Background: Knock-out of S1P lyase, the only enzyme cleaving the sphingoid backbone, causes a severe phenotype.
Results: S1P lyase deficiency affects sphingolipid and cholesterol metabolism and also expression of Orm1/3 proteins.
Conclusion: Accumulation of free and phosphorylated sphingoid bases by loss of S1P lyase causes readjustment of the balance between de novo biosynthesis and recycling to maintain (glyco)sphingolipid homeostasis.
Significance: Data provide the first evidence for a mechanism that enables maintenance of (glyco)sphingolipid homeostasis in the brain.
Keywords: Cholesterol, Neurodegeneration, Neurons, Sphingolipid, Sphingosine 1-phosphate, Orm Proteins, S1P lyase
Abstract
Sphingosine 1-phosphate lyase (S1P lyase) irreversibly cleaves sphingosine 1-phosphate (S1P) in the final step of sphingolipid catabolism. As sphingoid bases and their 1-phosphate are not only metabolic intermediates but also highly bioactive lipids that modulate a wide range of physiological processes, it would be predicted that their elevation might induce adjustments in other facets of sphingolipid metabolism and/or alter cell behavior. Indeed, we have previously reported that S1P lyase deficiency causes neurodegeneration and other adverse symptoms. We next asked the question whether and how S1P lyase deficiency affects the metabolism of (glyco)sphingolipids and cholesterol, two lipid classes that might be involved in the neurodegenerative processes observed in S1P lyase-deficient mice. As predicted, there was a considerable increase in free and phosphorylated sphingoid bases upon elimination of S1P lyase, but to our surprise, rather than increasing, the mass of (glyco)sphingolipids persisted at wild type levels. This was discovered to be due to reduced de novo sphingoid base biosynthesis and a corresponding increase in the recycling of the backbones via the salvage pathway. There was also a considerable increase in cholesterol esters, although free cholesterol persisted at wild type levels, which might be secondary to the shifts in sphingolipid metabolism. All in all, these findings show that accumulation of free and phosphorylated sphingoid bases by loss of S1P lyase causes an interesting readjustment of the balance between de novo biosynthesis and recycling to maintain (glyco)sphingolipid homeostasis. These changes, and their impact on the metabolism of other cellular lipids, should be explored as possible contributors to the neurodegeneration in S1P lyase deficiency.
Introduction
In mammals, the brain exhibits the highest lipid content in the body next to adipose tissue. Complex sphingolipids, such as glycosphingolipids (GSLs),2 especially the sialic acid-containing gangliosides, are particularly abundant in neuronal cells (1). Metabolic intermediates of these lipids, notably sphingosine, ceramide, and S1P, function as bioactive molecules by regulating numerous cellular signaling pathways (2).
Sphingolipid biosynthesis starts with the condensation of serine and palmitoyl-CoA to form 3-ketosphinganine catalyzed by serine palmitoyltransferase (SPT). 3-ketosphingosine is then reduced to dihydrosphingosine. The latter is N-acylated to dihydroceramide by a family of ceramide synthases (CerS), each of which uses a restricted subset of fatty acyl-CoAs (3). The formed dihydroceramide is finally desaturated to ceramide, the membrane anchor of all sphingolipids (1, 2). S1P is the common catabolic intermediate of all sphingolipids and is formed via deacylation of ceramide, yielding sphingosine. Sphingosine is then either phosphorylated by sphingosine kinases (SKs) to S1P or reused for ceramide formation. Until now, two isoforms of sphingosine kinase, SK1 and SK2, have been cloned and characterized (4, 5). They differ in their kinetic properties and are active at different subcellular sites. S1P phosphatase catalyzes dephosphorylation of S1P back to sphingosine that can be recycled for sphingolipid formation via a recycling or salvage pathway. A decisive step in the catabolism of sphingolipids is carried out by S1P lyase, encoded by the Sgpl1 gene (6). S1P lyase, which resides in the endoplasmic reticulum (ER) and is widely distributed in tissues, catalyzes the final catabolic step of the sphingolipid degradation pathway, cleaving S1P into phosphoethanolamine and hexadecenal (7). Notably, S1P lyase is the only enzyme that enables sphingoid substrate to irreversibly exit the sphingolipid metabolic pathway. The importance of S1P lyase was demonstrated in several publications wherein the phenotype of the S1P lyase-deficient mice was described and analyzed (8–11). S1P lyase-deficient mice already show retarding growth at day 6 after birth. In addition, life expectancy of these mice is generally reduced to 6–8 weeks. Their reduced body weight (to about half of their age-matched littermates) is accompanied by the complete absence of adipose tissue (8). However, the amount of lipids including sphingolipids and cholesterol is considerably elevated in serum and liver of these mice (8).
We showed that in neurons derived from S1P lyase-deficient mice, S1P is neurotoxic when generated by SK2 (10). In addition, we found that neurons that abundantly express S1P lyase in wild type mice are those that degenerate first in S1P lyase-deficient mice (12). We now asked the question whether and how S1P lyase deficiency affects the metabolism of sphingolipids and cholesterol, two lipid classes that might be involved in the neurodegenerative processes observed in these mice.
Our findings suggest that disruption of the Sgpl1 gene causes the expected elevation of sphingoid base phosphates and to a much lesser extent of free sphingoid bases but does not significantly alter the content of ceramide, sphingomyelin, and GSLs. However, de novo formation of sphingolipids is substantially decreased, whereas the recycling pathway is significantly increased in these neurons. Furthermore, the amount of free cholesterol in the brains of S1P lyase-deficient mice was unaffected, whereas the content of cholesterol esters doubled.
EXPERIMENTAL PROCEDURES
Cell Culture
Granule cells from cerebella of 6-day-old wild type and S1P lyase-deficient mice were cultured as described before (13). Briefly, cells were isolated by mild trypsinization (0.05% w/v) and dissociated by repeated passage through a constricted Pasteur pipette in a DNase solution (0.1% w/v). The cells were then suspended in Dulbecco's modified Eagle's medium (DMEM) (containing 10% heat-inactivated horse serum, 100 units/liter penicillin, and 100 mg/liter streptomycin) and plated onto poly-l-lysine-coated 8-cm2 Petri dishes (6 × 106 cells/dish).
