Background: Hyperoxia activates lung endothelial cell NADPH oxidase and generates oxidants.
Results: nmMLCK modulates hyperoxia-induced interaction between cortactin and p47phox, oxidant production, and vascular leak.
Conclusion: nmMLCK plays an important role in hyperoxia-induced NADPH oxidase activation and lung injury.
Significance: Targeting nmMLCK may provide a novel therapeutic intervention to manage bronchopulmonary dysplasia.
Keywords: Cell Biology, Cell Signaling, Inflammation, Oxygen Radicals, Permeability, Cytoskeleton, Hyperoxia, Lung Injury, NADPH oxidase, Non-muscle MLCK
Abstract
We recently demonstrated that hyperoxia (HO) activates lung endothelial cell NADPH oxidase and generates reactive oxygen species (ROS)/superoxide via Src-dependent tyrosine phosphorylation of p47phox and cortactin. Here, we demonstrate that the non-muscle ∼214-kDa myosin light chain (MLC) kinase (nmMLCK) modulates the interaction between cortactin and p47phox that plays a role in the assembly and activation of endothelial NADPH oxidase. Overexpression of FLAG-tagged wild type MLCK in human pulmonary artery endothelial cells enhanced interaction and co-localization between cortactin and p47phox at the cell periphery and ROS production, whereas abrogation of MLCK using specific siRNA significantly inhibited the above. Furthermore, HO stimulated phosphorylation of MLC and recruitment of phosphorylated and non-phosphorylated cortactin, MLC, Src, and p47phox to caveolin-enriched microdomains (CEM), whereas silencing nmMLCK with siRNA blocked recruitment of these components to CEM and ROS generation. Exposure of nmMLCK−/− null mice to HO (72 h) reduced ROS production, lung inflammation, and pulmonary leak compared with control mice. These results suggest a novel role for nmMLCK in hyperoxia-induced recruitment of cytoskeletal proteins and NADPH oxidase components to CEM, ROS production, and lung injury.
Introduction
The generation and potential role of reactive oxygen species (ROS),2 such as superoxide anion (O2˙̄) and hydrogen peroxide (H2O2), as intracellular second messengers modulating cellular responses is an important emerging theme in vascular physiology and pathophysiology (1). As one of the many potential sources of ROS, vascular NADPH oxidase plays a significant role in basal as well as agonist-mediated ROS/(O2˙̄) generation (2). Activation of phagocytic NADPH oxidase requires the assembly of the cytosolic p47phox, p67phox, p40phox, and Rac2 with membrane-associated cytochrome b558 reductase, which consists of p22phox and gp91phox also known as Nox2 (2, 3). Vascular cells express most of the subcomponents of phagocytic NADPH oxidase subunits, including Rac1. Recent studies have indicated that several novel Nox family isoforms, in addition to Nox2, are highly expressed in vascular endothelial and smooth muscle cells (3–6). We have recently demonstrated that exposure of human pulmonary artery endothelial cells (HPAECs) to hyperoxia (95% O2) increases ROS/O2˙̄ that is dependent on NADPH oxidase activation and independent of the mitochondrial electron transport or xanthine/xanthine oxidase system (5, 7–10).
Actin cytoskeleton and other cytoskeletal proteins play an important role in phagocytic and non-phagocytic assembly and the activation of NADPH oxidase. In phorbol ester-stimulated neutrophils, oxidase activity is known to co-sediment with the heavy plasma membrane fraction that contains actin and fodrin. Furthermore, the labile oxidase can be stabilized by chemical cross-linking that cannot be extracted by Triton X-100, suggesting interaction between the NADPH oxidase complex and actin filaments (11). Actin has recently been shown to enhance activation of NADPH oxidase in vitro, and actin seems to have a putative binding site for p47phox (12). In vascular smooth muscle cells the angiotensin II-mediated activation of NADPH oxidase and ROS generation is regulated in part by p47phox/actin interaction through cortactin (5). It seems that agonist-mediated cortactin and p47phox phosphorylation, translocation to cell periphery, and cortactin/p47phox co-localization to the plasma membrane (5, 9, 13–15) are all critically important events in human lung EC NADPH oxidase activation and ROS production. However, the mechanism(s) involving cytoskeletal proteins in the regulation NADPH-oxidase function is not completely understood.
