Background: The proprotein convertase, PC7, cycles between the trans-Golgi network (TGN) and the plasma membrane.
Results: Internalization of PC7 is mediated by clathrin-coated vesicles and depends on a Pro-Leu-Cys (PLC) motif in the cytoplasmic tail.
Conclusion: The PLC motif is essential and sufficient for internalization of PC7 from the plasma membrane.
Significance: New insight in the trafficking of PC7 provides further information concerning its physiological function.
Keywords: Endocytosis, Intracellular Trafficking, Protein Motifs, Proteolytic Enzymes, Trafficking, PC7
Abstract
Proprotein convertase 7 (PC7) is a member of the subtilisin-like proprotein convertase family, which is involved in the endoproteolysis of a variety of precursor proteins. Under steady state conditions, PC7 is mainly localized in the trans-Golgi network, but a small fraction is found at the cell surface. So far, no sorting signals for membrane trafficking have been identified in PC7. In this study, we have examined the internalization of PC7 from the plasma membrane. Our results show that internalization of PC7 is mediated by clathrin-coated vesicles. After inhibition of clathrin-mediated endocytosis using hypertonic conditions or the small molecule inhibitor, Pitstop 2, PC7 accumulated at the plasma membrane. Furthermore, PC7 was present in isolated clathrin-coated vesicles. To determine the internalization motif, constructs were generated in which parts of the N and C terminus of the cytoplasmic tail of PC7 were deleted, and chimeric proteins were constructed consisting of the luminal and transmembrane domains of Tac (CD25) and parts of the cytoplasmic domain of PC7. Antibody uptake experiments as well as surface biotinylation experiments demonstrated that the region between Ala713 and Cys726 in the cytoplasmic domain of PC7 is essential and sufficient for the internalization of PC7 but not for trans-Golgi network localization. Individual amino acids in this region were substituted with alanine, which identified Pro, Leu, and Cys as the essential amino acids. In conclusion, internalization of PC7 depends on a short transferable sequence in the cytoplasmic tail, which contains the three crucial amino acids PLC.
Introduction
Proprotein convertase 7 (PC7),3 also called PC8 or lymphoma PC (LPC), is the most distinct member of the subtilisin-like proprotein convertase family (1). Like the other family members PC1/3, PC2, furin, PC4, PC5/6, and PACE4, it cleaves precursors at the carboxyl-terminal side of basic amino acid residues motifs such as (K/R)X(K/R)R↓ (2). PCs are multidomain enzymes, consisting of a propeptide followed by a subtilisin-like catalytic domain, a P-domain (also called a middle domain), and one or more carboxyl-terminal domains. The first three domains are essential and sufficient for catalytic activity, and the carboxyl-terminal domains control intracellular trafficking (3, 4).
Human PC7 was first identified in the chromosome breakpoint region of a high grade lymphoma carrying a t(11;14)(q23;q32) translocation (5). Independently, human (6), mouse (7), and rat (8) PC7 were cloned from cell lines. PC7 binds to BiP in the endoplasmic reticulum to prevent aggregation and dissociates after folding (9). Its propeptide is removed autocatalytically. PC7 undergoes additional post-translational modifications (10), such as palmitoylation of 2 cysteines in the cytosolic tail (11), sulfation (12), and N-glycosylation (7). PC7 is widely expressed and has been demonstrated in early embryonic stages (8) as well as in several adult tissues (5, 7). The PC7 knock-out mouse does not show any obvious phenotypic abnormalities (13), and this in sharp contrast to knock-out mice generated for other PCs (14). This might be explained by redundancy provided by other PCs in the ability to process certain precursor proteins (14). Immunohistochemical studies have shown that PC7 (10), furin (15), and PC5/6B (16) each concentrate in the trans-Golgi network (TGN). Thus, furin and/or PC5/6B might as such compensate for the absence of PC7. Indeed, cleavage of a set of prorenin mutants with different cleavage sites indicated similar specificity, although furin appeared to have a broader activity (10). In addition, many other substrates can be processed by PC7 and by furin, such as platelet-derived growth factor (PDGF)-AA (17), PDGF-BB (18), bone morphogenic protein (BMP) (19), and vascular endothelial growth factor (VEGF)-C (20), among others. On the other hand, furin and PC5/6 knock-out mice are lethal, suggesting that the degree of redundancy for those enzymes is limited (21, 22). Substrate specificity under physiological conditions is also dependent on cell biological properties of the enzymes. Potential substrates should co-localize at least temporarily with the enzyme. It is therefore important to study the intracellular trafficking of the enzymes. Although the intracellular trafficking of furin and PC5/6B has been studied extensively, little is known about the trafficking of PC7.
Furin is concentrated in the TGN under steady state conditions. Nevertheless, it cycles between this compartment and the cell surface through the endocytic pathway. The cytoplasmic domain of furin is necessary and sufficient to direct TGN localization (15, 23) and to recycle the enzyme (24). The exit from the TGN to endosomes occurs via clathrin-coated vesicles (CCVs) and depends on the phosphorylation state of a pair of serines within a cluster of acidic residues, a tyrosine motif, and a leucine-isoleucine signal, which are all involved in the interaction of the cytoplasmic domain with AP-1 and its μ1 subunit (23, 25). TGN localization of furin is mediated by the interaction of the phosphorylated cytoplasmic domain with the connector protein PACS-1 (26). Recycling of furin from the plasma membrane occurs via an AP-2/clathrin-dependent pathway. The interaction of the cytoplasmic domain of furin with AP-2 depends on a tyrosine-based sorting signal, a phenylalanine signal, and to a lesser extent, on the leucine-isoleucine motif (24).
In contrast to furin, PC5/6B accumulates in a brefeldin A-dispersible and BaCl2-responsive perinuclear compartment and in the tips of AtT-20 cells. Thus, it has a distinct distribution within the TGN/endosomal pathway (27). Endocytosis is predominantly directed by a canonical tyrosine-based motif. Furthermore, two clusters of acidic amino acids within the cytoplasmic domain of PC5/6B contain differential sorting information. The membrane-proximal acidic amino acids direct TGN localization via PACS-1 but are not phosphorylation-dependent. The membrane-distal acidic amino acids promote the localization characteristic for full-length PC5/6B as described above. The differential localization of PC5/6B and furin may help explain the inability of PC5/6B to compensate for the absence of furin in knock-out mice (21) because it suggests that they might activate proproteins in distinct subcellular compartments. Similarly, a considerable proportion of PC7 is found in TGN-derived vesicles devoid of furin (28).