Labeling and Isolation of Cellular Sphingolipids
After 5 days in culture, cellular sphingolipids were metabolically labeled by adding [3-14C]serine (2 μCi/ml) or d-[1-14C]galactose (2 μCi/ml) into the culture medium (13). After 24 h, cells were harvested, and lipids were extracted from cell pellets by incubating the cells with 3 ml of chloroform/methanol/water (10:5:1, v/v) for 24 h at 48 °C. Phospholipids were degraded by mild alkaline hydrolysis with methanolic NaOH (100 mm) for 2 h at 37 °C. The lipid extracts were desalted by reversed-phase chromatography on RP18, applied to silica gel plates (Merck, Darmstadt, Germany), and resolved either in chloroform/methanol/0.22% CaCl2 (60:35:8, v/v) to separate gangliosides or in chloroform/methanol/acetic acid (190:9:1) to separate ceramide. Radioactively labeled sphingolipids were visualized by autoradiography and quantified using the bioimaging analyzer FUJIX Bas 1000 and Software TINA 2.09 (Raytest). Nonlabeled lipids were visualized by charring at 180 °C for 10 min. after dipping in 8% (w/v) H3PO4 containing 10% (w/v) CuSO4, and evaluated by photodensitometry (Shimadzu, Kyoto, Japan).
Serine-Palmitoyltransferase Activity
The enzymatic activity of serine palmitoyltransferase was measured using [14C]serine and palmitoyl-CoA as substrates (14). The assay mixture contained 0.1 m Hepes, pH 7.4, 5 mm dithiothreitol, 10 mm EDTA, 50 μm pyridoxal-5′-phosphate, 1.2 mm l-[14C]serine (1.6 μCi), 0.15 mm palmitoyl-CoA, and 100 μg of cell protein in a total volume of 100 μl. After incubation for 10 min at 37 °C, reactions were terminated by the addition of chloroform/methanol (5:3, v/v). The lipids were extracted by phase separation, applied to a silica gel plate (Merck), and resolved in chloroform/methanol/2 m NH4OH (40:10:1, v/v/v). The radiolabeled 3-ketosphinganine was quantified by phosphorimaging.
RNA Isolation and Real-time PCR
About 1 μg of total RNA (isolated using an RNA isolation kit from Qiagen) was used in reverse transcription with the SuperScript III First-Strand synthesis system (Invitrogen) and random primers (Invitrogen) as recommended by the manufacturer. The resulting total cDNA was then used in real-time PCR to measure mRNA. The RNA levels of 18 S rRNA were used as internal controls. The following primers and Universal ProbeLibrary probes (Roche Applied Science) were used for real-time PCR amplification: Sptlc1 (probe 108), 5′-CCTCCAGTCTCCAAGAACCA (forward) and 5′-ACCACGATGTTGTGGGTTG (reverse); Sptlc2 (probe 17), 5′-TCGGTGCTTCAGGAGGATAC (forward) and 5′-GAGAATGTGTGCGCAGGTAG (reverse); Ormdl1 (probe 17), 5′-GGGAATTGTCCTGTGACCAG (forward) and 5′-CACTGTGGGCAACTCCAAC (reverse); Ormdl2 (probe 92), 5′-TCCTGGAGACCACAGGTGTA (forward) and 5′-AGCTTGTTCCCCAGCTGTC (reverse); Ormdl3 (probe 109), 5′-CCCTCACCAACCTTATCCAC (forward) and 5′-GGACCCCGTAGTCCATCTG (reverse). The reactions were performed at 95 °C for 10 min, 95 °C for 10 s, 60 °C for 30 s, and 72 °C for 1 s.
Analysis of Cellular Sterols
Lipids were extracted with chloroform/methanol (2:1; v/v) and dried to constant weight in a SpeedVac® (Servant Instruments, Inc., Farmingdale, NY). 5α-cholestane (Serva Electrophoresis Inc., Heidelberg, Germany), epicoprostanol (Sigma-Aldrich), and stable isotope-labeled 24(R,S)hydroxycholesterol (Medical Isotopes Inc., Pelham, NH) were added as internal standards. After saponification, extraction, and derivatization, sterols were determined as trimethylsilyl ethers by using gas-liquid chromatography-flame ionization detection to analyze cholesterol and GC-mass spectrometry (GC-MS) to evaluate the cholesterol precursors lanosterol, lathosterol, and desmosterol, the cholesterol-derived metabolites cholestanol and 24(R,S)-hydroxycholesterol, and the plant sterols campesterol and sitosterol (15). The degree of esterification of cholesterol with fatty acids (lipoidal esters) was determined by quantifying the sterols before (free sterol) and after alkaline hydrolysis (total sterol) (16).
Mass Spectrometry of Sphingolipids
Sphingolipids were quantified by liquid chromatography (LC) electrospray ionization (ESI) tandem mass spectrometry, as described previously (17).
Data Analysis
All experiments were repeated at least three times with consistent results. Quantitative analysis was done from at least three different thin layer chromatography (TLC) plates. Data are expressed as means ± S.D. and normalized as indicated. Statistical analysis was performed using Student's t test. *, significantly different from wild type (p < 0.05).
