane but is compartmentalized. The AT1-R-luciferase prefers the MP-YFP-labeled microdomains (raft domains), contrary to KR-YFP-labeled non-raft microdomains. The examined mutations (DRY/AAY and TSTS/A) in the receptors did not change this uneven distribution pattern. Interestingly, the AngII stimulus evoked different distribution changes of the wild type and the mutated receptors with the YFP biomarkers. The data showed that Gq protein activation is essential for the proper compartmentalization of the AT1-R. Lack of Gq activation inhibits the appearance of the receptor in the non-raft microdomains (or the KR-YFP accumulation close to the receptor) and accelerates the association with Rab5. It also seems that the endocytosis of the receptor can occur by distinct mechanisms, depending on the Gq coupling. It is also possible that other mutations or pathological conditions can induce mislocalization of the receptors that can provoke altered signaling.
We also examined the compartmentalization of other receptors, such as 5HT-2C-R and EGF-R (supplemental Figs. 2 and 3). The 5HT-2C-R is also coupled to Gq protein, similarly to the AT1-R (30), whereas the EGF-R is a tyrosine kinase receptor and is widely used for the study of receptor internalization (31). It is important to note that the compartmentalization of AT1-R is strikingly different from those of 5HT-2C-R and EGF-R, which underlines the usefulness of the BRET-based approach to investigate the dynamics of receptor compartmentalization in living cell experiments. Taken together, these studies provided valuable information about the distribution and dynamics of AT1-R upon ligand binding among membrane microdomains.
Supplementary Material
Acknowledgments
We thank Dr. M. G. Caron for providing GRK2-YFP plasmid. The excellent technical assistance of Ilona Oláh, Judit Rácz, and Mártonné Schultz is greatly appreciated.
This work was supported by Hungarian Scientific Research Fund Grants OTKA PF63893, OTKA NK72661, and OTKA-Norway NNF78925 and National Development Agency of Hungary (NFU TAMOP) Grants 4.2.2-08/1/KMR and 4.2.1.B-09/1/KMR-2010-0001.

This article contains supplemental Figs. 1–3.
- MP-YFP
- myristoylated and palmitoylated YFP
- AngII
- angiotensin II
- BRET
- bioluminescence resonance energy transfer
- GPCR
- G protein-coupled receptor
- GRK
- GPCR kinase
- 5HT
- serotonin (5-hydroxytryptamine)
- AT1-R
- type 1 angiotensin receptor
- 5HT-2C-R
- type 2C serotonin receptor
- KR-YFP
- YFP-labeled CAAX domain of K-Ras
- PLC
- phospholipase C
- PH
- pleckstrin homology
- PLCδ1-PH-YFP
- YFP-tagged phospholipase Cδ1 PH domain
- PtdIns(4,5)P2
- phosphatidylinositol 4,5-bisphosphate
- HEK
- human embryonic kidney
- eYFP
- enhanced YFP
- EGF-R
- EGF receptor
- SI-AngII
- [Sar1,Ile8]AngII
- SII-AngII
- [Sar1,Ile4,Ile8]AngII
- DRY/AAY
- D125A/R126A mutation
- TSTS/A
- T332A/S335A/T336A/S338A mutation.
