Background: Dopamine D2 and D3 receptor subtypes are often co-expressed.
Results: Systems were established to allow concurrent detection of receptor homomers and heteromers.
Conclusion: Co-expressed D2 and D3 receptors form both homomers and heteromers and all are functional and present concurrently.
Significance: These observations are relevant to the pathogenesis and treatment of disorders in which D2 and D3 receptors are implicated.
Keywords: 7-Helix receptor, Dopamine, Fluorescence Resonance Energy Transfer (FRET), G Protein-coupled Receptors (GPCR), G Proteins
Abstract
Human dopamine D2long and D3 receptors were modified by N-terminal addition of SNAP or CLIP forms of O6-alkylguanine-DNA-alkyltransferase plus a peptide epitope tag. Cells able to express each of these four constructs only upon addition of an antibiotic were established and used to confirm regulated and inducible control of expression, the specificity of SNAP and CLIP tag covalent labeling reagents, and based on homogenous time-resolved fluorescence resonance energy transfer, the presence of cell surface D2long and D3 receptor homomers. Following constitutive expression of reciprocal constructs, potentially capable of forming and reporting the presence of cell surface D2long-D3 heteromers, individual clones were assessed for levels of expression of the constitutively expressed protomer. This was unaffected by induction of the partner protomer and the level of expression of the partner required to generate detectable cell surface D2long–D3 heteromers was defined. Such homomers and heteromers were found to co-exist and using a reconstitution of function approach both homomers and heteromers of D2long and D3 receptors were shown to be functional, potentially via trans-activation of associated G protein. These studies demonstrate the ability of dopamine D2long and D3 receptors to form both homomers and heteromers, and show that in cells expressing each subtype a complex mixture of homomers and heteromers co-exists at steady state. These data are of potential importance both to disorders in which D2long and D3 receptors are implicated, like schizophrenia and Parkinson disease, and also to drugs exerting their actions via these sites.
Introduction
The neurotransmitter dopamine and its receptors have been studied extensively because of their roles, among many others, in regulation of motor control, reward, and motivation (1). Five distinct genes encode the D1–D5 dopamine receptors, and with splice variations within the D2–D4 “D2-like” group the total number of isoforms is significantly larger (2).
Declining numbers of dopamine-producing neurons in the substantia nigra and loss of dopaminergic activity in the striatum are linked to motor dysfunction in Parkinson disease and alleviation of Parkinsonian symptoms can be achieved with agonists recruiting D2 receptors. A number of ligands employed clinically for this disorder, interestingly, actually have moderate selectivity for the D3 receptor over D2 receptors (3–5). However, because of the overlap of ligand recognition between the D2 and D3 receptors and co-expression of the two receptors in caudate, putamen, and striatum, their individual contributions are challenging to define (6). Drugs behaving as antagonists at the dopamine D2 receptor are universally employed to treat schizophrenia. Again, however, all clinically effective drugs display at least as high affinity for the D3 receptor as for the D2 receptor, complicating the determination of their respective roles (7).
As with other members of the G protein-coupled receptor subfamily (8), there has been much recent interest in the concept that monomeric, non-interacting proteins are not the only, or indeed predominant, forms of dopamine receptor subtypes. Given the quantitative prevalence of the D2-subtype a number of studies have explored dimerization or oligomerization of this receptor (9–13). As well as demonstrating the presence of such interactions in transfected cell systems a number of reports have provided evidence of D2–D2 interactions in native tissues (14). Furthermore, there may be a potential for alteration in the proportion of D2 receptor monomers versus dimers in schizophrenia (14) and cocaine self-administration may alter interactions between D2-receptor dimers (15). There is evidence that variants of the dopamine D4 receptor can form dimers or oligomers (16) and that the dopamine D3 receptor may form homomeric complexes (17).
There is also substantial evidence that a number of dopamine receptor heteromers may exist (18). For example, there is strong evidence for the presence of D1–D2 receptor heteromers in striatum (19) and upon co-expression in heterologous cell lines, and formation of D1–D2 heteromers modifies pharmacology and signaling versus the respective monomers (20). Therefore, it is pertinent to ask whether differences in the pharmacology of dopamine D2 and D3 receptors seen upon their co-expression may reflect such heteromeric interactions (21–24). Furthermore, it has been suggested that potential D2–D3 receptor heteromers might be an interesting and distinct therapeutic target (24). To date, however, previous studies have mostly used indirect methods to evaluate putative heteromers, and they have been limited to experiments employing transient co-transfection. A major challenge in studies examining the capacity of receptor pairs to form heteromers is that the corresponding homomers will likely also be present concurrently, although this is rarely explored. Herein, two approaches were taken to address these issues. The first, based on the recently developed SNAP and CLIP tags (25), was used to identify cell surface interactions between D2long and D3 receptors expressed stably in cells in which the expression of one or the other of the receptor pair could be varied in amount. The second approach involved functional complementation between two co-expressed but non-equivalent and non-functional dopamine receptor-G protein fusion constructs (26). Receptor-receptor interactions were shown to occur in each case and at expression levels similar to those found in striatum and caudate (6, 27). Furthermore, in these cells, both D2long and D3 homomers were observed to be present together with the heteromers.
EXPERIMENTAL PROCEDURES
Materials
[3H]Spiperone (65–140 Ci/mmol) was from GE Healthcare and [35S]GTPγS was from PerkinElmer Life Sciences. (+)-Butaclamol and dopamine were purchased from Sigma. All other compounds were synthesized by Servier.
DNA Constructs
SNAP- and CLIP-tagged Human Dopamine D2L and D3 Receptors
As described previously (27) the plasmids pSEMS1–26m (SNAP tag) and pCEMS1-CLIP10m (CLIP tag), as supplied by Covalys Biosciences AG (Witterswil, Switzerland), were modified by the addition of a small linker region encoding the metabotropic glutamate receptor 5 signal sequence, (MVLLLILSVLLLKEDVRGSAQS), and an epitope tag (either HA, YPYDVPDYA for the CLIP construct, or VSV-G, YTDIEMNRLGK for the SNAP construct) between the ClaI and EcoRI sites of the multiple cloning site upstream of the SNAP or CLIP tags (MCS1). The linker was made by annealing two complementary primers containing the sequences described above with the addition of a Kozak sequence, start codon, and appropriate nucleotides to generate ClaI and EcoRI “sticky” ends. The primers were annealed by combining 1 nm of each with 1× “multicore” buffer (Promega Corporation) in a final volume of 50 μl. This was then heated to 100 °C in a boiling water bath for 5 min, after which the bath was turned off and allowed to cool overnight. The annealed fragment was then purified by gel extraction and ligated into the plasmid by standard techniques. The human dopamine D2long (D2L) isoform and D3 receptors were PCR amplified using primers designed to add BamHI and NotI sites to the fragment termini. These were then ligated into the multiple cloning sites downstream of SNAP or CLIP tags (MCS2) of the modified plasmids described above. To create constructs that could be used to make Flp-InTM T-RExTM 293 inducible stable cell lines of these constructs, the entire insert from the ClaI site to the NotI site was cut out and ligated into a modified version of pcDNA5/FRT/TO (Invitrogen) with a ClaI site added to the multiple cloning site using a linker formed from two annealed primers as described above (28). To create the double stable cell lines, constructs containing the entire insert from the ClaI site to the NotI site were cut out and ligated back into pSEMS1–26m (SNAP tag) or pCEMS1-CLIP10m (CLIP tag).
Mutagenesis of Dopamine Receptor-G Protein Fusions
The Myc-D2L-C351I-Gαo protein was described in Ref. 29 and the Myc-D3-C351I-Gαo in Ref. 30. The Stratagene QuikChange method was used to introduce specific mutations. The primers used were as follows, with the mutated bases shown in bold italics; Val136 to Glu and Met140 to Asp in dopamine D2L, 5′-CAGGTACACAGCTGAGGCCATGCCCGACCTGTACAATACG-3′, Val132 to Glu and Val136 to Asp in dopamine D3, 5′-CAGGTACACTGCAGAGGTCATGCCCGATCACTACCAGCATGG-3′, and Gly204 to Ala in Gαo, 5′-CTGTTTGACGTTGGGGCCCAGCGATCTGAACG-3′. Template DNA was digested with DpnI to leave mutated plasmid and sequencing was carried out to confirm the introduction of the alterations.
Generation and Maintenance of Stable Flp-InTM T-RExTM 293 Cells
Cells were maintained in Dulbecco's modified Eagle's medium without sodium pyruvate, 4500 mg/liter of glucose, and l-glutamine, supplemented with 10% (v/v) fetal calf serum, 1% penicillin/streptomycin mixture, and 10 μg ml−1 of blasticidin in a humidified atmosphere. Flp-InTM T-RExTM 293 cells able to inducibly express the VSV-G-SNAP-tagged D2L or D3 receptor constructs or HA-CLIP-tagged D2L or D3 receptor constructs were generated as previously described (28, 31). Briefly, Flp-InTM T-RExTM 293 cells were co-transfected with plasmids pOG44 and pcDNA5/FRT/TO (Invitrogen) containing the desired cDNA, at a ratio of 9:1 using Lipofectamine according to the manufacturer's instructions (Invitrogen). After 48 h the medium was supplemented with 200 μg ml−1 of hygromycin B to select for stably transfected cells. Pools of cells were established and tested for inducible expression by the addition of 10 ng ml−1 of doxycycline for 24 h followed by screening for VSV-G, HA-, or SNAP/CLIP-tagged protein expression by Western blotting using membrane preparations.
Generation and Maintenance of Double-stable Flp-InTM T-RExTM 293 Cells
HA-CLIP-D3 or HA-CLIP-D2L receptor constructs in pCEMS1-CLIP10m were, respectively, co-transfected into Flp-InTM T-RExTM 293 cells expressing the reciprocal inducible VSV-G-SNAP-tagged D2L or D3 receptor constructs using Lipofectamine according to the manufacturer's instructions. After 48 h, the medium was changed to medium supplemented with 1 mg ml−1 of G418 (Roche Diagnostics) to initiate selection of stably co-transfected cells. All clones isolated were initially screened by fluorescent labeling with CLIP-Lumi4®Tb in the absence of doxycycline induction and SNAP-Lumi4®Tb following doxycycline treatment for receptor expression and subsequent specific binding of [3H]spiperone on cell membrane preparation and whole cells.