RESULTS
S1P Lyase Deficiency Affects Primarily Mass of Sphingoid Bases and Their Phosphates in Neurons
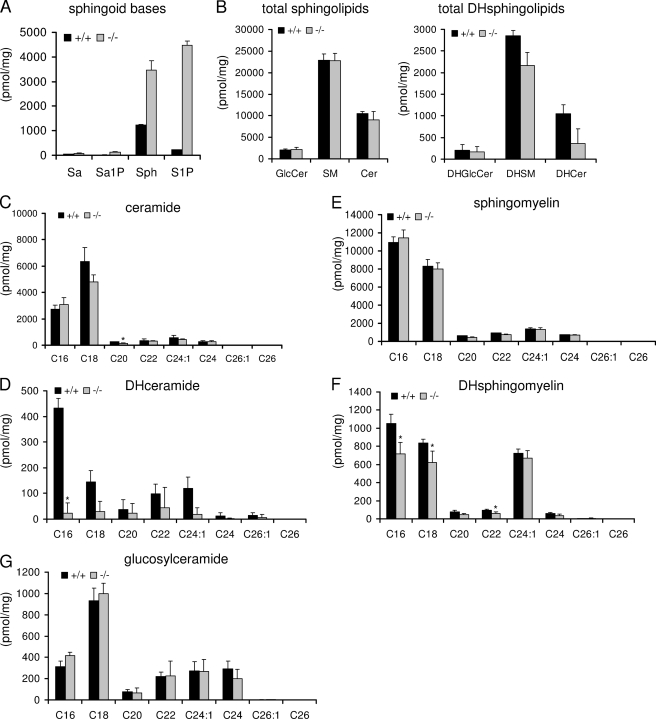
Using liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS), we have recently shown that the amount of sphingosine and especially that of S1P considerably increases in neurons lacking S1P lyase activity (10). We have now extended these measurements and observed that the amount of the saturated counterparts sphinganine and sphinganine 1-phosphate are less affected in these neurons (Fig. 1A). The amount of S1P increased from 200 pmol/mg in wild type neurons to about 4500 pmol/mg in S1P lyase-deficient neurons. Sphingosine levels increased about 3-fold (from 1200 pmol/mg in wild type neurons to 3500 pmol/mg in S1P lyase-deficient neurons). Thus, nearly similar amounts of sphingosine and S1P could be detected in S1P lyase-deficient neurons. Sphinganine and sphinganine 1-phosphate are much less abundant, amounting to 40 and 12 pmol/mg, respectively, in wild type neurons, and were elevated by about 2- and 10-fold, respectively, in S1P lyase-deficient neurons (Fig. 1A).
FIGURE 1.
S1P lyase deficiency affects primarily mass of sphingoid bases and their phosphates. Neuronal cultures were prepared from wild type (+/+) and S1P lyase-deficient (−/−) mouse cerebella. At day 5 in culture, cells were harvested, and lipids were extracted and evaluated by ESI-tandem mass spectrometry as described under “Experimental Procedures.” Data are averages of triplicate determinations. Numbers indicate carbon chain length followed by the number of double bounds in the fatty acid moiety. Sa, sphinganine; Sa1P, sphinganine 1-phosphate; Sph, sphingosine; SM, sphingomyelin; DHGlcCer, dihydroglucosylceramide; DHSM, dihydrosphingomyelin; DHCer, dihydroceramide; Cer, ceramide; DHsphingolipids, dihydrosphingolipids.
To find out whether the increased amounts of sphingosine in S1P lyase-deficient neurons affect the amount of other sphingolipid species, which are formed thereof via the recycling pathway, we determined the mass of ceramide, sphingomyelin, and glucosylceramide (GlcCer) and their dihydro counterparts in wild type and S1P lyase-deficient neurons, respectively. As shown in Fig. 1B, the unsaturated species of these sphingolipids are about 10 times more abundant in neurons than the respective saturated forms. Still, the mass of the unsaturated species was not affected by the absence of S1P lyase activity, whereas the amounts of dihydroceramide and of dihydrosphingomyelin were decreased to some extent (Fig. 1B). Analysis of individual molecular sphingolipid species that differ in the length of the fatty acid in their ceramide backbone revealed some additional particular differences (Fig. 1, C–G). As depicted in Fig. 1, C–G, cerebellar neurons exhibit several molecular species of ceramide, sphingomyelin, and GlcCer, of which those containing palmitic acid (C16:0) or stearic acid (C18:0) in amide linkage to their sphingosine moiety prevail. In the case of ceramide and GlcCer, molecular species containing stearic acid overbalance those with palmitic acid, whereas the reverse is true in the case of dihydroceramide and dihydrosphingomyelin, where molecular species containing palmitic acid overbalance those with stearic acid. Note that only in the case of dihydrosphingomyelin, a third molecular species, namely that containing a long unsaturated fatty acid C24:1 in its ceramide moiety, is nearly as abundant as that containing stearic acid (Fig. 1F). None of the saturated molecular species of ceramide, sphingomyelin, and GlcCer are significantly changed in S1P lyase-deficient neurons apart from a single exception (Fig. 1, C, E, and G). Only C20-ceramide is significantly reduced by 50% in neurons lacking S1P lyase activity (Fig. 1C). In contrast, the amounts of all saturated dihydro species of ceramide and sphingomyelin are reduced to some extent in S1P lyase-deficient neurons (Fig. 1, D and F). A maximal reduction of nearly 90% was observed in the case of C16-dihydroceramide in S1P lyase-deficient neurons. The corresponding C16-dihydrosphingomyelin was also significantly reduced by nearly 30% (Fig. 1F). Note that reproducibility of the amounts of dihydro-GlcCer that were close to the detection limit was problematic and is not shown.
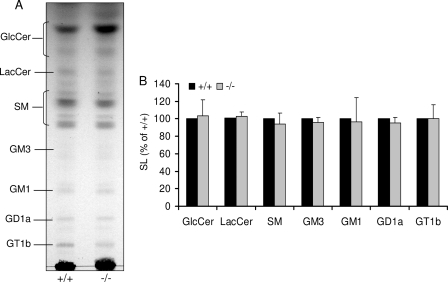
As shown in Fig. 2, similar results were obtained for GlcCer and sphingomyelin when separated by TLC and evaluated densitometrically. In addition, it becomes clear from this figure that there were no significant changes in the total amount of gangliosides in S1P lyase-deficient neurons (Fig. 2).
FIGURE 2.