REFERENCES
- 1. Jacobson K., Mouritsen O. G., Anderson R. G. (2007) Lipid Rafts. At a crossroad between cell biology and physics. Nat. Cell Biol. 9, 7–14 [DOI] [PubMed] [Google Scholar]
- 2. Hao M., Mukherjee S., Maxfield F. R. (2001) Cholesterol depletion induces large scale domain segregation in living cell membranes. Proc. Natl. Acad. Sci. U.S.A. 98, 13072–13077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Plowman S. J., Muncke C., Parton R. G., Hancock J. F. (2005) H-Ras, K-Ras, and inner plasma membrane raft proteins operate in nanoclusters with differential dependence on the actin cytoskeleton. Proc. Natl. Acad. Sci. U.S.A. 102, 15500–15505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Niv H., Gutman O., Kloog Y., Henis Y. I. (2002) Activated K-Ras and H-Ras display different interactions with saturable nonraft sites at the surface of live cells. J. Cell Biol. 157, 865–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prior I. A., Muncke C., Parton R. G., Hancock J. F. (2003) Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J. Cell Biol. 160, 165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zacharias D. A., Violin J. D., Newton A. C., Tsien R. Y. (2002) Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296, 913–916 [DOI] [PubMed] [Google Scholar]
- 7. Hunyady L., Catt K. J. (2006) Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol. Endocrinol. 20, 953–970 [DOI] [PubMed] [Google Scholar]
- 8. Shaw A. S. (2006) Lipid rafts. Now you see them, now you don't. Nat. Immunol. 7, 1139–1142 [DOI] [PubMed] [Google Scholar]
- 9. Ishizaka N., Griendling K. K., Lassègue B., Alexander R. W. (1998) Angiotensin II type 1 receptor. Relationship with caveolae and caveolin after initial agonist stimulation. Hypertension 32, 459–466 [DOI] [PubMed] [Google Scholar]
- 10. Linder A. E., Thakali K. M., Thompson J. M., Watts S. W., Webb R. C., Leite R. (2007) Methyl-beta-cyclodextrin prevents angiotensin II-induced tachyphylactic contractile responses in rat aorta. J. Pharmacol. Exp. Ther. 323, 78–84 [DOI] [PubMed] [Google Scholar]
- 11. Ushio-Fukai M., Alexander R. W. (2006) Caveolin-dependent angiotensin II type 1 receptor signaling in vascular smooth muscle. Hypertension 48, 797–803 [DOI] [PubMed] [Google Scholar]
- 12. Giepmans B. N., Adams S. R., Ellisman M. H., Tsien R. Y. (2006) The fluorescent toolbox for assessing protein location and function. Science 312, 217–224 [DOI] [PubMed] [Google Scholar]
- 13. Szidonya L., Cserzo M., Hunyady L. (2008) Dimerization and oligomerization of G-protein-coupled receptors. Debated structures with established and emerging functions. J. Endocrinol. 196, 435–453 [DOI] [PubMed] [Google Scholar]
- 14. Meyer B. H., Segura J. M., Martinez K. L., Hovius R., George N., Johnsson K., Vogel H. (2006) FRET imaging reveals that functional neurokinin-1 receptors are monomeric and reside in membrane microdomains of live cells. Proc. Natl. Acad. Sci. U.S.A. 103, 2138–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gao X., Lowry P. R., Zhou X., Depry C., Wei Z., Wong G. W., Zhang J. (2011) PI3K/Akt signaling requires spatial compartmentalization in plasma membrane microdomains. Proc. Natl. Acad. Sci. U.S.A. 108, 14509–14514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marullo S., Bouvier M. (2007) Trends Pharmacol. Sci. 28, 362–365 [DOI] [PubMed] [Google Scholar]
- 17. Turu G., Szidonya L., Gáborik Z., Buday L., Spät A., Clark A. J., Hunyady L. (2006) Differential β-arrestin binding of AT1 and AT2 angiotensin receptors. FEBS Lett. 580, 41–45 [DOI] [PubMed] [Google Scholar]