Transient Transfection of HEK293 Cells
Cells were maintained in Dulbecco's modified Eagle's medium without sodium pyruvate, 4500 mg liter−1 of glucose supplemented with 10% (v/v) newborn calf serum, 2 mm l-glutamine, and 1% penicillin/streptomycin mixture in a humidified atmosphere containing 5% CO2. Cells were transfected when 70–80% confluent. Cells were transfected with a total of 5 μg of DNA constructs using Lipofectamine following the manufacturer's instructions (Invitrogen). Following 24 h, the medium was replaced with one containing 25 ng ml−1 of pertussis toxin and cells were harvested for membrane preparations 24 h later.
Cell Membrane Preparation
Pellets of cells frozen at −80 °C for a minimum of 1 h, were thawed, and resuspended in ice-cold 10 mm Tris, 0.1 mm EDTA, pH 7.4 (TE buffer), supplemented with Complete protease inhibitors mixture (Roche Diagnostics). Cells were homogenized on ice by 40 strokes of a glass on Teflon homogenizer followed by centrifugation at 1,000 × g for 5 min at 4 °C to remove unbroken cells and nuclei. The supernatant fraction was transferred to ultracentrifuge tubes and subjected to centrifugation at 50,000 × g for 45 min at 4 °C. The resulting pellets were resuspended in ice-cold TE buffer and passed through a 25-gauge needle 3 times before being assessed for protein concentration. Membrane preparations were then aliquoted and stored at −80 °C until required.
Western Blotting
Membrane protein samples prepared as previously described were diluted to a final concentration of 2 mg ml−1 in TE buffer supplemented with complete protease inhibitors mixture (Roche Diagnostics). These protein samples were then diluted in Laemmli buffer (5 m urea, 0.17 m SDS, 0.4 m dithiothreitol, 50 mm Tris-HCl, pH 8.0, and 0.01% bromphenol blue) to a final concentration of 1 mg ml−1. Samples were heated at 100 °C for 5 min. 10 to 20 μg of protein of each sample was loaded into wells of 4 to 12% BisTris3 gels (NuPAGE; Invitrogen) and subjected to SDS-PAGE analysis using NuPAGE® MOPS SDS running buffer (NuPAGE; Invitrogen). After separation, the proteins were electrophoretically transferred onto nitrocellulose membrane, which was then blocked (5% fat-free milk powder in Tris-buffered solution (TBS) supplemented with 0.1% Tween 20 (TBS-Tween)) for 1 h at room temperature (RT) on a rotating shaker. The membrane was then rinsed with TBS-Tween (3 × 10 min) and further incubated with appropriate primary antibody (see figure legends) in 5% fat-free milk powder in TBS-Tween overnight at 4 °C on a rotating shaker. Following which the membrane was washed (3 × 10 min with PBS-Tween) and then incubated for 1 h with the appropriate secondary antibody (horseradish peroxidase (HRP)-linked donkey anti-rabbit IgG, HRP-linked sheep anti-mouse or HRP-linked goat anti-rat IgG, GE Healthcare) diluted 1:10,000 in 2% fat-free milk powder in TBS-Tween. After washing, proteins were detected by enhanced chemiluminescence (Pierce) according to the manufacturer's instructions.
[3H]Spiperone Binding Studies
Binding on Membrane Preparations
Binding studies were initiated by the addition of 2.5 or 5 μg (for D2L or D3 receptors membrane preparations, respectively) cell membranes in assay buffer (20 mm HEPES, 6 mm MgCl2, 1 mm EDTA, 1 mm EGTA, 40 μm ascorbic acid) to tubes containing [3H]spiperone (0.01–5 nm) for saturation bindings (28, 29). Nonspecific binding was determined by the addition of 10 μm (+)-butaclamol. Competition assays were carried out in the presence of ∼0.5 nm [3H]spiperone and increasing concentrations of the indicated compound. Reactions were incubated for 2 h at 30 °C and terminated by rapid vacuum filtration though GF/C glass fiber filters (AlphaBiotech, London, UK) followed by two washes with ice-cold PBS. The level of radioactivity associated with the filters was quantified using a TriCarb 2810 Tr scintillation counter (PerkinElmer Life Sciences).
Binding to Intact Cells
Cells were plated at 25,000 cells well−1 in 24- or 48-well plates (Corning, The Netherlands) 48 h before the assay. Twenty-four hours after plating, cells were treated or not with the appropriate amount of doxycycline for another 24 h. On the day of the experiment cells were washed with Hanks' balanced salt solution (3 times on ice) and, for saturations studies, 0.01–5 nm [3H]spiperone were added to appropriate wells containing or not 10 μm (+)-butaclamol to determine nonspecific binding. Competition assays were carried out in the presence of ∼0.5 nm [3H]spiperone and increasing concentrations of the indicated compound. Plates were incubated for 1 h at 37 °C in a humidified atmosphere. Reactions were terminated on ice followed by two washes with ice-cold Hanks' balanced salt solution. Cells were then incubated for 5 min with an ice-cold acid solution (0.2 m acetic acid, 0.5 m NaCl) to remove the bound fraction of the radioligand. The solution was collected and the radioactivity assessed using a TriCarb 2810 Tr scintillation counter.
[35S]GTPγS Binding Studies
[35S]GTPγS binding experiments were initiated by the addition of cell membranes (10 μg/assay) to assay buffer (20 mm HEPES, 100 mm NaCl, 6 mm MgCl2, 40 μm ascorbic acid, 3 μm guanosine 5′-diphosphate, 20 μg ml−1 of saponin, and 0.1 nm [35S]GTPγS) containing the indicated concentrations of ligand. Reactions were incubated for 2 h at 30 °C and terminated by rapid filtration through GF/C glass fiber filters followed by two washes with ice-cold PBS. The levels of [35S]GTPγS incorporated in cell membranes were then evaluated using a TriCarb 2810 Tr scintillation counter.
Homogeneous Time-resolved FRET Studies (htrFRET)
Cells expressing the receptors of interest were grown to 100,000 cells per well in solid black 96-well plates (Greiner Bio-One) coated with 0.1 mg ml−1 of poly-d-lysine. The htrFRET assays were conducted using Tag-LiteTM reagents from Cisbio Bioassays following the manufacturer's instructions (Cisbio Bioassays, Bagnols-sur-Cèze, France). Briefly, the growth medium was replaced with 100 μl of a mixture containing the fixed optimal concentrations of donor, Tag-Lite SNAP- or CLIP-Lumi4®Tb or donor and acceptor, Tag-Lite SNAP- or CLIP-Red (Cisbio Bioassays). Plates were incubated for 1 h at 37 °C in a humidified atmosphere (5% CO2), and subsequently washed four times in labeling medium (Cisbio Bioassays). Plates with 100 μl/well of fresh labeling medium (with or without compound) were then read on a PheraStar FS (BMG Labtechnologies, Offenburg, Germany) htrFRET compatible reader, following different incubation times at 37 °C. Both the emission signal from the Tag-Lite SNAP- or CLIP-Lumi4®Tb cryptate (620 nm) and the FRET signal resulting from the acceptor Tag-Lite SNAP- or CLIP-Red (665 nm) were recorded following excitation at 337 nm (31).
Epifluorescence Imaging of SNAP-tag Proteins in Live Cells
Cells stably expressing the receptor of interest were grown on coverslips pre-treated with 0.1 mg ml−1 of poly-d-lysine. Fluorescently labeled SNAP and CLIP tag-specific substrates (SNAP-SurfaceTM 488 and CLIP-SurfaceTM 547) (31) were diluted in complete Dulbecco's modified Eagle's medium from a stock solution yielding a labeling solution of 2.5 μm dye substrate. The medium on the cells expressing a SNAP or CLIP tag fusion protein was replaced with the labeling solution and incubated at 37 °C (5% CO2) for 30 or 45 min, respectively. Cells were washed three times with HEPES physiological saline solution (130 mm NaCl, 5 mm KCl, 1 mm CaCl2, 1 mm MgCl2, 20 mm HEPES, and 10 mm d-glucose, pH 7.4). Coverslips were then transferred to a microscope chamber where they were imaged using an inverted Nikon TE2000-E microscope (Nikon Instruments, Melville, NY) equipped with a ×40 (numerical aperture-1.3) oil-immersion Pan Fluor lens and a cooled digital photometrics Cool Snap-HQ charge-coupled device camera (Roper Scientific, Trenton, NJ).
Data Analysis
All data were quantified and analyzed using GraphPad Prism 5.0. Specifically, saturation curves were fit using the nonlinear regression analysis of one site binding. Displacement curves were fitted using the nonlinear regression analysis of competitive binding.
RESULTS
Pharmacological and Functional Characterization of Modified Human Dopamine D2L and D3 Receptors
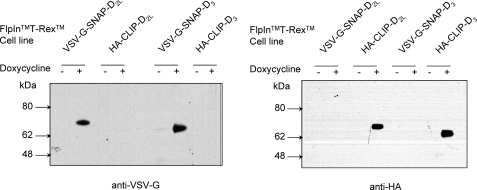
Both the human dopamine D2long isoform (D2L) and dopamine D3 receptors were modified at the N terminus to incorporate either a combination of the VSV-G epitope tag and the “SNAP” variant of O6-alkylguanine-DNA-alkyltransferase or the HA epitope tag and the “CLIP” variant of this enzyme (25, 28) (Fig. 1). Each of these constructs was then cloned into the Flp-InTM locus of Flp-InTM T-RExTM 293 cells and populations of cells harboring each of the constructs were isolated. This system allows control of expression of DNA at this locus upon addition of either tetracycline or doxycycline. Preliminary studies indicated that expression of the receptor constructs was indeed dependent on the presence of doxycycline, with maximal expression obtained over a 24-h period in the presence of 10 ng ml−1 of the inducer (not shown). Membranes from untreated cells and from those induced to express the relevant construct by addition of doxycycline (10 ng ml−1, 24 h) were resolved by SDS-PAGE and immunoblotted with either anti-VSV-G or anti-HA (Fig. 2). As anticipated, anti-VSV-G immunoreactivity was detected only in membranes of cells induced to express either VSV-G-SNAP-D2L or VSV-G-SNAP-D3, whereas anti-HA immunoreactivity was detected only in cells induced to express HA-CLIP-D2L or HA-CLIP-D3 (Fig. 2). The migration of either D2L or D3 receptors in such gels was similar whether they contained the VSV-G-SNAP or HA-CLIP tag combination, and the apparent molecular mass of the tagged receptors was consistent with the values expected in the presence of tags. In this regard, both forms of tagged D2L receptor exhibited a slightly higher apparent molecular mass than the corresponding D3 receptors (∼70 versus ∼65 kDa, respectively) (Fig. 2). For both receptor subtypes essentially all of the immunoreactive material detected in such reducing gels corresponded to monomers of D2L and D3 receptors (Fig. 2).