Ganglioside profile is not changed in absence of S1P lyase. Cultured wild type (+/+) and S1P lyase-deficient (−/−) neurons were harvested, and lipids were extracted. A, isolated lipids were separated by TLC in chloroform/methanol/0.22% CaCl2 (60:35:8) and (B) quantified as described under “Experimental Procedures.” SM, sphingomyelin. The terminology used for gangliosides is that of Svennerholm (42). Data are averages of triplicate determination and given relative to wild type controls.
S1P Lyase Deficiency Results in Reduction of de Novo Formation of GSLs and of Sphingomyelin with Simultaneous Enhancement of Recycling Pathway in Neurons
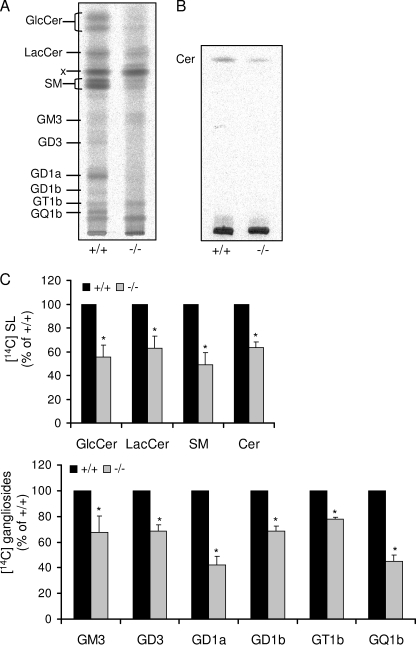
The massive reduction of the amount of C16-dihydroceramide and also of C18-dihydroceramide (Fig. 1D), both representing crucial biosynthetic intermediates of more complex sphingolipids with nearly no changes in the total amount of ceramides (including dihydroceramides, see Fig. 1B) in S1P lyase-deficient neurons prompted us to carry out metabolic studies in these cells. First we followed the incorporation of [14C]serine into newly formed cellular sphingolipids. As depicted in Fig. 3, incorporation of [14C]serine into cellular sphingolipids of S1P lyase-deficient neurons was considerably reduced. The content of GlcCer, lactosylceramide (LacCer), sphingomyelin, and ceramide (Cer) was greatly decreased by about 40% (Fig. 3, A and C). As a consequence, the de novo formation of complex GSL was also significantly reduced in S1P lyase-deficient neurons. The amount of GD1a and of GQ1b representing the end products of a- and b-series of gangliosides, respectively, in these cells decreased by more than 50%, whereas that of intermediate gangliosides such as GM3, GD3, and GD1b, was reduced by about 30% (Fig. 2B). Note that some intermediate gangliosides of the biosynthetic pathway including GM2 and GM1 were hardly detectable even in wild type neurons (Fig. 3A).
FIGURE 3.
De novo sphingolipid synthesis is reduced in S1P lyase-deficient neurons. Cultured wild type (+/+) and S1P lyase-deficient (−/−) neurons were labeled with [14C]serine (2 μCi/ml) for 24 h. Then, cells were harvested, and lipids were extracted, isolated, separated by TLC in (A) chloroform/methanol/0.22% CaCl2 (60:35:8) or (B) chloroform/methanol/acetic acid (190:9:1), and (C) quantified as described under “Experimental Procedures.” x, unknown compound; SM, sphingomyelin; Cer, ceramide; SL, sphingolipids. Data are averages of triplicate determination and given relative to wild type controls.
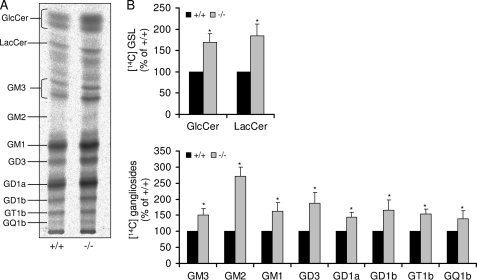
It has been reported previously that in neuronal cells, the salvage pathway in which sphingosine resulting from the degradation of cellular sphingolipids is recycled via ceramide to all sphingolipids contributes considerably to the mass of cellular sphingolipids (18). To assess this pathway, we evaluated the incorporation of [1-14C]galactose into cellular GSLs. As illustrated in Fig. 4, the amount of radioactively labeled GSLs was significantly higher in S1P lyase-deficient neurons than in wild type neurons. GlcCer and LacCer, both precursors of complex GSLs, amounted to about ∼150% in neurons derived from S1P lyase-deficient mice when compared with wild type neurons (Fig. 4B). Accordingly, incorporation of radioactivity in all ganglioside species was 30–50% higher in S1P lyase-deficient neurons. Note that ganglioside GM2, an intermediate of the a-series gangliosides, which is hardly detectable in wild type neurons, is clearly evident in S1P lyase-deficient neurons (Fig. 4).
FIGURE 4.
Sphingolipid recycling pathway is elevated in S1P lyase-deficient neurons. Cultured wild type (+/+) and S1P lyase-deficient (−/−) neurons were labeled with [14C]galactose (2 μCi/ml) for 24 h. Then, cells were harvested, and lipids were extracted, isolated (A), separated by TLC in chloroform/methanol/0.22% CaCl2 (60:35:8), and (B) quantified as described under “Experimental Procedures.” Data are averages of triplicate determination and given relative to wild type controls.