We recently demonstrated that hyperoxia induces recruitment of cortactin, p47phox, Rac1, and dynamin2 to caveolin-enriched microdomains (CEM), and silencing of dynamin2 with siRNA attenuated hyperoxia-induced ROS/O2˙̄ production and recruitment of p47phox to CEMs (16). CEMs are cholesterol- and sphingolipid-rich membrane rafts that are important for various receptor- and integrin-mediated transmembrane cell signaling (17–19), and cytoskeletal proteins cortactin and actin play an important role in CEM organization, clustering, and function (18, 20, 21). More recently, we observed increased recruitment of phosphorylated myosin light chain (p-MLC) to CEMs by hyperoxia; however, the role of myosin phosphorylation by myosin light chain kinase (MLCK) in endothelial NADPH oxidase activation is unclear. In endothelial cells, non-skeletal muscle (nm) MLCK exists as a high molecular mass (∼210 kDa) protein compared with the low molecular mass (∼130–160 kDa) isoform that is present in smooth muscle cells (22). nmMLCK has been shown to play a critical role in endothelial barrier function and vascular homeostasis (9, 12, 14, 21); however, very little is known on regulation of NADPH oxidase activation and ROS generation by nmMLCK.
This study demonstrates for the first time that hyperoxia-induced NADPH oxidase activation and ROS generation is dependent on nmMLCK. Our results demonstrate that (i) hyperoxia-induced ROS/O2˙̄ production is attenuated by down-regulation or inhibition of nmMLCK in lung ECs, (ii) nmMLCK is essential for the translocation and association of cortactin and p47phox, (iii) down-regulation of nmMLCK expression with siRNA blocks recruitment of Src, cortactin, and p47phox and phosphorylated Src, cortactin, and MLC to CEMs, and (iv) nmMLCK knock-out mice show decreased ROS production, phosphorylation of Src, cortactin, and MLC and pulmonary leak. These results provide strong evidence for the involvement of ∼214-kDa non-muscle MLCK in the assembly and activation of non-phagocytic NADPH oxidase and ROS/(O2˙̄) production in response to hyperoxia in lung endothelium.
EXPERIMENTAL PROCEDURES
Materials
HPAECs, endothelial basal media (EBM-2), and bullet kits were obtained from Lonza (San Diego, CA). Phosphate-buffered saline (PBS) was obtained from Biofluids Inc. (Rockville, MD). Dihydroethidine, DCFDA (6-carboxy-2′, 7′–dichlorodihydrofluorescein diacetate), Alexa Fluor 568 Phalloidin, Alexa Fluor 488, 568, or 594 secondary antibodies, Prolong Gold maintain media, and precast Tris-Glycine PAAG were purchased from Invitrogen. Antibodies for MLCK, MLC, cortactin, and p47, protein A/G plus agarose, and BSA were from Santa Cruz Biotechnology (Santa Cruz, CA); scrambled RNA and siRNA MLCK were from Dharmacon (Lafayette, CO); shMLCK-GFP was from Open Biosystems (Huntsville, AL); adenoviral construct nmMLCK-FLAG (WT) was generated at the services of the University of Iowa Gene Transfer Vector Core (Iowa City, IA); Rac1 antibody was from BD Biosciences; Gene silencer was from Genlantis (San Diego, CA); FuGENE HD transfection reagent was from Roche Applied Science; phosphatase inhibitor mixture, ML-7, and antibodies for actin, phospho-Src (Tyr-418), and FLAG were from Sigma; anti-phospho-cortactin (Tyr-486) antibody was from Chemicon (Boronia, Australia); microscopy Lab-Tek slide chambers were from Electron Microscopy Sciences (Hatfield, PA); glass bottom 35-mm dishes were from MatTek Corp. (Ashland, MA); incubator chamber for hyperoxia exposure was from Billups-Rothenberg (Del Mar, CA); cell lysis buffer and antibodies for p-MLC and caveolin-1 were from Cell Signaling Technology (Danvers, MA). Polyclonal goat anti-p47phox antibody and wild type p47-GFP were kind gifts from Drs. Leto (NIH) and Kleinberg (University of Maryland, Baltimore). Stably expressing wild type cortactin-GFP was generated as described previously (23).
Endothelial Cell Culture
HPAECs (passage 5–7) were grown in EGM-2 complete medium with 10% FBS, 100 units/ml penicillin, and streptomycin in a 37 °C incubator under a 5% CO2, 95% air atmosphere. Once they formed contact-inhibited monolayers with typical cobblestone morphology, the cells were detached from T-75 flasks with 0.05% trypsin and resuspended in fresh complete medium, then cultured in 35-, 60-, or 100-mm dishes on slide chambers or glass bottom 35-mm dishes. Before exposure to normoxia or hyperoxia, cells were starved overnight in EGM-2 medium containing 1% FBS.
Isolation of Mouse Lung Endothelial Cells and Culture
Mouse lung endothelial cells from 4–5-week-old C57BL/6 and nmMLCK−/− null mice were isolated using collagenase A digestion of lungs for 60 min at 37 °C with gentle shaking essentially as described previously (24). Endothelial cells were characterized by cobblestone morphology, staining positive for platelet/endothelial cell adhesion molecule-1 expression and indirect immunofluorescent staining for Von Willebrand factor and Dil-Ac-LDL uptake. Cells were cultured in EGM-2 complete medium containing 10% FBS and used at passages 4–7.