Trafficking of PC7 has not yet been studied into detail. It has been demonstrated that PC7 reaches the cell surface via the conventional route as well as via a brefeldin A- and coat protein complex II (COPII)-independent unconventional secretory pathway (12). Furthermore, it has been suggested that pro-EGF processing by full-length membrane-bound PC7 does not occur at the cell surface but that it requires endocytosis into clathrin-coated pits (29). In this study, the endocytosis of PC7 from the plasma membrane was investigated by means of cell fractionation, antibody uptake experiments, and cell surface biotinylation.
EXPERIMENTAL PROCEDURES
Plasmids and Mutagenesis
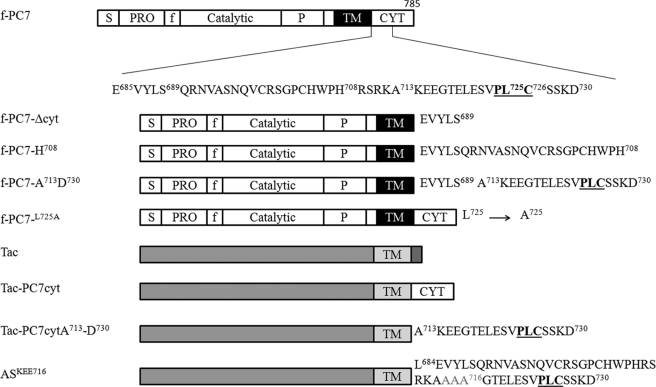
The cloning of the 2.6-kb human PC7 cDNA in the mammalian expression vector pcDNA3 (Invitrogen), the introduction of the FLAG epitope (amino acids DYKDDDDK) between the pro- and catalytic domain, and the mutants f-PC7-Δcyt (lacking the cytoplasmic tail; stop codon after Ser689) and f-PC7-H708 (stop codon after His708) have been described previously (10, 11). PC7 constructs carrying the FLAG epitope are indicated as f-PC7. New mutations were generated using the QuikChange site-directed mutagenesis kit (Stratagene) according to the guidelines of the supplier, standard polymerase chain reactions, or a combination of both. Mutations were confirmed by nucleotide sequence analysis, and an overview of the crucial constructs is given in Fig. 1. To facilitate the subcloning of the mutants, an XhoI site was created from the Leu684–Glu685 codons, making use of codon degeneracy. In mutants f-PC7-L725, f-PC7-C726, and f-PC7-D730, a stop codon was introduced immediately following the indicated amino acid. Construct f-PC7-A713-D730 encodes a PC7 mutant in which amino acids Ala713 until Asp730 are fused to the carboxyl terminus of f-PC7-Δcyt, and in the f-PC7L725A residue, Leu725 is replaced by an alanine using full-length f-PC7 as template.
FIGURE 1.
Schematic overview of different types of constructs. The different domain structures are indicated (S = signal peptide; PRO = propeptide; P = processing domain, f = FLAG tag; TM = transmembrane; CYT = cytoplasmic). In deletion constructs, a stop codon was introduced after the indicated amino acid (f-PC7-H708 is included as example). In the chimeric proteins, the cytoplasmic tail of Tac was swapped with (part of) the cytoplasmic tail of PC7. In the AS constructs, individual amino acids or groups of three amino acids were substituted with alanine. ASKEE716 is shown as example.
The human cDNA encoding the α-chain of the interleukin-2 receptor, Tac (CD25), was a gift from Dr. Bonifacino (Bethesda, MD) and was subcloned as an EcoRI-XbaI fragment in pcDNA3. In Tac-PC7cyt, only the cytoplasmic tail was swapped (Leu684–Cys785). TacPC7cyt-D730 and TacPC7cyt-A713-D730 contained only parts of the cytoplasmic tail of PC7 (Leu684–Asp730 and Ala713–Asp730, respectively). The alanine-scan of residues Lys714–Cys726 was performed using TacPC7cyt-D730 as template and the mutants named AS (alanine scan) with the substituted amino acid(s) indicated (e.g. ASC726). Amino acids were individually substituted by alanines, except for ASKEE716 and ASGTE719, in which three amino acids were replaced simultaneously.4
Cell Lines and DNA Transfer
Medium, serum, and supplements used for the maintenance of cells were obtained from Invitrogen. Generation of the CHO-DHFR− cell line stably overexpressing PC7 (CHO-PC7) has been described before (10). The cells were cultured in minimal essential medium α containing ribonucleosides and deoxyribonucleosides supplemented with 10% fetal calf serum and 250 μg/ml G418. CHO-K1 cells were grown in Dulbecco's modified Eagle's medium/Ham's F12 (1:1) supplemented with 10% fetal calf serum.
CHO-K1 cells were plated 1 day before transfection. 8–10 × 105 cells/10-cm2 culture plates were transfected with 2 μg of DNA and 6 μl of FuGENE (Roche Diagnostics) and used for experiments the next day. All experiments were performed at least twice.
Antibody Uptake/Immunofluorescence Experiments
Transfected CHO-K1 cells were washed and incubated with serum-free medium containing 1 μg/ml anti-FLAG antibody M2 (Sigma) or anti-Tac (BIOSOURCE) for 30 min at 4 °C followed by 15 or 60 min at 37 °C, as indicated. The effect of sucrose and the small molecule inhibitor of clathrin-mediated endocytosis (Pitstop 2, Ascent Scientific) was studied by preincubation of the cells with 0.3 m sucrose (final concentration) in serum-free medium or with serum-free medium containing 30 μm Pitstop 2 for 30 min at 37 °C. Subsequently, the cells were incubated for 30 min at 4 °C and 15 min at 37 °C in the same solutions containing 1 μg/ml anti-FLAG antibody M2. Anti-TGN38 antibody was a generous gift of Dr. Luzio (Cambridge, UK).
After incubation with antibody, the cells were washed in ice-cold PBS, fixed in 4% freshly prepared formaldehyde, and quenched with 50 mm NH4Cl. To facilitate rapid and conclusive screening of the mutants, two different methods were used for the processing of anti-FLAG (M2) and anti-Tac antibody uptake for immunofluorescence. The cells incubated with M2 were incubated for 1 h at room temperature with a rabbit anti-mouse Fab fragment (Jackson ImmunoResearch Laboratories) in PBS-B (PBS containing 0.5% blocking reagent (Roche Diagnostics)) before permeabilization with PBS-BT (PBS-B containing 0.2% Triton X-100). Finally, the cells were incubated with FITC-conjugated donkey anti-rabbit and TRITC-conjugated sheep anti-mouse antibodies (GE healthcare). Using this two-step method, surface-localized M2 was labeled with FITC, whereas internalized M2 was labeled with TRITC. Cells incubated with anti-Tac antibody were immediately permeabilized with PBS-BT and incubated only with TRITC-conjugated sheep anti-mouse antibodies. Slides were analyzed with a Zeiss Axiophot microscope equipped with UV optics. A large number of positive cells were analyzed (>100), and representative cells are shown in the figures. Only cells overexpressing low to moderate levels of recombinant protein were analyzed. Images were recorded with a CE2000A charge-coupled device camera (Photometrics).