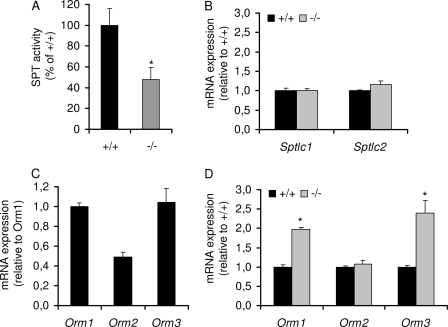
Reduction of de Novo Formation of GSLs in S1P Lyase-deficient Neurons Is Accompanied by Reduced SPT Activity and Up-regulation of Orm Protein Expression
The rate-limiting step of de novo sphingolipid biosynthesis is catalyzed by SPT. As depicted in Fig. 5A, SPT activity was considerably reduced (by about 50%) in S1P lyase-deficient neurons. However, the transcript amounts of both SPT subunits were similar in wild type and lyase-deficient cells (Fig. 5B). Recently, Orm1/2 proteins were reported to negatively regulate sphingolipid synthesis in yeast (19, 20). We therefore studied the expression of this evolutionarily conserved family of ER-resident proteins in cerebellar neurons. As shown in Fig. 5C, mRNAs of Orm1/2/3 could be identified in neurons (Fig. 5C). Moreover, in lyase-deficient neurons, low SPT activity was paralleled by considerably elevated transcription levels of Orm1 and Orm3 (Fig. 5D).
FIGURE 5.
S1P lyase deficiency is accompanied by decreased SPT activity and up-regulation of Orm protein expression. Cultured wild type (+/+) and S1P lyase-deficient (−/−) neurons were harvested. A, SPT activity was assayed in cell homogenate as described under “Experimental Procedures.” B–D, alternatively, mRNA content of SPT subunits (B) and Orm proteins (C and D) was quantified by RT-PCR using 18 S mRNA as reference.
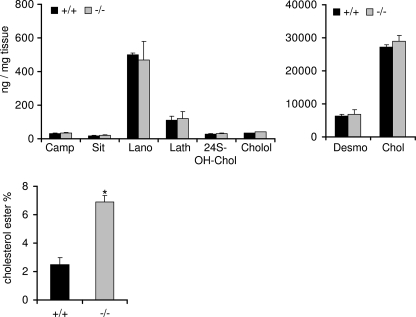
S1P Lyase Deficiency Is Associated with Increase of Cholesterol Esters but Persisting Free Cholesterol Levels in Nerve Tissue
The close interaction of sphingolipids and especially of sphingomyelin with cholesterol in cellular plasma membranes is well documented (21, 22). Because our results indicated no changes in the amount of plasma membrane-associated sphingolipid species in neurons lacking S1P lyase activity, we evaluated the amounts of free cholesterol and some of its metabolites as well as of cholesterol esters in brains of S1P lyase-deficient and of wild type mice, respectively. In addition, we determined some plant sterols. As becomes clear from Fig. 6, free cholesterol is by far the most abundant sterol fraction in the brain, amounting to nearly 30 μg/mg of tissue. Its immediate metabolic precursor desmosterol occurs in considerably lower amounts (about 7 μg/mg), whereas two other precursors, lathosterol and lanosterol, amount to only about 0.5 and 0.1 μg/mg, respectively. The amounts of the cholesterol-derived metabolites 24(S,R)-hydroxy-cholesterol and cholestanol are even much lower (about 20 ng/mg). In the same range are the determined plant sterols, campesterol and sitosterol, which are most probably derived from the ingested animal food. No significant differences of these sterols were observed in brains from S1P lyase-deficient and wild type mice (Fig. 6). By contrast, the levels of cholesterol esters were increased more than 2-fold in brains of S1P lyase knock-out mice when compared with wild type mice (Fig. 6). Note that in wild type animals, esterified cholesterol constitutes less than 5% of total brain cholesterol.
FIGURE 6.
Sterol profiles except cholesterol esters are unchanged in S1P lyase-deficient nervous tissue. Sterols were determined in brains of wild type (+/+) and S1P lyase-deficient (−/−) mice as described under “Experimental Procedures.” Camp, campesterol; Sit, sitosterol; Lano, lanosterol; Lath, lathosterol; 24S-OH-Chol, 24(R,S)-hydroxycholesterol; Cholol, cholestanol; Desmo, desmosterol; Chol, cholesterol.
DISCUSSION
In a recent study, we showed that S1P lyase deficiency causes neurodegeneration, which was induced by the accumulation of its substrate S1P in the ER (12). We now show that S1P lyase deficiency does not affect the total amount of more complex sphingolipids in neurons despite a significant accumulation of sphingoid bases and their phosphates. By contrast, S1P lyase deficiency causes a considerable elevation of the mass of ceramide and sphingomyelin in liver (8). Note that in liver, a 472-fold increase of S1P and a 42-fold elevation of sphingosine have been reported (8). These amounts are remarkably higher than those obtained in cerebellar neurons in which S1P increased 20-fold and sphingosine increased 3-fold relative to wild type. Thus, the inability to cleave the sphingoid structure has different consequences in different tissues. In neurons, free sphingosine and phosphorylated sphingosine amount to almost equal concentrations with no changes in complex (glyco)sphingolipids, whereas in liver, the content of sphingosine exceeds by far that of S1P and the mass of sphingolipids is considerably elevated (8). Obviously, nervous tissue has a better ability to overcome perturbations of sphingolipid metabolism.
Cell type- and tissue-specific differences in sphingolipid metabolism have been well known for many years. Don Marcus and co-workers (18) suggested in the nineties that in slowly dividing cells in which the need for synthesis is relatively low, GSLs are synthesized predominantly from sphingoid bases recycled from the hydrolytic pathway. Accordingly, in more rapidly dividing cells, the need for increased synthesis is met by up-regulating the de novo pathway (18). Hence, in terminally differentiated, postmitotic neurons, recycling of sphingosine for sphingolipid formation is a well developed common mechanism. The inability to degrade S1P clearly promotes this pathway in neurons. As described earlier, this salvage pathway utilizes S1P as a starting point (23). S1P is dephosphorylated by S1P phosphatase, which resides in the ER to produce sphingosine. Ceramide is then synthesized via acylation of sphingosine by ceramide synthases from which CerS1 prevails in nervous tissue (24). Ceramide synthases probably trap free sphingosine released from lysosomes at the surface of the ER or in ER-associated membranes. In cell culture models, exogenous sphingosine is taken up by the cells and is largely utilized for the biosynthesis of complex sphingolipids (25–28). Note that sphingolipids have a turnover of several hours in neuronal cells (29).