Exposure of Cells to Hyperoxia
HPAECs were placed in a humidity-controlled airtight incubator chamber and flushed continuously with 95% O2, 5% CO2 for 30 min until the oxygen level inside the chamber reached ∼95%. The chamber was then placed in a cell culture incubator at 37 °C for 3 h. The concentration of O2 inside the chamber was monitored with a digital oxygen monitor. The buffering capacity of the cell culture medium did not change significantly during the period of hyperoxia and the pH was maintained at ∼7.4 (7).
Generation of MLCK-FLAG (WT) cDNA
3×FLAG-tagged human non-muscle MLCK1 (GenBankTM U48959) open reading frame driven by a CMV promoter (plasmid pJM1) was made by following exactly the same strategy as described earlier for human MLCK2 (plasmid pJM2) (25). To make an adenoviral expression vector, an XhoI-EcoRI fragment from the plasmid pJM1 was inserted into the matching sites in the vector pacAd5 CMV K-N pA (Gene Transfer Vector Core, University of Iowa), and recombinants were verified by restriction digestion followed by agarose gel electrophoresis as well as sequencing both strands at the cloning junctions.
Transfection and Infection of HPAECs
To knock down endogenous nmMLCK, HPAECs grown to ∼60–70% confluence were transfected with Gene Silencer transfection agent plus scrambled RNA or MLCK siRNA (50 nm) (Fl-luciferase GL2 duplex siRNA target sequence, 5′-CGTACGCGGAATACTTCGA-3) in serum-free EBM-2 medium according to manufacturer's recommendations. At 3 h post-transfection, fresh complete EGM-2 medium was added, and the cells were cultured for an additional 72 h before experiments.
In contrast to above, to overexpress MLCK, HPAECs grown to ∼50% confluence were transfected with 1 μg/ml Vector control or FLAG-tagged MLCK wild type plasmid using FuGENE HD (3 μg/ml) transfection reagent in serum-free EGM-2 medium according to the manufacturer's recommendation. After 3 h the medium was replaced by complete EGM-2, and the cells were incubated for 72 h. Real time PCR was performed according to manufacturer protocol as described earlier (10).
Determination of Hyperoxia-induced ROS Formation
ROS and superoxide production in HPAECs exposed to either normoxia or hyperoxia were determined by loading the cells with 10 μm DCFDA or hydroethidine for 30 min in serum-free medium at 37 °C in a 95% air, 5% CO2 environment. At the end of incubation, the medium containing fluorescent dyes was aspirated, and cells were washed once with complete medium followed by the addition of fresh complete medium. They were then exposed to normoxia or hyperoxia. Cells were then washed twice with Phenol Red-free basal EBM-2, and the fluorescence of oxidized DCFDA or hydroethidine was determined using a Nikon Eclipse TE 2000-S fluorescence microscopy with a Hamamatsu digital CCD camera (Japan) and a 20× objective lens. We also carried out ex vivo experiments using cells grown in a glass bottom dish (35-mm) and a 60× objective lens. The relative fluorescence values were calculated using entire images and MetaVue software (Universal Imaging Corp.) and expressed as % of control.
Immunofluorescence Microscopy
HPAECs grown on slide chambers were exposed to either normoxia or HO and immediately fixed with 3.7% paraformaldehyde in PBS for 10 min followed by permeabilization for 4 min in 3.7% paraformaldehyde containing 0.25% Triton X-100. The cells were then rinsed 3 times with PBS and incubated for 30 min at room temperature in TBST blocking buffer containing 1% BSA followed by incubation with primary antibodies (1:200 dilution in blocking buffer for 1 h). After thoroughly rinsing with TBST, cells were then stained with Alexa Fluor secondary antibodies (1:200 dilutions in blocking buffer for 1 h). After washing, slides were prepared with maintain media and examined with a Nikon Eclipse TE 2000-S fluorescence microscope, and the images were recorded with a Hamamatsu digital camera (Japan) using a 60× oil immersion objective lens. Alternatively, we also used Leica DMI6000 and a 63× (NA 1.40) oil objective on a SP5 resonant scanner laser scanning confocal microscope (Leica Microsystems, Wetzlar, Germany). Quantification of cell periphery colocalization was performed by intensity correlation analysis as described previously (26). Briefly, for each image, background signal was subtracted by drawing a region of interest around the cell periphery of individual cells. All areas outside the cell were cleared to best visualize the leading edges including cell periphery, and the fluorescence intensity within the entire cell was summed by MBF ImageJ bundle (Tony Collins, McMaster University, and Wayne Rasband, NIH, rsb.info.nih.gov/ij) with an isodata algorithm. Threshold pixels in cell periphery were selected by drawing regions of interest, and fluorescence intensities within the selected cell periphery regions were summed. The ratio of cortactin or p47phox to FLAG-tagged nmMLCK in cell periphery was calculated from the summed fluorescence intensities within selected cell periphery region to the summed fluorescence intensity of the entire cell.