Surface Biotinylation
8–10 × 105 transfected CHO-K1 cells were placed on ice, washed four times with PBS, and incubated for 20 min with 1 mg/ml sulfo-NHS-LC-biotin (Pierce) in PBS. Unreacted sulfo-NHS-LC-biotin was quenched with complete culture medium (10 min). After two additional washes with PBS, cells were lysed in PBS containing 1% Triton X-100 and Complete protease inhibitor mixture (Roche Diagnostics). Biotinylated proteins were precipitated using ImmunoPure immobilized streptavidin (Pierce), and nonbiotinylated proteins in the supernatant were precipitated using 4 volumes of methanol. Both samples were separated on SDS-PAGE and analyzed by Western blotting using either M2 (n = 5–10) or anti-Tac (n = 4–6) antibody. Quantification of biotinylated and unbiotinylated PC7 or Tac chimeras was performed using the Image Station 440 (Kodak Digital Science).
Isolation of CCVs
CCVs were isolated from CHO-K1 cells stably overexpressing PC7 (10) essentially as described previously (23). Briefly, cells were washed in PBS containing 0.1 mm CaCl2 and 1 mm MgCl2, scraped, and spun for 10 min at 2000 × g. The cell pellet was resuspended in vesicle buffer (140 mm sucrose, 75 mm potassium acetate, 10 mm MES, pH 6.7, 1 mm EGTA, 0.5 mm magnesium acetate) containing 1 mm dithiothreitol, 1 mm PMSF, and Complete protease inhibitor mixture and homogenized by 15 passages through a 24-gauge needle. From a postnuclear supernatant, a postmitochondrial supernatant was prepared, which was fractionated by two gradient centrifugations. First a microsomal fraction was prepared on a 10-ml continuous gradient of 10–90% (w/v) 2H2O (Sigma) in vesicle buffer followed by a 10-ml continuous gradient from 2% (w/v) Ficoll, 9% (w/v) 2H2O to 20% Ficoll, 90% 2H2O in vesicle buffer. 1-ml fractions were collected from the bottom of the second gradient, precipitated with trichloroacetic acid (10% final concentration), and analyzed by Western blotting.
RESULTS
PC7 Shuttles from Plasma Membrane (PM) to TGN
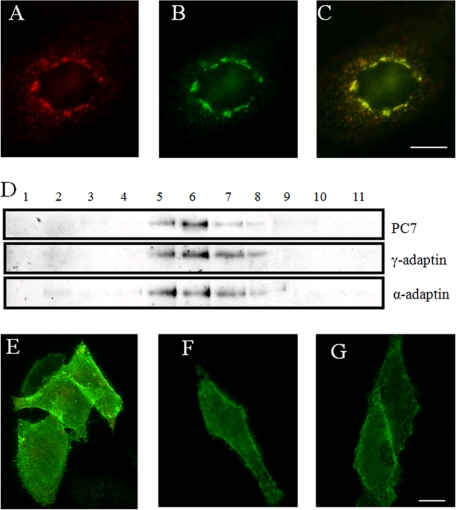
Under steady state conditions, PC7 is concentrated in the TGN, frequently associated with coated buds and vesicles also positive for the AP-1 subunit γ-adaptin (10). To demonstrate that PC7, like furin and PC5/6B, recycles from the PM to the TGN, antibody uptake experiments were performed. CHO-K1 cells were transfected with f-PC7. 24 h later, the cells were incubated with the anti-FLAG antibody M2 for 30 min at 4 °C to allow binding of the antibody without internalization and for 1 h at 37 °C to allow internalization of the membrane-bound PC7. After incubation with the antibody, the cells were fixed, and PC7 was visualized with a TRITC-conjugated secondary antibody (Fig. 2A). After 1 h of endocytosis, the majority of PC7 had been internalized and transported to the TGN as demonstrated by its co-localization with TGN38, an endogenous marker protein for the TGN (Fig. 2, B and C). This demonstrates that PC7 can recycle from the plasma membrane to the TGN.
FIGURE 2.
PC7 recycles from PM to TGN via CCVs. A, CHO-K1 cells transfected with f-PC7 were incubated with the anti-FLAG antibody M2 for 30 min at 4 °C and 1 h at 37 °C, fixed, and stained with a TRITC-conjugated secondary antibody to visualize PC7. B, immunofluorescent post-fix staining of the same cells for TGN38 to visualize the TGN. C, merged picture of those cells demonstrates that f-PC7 was transported from the PM to the TGN. D, CCVs were purified using standard procedures as described under ”Experimental Procedures.“ Each fraction from the second density gradient was separated by SDS-PAGE and analyzed by Western blot analysis for α- and γ-adaptin and PC7. E, antibody uptake experiment (30 min 4 °C, 15 min 37 °C) in hypertonic medium on CHO-K1 cells transfected with f-PC7 using the anti-FLAG antibody M2. A double immunofluorescent staining was performed to visualize intracellular PC7 in red and to visualize cell surface-localized PC7 in green. PC7 mainly localizes at the PM under these conditions. F, antibody uptake experiment (30 min 4 °C, 15 min 37 °C) on CHO-K1 cells transfected with f-PC7 and incubated with 30 μm Pitstop 2, using the anti-FLAG antibody M2. A double immunofluorescent staining was performed to visualize intracellular PC7 in red and to visualize cell surface-localized PC7 in green. PC7 mainly localizes at the PM under these conditions. G, CHO-K1 cells transfected with f-PC7 were incubated for 30 min at 4 °C with serum-free medium containing anti-FLAG antibody M2. A double immunofluorescent staining was performed to visualize intracellular PC7 in red and cell surface-localized PC7 in green. PC7 mainly localizes at the PM under these conditions. Scale bar = 10 μm.