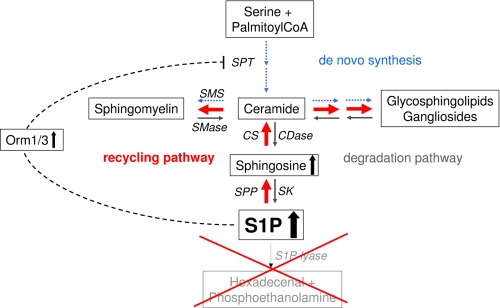
Our results document that in S1P lyase-deficient neurons, increased recycling of sphingoid bases is accompanied by a considerable reduction of de novo sphingolipid biosynthesis. This was not surprising as we have observed earlier that accumulation of the semisynthetic S1P analog cis-4-methysphingosine-phosphate is accompanied by down-regulation of de novo sphingolipid biosynthesis (13). In addition, we showed that sphingoid bases modulate de novo sphingolipid biosynthesis by down-regulating SPT activity in neurons (30, 31). The mechanism underlying this feedback regulation is, however, not known. Nevertheless, in yeast, Orm1/2 proteins were identified as negative regulators of SPT, the enzyme catalyzing the committed step of de novo sphingolipid synthesis located in the ER (19, 20). The expression of these proteins is dependent on sphingoid base levels (19, 20). Orm1/2/3 are members of a family of evolutionarily conserved ER-resident proteins that were initially associated with ER stress (32). Likewise, high expression levels of Orm1/3 in S1P lyase-deficient neurons might be aimed to reduce the elevated amounts of free and phosphorylated sphingoid bases. This is of vital importance because high amounts of S1P in the ER were shown to cause neuronal death (12). Thus, although the high levels of free and phosphorylated sphingoid bases in S1P lyase-deficient neurons reduce the de novo sphingolipid formation, this appears to be compensated for by increased utilization of the recycling pathway (Fig. 7).
FIGURE 7.
Scheme of sphingolipid metabolism and consequences of S1P lyase deficiency. S1P lyase deficiency leads to an increase of cellular S1P and of sphingosine (to a lesser extent). Consequently, de novo sphingolipid biosynthesis (blue arrows) is reduced, possibly by up-regulation of Orm1/3 expression. Simultaneously, the recycling pathway (red arrows) is elevated. Thus, the total mass of sphingolipids is hardly affected by S1P lyase deficiency in neurons. SMS, sphingomyelin synthases; SMase, sphingomyelinases; CS, ceramide synthases; CDase, ceramidases; SPP, S1P phosphatase; SK, sphingosine kinases.
The recycling of long chain sphingoid bases, leading to the regeneration of sphingolipids, has been estimated to contribute from 50 to 90% of sphingolipid biosynthesis depending on the cell type and the stage of differentiation (18, 33, 34). These metabolic events suggest a critical role for sphingolipid breakdown in sphingolipid biosynthesis and turnover as well as in cellular signal transduction. Interestingly, the majority of endogenous sphingosine is recycled (35). Nevertheless, phosphorylation of 1–3% sphingosine may be sufficient to double the levels of S1P (2). Enlarged levels of intracellular S1P constitute an increase of a bioactive molecule and therefore represent a possible risk for the cell. Activation of the recycling pathway is an opportunity to reduce the intracellular amount of S1P. Our results demonstrate that despite a considerable increase of the recycling pathway, accumulation of intracellular S1P still occurred, suggesting that the capacity of this pathway is not sufficient to counterbalance the accumulating amount of S1P. As reported recently, this elevated amount of S1P is apparently harmful by inducing neuronal apoptosis via a calcium-triggered calpain-mediated mechanism (12).
Although the changes in total sphingolipid amounts were rather small, the shift in sphingolipid metabolism might affect the metabolism of other lipids. Indeed, elevation of cholesterol esters was found. Although cholesterol esters represent only a minor percentage of total cholesterol in the brain, this result might be of pathophysiological relevance. About a decade ago, a direct correlation between cholesterol ester levels and the production of amyloid β-peptide, which characteristically accumulates in all forms of Alzheimer disease, was reported (36). Note that investigation of amyloid body formation implies the generation of a conditional S1P lyase knock-out mouse model given the very limited life span of the S1P lyase-deficient mice. We have, however, shown recently (12) that sphingoid base phosphate-dependent neurotoxicity shows similarity with that of the amyloid β-peptide in Alzheimer disease (37, 38). Both S1P (12) and amyloid β-peptide (38) induce an aberrant reactivation of cell cycle events and activation of CDK5 (cyclin-dependent kinase). In addition, S1P (12) and amyloid β-peptide (37, 39) neurotoxicity involves calpain and procaspase-12 activation by disruption of ER calcium homeostasis but not by membrane- or mitochondria-targeted signals. In addition, it has been shown recently that cell-associated S1P stimulates the activity of BACE1, the rate-limiting enzyme for amyloid β-peptide production (40).
Free cholesterol, which constitutes about 95% of total brain cholesterol, persisted at wild type levels in the brains of S1P lyase-deficient mice, although elevated levels of cholesterol were found in serum and liver of these animals (8). This is, however, not surprising because stability of cholesterol homeostasis in the brain has already been demonstrated experimentally. Total brain cholesterol content and thus cholesterol homeostasis were maintained in guinea pigs despite administration of high doses of statins or high dietary cholesterol intake (41).
Our results show that the impact of S1P lyase deficiency on sphingolipid and cholesterol metabolism is less dramatic in neurons than in liver. (Glyco)sphingolipid homeostasis is maintained in neurons even in the absence of S1P lyase activity due to an interesting readjustment of the balance between de novo biosynthesis and recycling. Nonetheless, the changes in S1P and cholesterol esters should be explored further as possible contributors to the neurodegeneration reported in S1P lyase-deficient mice.