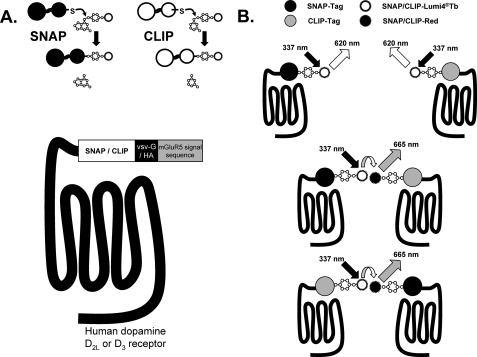
FIGURE 1.
Organization and applications of SNAP- and CLIP-tagged forms of the D2L and D3 dopamine receptors. A, SNAP and CLIP tags allow the covalent incorporation of fluorophores and other reagents that are linked to either benzylguanine (SNAP) or benzylcytosine (CLIP) (upper panel). These tags were linked in-frame with both a leader sequence derived from the metabotropic glutamate receptor 5 and either the VSV-G or HA peptide epitope tag sequence at the N terminus of either the human D2L or D3 dopamine receptors (lower panel). B, addition and covalent attachment of SNAP-Lumi4®Tb or CLIP-Lumi4®Tb allows detection and quantification of cell surface receptors by measuring fluorescence emission at 620 nm following excitation at 337 nm (upper panel). As SNAP-Lumi4®Tb or CLIP-Lumi4®Tb can also act as energy donors in htrFRET studies, co-addition of an appropriate energy acceptor, e.g. SNAP/CLIP Red can allow the detection of protein homo- or hetero-interactions as fluorescence emission at 665 nm following excitation at 337 nm (lower panel).
FIGURE 2.
Regulated control and selective detection of VSV-G-SNAP and HA-CLIP tagged forms of the D2L and the D3 receptor. Populations of Flp-InTM T-RExTM 293 cells with VSV-G-SNAP-D2L, VSV-G-SNAP-D3, HA-CLIP-D2L, or VSV-G-SNAP-D3 located at the Flp-InTM locus were untreated (−) or treated (+) with doxycycline (10 ng ml−1, 24 h). Membranes were subsequently prepared, resolved by SDS-PAGE, and immunoblotted with anti-VSV-G (left) or anti-HA (right).
Saturation binding experiments in membrane preparations from each cell line showed that the radiolabeled antagonist [3H]spiperone bound each of these constructs with subnanomolar affinity (Table 1). Subsequent competition binding studies performed on membranes of cells expressing each of the D2L receptor constructs indicated that all ligands evaluated displaced [3H]spiperone with estimated affinities similar to those previously obtained in membranes expressing non-tagged D2 receptors. A distinct rank order of affinity was observed for a number of the ligands studied, with dopamine, quinelorane, S33084, and pramipexole being selective for the D3 receptor, whereas butaclamol was selective for the D2L receptor (Fig. 3). Each of the constructs was also functional and able to induce G protein activation upon addition of dopamine as assessed in [35S]GTPγS binding assays. With maximal expression of each construct dopamine increased binding of [35S]GTPγS in a concentration-dependent fashion and no substantial differences in potency were observed between VSV-G-SNAP- and HA-CLIP-tagged forms of the same receptor (Table 2). As anticipated from the binding assays, dopamine was more potent at the D3 receptor (Table 2). Interestingly, the potency of dopamine in such assays was lower when expression of the constructs was constrained by induction with lower concentrations of doxycycline (data not shown), suggesting that in such [35S]GTPγS binding assays a receptor reserve (32) can be produced with high level expression of the constructs.
TABLE 1.
Expression and [3H]spiperone binding characteristics of dopamine D2L and D3 receptor constructs
Expression of each of the constructs noted was induced in Flp-InTM T-RExTM 293 cells harboring these by treatment with doxycycline (10 ng ml−1 for 24 h). Membranes prepared from these cells were then used in [3H]spiperone saturation binding studies. Data are mean ± S.E. from n = 4 experiments performed in duplicate.
|
Bmax (pmol mg membrane protein−1) |
Kd |
|||
|---|---|---|---|---|
| Mean | ±S.E. | Mean | ±S.E. | |
| nm | ||||
| VSV-SNAP-D2L | 20.4 | 7.1 | 0.31 | 0.07 |
| HA-CLIP-D2L | 26.7 | 5.2 | 0.4 | 0.06 |
| VSV-SNAP-D3 | 9.9 | 3.8 | 0.35 | 0.08 |
| HA-CLIP-D3 | 8.8 | 2.5 | 0.33 | 0.04 |
FIGURE 3.
Pharmacological characterization of VSV-G-SNAP-tagged forms of D2L and D3 receptors. Membranes from Flp-InTM T-RExTM 293 cells induced to express either VSV-G-SNAP-D2L (A) or VSV-G-SNAP-D3 (B) were employed in competition binding studies with [3H]spiperone and a range of dopaminergic ligands. Data are presented as % specific binding of [3H]spiperone and are mean ± S.E. from n = 2–3 experiments performed in duplicate.
TABLE 2.
The N-terminal SNAP- and CLIP-tagged forms of the D2L and D3 receptors are functional
Membranes prepared from Flp-InTM T-RExTM 293 cells harboring each of the constructs following treatment with doxycycline (10 ng ml−1 for 24 h) were used in [35S]GTPγS binding studies with a range of concentrations of dopamine. Data are mean ± S.E. from n = 3 experiments performed in duplicate. Statistical analysis was performed by one-way analysis of variance with Tukey's multiple comparison post test. VSV-G-SNAP-D3 significantly different from VSV-G-SNAP-D2L.
| Construct | Dopamine(pEC50) |
|
|---|---|---|
| Mean | ±S.E. | |
| VSV-SNAP-D2L | 8.25 | 0.13 |
| HA-CLIP-D2L | 8.14 | 0.25 |
| VSV-SNAP-D3 | 9.26a | 0.30 |
| HA-CLIP-D3 | 8.60 | 0.33 |
a p < 0.01.
Homomers of Modified D2L and D3 Receptors Are Present at the Cell Surface
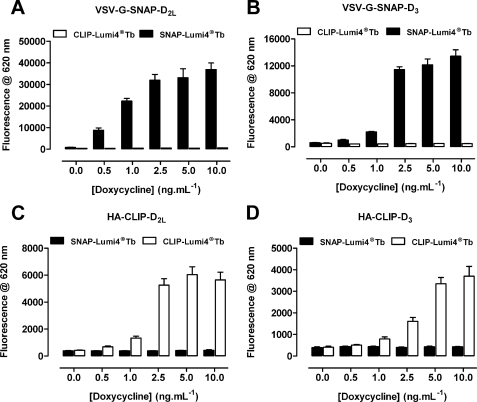
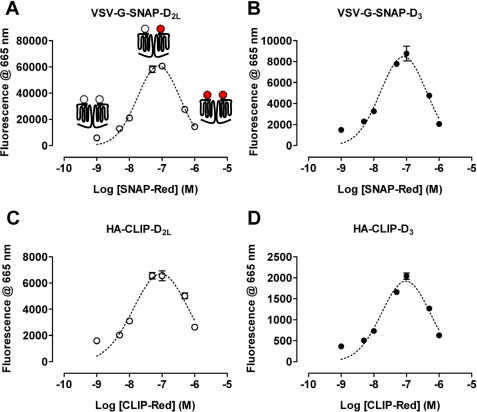
To assess the suitability of these tagged constructs to report on cell surface delivery and potential protein-protein interactions, cells were untreated or induced to express each variant by addition of differing concentrations of doxycycline for 24 h. Subsequently the binding of either SNAP-Lumi4®Tb (10 nm) or CLIP-Lumi4®Tb (20 nm) was assessed in intact cells by monitoring fluorescence emission at 620 nm following excitation at 337 nm (Fig. 4). The specificity of these reagents was shown because in cells induced to express the SNAP-tagged receptors, CLIP-Lumi4®Tb was unable to label the cells (Fig. 4, A and B), whereas SNAP-Lumi4®Tb binding was minimal in the absence of doxycycline but increased greatly following addition of doxycycline, with maximal levels achieved for both receptor constructs with 2.5–10 ng ml−1 of the antibiotic and with more modest levels produced by treatment with between 0.25 and 1.0 ng ml−1 (Fig. 4, A and B). In cells able to express the CLIP-tagged receptors, SNAP-Lumi4®Tb was unable to bind (Fig. 4, C and D), but now, CLIP-Lumi4®Tb labeled these cells following treatment with doxycycline and, again, the extent of labeling was dependent on the concentration of doxycycline used as inducer (Fig. 4, C and D). Whether labeling with SNAP-Lumi4®Tb or CLIP-Lumi4®Tb a greater maximal signal could be achieved for the D2L receptor constructs compared with the equivalent forms of the D3 receptor at equivalent doxycycline concentrations, whereas CLIP-Lumi4®Tb produced less signal output than SNAP-Lumi4®Tb (Fig. 4). Cells induced with 10 ng ml−1 of doxycycline were then treated with a fixed concentration of either SNAP-Lumi4®Tb (10 nm) or CLIP-Lumi4®Tb (20 nm) as potential energy donors, along with increasing concentrations of the corresponding htrFRET energy acceptor partner SNAP-Red or CLIP-Red to detect potential homomeric interactions between receptors. Following excitation at 337 nm, the binding of SNAP/CLIP-Lumi4®Tb and energy transfer to SNAP/CLIP-Red was assessed through measurement of fluorescence emission at 665 nm (Fig. 5). In each case, fluorescence output at 665 nm initially increased as SNAP/CLIP-Red concentrations were increased, reached a maximum in the presence of ∼100 nm SNAP/CLIP-Red, and subsequently declined as concentrations of SNAP/CLIP-Red were further increased. These data are consistent with the SNAP/CLIP-Red energy acceptor being able to compete with the corresponding SNAP/CLIP-Lumi4®Tb energy donor to bind to the appropriately tagged receptor and that individual copies of each variant of the D2L or D3 receptors are close enough to enable the resonant transfer of energy unveiling the presence of homodimers or homo-oligomers at the cell surface (Fig. 5).
FIGURE 4.