PC7 Recycles to TGN via Clathrin-mediated Endocytosis
Within 1 h, the majority of PC7 recycled from the PM to the TGN. This recycling might occur through clathrin-mediated endocytosis, which can occur within a time frame of 4 min (30). Therefore, to demonstrate that PC7 localizes to CCVs, CCVs were prepared from CHO-K1 cells stably overexpressing PC7 using a standard fractionation protocol (23). The last density gradient of the purification procedure was analyzed by Western blot analysis. PC7 was mainly observed in fractions 5–7. These fractions also contained γ-adaptin and α-adaptin, which are marker proteins for CCVs (Fig. 2D), indicating that PC7 is present in CCVs.
To substantiate that PC7 recycles from the plasma membrane to the TGN via CCVs, antibody uptake experiments were performed on CHO-K1 cells transfected with f-PC7 in the presence or absence of hypertonic medium (0.3 m sucrose). Hypertonic treatment induces abnormal clathrin polymerization into empty microcages, rendering clathrin unavailable for assembly, and as such, the clathrin-coated pits disappear, inhibiting endocytosis (31). If PC7 recycles to the TGN via CCVs, PC7 should accumulate at the PM upon hypertonic treatment. Surface-localized PC7 was stained green using an FITC antibody, whereas intracellular PC7 was stained red using a TRITC antibody, using a double labeling method as described by Franzusoff et al. (32). Upon treatment of the cells with hypertonic medium, PC7 accumulated at the PM (Fig. 2E), whereas without sucrose treatment, the majority of PC7 either was internalized or had accumulated in punctate structures at the cell surface, probably clathrin-coated pits (Fig. 3). Although the 15-min incubation at 37 °C is long enough for endocytosis, it is too short for recycling to the TGN. Hypertonic solution is not specific for the disruption of clathrin-mediated endocytosis. Therefore, to formally prove the involvement of clathrin-mediated endocytosis, we selectively blocked endocytic ligand association with the clathrin terminal domain, using the small molecule inhibitor Pitstop 2 (33). Similarly as for cells treated with hypertonic solution, PC7 also accumulated at the PM upon incubation with Pitstop 2 (Fig. 2F). As a negative control, cells were incubated for 30 min at 4 °C with anti-FLAG M2 antibody without any chase period. In those experiments, no internalization of PC7 could be observed (Fig. 2G). Taken together, these experiments demonstrate that PC7 is endocytosed via CCVs.
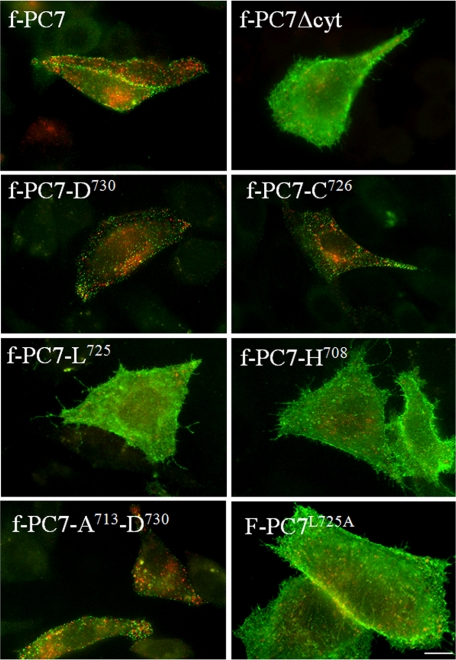
FIGURE 3.
Region between Ala713 and Cys726 in cytoplasmic domain of PC7 is essential for endocytosis of PC7. Antibody uptake experiments (30 min 4 °C, 15 min 37 °C) using the anti-FLAG antibody M2 were performed on CHO-K1 cells transfected with f-PC7 or different truncation constructs in which parts of the cytoplasmic tail of PC7 were deleted. Construct f-PC7L725A contains the entire cytoplasmic tail in which Leu725 was substituted with alanine. Double immunofluorescent staining was performed to stain cell surface-localized PC7 in green and to stain internalized PC7 in red. Scale bar = 10 μm.
Cytoplasmic Domain of PC7 Is Required for Recycling of PC7 from PM to TGN
In the case of furin and PC5/6B, the motif required for the trafficking of proteins from the PM to the TGN is located in the cytoplasmic tail (24, 27). To investigate whether this is also the case for PC7, an antibody uptake experiment was performed using a construct lacking the cytoplasmic domain of PC7 (Fig. 3). f-PC7Δcyt accumulated at the plasma membrane with only a few weakly stained internalized vesicles. Moreover, surface staining was mostly diffuse with few punctated structures such as f-PC7. This demonstrates that the cytoplasmic domain is indeed essential for endocytosis. To determine the region within the cytoplasmic domain that is required for endocytosis of PC7 from the PM, antibody uptake experiments were performed using constructs in which parts of the cytoplasmic domain were truncated. Antibody uptake experiments performed with f-PC7-D730 and f-PC7-C726 showed efficient endocytosis of PC7, similar to that of the full-length f-PC7 construct (Fig. 3). In contrast, when one extra amino acid was deleted from the C terminus (construct f-PC7-L725) or when even more amino acids were removed using the construct f-PC7-H708, PC7 accumulated at the PM (Fig. 3). To further narrow down the region within the cytoplasmic domain that contains the signal for endocytosis, a construct encoding a PC7 mutant in which amino acids Ala713 until Asp730 are fused to the carboxyl terminus of f-PC7-Δcyt was generated. This construct was also endocytosed (Fig. 3). Finally, when Leu725 was mutated to alanine using full-length f-PC7 as template, PC7 accumulated at the PM, suggesting that this amino acid is an essential part of the major, if not only, endocytic motif of PC7 (Fig. 3). Altogether, those results demonstrate that a motif in the region between Ala713 and Cys726 in the cytoplasmic domain of PC7 is essential for the trafficking of PC7 from the PM to the TGN.
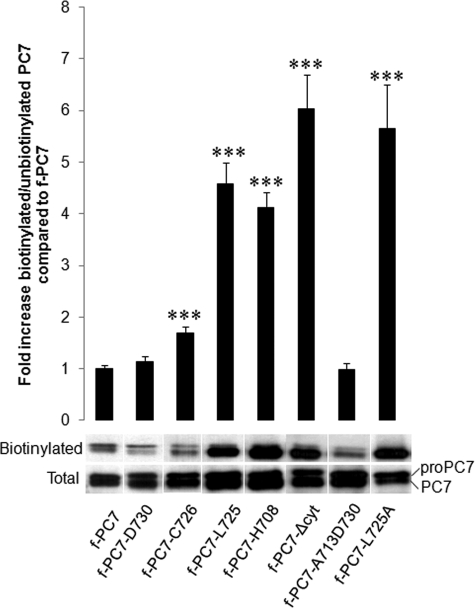
To confirm the results obtained by the immunofluorescence experiments, surface biotinylation experiments were performed. Therefore, CHO-K1 cells were transfected with the same constructs, and the surface proteins were biotinylated using sulfo-NHS-LC-biotin. The ratio between biotinylated and unbiotinylated PC7 was determined as described under “Experimental Procedures.” If PC7 accumulates at the PM, more biotinylated PC7 should be detected, resulting in an increased ratio of biotinylated/unbiotinylated PC7. Indeed, this ratio significantly increased severalfold after transfection with the constructs f-PC7-L725, f-PC7-H708 f-PC7-Δcyt, and f-PC7L725A (Fig. 4). This confirmed the results obtained by the immunofluorescent staining. A small but significant increase was observed with construct f-PC7-C726, indicating that truncation after this cysteine already affected the internalization signal slightly. This minor effect could not be observed in the antibody uptake experiment.