Acknowledgments
We thank Paul P. Van Veldhoven for providing S1P lyase-deficient mice and Andrea Raths for technical assistance.
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (EC 118/6-1) (to G. v. E.-D.) and by NIH Grant GM069338 (to A. H. M.).
- GSL
- glycosphingolipid
- ER
- endoplasmic reticulum
- S1P
- sphingosine 1-phosphate
- S1P lyase
- sphingosine 1-phosphate lyase
- SK
- sphingosine kinase
- SPT
- serine palmitoyltransferase
- GlcCer
- glucosylceramide
- LacCer
- lactosylceramide.
REFERENCES
- 1. van Echten-Deckert G., Herget T. (2006) Sphingolipid metabolism in neural cells. Biochim. Biophys. Acta 1758, 1978–1994 [DOI] [PubMed] [Google Scholar]
- 2. Hannun Y. A., Obeid L. M. (2008) Principles of bioactive lipid signaling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9, 139–150 [DOI] [PubMed] [Google Scholar]
- 3. Stiban J., Tidhar R., Futerman A. H. (2010) Ceramide synthases: roles in cell physiology and signaling. Adv. Exp. Med. Biol. 688, 60–71 [DOI] [PubMed] [Google Scholar]
- 4. Kohama T., Olivera A., Edsall L., Nagiec M. M., Dickson R., Spiegel S. (1998) Molecular cloning and functional characterization of murine sphingosine kinase. J. Biol. Chem. 273, 23722–23728 [DOI] [PubMed] [Google Scholar]
- 5. Liu H., Sugiura M., Nava V. E., Edsall L. C., Kono K., Poulton S., Milstien S., Kohama T., Spiegel S. (2000) Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 275, 19513–19520 [DOI] [PubMed] [Google Scholar]
- 6. Van Veldhoven P. P. (2000) Sphingosine-1-phosphate lyase. Methods Enzymol. 311, 244–254 [DOI] [PubMed] [Google Scholar]
- 7. Fyrst H., Saba J. D. (2008) Sphingosine-1-phosphate lyase in development and disease: sphingolipid metabolism takes flight. Biochim. Biophys. Acta 1781, 448–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bektas M., Allende M. L., Lee B. G., Chen W., Amar M. J., Remaley A. T., Saba J. D., Proia R. L. (2010) Sphingosine-1-phosphate lyase deficiency disrupts lipid homeostasis in liver. J. Biol. Chem. 285, 10880–10889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Veldhoven P. P. (2005) Sphingosine 1-phosphate lyase deficient mice. Chem. Phys. Lipids 136, 164–165 [DOI] [PubMed] [Google Scholar]
- 10. Hagen N., Van Veldhoven P. P., Proia R. L., Park H., Merrill A. H., Jr., van Echten-Deckert G. (2009) Subcellular origin of sphingosine 1-phosphate is essential for its toxic effect in lyase-deficient neurons. J. Biol. Chem. 284, 11346–11353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weber C., Krueger A., Münk A., Bode C., Van Veldhoven P. P., Gräler M. H. (2009) Discontinued postnatal thymocyte development in sphingosine-1-phosphate lyase-deficient mice. J. Immunol. 183, 4292–4301 [DOI] [PubMed] [Google Scholar]
- 12. Hagen N., Hans M., Hartmann D., Swandulla D., van Echten-Deckert G. (2011) Sphingosine 1-phosphate links glycosphingolipid metabolism to neurodegeneration via a calpain-mediated mechanism. Cell Death Differ. 18, 1356–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Echten-Deckert G., Zschoche A., Bär T., Schmidt R. R., Raths A., Heinemann T., Sandhoff K. (1997) cis-4-Methylsphingosine decreases sphingolipid biosynthesis by specifically interfering with serine palmitoyltransferase activity in primary cultured neurons. J. Biol. Chem. 272, 15825–15833 [DOI] [PubMed] [Google Scholar]
- 14. Herget T., Esdar C., Oehrlein S. A., Heinrich M., Schütze S., Maelicke A., van Echten-Deckert G. (2000) Production of ceramides causes apoptosis during early neural differentiation in vitro. J. Biol. Chem. 275, 30344–30354 [DOI] [PubMed] [Google Scholar]
- 15. Päivä H., Thelen K. M., Van Coster R., Smet J., De Paepe B., Mattila K. M., Laakso J., Lehtimäki T., von Bergmann K., Lütjohann D., Laaksonen R. (2005) High-dose statins and skeletal muscle metabolism in humans: a randomized, controlled trial. Clin. Pharmacol. Ther. 78, 60–68 [DOI] [PubMed] [Google Scholar]
- 16. Lütjohann D., Breuer O., Ahlborg G., Nennesmo I., Sidén A., Diczfalusy U., Björkhem I. (1996) Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24(S)-hydroxycholesterol from the brain into the circulation. Proc. Natl. Acad. Sci. U.S.A. 93, 9799–9804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shaner R. L., Allegood J. C., Park H., Wang E., Kelly S., Haynes C. A., Sullards M. C., Merrill A. H., Jr. (2009) Quantitative analysis of sphingolipids for lipidomics using triple quadrupole and quadrupole linear ion trap mass spectrometers. J. Lipid Res. 50, 1692–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gillard B. K., Clement R. G., Marcus D. M. (1998) Variations among cell lines in the synthesis of sphingolipids in de novo and recycling pathways. Glycobiology 8, 885–890 [DOI] [PubMed] [Google Scholar]
- 19. Breslow D. K., Collins S. R., Bodenmiller B., Aebersold R., Simons K., Shevchenko A., Ejsing C. S., Weissman J. S. (2010) Orm family proteins mediate sphingolipid homeostasis. Nature 463, 1048–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han S., Lone M. A., Schneiter R., Chang A. (2010) Orm1 and Orm2 are conserved endoplasmic reticulum membrane proteins regulating lipid homeostasis and protein quality control. Proc. Natl. Acad. Sci. U.S.A. 107, 5851–5856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Puri V., Jefferson J. R., Singh R. D., Wheatley C. L., Marks D. L., Pagano R. E. (2003) Sphingolipid storage induces accumulation of intracellular cholesterol by stimulating SREBP-1 cleavage. J. Biol. Chem. 278, 20961–20970 [DOI] [PubMed] [Google Scholar]