Regulated expression of VSV-G-SNAP- and HA-CLIP-tagged forms of D2L and D3 receptor is detected by the binding of SNAP/CLIP-Lumi4®Tb. Cells as described in the legend to Fig. 2 were untreated or induced with varying concentration of doxycycline. SNAP-Lumi4®Tb (10 nm) or CLIP-Lumi4®Tb (20 nm) was added and fluorescence emission at 620 nm was measured. Data are mean ± S.E. from n = 3 experiments performed in duplicate.
FIGURE 5.
Detection of cell surface D2L and D3 receptor homomers. Cells as described in the legend to Fig. 4, induced with 10 ng ml−1 of doxycycline were labeled with either SNAP-Lumi4®Tb (10 nm) (A and B) or CLIP-Lumi4®Tb (20 nm) (C and D) as potential energy donor along with varying concentrations of the partner energy acceptor, SNAP-Red or CLIP-Red. Following excitation at 337 nm, fluorescence emission at 665 nm assessed the binding of SNAP/CLIP-Lumi4®Tb and energy transfer to receptors labeled with SNAP/CLIP-Red. Panel A illustrates that, at low energy acceptor levels, most SNAP-D2L molecules will bind SNAP-Lumi4®Tb (open schematic) and a low energy transfer signal will be generated, at moderate levels of SNAP-Red the prospect of one protomer of a receptor dimer binding a molecule of SNAP-Lumi4®Tb and the other a molecule of SNAP-Red (filled schematic) will be greatest and the potential energy transfer should be maximal, whereas addition of further SNAP-Red will outcompete SNAP-Lumi4®Tb and limit energy transfer signals. In each case ∼100 nm of the SNAP/CLIP-Red energy acceptor resulted in the highest energy transfer signal (A–D).
Heteromers of D2L and D3 Receptors Are Present at the Cell Surface of Cells Co-expressing These Receptors at Modest Levels
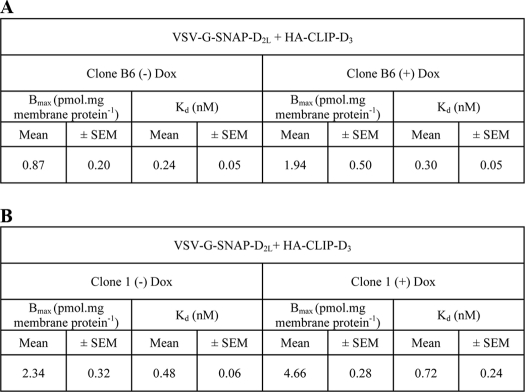
To explore potential formation of heteromers between D2L and D3 receptors, each of the cell populations detailed above were then further transfected but with the alternate constructs. For example, HA-CLIP-D3 was introduced constitutively into cells able to express VSV-G-SNAP-D2L upon addition of doxycyline, whereas HA-CLIP-D2L was introduced into cells able to express VSV-G-SNAP-D3 following addition of the antibiotic. Individual clones were subsequently isolated. A substantial number of clones were selected in which HA-CLIP-D2L was present constitutively. However, preliminary screens, performed on cell membrane preparations and measuring specific binding of [3H]spiperone, indicated high levels of D2L receptor expression in many of these clones. Furthermore, subsequent addition of doxycyline to induce VSV-G-SNAP-D3 resulted in the appearance of limited numbers of additional specific [3H]spiperone binding sites that should reflect the D3 receptor (not shown). These clones were considered inappropriate for studies on receptor heteromerization both because of the high total receptor population and the poor ratio of D3 to D2L expression that could be achieved and regulated. By contrast, following transfection of HA-CLIP-D3 into cells able to express VSV-G-SNAP-D2L in an inducible manner a number of clones were identified with modest constitutive [3H]spiperone binding levels in the absence of doxycycline treatment and, therefore, corresponding to the D3 receptor. Furthermore, although addition of maximally effective concentrations of doxycycline resulted in the production of substantial levels of the VSV-G-SNAP-D2L receptor, the inducible nature of this receptor construct meant that addition of low concentrations of doxycycline resulted in only a modest increase in specific [3H]spiperone binding sites that should potentially correspond to the D2L construct. One of these clones (B6) was initially selected for detailed analysis. In membranes from these cells, generated without doxycycline treatment, the specific binding of [3H]spiperone was 0.87 ± 0.20 pmol mg−1 of protein, whereas after treatment with 1 ng ml−1 of doxycycline, it increased to 1.94 ± 0.5 pmol mg−1 of protein (mean ± S.E., n = 3) (Table 3, part A). Similar observations were recorded for a further clone with somewhat higher constitutive expression of the D3 receptor (Table 3, part B). Clone B6 cells were therefore selected for detailed analysis because there are concerns that artifacts may be generated in protein-protein interaction studies if expression levels are not restricted to modest levels. Subsequently, intact cell studies measuring the specific binding of [3H]spiperone were performed in clone B6 cells to define cell surface receptor levels. In the absence of doxycycline, the specific binding was 1.29 ± 0.12 × 10−19 mol cell−1 (mean ± S.E.) corresponding to 77,697 ± 7,228 receptors per cell and then increased to 2.57 ± 0.25 × 10−19 mol cell−1 (mean ± S.E.), corresponding to 154,791 ± 15,058 receptors per cell, following treatment with 1 ng ml−1 of doxycycline for 24 h (Fig. 6). The concentration of doxycycline used in further experiments was then constrained to 1 ng ml−1 to achieve a ratio of D2L/D3 receptor expression levels close to 1:1.
TABLE 3.
[3H]Spiperone binding sites in clonal cell lines constitutively expressing the D3 receptor and expressing the D2L receptor following treatment with doxycycline
Clone B6 and clone 1 cells express HA-CLIP D3 constitutively and harbor VSV-G-SNAP-D2L at the inducible locus. Saturation [3H]spiperone binding studies were performed on membranes of these cells that had been treated (+) or not (−) with doxycycline (1 ng mL−1) for 24 h. Binding sites detected in the absence of doxycycline potentially represent HA-CLIP D3; although the additional sites identified following doxycycline treatment should reflect VSV-G-SNAP-D2L. Confirmation of this for clone B6 is provided in Fig. 7 and 8. Data are mean ± S.E. from n = 3 experiments performed in duplicate.
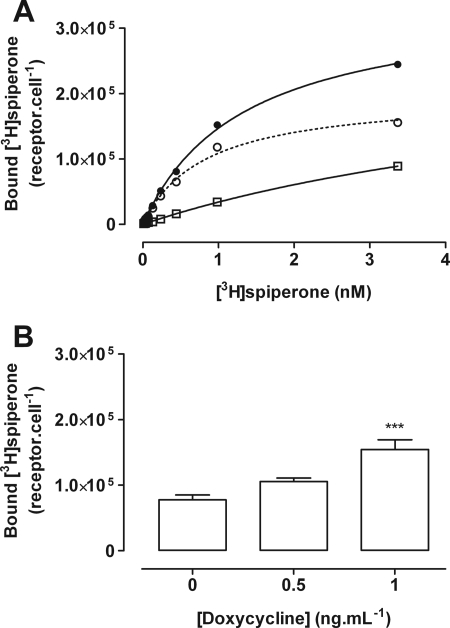
FIGURE 6.
Cell surface binding of [3H]spiperone in cells of clone B6. A, total (filled circles) and nonspecific (open squares) binding of varying concentrations of [3H]spiperone was assessed on whole cells of clone B6 that were untreated and thus expressed only HA-CLIP-D3 to allow measurement of specific (open circles) binding. B, specific binding of [3H]spiperone was assessed in the absence of doxycycline and following addition of either 0.5 or 1.0 ng ml−1 of doxycycline for 24 h to assess the total amount of HA-CLIP-D3 + VSV-G-SNAP-D2L. Data are mean ± S.E. from n = 3 experiments performed in duplicate. ***, significantly greater than absence of doxycycline p < 0.001.
Doxycycline Treatment Regulates Expression Levels Only of a Construct Harbored at the Inducible Locus
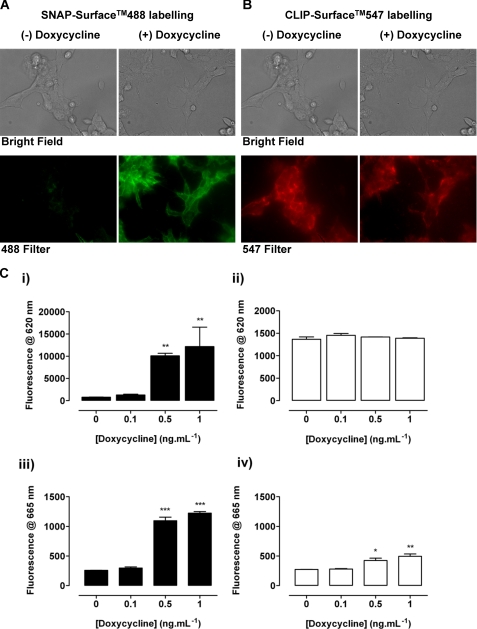
The [3H]spiperone binding studies in clone B6 cells could not directly define that the extra binding sites detected after addition of doxycycline reflect only the D2L receptor without some indirect effect on the D3 receptor population. As such, two further and distinct approaches were chosen to quantify the density of HA-CLIP-D3 receptors in clone B6 cells, to characterize any regulation of the cell surface density of D3 sites upon induction of D2L receptor expression, and to confirm the appearance of VSV-G-SNAP-D2L receptors following treatment with doxycycline. First, the cell impermeant SNAP- and CLIP-fluorophore substrates SNAP-Surface 488 and CLIP-Surface 547 were co-added to cells treated or not with doxycycline. This resulted in cell fluorescence representing the covalent attachment of CLIP-Surface 547 to D3 receptors at the cell surface in both the presence and absence of doxycycline treatment and this was similar in each case (Fig. 7, A and B). By contrast, fluorescence corresponding to the covalent attachment of SNAP-Surface 488 to D2L receptors was only observed following treatment with the antibiotic (Fig. 7A). Second, cells were labeled with SNAP-Lumi4®Tb and fluorescence emitted at 620 nm after excitation at 337 nm was recorded. Quantitatively, fluorescence emitted at 620 nm by the D2L receptor construct following covalent labeling with SNAP-Lumi4®Tb was near background levels both in the absence and presence of 0.1 ng ml−1 of doxycycline. However, when the same cells were treated with 0.5 or 1 ng ml−1 of this antibiotic for 24 h, the signal increased substantially (Fig. 7C, i). In contrast, the fluorescence emitted at 620 nm after addition of CLIP-Lumi4®Tb, which covalently labels HA-CLIP-D3, was substantial without doxycycline treatment and unchanged following treatment (Fig. 7C, ii). To detect the presence of potential cell surface expression of D2L-D3 heteromers, a combination of SNAP-Lumi4®Tb (10 nm) and CLIP-Red (100 nm) was added to untreated and doxycycline-treated B6 cells (Fig. 7C, iii). Only background fluorescence emission at 665 nm was observed without doxycycline or following treatment with a concentration of antibiotic (0.1 ng ml−1 as reported in Fig. 7C, i) that did not result in significant expression of the D2L construct (Fig. 7C, iii). However, when cells were treated with either 0.5 or 1 ng ml−1 of doxycycline, a marked increase in fluorescence emission at 665 nm was observed, representing resonance energy transfer from SNAP-Lumi4®Tb to CLIP-Red and subsequent emission, which is consistent with the presence of dopamine D2L–D3 heteromers (Fig. 7C, iii). Equivalent results were obtained when the reverse combination of energy donor and energy acceptor, i.e. CLIP-Lumi4®Tb (20 nm) and SNAP-Red (100 nm), was added to untreated and doxycycline-treated B6 cells (Fig. 7C, iv) except that the signal to background emission at 665 nm was rather lower than when using the SNAP-Lumi4®Tb/CLIP-Red combination and, therefore, the D2L receptor as the energy donor.