FIGURE 4.
Truncation of cytoplasmic tail of PC7 beyond Ser727 results in increased surface localization. CHO-K1 cells transfected with the same constructs as in Fig. 3. Surface molecules were biotinylated with sulfo-NHS-LC-biotin, and the ratio of biotinylated/unbiotinylated PC7 was determined by Western blot analysis. A representative figure of this Western blot analysis is shown. Quantification of biotinylated and unbiotinylated PC7 was performed using the Image Station 440 (Kodak Digital Science). Statistical analysis was performed using the Student's t test. Values were considered to be significantly different from f-PC7 when p value < 0.05. ***, p value < 0.001. Error bars indicate S.E.
Region Encoded by Amino Acids Ala713–Asp730 Is Essential and Sufficient for Endocytosis
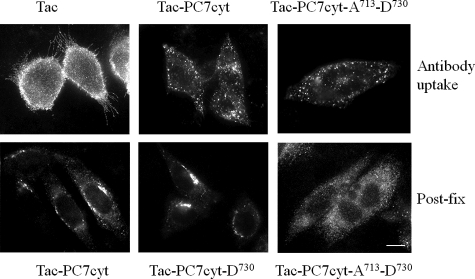
The above described experiments demonstrated that the region between Ala713 and Cys726 in the cytoplasmic domain of PC7 is essential for endocytosis of PC7. To demonstrate that this region is also sufficient for endocytosis, chimeric proteins were constructed consisting of the luminal and transmembrane domains of Tac (CD25) and parts of the cytoplasmic domain of PC7. The Tac luminal and transmembrane domains have been used previously to assess the sorting capacity of cytoplasmic sequences (34). Tac is the α-chain of the IL-2 receptor. It is a type 1 transmembrane protein present on the PM of activated T and B cells, some thymocytes, myeloid precursors, and oligodendrocytes. Antibody uptake experiments using an anti-Tac antibody indeed demonstrated that Tac was mainly localized at the plasma membrane (Fig. 5). However, when the cytoplasmic tail of Tac was swapped with the cytoplasmic tail of PC7, Tac-PC7cyt was endocytosed, as demonstrated by antibody uptake experiments (Fig. 5). Similar results were obtained when the cytoplasmic tail of Tac was swapped with only part of the cytoplasmic tail of PC7 (between Ala713 and Asp730) (Fig. 5). This demonstrates that this part of the cytoplasmic tail of PC7 is not only essential but also sufficient for endocytosis. To determine whether this region is also sufficient to confer TGN localization, transfected cells were analyzed by immunofluorescent staining (post-fix). Both Tac-PC7cyt and Tac-PC7cyt-D730 were found to be concentrated in the TGN, but Tac-PC7cyt-A713-D730 was not (Fig. 5). Instead a dispersed staining was observed. These results indicated that the region Ala713–Asp730 is sufficient for endocytosis but not for TGN localization. TGN localization requires the presence of additional sequences, present in the proximal part of the cytoplasmic tail.
FIGURE 5.
Region encoded by amino acids Ala713–Asp730 is sufficient for endocytosis but not for TGN localization. Upper panels, Chimeric proteins were constructed composed of the luminal and transmembrane domains of Tac (CD25) and (parts of) the cytoplasmic domain of PC7, and antibody uptake experiments were performed (30 min 4 °C, 15 min 37 °C). When the cytoplasmic tail of Tac was swapped with the cytoplasmic tail of PC7 or only with part of the cytoplasmic tail of PC7 (between Ala713 and Asp730), PC7 was still endocytosed. Lower panels, transfected cells were fixed, and Tac was subsequently visualized by immunofluorescent staining. Both Tac-PC7cyt and TaPC7cyt-D730 were found to be concentrated in the TGN, but Tac-PC7cyt-A713-D730 was not. Scale bar = 10 μm.
A Newly Defined PLC Motif Is Essential for Endocytosis
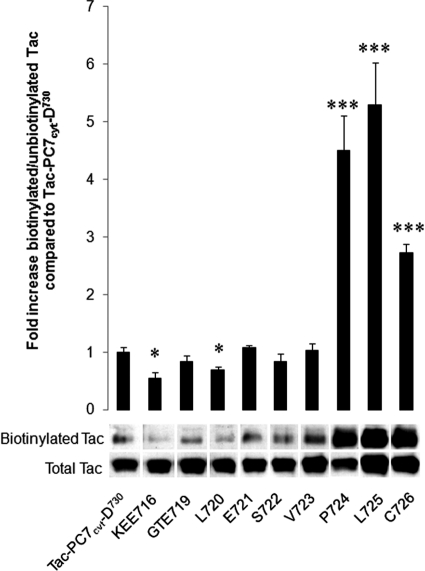
To determine the exact amino acids that are required for endocytosis, we substituted individual amino acids or groups of three amino acids with alanine(s) in Tac-PC7cyt-D730 and performed similar antibody uptake experiments. These AS constructs ASKEE716, ASGTE719, ASL720, ASE721, ASS722, and ASV723 were internalized similar to Tac-PC7cyt-D730 (Fig. 6). In contrast, when amino acids Pro724, Leu725, or Cys726 were substituted with an alanine, the constructs remained mainly diffusely localized at the PM. Those results were confirmed with surface biotinylation experiments, as described above (Fig. 7). Indeed, biotinylated/unbiotinylated PC7 was significantly higher after transfection with the constructs ASP724, ASL725, and ASC726 as compared with TAC-PC7cyt. The other constructs showed surface biotinylation, comparable with TAC-PC7cyt. Even slightly less surface biotinylation was detected for constructs ASKEE716 and ASL720. Altogether, those results demonstrate that Pro724, Leu725, and Cys726 are the only three amino acids essential for endocytosis.