- 18. Várnai P., Balla T. (1998) Visualization of phosphoinositides that bind pleckstrin homology domains. Calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J. Cell Biol. 143, 501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hunyady L., Baukal A. J., Gaborik Z., Olivares-Reyes J. A., Bor M., Szaszak M., Lodge R., Catt K. J., Balla T. (2002) Differential PI 3-kinase dependence of early and late phases of recycling of the internalized AT1 angiotensin receptor. J. Cell Biol. 157, 1211–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gáborik Z., Jagadeesh G., Zhang M., Spät A., Catt K. J., Hunyady L. (2003) The role of a conserved region of the second intracellular loop in AT1 angiotensin receptor activation and signaling. Endocrinology 144, 2220–2228 [DOI] [PubMed] [Google Scholar]
- 21. Qian H., Pipolo L., Thomas W. G. (2001) Association of β-arrestin 1 with the type 1A angiotensin II receptor involves phosphorylation of the receptor carboxyl terminus and correlates with receptor internalization. Mol. Endocrinol. 15, 1706–1719 [DOI] [PubMed] [Google Scholar]
- 22. Kim J., Ahn S., Ren X. R., Whalen E. J., Reiter E., Wei H., Lefkowitz R. J. (2005) Functional antagonism of different G protein-coupled receptor kinases for β-arrestin-mediated angiotensin II receptor signaling. Proc. Natl. Acad. Sci. U.S.A. 102, 1442–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hasbi A., Devost D., Laporte S. A., Zingg H. H. (2004) Real-time detection of interactions between the human oxytocin receptor and G protein-coupled receptor kinase-2. Mol. Endocrinol. 18, 1277–1286 [DOI] [PubMed] [Google Scholar]
- 24. Hunyady L., Bor M., Balla T., Catt K. J. (1994) Identification of a cytoplasmic Ser-Thr-Leu motif that determines agonist-induced internalization of the AT1 angiotensin receptor. J. Biol. Chem. 269, 31378–31382 [PubMed] [Google Scholar]
- 25. Noda K., Feng Y. H., Liu X. P., Saad Y., Husain A., Karnik S. S. (1996) The active state of the AT1 angiotensin receptor is generated by angiotensin II induction. Biochemistry 35, 16435–16442 [DOI] [PubMed] [Google Scholar]
- 26. Wei H., Ahn S., Shenoy S. K., Karnik S. S., Hunyady L., Luttrell L. M., Lefkowitz R. J. (2003) Independent β-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc. Natl. Acad. Sci. U.S.A. 100, 10782–10787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holloway A. C., Qian H., Pipolo L., Ziogas J., Miura S., Karnik S., Southwell B. R., Lew M. J., Thomas W. G. (2002) Side chain substitutions within angiotensin II reveal different requirements for signaling, internalization, and phosphorylation of type 1A angiotensin receptors. Mol. Pharmacol. 61, 768–777 [DOI] [PubMed] [Google Scholar]
- 28. Aumelas A., Sakarellos C., Lintner K., Fermandjian S., Khosla M. C., Smeby R. R., Bumpus F. M. (1985) Studies on angiotensin II and analogs. Impact of substitution in position 8 on conformation and activity. Proc. Natl. Acad. Sci. U.S.A. 82, 1881–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Conchon S., Monnot C., Teutsch B., Corvol P., Clauser E. (1994) Internalization of the rat AT1a and AT1b receptors. Pharmacological and functional requirements. FEBS Lett. 349, 365–370 [DOI] [PubMed] [Google Scholar]
- 30. Becamel C. (2008) 5-Hydroxytryptamine receptor 2C. UCSD-Nature Molecule Pages, doi: 10.1038/mp.a000152.000101 [DOI] [Google Scholar]
- 31. Sorkin A., Goh L. K. (2008) Endocytosis and intracellular trafficking of ErbBs. Exp. Cell Res. 314, 3093–3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Szidonya L., Süpeki K., Karip E., Turu G., Várnai P., Clark A. J., Hunyady L. (2007) AT1 receptor blocker-insensitive mutant AT1A angiotensin receptors reveal the presence of G protein-independent signaling in C9 cells. Biochem. Pharmacol. 73, 1582–1592 [DOI] [PubMed] [Google Scholar]
- 33. Bonde M. M., Hansen J. T., Sanni S. J., Haunsø S., Gammeltoft S., Lyngsø C., Hansen J. L. (2010) Biased signaling of the angiotensin II type 1 receptor can be mediated through distinct mechanisms. PloS One 5, e14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.