FIGURE 7.
The D3 receptor is expressed constitutively but the D2L receptor only upon addition of doxycycline in clone B6 cells and when co-expressed they form heteromers. Clone B6 cells grown on glass coverslips were untreated ((−) Doxycycline) or treated ((+) Doxycycline) with doxycycline (1 ng ml−1, 24 h). A combination of CLIP-Surface 547 and SNAP-Surface 488 was added for 45 min and images were taken to identify covalent labeling of HA-CLIP-D3 (547 filter, red) and VSV-G-SNAP-D2L (488 filter, green) (A and B). Brightfield images demonstrate that all cells express the appropriate construct. Representative examples of images are displayed. C, the binding and fluorescence emission at 620 nm of either SNAP-Lumi4®Tb (10 nm) (i) or CLIP-Lumi4®Tb (20 nm) (ii) was assessed in B6 cells grown in various concentrations of doxycycline (0–1.0 ng ml−1 for 24 h). Following co-addition of SNAP-Lumi4®Tb (10 nm) and CLIP-Red (100 nm) (iii) or CLIP-Lumi4®Tb (20 nm) and SNAP-Red (100 nm) (iv) fluorescence at 665 nm was measured to detect D2L–D3 receptor heteromers. Data are mean ± range from a single experiment representative of n = 3 performed in duplicate. Significantly greater than no doxycycline are indicated, p < 0.05 (*), p < 0.01 (**), or p < 0.001 (***).
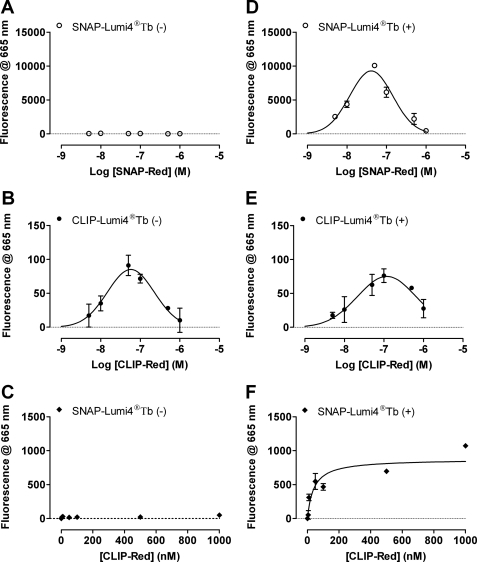
Homomers and Heteromers of D2L and D3 Receptors Co-exist
To examine whether D2L–D3 receptor heteromers in these cell lines co-existed with the corresponding homomers or replaced them upon induction of expression of the second receptor, B6 cells were labeled with SNAP-Lumi4®Tb (10 nm) and various concentrations of SNAP-Red. As anticipated from the studies outlined above, in the absence of doxycycline at all concentrations of SNAP-Red, minimal emission of fluorescence was measured at 665 nm reflecting the absence of VSV-G-SNAP-D2L receptors (Fig. 8A). By contrast, labeling with CLIP-Lumi4®Tb (20 nm) and increasing concentrations of CLIP-Red, resulted in a bell-shaped increase of fluorescence at 665 nm, characterized by a weak signal at low concentrations of CLIP-Red, which reached a maximum at ∼100 nm and then decreased at higher concentrations of CLIP-Red (Fig. 8B). As in Fig. 5, these data are consistent with the presence of homodimers/oligomers of HA-CLIP-D3 receptors at the cell surface in the absence of VSV-G-SNAP-D2L (Fig. 8B). Furthermore, addition of SNAP-Lumi4®Tb (10 nm) and varying concentrations of CLIP-Red also failed to generate fluorescence at 665 nm. This lack of signal is consistent with the absence of detectable D3 homodimers due to the inability of SNAP-Lumi4®Tb to bind HA-CLIP-D3 receptors and also with the absence of D2L–D3 receptor heteromers because no D2L receptor was expressed without doxycycline (Fig. 8C). After induction of D2L receptor expression with 1 ng ml−1 of doxycycline, co-application of SNAP-Lumi4®Tb (10 nm) with increasing concentrations of SNAP-Red now resulted in a bell-shaped elevation of fluorescence at 665 nm reflecting identification of D2L–D2L receptor interactions (Fig. 8D). Similarly, when CLIP-Lumi4®Tb (10 nm) was co-applied with increasing concentrations of CLIP-Red, the resulting bell-shaped increase of energy transfer observed was consistent with the continuing presence of D3 receptor homomers (Fig. 8E). Finally, the combination of SNAP-Lumi4®Tb (10 nm) with increasing concentrations of CLIP-Red also resulted in emission of fluorescence at 665 nm. Unlike the SNAP/CLIP-Lumi4®Tb + SNAP/CLIP-Red co-additions, after co-addition of SNAP-Lumi4®Tb and increasing concentrations of CLIP-Red, fluorescence at 665 nm initially increased, reached a maximal level, and was then maintained. This is consistent with the presence of D2L–D3 receptor heteromers as CLIP-Red binds to the D3 receptor but does not compete with SNAP-Lumi4®Tb to bind the D2L receptor and, therefore, energy transfer is anticipated to saturate when all molecules of the D3 receptor have bound CLIP-Red (Fig. 8F).
FIGURE 8.
D2L–D3 heteromers co-exist with D2L receptor homomers and D3 receptor homomers at the surface of clone B6 cells. Clone B6 cells were untreated (A–C) or treated (D–F) with doxycycline (1.0 ng ml−1, 24 h). Combinations of SNAP-Lumi4®Tb (10 nm) and various concentrations of SNAP-Red to identify D2L-D2L homomers (A and D), CLIP-Lumi4®Tb (20 nm), and increasing concentrations of CLIP-Red to identify D3–D3 homomers (B and E), or a combination of SNAP-Lumi4®Tb (10 nm) and a range of concentrations of CLIP-Red to identify D2L–D3 heteromers (C and F) were added and htrFRET was assessed as fluorescence emission at 665 nm. Data are mean ± S.E. from a single experiment representative of n = 3 performed in triplicate. In C and F the concentration of energy acceptor is displayed on a linear scale to demonstrate clear saturation of the energy transfer signal.
Detection of Homomers and Heteromers of D2L and D3 Receptors Is Unaffected by Presence of Dopaminergic Agonists
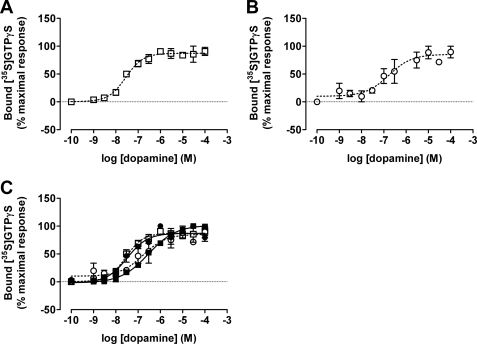
In cells induced to express each of VSV-G-SNAP-D2L, VSV-G-SNAP-D3, HA-CLIP-D2L, or HA-CLIP-D3 individually by treatment with 1 ng ml−1 of doxycycline, no significant effect was observed on the presence of the corresponding homomers following treatment between 5 and 30 min with dopamine, (+)-butaclamol, or pergolide (10 μm) (supplemental Fig. S1). Similarly, no significant effect on the presence of dopamine D2L–D3 heteromers or the corresponding homomers was detected in clone B6 cells treated with 1 ng ml−1 of doxycycline in response to treatment for up to 30 min with either dopamine or pramipexole (supplemental Fig. S2).
Functional Complementation as an Alternative Approach to Identify Homo- and Heterodimerization of D2L and D3 Receptors and to Assess Functionality
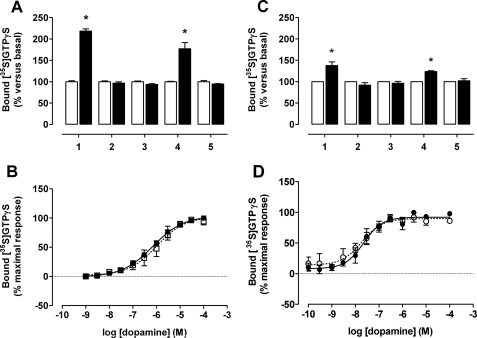
Although the studies above clearly defined interactions between D2L and D3 receptors they did not address the functionality of such complexes. To do so we employed the potential for functional complementation following co-expression of pairs of nonequivalent and nonfunctional fusions between dopamine receptors and a G protein they are usually able to activate. Although the D2L receptor interacts effectively with each member of the pertussis toxin-sensitive Gαi family of G proteins (29), the D3 receptor appears to be more selective, showing greatest activation of Gαo (30, 33). We therefore generated fusion proteins containing either the D2L or D3 receptors linked to a pertussis toxin-insensitive, C351I, a variant of Gαo that both receptors still activate (29, 30). To produce the first set of inactive fusions, modifications were made at the G protein level where a further G204A mutation was introduced into C351I-Gαo. This prevents effective GDP-GTP exchange in response to receptor occupancy and activation by agonists. G204A,C351I-Gαo was then linked to either the wild type D2L or D3 receptors (29, 30). For the second set, mutations were introduced into the receptors such that a pair of hydrophobic residues located at equivalent positions in the 2nd intracellular loop of each of the D2L and D3 receptors was converted to acidic residues. As shown previously this provides a generic means in the rhodopsin family G protein-coupled receptors to eliminate G protein activation in response to agonists without alteration of the ligand binding pocket (33). These changes produced V136E,M140D-D2L and V132E,V136D-D3 receptors and each of these was then linked to C351I-Gαo.