FIGURE 6.
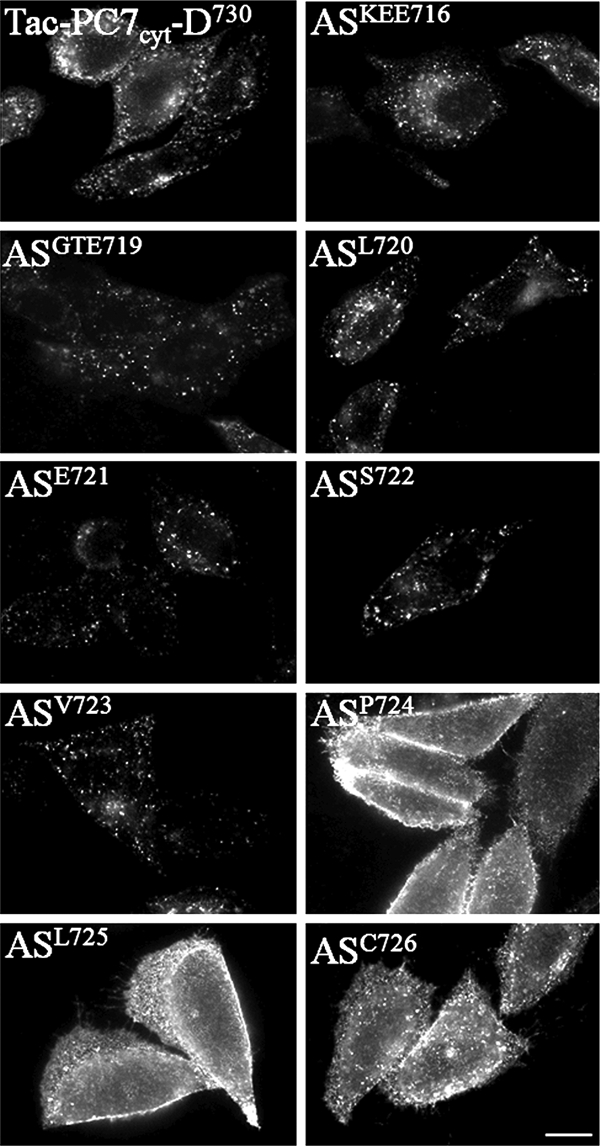
A newly defined PLC motif is essential and sufficient for endocytosis. Chimeric proteins were constructed consisting of the luminal and transmembrane domains of Tac (CD25) and part of the cytoplasmic domain of PC7 and in which individual amino acids or groups of three amino acids were substituted with alanine to determine the exact TGN sorting motif. Antibody uptake experiments (30 min 4 °C, 15 min 37 °C) using an anti-Tac antibody were performed on CHO-K1 cells transfected with those constructs. Three amino acids (PLC) were essential for the internalization of the chimeric construct. Scale bar = 10 μm.
FIGURE 7.
Surface biotinylation assay. CHO-K1 cells transfected with the same constructs as in Fig. 6. Surface molecules were biotinylated with sulfo-NHS-LC-biotin, and the ratio of biotinylated/unbiotinylated Tac chimeras was determined by Western blot analysis. A representative figure of this Western blot analysis is shown. Quantification of biotinylated and unbiotinylated Tac chimeras was performed using the Image Station 440 (Kodak Digital Science). Statistical analysis was performed using the Student's t test. Values were considered to be significantly different from Tac-PC7cyt-D730 when p value < 0.05. *, p value < 0.05, ***, p value < 0.001. Error bars indicate S.D.
DISCUSSION
In this study, we show that PC7 cycles between the TGN and the PM using motifs in the cytoplasmic tail. Internalization is mediated by CCVs and depends on a short cytoplasmic region containing the crucial amino acid sequence PLC. This short region is essential and sufficient for endocytosis but not sufficient to confer TGN localization.
PC7 is localized, at least in part, in the TGN. Overexpression studies have shown partial co-localization with TGN markers, but a less confined expression pattern (10, 11). Ultrastructural analysis showed immunoreactivity associated with coated buds and vesicles adjacent to the Golgi stack. These overexpression studies are hampered by the large relative proportion of pro-PC7 in the endoplasmic reticulum, which obfuscates the juxtanuclear TGN staining. The high proportion of pro-PC7 is caused by the slow maturation and tendency to aggregate (9). The subcellular localization of endogenous PC7 has been studied in rat liver and the lymphoblastoid cell line T2, and these studies confirm the partial TGN localization and suggest that the remainder is localized in post-TGN structures (28, 35). In this study, we show that recombinant PC7 recycles in Chinese hamster ovary CHO cells to a juxtanuclear region that co-localizes to a large extend with the TGN marker TGN38. It has been shown that the TGN localization of furin is only partly overlapping with PC7 and PC5/6B (27, 28, with part of PC7 and PC5/6B in a post-TGN compartment. To what extent PC7 and PC5/6B co-localize has not been studied yet.
The involvement of CCVs in the endocytosis of PC7 is supported by the presence of PC7 in fractions enriched for CCVs and containing the markers α- and γ-adaptin. Furthermore, after hypertonic treatment of the cells or incubation with Pitstop 2, which both interfere with clathrin-mediated endocytosis, PC7 accumulates at the PM. After furin (23) and PC5/6B (27), PC7 is now the third member of the PC family that has been shown to be internalized in CCVs.
The information that is required for internalization and TGN localization of PC7 is contained within the cytoplasmic tail and is transferable to a reporter construct. Sequence analysis showed that no tyrosine is present in the cytoplasmic tail, and therefore, no YXX(Y = Tyr, X = any amino acid and Ø = hydrophobic amino acid) motif, which is found in both furin and PC5/6B. On the other hand, the commonly found dileucine trafficking signal is present twice (Leu747-Leu748 and Leu752-Leu753), albeit not in the context of the extended motif (ED)XXXL(LI) or DXXLL (36). Deletion of the region containing these dileucine sequences did not appear to affect internalization, confirming that they do not play a major role in this trafficking step.