- 22. Worgall T. S. (2008) Regulation of lipid metabolism by sphingolipids. Subcell. Biochem. 49, 371–385 [DOI] [PubMed] [Google Scholar]
- 23. Le Stunff H., Giussani P., Maceyka M., Lépine S., Milstien S., Spiegel S. (2007) Recycling of sphingosine is regulated by the concerted actions of sphingosine-1-phosphate phosphohydrolase 1 and sphingosine kinase 2. J. Biol. Chem. 282, 34372–34380 [DOI] [PubMed] [Google Scholar]
- 24. Levy M., Futerman A. H. (2010) Mammalian ceramide synthases. IUBMB Life 62, 347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chigorno V., Riva C., Valsecchi M., Nicolini M., Brocca P., Sonnino S. (1997) Metabolic processing of gangliosides by human fibroblasts in culture: formation and recycling of separate pools of sphingosine. Eur. J. Biochem. 250, 661–669 [DOI] [PubMed] [Google Scholar]
- 26. Riboni L., Prinetti A., Bassi R., Tettamanti G. (1994) Formation of bioactive sphingoid molecules from exogenous sphingomyelin in primary cultures of neurons and astrocytes. FEBS Lett. 352, 323–326 [DOI] [PubMed] [Google Scholar]
- 27. Prinetti A., Chigorno V., Tettamanti G., Sonnino S. (2000) Sphingolipid-enriched membrane domains from rat cerebellar granule cells differentiated in culture: a compositional study. J. Biol. Chem. 275, 11658–11665 [DOI] [PubMed] [Google Scholar]
- 28. van Echten G., Iber H., Stotz H., Takatsuki A., Sandhoff K. (1990) Uncoupling of ganglioside biosynthesis by brefeldin A. Eur. J. Cell Biol. 51, 135–139 [PubMed] [Google Scholar]
- 29. Riboni L., Bassi R., Prinetti A., Tettamanti G. (1996) Salvage of catabolic products in ganglioside metabolism: a study on rat cerebellar granule cells in culture. FEBS Lett. 391, 336–340 [DOI] [PubMed] [Google Scholar]
- 30. van Echten G., Birk R., Brenner-Weiss G., Schmidt R. R., Sandhoff K. (1990) Modulation of sphingolipid biosynthesis in primary cultured neurons by long chain bases. J. Biol. Chem. 265, 9333–9339 [PubMed] [Google Scholar]
- 31. Mandon E. C., van Echten G., Birk R., Schmidt R. R., Sandhoff K. (1991) Sphingolipid biosynthesis in cultured neurons: down-regulation of serine palmitoyltransferase by sphingoid bases. Eur. J. Biochem. 198, 667–674 [DOI] [PubMed] [Google Scholar]
- 32. Hjelmqvist L., Tuson M., Marfany G., Herrero E., Balcells S., Gonzàlez-Duarte R. (2002) ORMDL proteins are a conserved new family of endoplasmic reticulum membrane proteins. Genome Biol. 3, RESEARCH0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tettamanti G., Bassi R., Viani P., Riboni L. (2003) Salvage pathways in glycosphingolipid metabolism. Biochimie 85, 423–437 [DOI] [PubMed] [Google Scholar]
- 34. Chigorno V., Giannotta C., Ottico E., Sciannamblo M., Mikulak J., Prinetti A., Sonnino S. (2005) Sphingolipid uptake by cultured cells: complex aggregates of cell sphingolipids with serum proteins and lipoproteins are rapidly catabolized. J. Biol. Chem. 280, 2668–2675 [DOI] [PubMed] [Google Scholar]
- 35. Kitatani K., Idkowiak-Baldys J., Hannun Y. A. (2008) The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell. Signal. 20, 1010–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Puglielli L., Konopka G., Pack-Chung E., Ingano L. A., Berezovska O., Hyman B. T., Chang T. Y., Tanzi R. E., Kovacs D. M. (2001) Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid β-peptide. Nat. Cell Biol. 3, 905–912 [DOI] [PubMed] [Google Scholar]
- 37. Nakagawa T., Zhu H., Morishima N., Li E., Xu J., Yankner B. A., Yuan J. (2000) Caspase-12 mediates endoplasmic reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 403, 98–103 [DOI] [PubMed] [Google Scholar]
- 38. Lopes J. P., Oliveira C. R., Agostinho P. (2010) Neurodegeneration in an Aβ-induced model of Alzheimer disease: the role of Cdk5. Aging Cell 9, 64–77 [DOI] [PubMed] [Google Scholar]
- 39. Nakagawa T., Yuan J. (2000) Cross-talk between two cysteine protease families: activation of caspase-12 by calpain in apoptosis. J. Cell Biol. 150, 887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takasugi N., Sasaki T., Suzuki K., Osawa S., Isshiki H., Hori Y., Shimada N., Higo T., Yokoshima S., Fukuyama T., Lee V. M., Trojanowski J. Q., Tomita T., Iwatsubo T. (2011) BACE1 activity is modulated by cell-associated sphingosine 1-phosphate. J. Neurosci. 31, 6850–6857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lütjohann D., Stroick M., Bertsch T., Kühl S., Lindenthal B., Thelen K., Andersson U., Björkhem I., Bergmann Kv K., Fassbender K. (2004) High doses of simvastatin, pravastatin, and cholesterol reduce brain cholesterol synthesis in guinea pigs. Steroids 69, 431–438 [DOI] [PubMed] [Google Scholar]
- 42. Svennerholm L. (1963) Chromatographic separation of human gangliosides. J. Neurochem. 10, 613–623 [DOI] [PubMed] [Google Scholar]