Following transient transfection of these constructs in HEK293 cells and treatment of the cells with 25 ng ml−1 of pertussis toxin for 24 h to prevent any possible interactions with endogenously expressed Gi family G proteins, membranes were prepared. Specific [3H]spiperone binding was measured to define expression of each construct and affinity for the ligand. [35S]GTPγS binding studies were then performed using membrane amounts containing the same number of [3H]spiperone binding sites. Addition of dopamine (10 μm) to membranes expressing D2L-C351I-Gαo resulted in a robust increase in binding of [35S]GTPγS, consistent with activation of the G protein within the fusion (Fig. 9A). By contrast, no such increase was observed in membranes expressing equal numbers of D2L-G204A,C351I-Gαo or V136E,M140D D2L-C351I-Gαo [3H]spiperone binding sites (Fig. 9A). Although mixing together membrane preparations expressing each of D2L-G204A,C351I-Gαo and V136E,M140D D2L-C351I-Gαo equally did not allow dopamine to stimulate binding of [35S]GTPγS (Fig. 9A), following co-expression of these two individually nonfunctional constructs, at this point dopamine did produce a robust stimulation (Fig. 9A). This is consistent with the presence of functional D2L homodimers. Importantly, the potency of dopamine to enhance binding of [35S]GTPγS was the same in membranes expressing either D2L-C351I-Gαo alone or the combination of D2L-G204A,C351I-Gαo with V136E,M140D D2L-C351I-Gαo (Fig. 9B, Table 4). Equivalent results were obtained with the corresponding D3 receptor constructs, except that the extent of stimulation of [35S]GTPγS binding produced by dopamine was substantially lower than that produced by the same number of D2L receptor binding sites (Fig. 9C). Nonetheless, the higher potency of dopamine at the D3 receptor compared with the D2L receptor, observed with the SNAP-tagged constructs, and previously shown for unmodified forms of these receptors, was preserved in the fusion proteins and upon reconstitution of the functional homodimers (Fig. 9D, Table 4).
FIGURE 9.
Functional complementation identifies both dopamine D2L and D3 homomers. HEK293 cells that were transfected with each of the human D2L or D3 receptor-G protein constructs or the combination of nonequivalent and nonfunctional receptor-G-protein fusion constructs were treated with pertussis toxin prior to membrane preparation. Following [3H]spiperone binding studies to assess expression levels, membranes containing 10 fmol of binding sites were used in [35S]GTPγS binding studies performed in the absence (open bars) or presence (filled bars) of dopamine (10 μm). A shows human D2L receptor-G protein fusion constructs, D2L-C351I-Gαo (1), V136E,M140D D2L-C351I-Gαo (2), D2L-G204A,C351I-Gαo (3), or the combination of D2L-G204A,C351I-Gαo with V136E,M140D D2L-C351I-Gαo (4), whereas 5 reflects membranes expressing either D2L-G204A,C351I-Gαo or V136E,M140D D2L-C351I-Gαo individually that were combined prior to the assay. B, evaluation of the function of D2L homomers in [35S]GTPγS binding assays performed in the presence of varying concentrations of dopamine on membranes expressing either the combination of D2L-G204A,C351I-Gαo and V136E,M140D D2L-C351I-Gαo (open squares) or D2L-C351I-Gαo (filled squares). C and D represent equivalent studies but using the equivalent D3 receptor constructs where in C, D3-C351I-Gαo (1), V132E,V136D D3-C351I-Gαo (2), D3-G204A, C351I-Gαo (3), or the combination of D3-G204A,C351I-Gαo with V132E,V136D D3-C351I-Gαo (4), and 5 reflects membranes expressing either D3-G204A,C351I-Gαo, or V132E,V136D D3-C351I-Gαo individually that were combined prior to the assay. D, assessment of the functionality of D3 homomers as measured in [35S]GTPγS binding experiments on membranes expressing either the combination of D3-G204A,C351I-Gαo, and V132E,V136D D3-C351I-Gαo (open circles) or D3-C351I-Gαo (filled circles). Data are mean ± S.E. from n = 4 experiments performed in duplicate. Significantly greater than basal, p < 0.05 (*).
TABLE 4.
[35S]GTPγS binding in response to dopamine of dopamine receptor-G protein fusions expressed transiently in HEK293 cells
Dopamine receptor-G protein fusion proteins were expressed individually or in combination in HEK293 cells and [35S]GTPγS binding studies performed in membranes derived from pertussis toxin-treated cells. Statistical analysis was performed by one-way analysis of variance with Dunnett's post test. Data are mean ± S.E. from n = 3–4 experiments performed in duplicate.
| Dopamine (pEC50) |
||
|---|---|---|
| Mean | ±S.E. | |
| D2L-C351I-Gαo | 6.53 | 0.04 |
| V136E,M140D-D2L-C351I-Gαo | NDa | ND |
| D2L-G204A,C351I-Gαo | ND | ND |
| V136E,M140D-D2L-C351I-Gαo + D2L-G204A,C351I-Gαo | 5.99 | 0.14 |
| V136E,M140D-D2L-Gαo + D3-G204A,C351I-Gαo | 7.55b | 0.1 |
| D3-C351I-Gαo | 7.49c | 0.14 |
| V132E,V136D-D3-C351I-Gαo | ND | ND |
| D3-G204A,C351I-Gαo | ND | ND |
| V132E,V136D-D3-C351I-Gαo + D3-G204A,C351I-Gαo | 7.72b | 0.17 |
| V132E,V136D-D3-C351I-Gαo + D2L- G204A,C351I-Gαo | 6.78 | 0.27 |
a ND, activation not detected.
b Significantly different from D2L-C351I-Gαo p < 0.001.
c Significantly different from D2L-C351I-Gαo p < 0.01.
Based on these data, we used the same approach to define the functionality of D2L-D3 heteromers. Following co-expression of V136E,M140D D2L-C351I-Gαo along with D3-G204A,C351I-Gαo, dopamine stimulated the binding of [35S]GTPγS in a concentration-dependent fashion (Fig. 10A). The same was true following the co-expression of V132E,V136D D3-C351I-Gαo with D2L-G204A,C351I-Gαo (Fig. 10B). Of particular interest, the higher potency of dopamine at the D3 versus D2L receptor was recapitulated following co-expression of the fusion containing the wild type D3 receptor linked to the inactive G protein with the inactive D2L receptor linked to wild type G protein (Fig. 10C, Table 4), indicative of a likely trans-activation process (34). Likewise, the lower potency of dopamine at the D2L receptor was reiterated in the heterodimer containing wild type D2L receptors linked to the inactive G protein plus inactive modified D3 receptors linked to an active G protein (Fig. 10C, Table 4).
FIGURE 10.
Functional complementation identifies and demonstrates the function of D2L–D3 heteromers. Studies akin to those of Fig. 9 were performed in response to various concentrations of dopamine following co-expression of V136E,M140D D2L-C351I-Gαo with D3-G204A,C351I-Gαo (A, open squares) or V132E,V136D D3-C351I-Gαo with D3-G204A,C351I-Gαo (B, open circles). In C, the potency of dopamine to activate the reconstituted heteromers is compared directly with the corresponding active single D2L-C351I-Gαo (filled squares) and D3-C351I-Gαo (filled circles) fusion proteins. Data are mean ± S.E. from n = 3–4 experiments performed in duplicate. See Table 4 for quantitative details.
DISCUSSION
Existence of GPCR dimers has been widely reported (35–38). Individual dopamine receptors have been reported to form homomers and heteromers with partners within the dopaminergic family (7, 16, 17, 19, 21, 24) but also with GPCRs which respond to different ligands (7, 9–11, 16, 17, 19, 21, 24, 40). However, these reports have generally been limited to transiently transfected cells where expression of each subtype can be high, is frequently not reported, and where both the proportion of each receptor and effective cell surface delivery is difficult to estimate. Recently, the use of SNAP and CLIP tagging of proteins has proven to be a useful method to explore protein-protein interactions via htrFRET (28, 41). This technology is based on the enzymatic activity of the SNAP and CLIP tags that allows covalent attachment of small molecules or other reagents that are labeled in various ways. In these studies, we have used the SNAP and CLIP Tag-Lite technology in combination with htrFRET to report and study interactions between human dopamine D2L and D3 receptors. The modified receptors were targeted effectively to the cell surface as visualized via fluorescent microscopy. Their function and the rank order affinity of several D3 receptor selective ligands were also similar to values previously reported for unmodified receptors (5, 42, 43). Furthermore, we generated cell lines able to individually express various forms of the SNAP- and CLIP-tagged receptors to relatively high levels to test and ensure the specificity of the labeling reagents. In cells induced to express a single SNAP/CLIP-modified D2L or D3 dopamine receptor, they exist as homomers. In addition, even at relatively low receptor expression, similar to those detected in vivo, D2L and D3 co-expressed in Flp-InTM T-RExTM 293 cells were able to form heteromers and we demonstrated that such heteromers co-exist at steady state with homomers of each individual receptor. Although the likely co-existence of homomers alongside heteromers of co-expressed receptors is frequently discussed (31), we are unaware of other studies that have examined this possibility directly. One of the major advantages of the system described in these studies is that the observations reported were acquired in cell lines stably expressing one receptor and in which the pharmacology of this receptor could be characterized before the second, entirely inducible, receptor was then expressed.