Our results show that internalization of PC7 depends on a short region containing the essential contiguous amino acid motif proline, leucine, and cysteine. The PLC motif was found to be the dominant, if not only, motif for internalization. Mutation of the leucine into an alanine in full-length PC7 blocked internalization to the same extent as deletion of the entire cytoplasmic tail. This motif is different from all previously identified internalization motifs. The amino acids are also found in the KCPL motif of P-selectin, which mediates sorting from endosomes to lysosomes (37). However, because the order of the amino acids is not conserved and no interacting proteins are currently known, the possible relevance of this amino acid similarity remains to be determined. Comparison of the PLC motif in other species shows that the sequence ESVPLC is highly conserved (Fig. 8). Nevertheless, the cysteine residue is replaced by a phenylalanine in Xenopus laevis, and the valine residue is replaced by methionine in mouse and rat. Single amino acid replacement of the first three amino acids of this sequence did not show a deleterious effect on internalization, so it is remarkable that these amino acids are highly conserved as well. The cysteine to phenylalanine substitution is nonconservative and suggests that this residue might be somewhat less important. This is consistent with the cell surface biotinylation experiment, which detected slightly less PC7 at the PM after mutation of the cysteine as compared with mutations of the proline or leucine.
FIGURE 8.
Sequence alignment of part of cytoplasmic tail of PC7, containing internalization motif, from different organisms. Alignment of the amino acid sequence corresponding to the region Lys714–Asp730 of the cytoplasmic tail of human PC7 in mouse, rat, dog, Xenopus, cat, rabbit, and guinea pig is shown.
Although the short region containing the PLC motif is sufficient for internalization, additional sequences in the cytoplasmic tail, proximal to the TM domain, are needed for TGN localization. This region of ∼25 amino acids contains the two cysteines that are reversibly palmitoylated (10). However, these cysteines have been shown to affect the half-life of PC7 but not its TGN localization (11). Another remarkable feature is the presence of seven basic amino acids near these cysteines, of which five are clustered in the sequence HRSRKAK. Furin and PC5/6B contain clusters of acidic amino acids that are necessary for TGN localization and interact with the connector protein PACS-1 (23, 26, 27). No acidic cluster is present in PC7, but the basic cluster might function as the binding site for a TGN-directing protein.
The need for seven PCs is not fully understood, given the similar substrate specificity and overlapping expression patterns. However, the severe phenotypes in most PC KOs (14) indicate that there is limited or no redundancy for the processing of some substrates. This may be related to the specific cleavage site of a substrate or the unique temporospatial coexpression of the substrate and the PC. Characterizing the intracellular trafficking of PCs may contribute to understanding the specific PC-substrate pairs and hence the identification of a PC for possible therapeutic intervention. In this study, we have demonstrated that PC7 cycles between the PM to the TGN in CCVs using a novel transferable PLC motif in its cytoplasmic tail. This transferable motif is essential for internalization but not sufficient for TGN localization.
Acknowledgment
We thank Meike Teuchert for suggestions and practical contribution.
This work was supported in part by grants from the “Fonds voor Wetenschappelijk Onderzoek Vlaanderen” (FWO) and “Geconcerteerde Onderzoeksacties“ (GOA 2008/16 and GOA/12/016).
The multiple mutants used in this study are defined as follows: ASKEE716 = ASK714A,E715A,E716A; and ASGTE719 = ASG717A,T718A,E719A.
- PC7
- proprotein convertase 7
- AS
- alanine scan
- CCV
- clathrin-coated vesicle
- PACS-1
- phosphofurin acidic cluster sorting protein 1
- PLC
- proline, leucine, cysteine
- PM
- plasma membrane
- TGN
- trans-Golgi network
- TRITC
- tetramethylrhodamine isothiocyanate.
REFERENCES
- 1. Seidah N. G. (2011) What lies ahead for the proprotein convertases? Ann. N.Y. Acad. Sci. 1220, 149–161 [DOI] [PubMed] [Google Scholar]
- 2. Taylor N. A., Van De Ven W. J., Creemers J. W. (2003) Curbing activation: proprotein convertases in homeostasis and pathology. FASEB J. 17, 1215–1227 [DOI] [PubMed] [Google Scholar]
- 3. Creemers J. W., Siezen R. J., Roebroek A. J., Ayoubi T. A., Huylebroeck D., Van de Ven W. J. (1993) Modulation of furin-mediated proprotein processing activity by site-directed mutagenesis. J. Biol. Chem. 268, 21826–21834 [PubMed] [Google Scholar]
- 4. Thomas G. (2002) Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 3, 753–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meerabux J., Yaspo M. L., Roebroek A. J., Van de Ven W. J., Lister T. A., Young B. D. (1996) A new member of the proprotein convertase gene family (LPC) is located at a chromosome translocation breakpoint in lymphomas. Cancer Res. 56, 448–451 [PubMed] [Google Scholar]
- 6. Bruzzaniti A., Goodge K., Jay P., Taviaux S. A., Lam M. H., Berta P., Martin T. J., Moseley J. M., Gillespie M. T. (1996) PC8, a new member of the convertase family. Biochem. J. 314, 727–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seidah N. G., Hamelin J., Mamarbachi M., Dong W., Tardos H., Mbikay M., Chretien M., Day R. (1996) cDNA structure, tissue distribution, and chromosomal localization of rat PC7, a novel mammalian proprotein convertase closest to yeast kexin-like proteinases. Proc. Natl. Acad. Sci. U.S.A. 93, 3388–3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Constam D. B., Calfon M., Robertson E. J. (1996) SPC4, SPC6, and the novel protease SPC7 are coexpressed with bone morphogenetic proteins at distinct sites during embryogenesis. J. Cell Biol. 134, 181–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Creemers J. W., van de Loo J. W., Plets E., Hendershot L. M., Van De Ven W. J. (2000) Binding of BiP to the processing enzyme lymphoma proprotein convertase prevents aggregation, but slows down maturation. J. Biol. Chem. 275, 38842–38847 [DOI] [PubMed] [Google Scholar]
- 10. van de Loo J. W., Creemers J. W., Bright N. A., Young B. D., Roebroek A. J., Van de Ven W. J. (1997) Biosynthesis, distinct post-translational modifications, and functional characterization of lymphoma proprotein convertase. J. Biol. Chem. 272, 27116–27123 [DOI] [PubMed] [Google Scholar]