However, these results generate a further series of questions. Although we clearly identified signals corresponding to each of D2L–D2L and D3–D3 homomers, respectively, as well as the corresponding heteromer in cells co-expressing the two receptors, the current studies do not provide an obvious response to the question of the relative proportions of each species. Furthermore, growing evidence suggests that not all receptor-receptor interactions generate stable complexes, rather that certain GPCRs may fluctuate between monomers, dimers, and higher oligomers, potentially dependent upon expression levels (41, 44, 45), rapidly (44, 46) and in response to both ligand challenge (47), and even alterations in physiological conditions (48, 49). Apart from metabotropic glutamate-like class C receptors that are constitutive dimers or oligomers, the proportion of such complexes is challenging to assess. For example, although a number of heteromer-selective antibodies have been described and used to visualize such complexes (48, 49) they are not able to provide information on the proportion of these pairings. This key issue also challenges efforts to define potential unique pharmacology corresponding to heteromers. It is anticipated that allosteric interactions between the distinct protomers will modulate pharmacological characteristics and function (50, 51), and such observations have been noted in many situations in which pairs of GPCRs that can form heteromers have been co-expressed (52, 53). This has also promoted the idea that GPCR heteromers may provide a unique group of potential therapeutic targets (48–51, 54). Indeed, following previous studies involving co-transfection of pairs of dopamine receptors, distinct function and pharmacology has been observed (19, 22–24, 55). However, full definition of heteromer pharmacology may require the development of new systems that, for example, only allow heteromer pairs to be activated or delivered to the surface of cells. Equally, the growing evidence that GPCR complexes may be transitory rather than stable agglomerates suggests that it may be possible to disrupt these selectively. Although we did not observe effects of a number of dopamineric ligands on the presence of homomers and heteromers in these studies it is possible that rapid fluctuation between states would not be measured by the approaches used herein as these are best suited to assess the situation at steady state. It has been reported that heteromers of D2 and D3 receptors respond differently to certain partial agonists and antiparkinson drugs as compared with constituent homomers, and that such agents may promote formation of homomers and heteromers (21, 22). If this is the case, this implicates another level of fine-tuning because, in vivo, the distribution of D2 and D3 receptors overlaps (56). Furthermore, their proportions vary in patients suffering from schizophrenia or Parkinson disease (14), conditions treated by ligands that probably recognize heteromers. There has been considerable interest in the contribution of D3 receptors to the action of antipsychotic drugs because, for example, schizophrenia is associated with an elevation of mesolimbic D3 receptors (14). Despite these examples, the dopaminergic agonists explored in this study do not seem to affect steady state homomer and heteromer levels. However, it is clearly possible that rapid fluctuations between states occur that would not be detected by the approaches used herein.
G protein coupling of D2 receptors has been well characterized with a general consensus for promiscuous coupling to Gαi1, Gαi2, Gαi3, and Gαo1 (29, 33, 57). By contrast, the G protein coupling profile of D3 receptors has proven more challenging to define: they appear to be most efficient in coupling to Gαo but may also recruit other G protein subtypes (58, 59). To examine the functionality of D2L–D3 heteromers, we employed a functional reconstitution strategy based on the co-expression of pairs of molecularly distinct but inactive GPCR-G protein α subunit fusion proteins (26, 60). Initially, as for other such constructs, we demonstrated homomeric interactions using such fusions incorporating an engineered, pertussis toxin-resistant variant of Gαo. Importantly, the potency of dopamine to enhance binding of [35S]GTPγS in membrane preparations co-expressing a pair of such individually inactive fusions was not different from that observed following expression of the equivalent single, active fusion protein. Furthermore, as anticipated from earlier studies, the potency of dopamine to activate the D3-C351I-Gαo fusion protein was greater than that observed for the D2L-C351I-Gαo fusion protein. Potency of dopamine in membranes transfected to allow expression of active D2L–D3 heteromers was, in each case, consistent with the potency of dopamine at the receptor linked to the inactive G protein and, hence, trans-activation of the G protein linked to the inactive protomer of the heterodimer. This concept of trans-activation has previously been supported by such functional reconstitution studies employing GPCRs that respond to distinct ligands (34, 39, 61).
GPCR oligomerization has been reported to influence important receptor functions such as biosynthesis, maturation, targeting, pharmacology, and signaling (36, 39, 54). Given that we have been able to record the concurrent presence of each of dopamine receptor homomers and heteromers in these studies, it is conceivable that variations in the proportion of homomers and heteromers is implicated in the pathogenesis and symptoms of disorders known to reflect disrupted dopaminergic transmission and responsive to agents acting at D2 and D3 receptors, notably schizophrenia, and requires evaluation in future work, not least because D2L–D3 heteromers might provide the opportunity for developing novel classes of ligands with advantages relative to existing agents acting at their respective monomers-homomers. Irrespective of the outcome of such work, the present study provides the first physical evidence both for the existence of D2L–D3 heteromers, and that they can co-exist with their corresponding homomers at the cell surface.
Supplementary Material

This article contains supplemental Figs. S1 and S2.
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- htrFRET
- homogeneous time-resolved FRET
- VSV
- vesicular stomatitis virus.
REFERENCES
- 1. Gardner E. L. (2011) Addiction and brain reward and antireward pathways. Adv. Psychosom. Med. 30, 22–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schetz J. A., Sibley D. R. (2007) in Handbook of Contemporary Neuropharmacology (Sibley D. R., Hanin I., Kuhar M. J., Skolnik P., eds) pp. 221–256, John Wiley & Sons, Inc., New York [Google Scholar]
- 3. Joyce J. N. (2001) D2 but not D3 receptors are elevated after 9 or 11 months chronic haloperidol treatment. Influence of withdrawal period. Synapse 40, 137–144 [DOI] [PubMed] [Google Scholar]
- 4. Joyce J. N., Millan M. J. (2005) Dopamine D3 receptor antagonists as therapeutic agents. Drug Discov. Today 10, 917–925 [DOI] [PubMed] [Google Scholar]
- 5. Millan M. J., Maiofiss L., Cussac D., Audinot V., Boutin J. A., Newman-Tancredi A. (2002) Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J. Pharmacol. Exp. Ther. 303, 791–804 [DOI] [PubMed] [Google Scholar]
- 6. Xu J., Hassanzadeh B., Chu W., Tu Z., Jones L. A., Luedtke R. R., Perlmutter J. S., Mintun M. A., Mach R. H. (2010) [3H]4-(Dimethylamino)-N-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)benzamide. A selective radioligand for dopamine D(3) receptors. II. Quantitative analysis of dopamine D3 and D2 receptor density ratio in the caudate-putamen. Synapse 64, 449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perreault M. L., O'Dowd B. F., George S. R. (2011) Dopamine receptor homooligomers and heterooligomers in schizophrenia. CNS Neurosci. Ther. 17, 52–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Milligan G., McGrath J. C. (2009) GPCR theme editorial. Br. J. Pharmacol. 158, 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Armstrong D., Strange P. G. (2001) Dopamine D2 receptor dimer formation. Evidence from ligand binding. J. Biol. Chem. 276, 22621–22629 [DOI] [PubMed] [Google Scholar]
- 10. Lee S. P., O'Dowd B. F., Rajaram R. D., Nguyen T., George S. R. (2003) D2 dopamine receptor homodimerization is mediated by multiple sites of interaction, including an intermolecular interaction involving transmembrane domain 4. Biochemistry 42, 11023–11031 [DOI] [PubMed] [Google Scholar]
- 11. Guo W., Urizar E., Kralikova M., Mobarec J. C., Shi L., Filizola M., Javitch J. A. (2008) Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J. 27, 2293–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Han Y., Moreira I. S., Urizar E., Weinstein H., Javitch J. A. (2009) Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat. Chem. Biol. 5, 688–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Albizu L., Cottet M., Kralikova M., Stoev S., Seyer R., Brabet I., Roux T., Bazin H., Bourrier E., Lamarque L., Breton C., Rives M. L., Newman A., Javitch J., Trinquet E., Manning M., Pin J. P., Mouillac B., Durroux T. (2010) Time-resolved FRET between GPCR ligands reveals oligomers in native tissues. Nat. Chem. Biol. 6, 587–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang M., Pei L., Fletcher P. J., Kapur S., Seeman P., Liu F. (2010) Schizophrenia, amphetamine-induced sensitized state, and acute amphetamine exposure all show a common alteration. Increased dopamine D2 receptor dimerization. Mol. Brain 3, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Franco R., Seeman P., Barrera C., Aymerich M. S. (2010) Cocaine self-administration markedly increases dopamine D2 receptor negative cooperativity for dopamine binding. A receptor dimer-based analysis. Synapse 64, 566–569 [DOI] [PubMed] [Google Scholar]
- 16. Van Craenenbroeck K., Borroto-Escuela D. O., Romero-Fernandez W., Skieterska K., Rondou P., Lintermans B., Vanhoenacker P., Fuxe K., Ciruela F., Haegeman G. (2011) Dopamine D4 receptor oligomerization. Contribution to receptor biogenesis. FEBS J. 278, 1333–1344 [DOI] [PubMed] [Google Scholar]
- 17. Nimchinsky E. A., Hof P. R., Janssen W. G., Morrison J. H., Schmauss C. (1997) Expression of dopamine D3 receptor dimers and tetramers in brain and transfected cells. J. Biol. Chem. 272, 29229–29237 [DOI] [PubMed] [Google Scholar]
- 18. Maggio R., Aloisi G., Silvano E., Rossi M., Millan M. J. (2009) Heterodimerization of dopamine receptors. New insights into functional and therapeutic significance. Parkinsonism Relat. Disord. 15, S2–S7 [DOI] [PubMed] [Google Scholar]
- 19. Hasbi A., O'Dowd B. F., George S. R. (2011) Dopamine D1–D2 receptor heteromer signaling pathway in the brain. Emerging physiological relevance. Mol. Brain 4, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rashid A. J., O'Dowd B. F., Verma V., George S. R. (2007) Neuronal Gq/11-coupled dopamine receptors. An uncharted role for dopamine. Trends Pharmacol. Sci. 28, 551–555 [DOI] [PubMed] [Google Scholar]
- 21. Maggio R., Scarselli M., Novi F., Millan M. J., Corsini G. U. (2003) Potent activation of dopamine D3/D2 heterodimers by the antiparkinsonian agents, S32504, pramipexole, and ropinirole. J. Neurochem. 87, 631–641 [DOI] [PubMed] [Google Scholar]
- 22. Maggio R., Novi F., Rossi M., Corsini G. U., Millan M. J. (2008) Partial agonist actions at dopamine D2L receptors are modified by co-transfection of D3 receptors. potential role of heterodimer formation. Parkinsonism Relat. Disord. 14, S139-S144 [DOI] [PubMed] [Google Scholar]
- 23. Novi F., Millan M. J., Corsini G. U., Maggio R. (2007) Partial agonist actions of aripiprazole and the candidate antipsychotics S33592, bifeprunox, N-desmethylclozapine, and preclamol at dopamine D2L receptors are modified by co-transfection of D3 receptors. Potential role of heterodimer formation. J. Neurochem. 102, 1410–1424 [DOI] [PubMed] [Google Scholar]
- 24. Maggio R., Millan M. J. (2010) Dopamine D2–D3 receptor heteromers. Pharmacological properties and therapeutic significance. Curr. Opin. Pharmacol. 10, 100–107 [DOI] [PubMed] [Google Scholar]
- 25. Maurel D., Comps-Agrar L., Brock C., Rives M. L., Bourrier E., Ayoub M. A., Bazin H., Tinel N., Durroux T., Prézeau L., Trinquet E., Pin J. P. (2008) Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies. Application to GPCR oligomerization. Nat. Methods 5, 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Milligan G., Parenty G., Stoddart L. A., Lane J. R. (2007) Novel pharmacological applications of G-protein-coupled receptor-G protein fusions. Curr. Opin. Pharmacol. 7, 521–526 [DOI] [PubMed] [Google Scholar]
- 27. Kellendonk C., Simpson E. H., Polan H. J., Malleret G., Vronskaya S., Winiger V., Moore H., Kandel E. R. (2006) Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron 49, 603–615 [DOI] [PubMed] [Google Scholar]
- 28. Ward R. J., Pediani J. D., Milligan G. (2011) Ligand-induced internalization of the orexin OX1 and cannabinoid CB1 receptors assessed via N-terminal SNAP and CLIP-tagging. Br. J. Pharmacol. 162, 1439–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lane J. R., Powney B., Wise A., Rees S., Milligan G. (2007) Protean agonism at the dopamine D2 receptor. (S)-3-(3-Hydroxyphenyl)-N-propylpiperidine is an agonist for activation of Go1 but an antagonist/inverse agonist for Gi1, Gi2, and Gi3. Mol. Pharmacol. 71, 1349–1359 [DOI] [PubMed] [Google Scholar]
- 30. Lane J. R., Powney B., Wise A., Rees S., Milligan G. (2008) G protein coupling and ligand selectivity of the D2L and D3 dopamine receptors. J. Pharmacol. Exp. Ther. 325, 319–330 [DOI] [PubMed] [Google Scholar]
- 31. Ward R. J., Pediani J. D., Milligan G. (2011) Heteromultimerization of cannabinoid CB1 receptor and orexin OX1 receptor generates a unique complex in which both protomers are regulated by orexin A. J. Biol. Chem. 286, 37414–37428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burt A. R., Carr I. C., Mullaney I., Anderson N. G., Milligan G. (1996) Agonist activation of p42 and p44 mitogen-activated protein kinases following expression of the mouse δ-opioid receptor in Rat-1 fibroblasts. Effects of receptor expression levels and comparisons with G-protein activation. Biochem. J. 320, 227–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carrillo J. J., Pediani J., Milligan G. (2003) Dimers of class A G protein-coupled receptors function via agonist-mediated trans-activation of associated G proteins. J. Biol. Chem. 278, 42578–42587 [DOI] [PubMed] [Google Scholar]
- 34. Pfeiffer M., Koch T., Schröder H., Klutzny M., Kirscht S., Kreienkamp H. J., Höllt V., Schulz S. (2001) Homo- and heterodimerization of somatostatin receptor subtypes. Inactivation of sst3 receptor function by heterodimerization with sst2A. J. Biol. Chem. 276, 14027–14036 [DOI] [PubMed] [Google Scholar]
- 35. Milligan G. (2004) G protein-coupled receptor dimerization. Function and ligand pharmacology. Mol. Pharmacol. 66, 1–7 [DOI] [PubMed] [Google Scholar]
- 36. Milligan G., Pediani J., Fidock M., López-Giménez J. F. (2004) Dimerization of α1-adrenoceptors. Biochem. Soc. Trans. 32, 847–850 [DOI] [PubMed] [Google Scholar]
- 37. Pfeiffer M., Koch T., Schröder H., Laugsch M., Höllt V., Schulz S. (2002) Heterodimerization of somatostatin and opioid receptors cross-modulates phosphorylation, internalization, and desensitization. J. Biol. Chem. 277, 19762–19772 [DOI] [PubMed] [Google Scholar]
- 38. Baragli A., Alturaihi H., Watt H. L., Abdallah A., Kumar U. (2007) Heterooligomerization of human dopamine receptor 2 and somatostatin receptor 2. Co-immunoprecipitation and fluorescence resonance energy transfer analysis. Cell. Signal. 19, 2304–2316 [DOI] [PubMed] [Google Scholar]
- 39. Margeta-Mitrovic M., Jan Y. N., Jan L. Y. (2001) Function of GB1 and GB2 subunits in G protein coupling of GABA(B) receptors. Proc. Natl. Acad. Sci. U.S.A. 98, 14649–14654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alvarez-Curto E., Pediani J. D., Milligan G. (2010) Applications of fluorescence and bioluminescence resonance energy transfer to drug discovery at G protein-coupled receptors. Anal. Bioanal. Chem. 398, 167–180 [DOI] [PubMed] [Google Scholar]
- 41. List S. J., Seeman P. (1981) Resolution of dopamine and serotonin receptor components of [3H]spiperone binding to rat brain regions. Proc. Natl. Acad. Sci. U.S.A. 78, 2620–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tadori Y., Forbes R. A., McQuade R. D., Kikuchi T. (2011) Functional potencies of dopamine agonists and antagonists at human dopamine D2 and D3 receptors. Eur. J. Pharmacol. 666, 43–52 [DOI] [PubMed] [Google Scholar]
- 43. Hern J. A., Baig A. H., Mashanov G. I., Birdsall B., Corrie J. E., Lazareno S., Molloy J. E., Birdsall N. J. (2010) Formation and dissociation of M1 muscarinic receptor dimers seen by total internal reflection fluorescence imaging of single molecules. Proc. Natl. Acad. Sci. U.S.A. 107, 2693–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dorsch S., Klotz K. N., Engelhardt S., Lohse M. J., Bünemann M. (2009) Analysis of receptor oligomerization by FRAP microscopy. Nat. Methods 6, 225–230 [DOI] [PubMed] [Google Scholar]
- 45. Kasai R. S., Suzuki K. G., Prossnitz E. R., Koyama-Honda I., Nakada C., Fujiwara T. K., Kusumi A. (2011) Full characterization of GPCR monomer-dimer dynamic equilibrium by single molecule imaging. J. Cell Biol. 192, 463–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. O'Dowd B. F., Ji X., Alijaniaram M., Nguyen T., George S. R. (2011) Separation and reformation of cell surface dopamine receptor oligomers visualized in cells. Eur. J. Pharmacol. 658, 74–83 [DOI] [PubMed] [Google Scholar]
- 47. Gupta A., Mulder J., Gomes I., Rozenfeld R., Bushlin I., Ong E., Lim M., Maillet E., Junek M., Cahill C. M., Harkany T., Devi L. A. (2010) Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci. Signal. 3, ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rozenfeld R., Gupta A., Gagnidze K., Lim M. P., Gomes I., Lee-Ramos D., Nieto N., Devi L. A. (2011) AT1R-CB1 R heteromerization reveals a new mechanism for the pathogenic properties of angiotensin II. EMBO J. 30, 2350–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smith N. J., Milligan G. (2010) Allostery at G protein-coupled receptor homo- and heteromers. Uncharted pharmacological landscapes. Pharmacol. Rev. 62, 701–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maurice P., Kamal M., Jockers R. (2011) Asymmetry of GPCR oligomers supports their functional relevance. Trends Pharmacol. Sci. 32, 514–520 [DOI] [PubMed] [Google Scholar]
- 51. Ellis J., Pediani J. D., Canals M., Milasta S., Milligan G. (2006) Orexin-1 receptor-cannabinoid CB1 receptor heterodimerization results in both ligand-dependent and -independent coordinated alterations of receptor localization and function. J. Biol. Chem. 281, 38812–38824 [DOI] [PubMed] [Google Scholar]
- 52. Liu X. Y., Liu Z. C., Sun Y. G., Ross M., Kim S., Tsai F. F., Li Q. F., Jeffry J., Kim J. Y., Loh H. H., Chen Z. F. (2011) Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell 147, 447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Urizar E., Yano H., Kolster R., Galés C., Lambert N., Javitch J. A. (2011) CODA-RET reveals functional selectivity as a result of GPCR heteromerization. Nat. Chem. Biol. 7, 624–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bouthenet M. L., Souil E., Martres M. P., Sokoloff P., Giros B., Schwartz J. C. (1991) Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry. Comparison with dopamine D2 receptor mRNA. Brain Res. 564, 203–219 [DOI] [PubMed] [Google Scholar]
- 55. Leck K. J., Blaha C. D., Matthaei K. I., Forster G. L., Holgate J., Hendry I. A. (2006) Gz proteins are functionally coupled to dopamine D2-like receptors in vivo. Neuropharmacology 51, 597–605 [DOI] [PubMed] [Google Scholar]
- 56. Nickolls S. A., Strange P. G. (2004) The influence of G protein subtype on agonist action at D2 dopamine receptors. Neuropharmacology 47, 860–872 [DOI] [PubMed] [Google Scholar]
- 57. Newman-Tancredi A., Cussac D., Audinot V., Pasteau V., Gavaudan S., Millan M. J. (1999) G protein activation by human dopamine D3 receptors in high-expressing Chinese hamster ovary cells. A guanosine 5′-O-(3-[35S]thio)-triphosphate binding and antibody study. Mol. Pharmacol. 55, 564–574 [PubMed] [Google Scholar]
- 58. Pascal G., Milligan G. (2005) Functional complementation and the analysis of opioid receptor homodimerization. Mol. Pharmacol. 68, 905–915 [DOI] [PubMed] [Google Scholar]
- 59. Milligan G. (2009) G protein-coupled receptor heterodimerization. Contribution to pharmacology and function. Br. J. Pharmacol. 158, 5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Milligan G. (2007) G protein-coupled receptor dimerisation. Molecular basis and relevance to function. Biochim. Biophys. Acta 1768, 825–835 [DOI] [PubMed] [Google Scholar]
- 61. Duthey B., Caudron S., Perroy J., Bettler B., Fagni L., Pin J. P., Prézeau L. (2002) A single subunit (GB2) is required for G-protein activation by the heterodimeric GABA(B) receptor. J. Biol. Chem. 277, 3236–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.