- 11. van de Loo J. W., Teuchert M., Pauli I., Plets E., Van de Ven W. J., Creemers J. W. (2000) Dynamic palmitoylation of lymphoma proprotein convertase prolongs its half-life, but is not essential for trans-Golgi network localization. Biochem. J. 352, 827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rousselet E., Benjannet S., Hamelin J., Canuel M., Seidah N. G. (2011) The proprotein convertase PC7: unique zymogen activation and trafficking pathways. J. Biol. Chem. 286, 2728–2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Villeneuve P., Feliciangeli S., Croissandeau G., Seidah N. G., Mbikay M., Kitabgi P., Beaudet A. (2002) Altered processing of the neurotensin/neuromedin N precursor in PC2 knockdown mice: a biochemical and immunohistochemical study. J. Neurochem. 82, 783–793 [DOI] [PubMed] [Google Scholar]
- 14. Creemers J. W., Khatib A. M. (2008) Knock-out mouse models of proprotein convertases: unique functions or redundancy? Front. Biosci. 13, 4960–4971 [DOI] [PubMed] [Google Scholar]
- 15. Molloy S. S., Thomas L., VanSlyke J. K., Stenberg P. E., Thomas G. (1994) Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J. 13, 18–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Bie I., Marcinkiewicz M., Malide D., Lazure C., Nakayama K., Bendayan M., Seidah N. G. (1996) The isoforms of proprotein convertase PC5 are sorted to different subcellular compartments. J. Cell Biol. 135, 1261–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Siegfried G., Khatib A. M., Benjannet S., Chrétien M., Seidah N. G. (2003) The proteolytic processing of pro-platelet-derived growth factor-A at RRKR86 by members of the proprotein convertase family is functionally correlated to platelet-derived growth factor-A-induced functions and tumorigenicity. Cancer Res. 63, 1458–1463 [PubMed] [Google Scholar]
- 18. Siegfried G., Basak A., Prichett-Pejic W., Scamuffa N., Ma L., Benjannet S., Veinot J. P., Calvo F., Seidah N., Khatib A. M. (2005) Regulation of the stepwise proteolytic cleavage and secretion of PDGF-B by the proprotein convertases. Oncogene 24, 6925–6935 [DOI] [PubMed] [Google Scholar]
- 19. Constam D. B., Robertson E. J. (1999) Regulation of bone morphogenetic protein activity by pro domains and proprotein convertases. J. Cell Biol. 144, 139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Siegfried G., Basak A., Cromlish J. A., Benjannet S., Marcinkiewicz J., Chrétien M., Seidah N. G., Khatib A. M. (2003) The secretory proprotein convertases furin, PC5, and PC7 activate VEGF-C to induce tumorigenesis. J. Clin. Invest. 111, 1723–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roebroek A. J., Umans L., Pauli I. G., Robertson E. J., van Leuven F., Van de Ven W. J., Constam D. B. (1998) Failure of ventral closure and axial rotation in embryos lacking the proprotein convertase furin. Development 125, 4863–4876 [DOI] [PubMed] [Google Scholar]
- 22. Essalmani R., Hamelin J., Marcinkiewicz J., Chamberland A., Mbikay M., Chrétien M., Seidah N. G., Prat A. (2006) Deletion of the gene encoding proprotein convertase 5/6 causes early embryonic lethality in the mouse. Mol. Cell. Biol. 26, 354–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Teuchert M., Schäfer W., Berghöfer S., Hoflack B., Klenk H. D., Garten W. (1999) Sorting of furin at the trans-Golgi network: interaction of the cytoplasmic tail sorting signals with AP-1 Golgi-specific assembly proteins. J. Biol. Chem. 274, 8199–8207 [DOI] [PubMed] [Google Scholar]
- 24. Teuchert M., Berghöfer S., Klenk H. D., Garten W. (1999) Recycling of furin from the plasma membrane: functional importance of the cytoplasmic tail sorting signals and interaction with the AP-2 adaptor medium chain subunit. J. Biol. Chem. 274, 36781–36789 [DOI] [PubMed] [Google Scholar]
- 25. Jones B. G., Thomas L., Molloy S. S., Thulin C. D., Fry M. D., Walsh K. A., Thomas G. (1995) Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. EMBO J. 14, 5869–5883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wan L., Molloy S. S., Thomas L., Liu G., Xiang Y., Rybak S. L., Thomas G. (1998) PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell 94, 205–216 [DOI] [PubMed] [Google Scholar]
- 27. Xiang Y., Molloy S. S., Thomas L., Thomas G. (2000) The PC6B cytoplasmic domain contains two acidic clusters that direct sorting to distinct trans-Golgi network/endosomal compartments. Mol. Biol. Cell 11, 1257–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wouters S., Leruth M., Decroly E., Vandenbranden M., Creemers J. W., Van de Loo J. W., Ruysschaert J. M., Courtoy P. J. (1998) Furin and proprotein convertase 7 (PC7)/lymphoma PC endogenously expressed in rat liver can be resolved into distinct post-Golgi compartments. Biochem. J. 336 311–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rousselet E., Benjannet S., Marcinkiewicz E., Asselin M. C., Lazure C., Seidah N. G. (2011) Proprotein convertase PC7 enhances the activation of the EGF receptor pathway through processing of the EGF precursor. J. Biol. Chem. 286, 9185–9195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rappoport J. Z., Kemal S., Benmerah A., Simon S. M. (2006) Dynamics of clathrin and adaptor proteins during endocytosis. Am. J. Physiol. Cell Physiol. 291, C1072–C1081 [DOI] [PubMed] [Google Scholar]
- 31. Heuser J. E., Anderson R. G. (1989) Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 108 389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Franzusoff A., Redding K., Crosby J., Fuller R. S., Schekman R. (1991) Localization of components involved in protein transport and processing through the yeast Golgi apparatus. J. Cell Biol. 112, 27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. von Kleist L., Stahlschmidt W., Bulut H., Gromova K., Puchkov D., Robertson M. J., MacGregor K. A., Tomilin N., Pechstein A., Chau N., Chircop M., Sakoff J., von Kries J. P., Saenger W., Kräusslich H. G., Shupliakov O., Robinson P. J., McCluskey A., Haucke V. (2011) Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell 146, 471–484 [DOI] [PubMed] [Google Scholar]
- 34. Voorhees P., Deignan E., van Donselaar E., Humphrey J., Marks M. S., Peters P. J., Bonifacino J. S. (1995) An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO J. 14, 4961–4975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leonhardt R. M., Fiegl D., Rufer E., Karger A., Bettin B., Knittler M. R. (2010) Post-endoplasmic reticulum rescue of unstable MHC class I requires proprotein convertase PC7. J. Immunol. 184, 2985–2998 [DOI] [PubMed] [Google Scholar]
- 36. Bonifacino J. S., Traub L. M. (2003) Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72 395–447 [DOI] [PubMed] [Google Scholar]
- 37. Blagoveshchenskaya A. D., Norcott J. P., Cutler D. F. (1998) Lysosomal targeting of P-selectin is mediated by a novel sequence within its cytoplasmic tail. J. Biol. Chem. 273, 2729–2737 [DOI] [PubMed] [Google Scholar]