CEM Isolation
CEMs were isolated from HPAECs as described earlier (16). Briefly, ECs were scraped in PBS, centrifuged at 2000 rpm at 4 °C, and lysed with 0.2 ml of TN solution (25 mm Tris-HCl, pH 7.5, containing 150 mm NaCl, 1 mm DTT, protease inhibitors, 10% sucrose, 1% Triton X-100) for 30 min on ice. Triton X-100-insoluble materials were then mixed with 0.6 ml of cold 60% OptiprepTM and overlaid with 0.6 ml of 40, 30, and 20% OptiprepTM in TN solution. The gradients were centrifuged at 35,000 rpm in SW60 rotor for 12 h at 4 °C, and different fractions were collected. Cellular proteins associated with the 20% OptiprepTM fraction were analyzed by SDS-PAGE and Western blotting.
Preparation of Cell Lysates, Immunoprecipitation, and Western Blotting
HPAECs were serum-deprived for ∼18 h in EBM-2 containing 1% FBS. After exposure to normoxia or hyperoxia, cells were washed with ice-cold PBS containing 1 mm vanadate, scraped into 1 ml of lysis buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, 1 mm EDTA, 1 mm PMSF, 1 mm Na3VO4, 1 mm NaF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 μg/ml pepstatin), sonicated on ice with a probe sonicator (15 s), and centrifuged at 5000 × g in a microcentrifuge (4 °C) for 5 min (9). Equal volumes of the supernatants adjusted to 1 mg protein/ml were denatured by boiling in 6× SDS sample buffer for 5 min, subjected to SDS-PAGE, and immunoblotted with antibodies as indicated in the figures.
Exposure of Mice to Hyperoxia and Animal Procedures
nmMLCK−/− in C57BL/6 background were generated and phenotyped as described earlier (27, 28). C57BL/6 wild type or MLCK−/− male mice of 7 weeks of age were exposed to either normoxia or HO for 72 h in a plexiglass chamber designed for animal procedures. The chamber was supplied with 95% oxygen and 5% CO2, and oxygen saturation inside the chamber was monitored by an oxygen sensor (Pro ox 110, BioSpherix Inc., Redfield, NY). Mice were supplied with fresh water and a Harlan Teklad diet. Experiments conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (publication no. 85-23) and were approved by the Institutional Animal Care and Use Committee of The University of Chicago. Bronchoalveolar (BAL) lavage fluids were collected on ice from control, and nmMLCK knock-out mice were exposed to either normoxia or hyperoxia; cells were isolated by low speed centrifugation at 500 × g and were analyzed by cytological techniques. BAL fluids devoid of cells were also analyzed for total protein, and hydrogen peroxide content was determined by Amplex red assay with absorption read at 560 nm according to the manufacturer's protocol on a Bio-Rad iMark plate reader. Whole lungs without trachea were stored in liquid nitrogen and were processed for paraffin embedment, sectioning, and staining by hematoxylin and eosin and for Alexa Fluor antibodies by the histology core facility at University of Illinois at Chicago. To clarify the auto fluorescence, we analyzed non-stained paraffin sections by fluorescent microscopy using same software settings as for stained slides (image, gamma-1.00; exposure time, 300 ms; image scaling, low/high, not in autoscale mode).
Statistics
Analysis of variance and Student-Newman-Keuls tests were used to compare the means of two or more different treatment groups. The level of significance was set to p < 0.05 unless otherwise stated. Data are expressed as the mean ± S.E.
RESULTS
Role of nmMLCK in Hyperoxia-mediated ROS and Superoxide Production in HPAECs
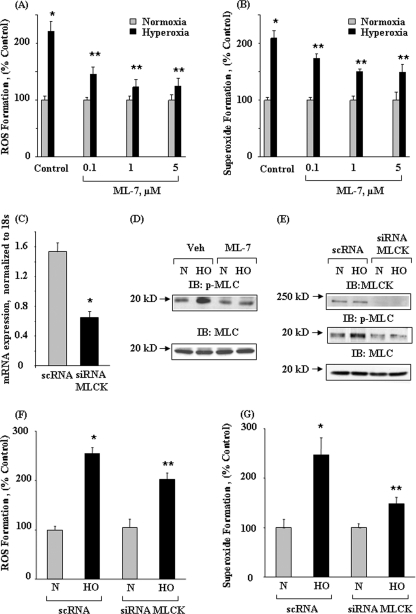
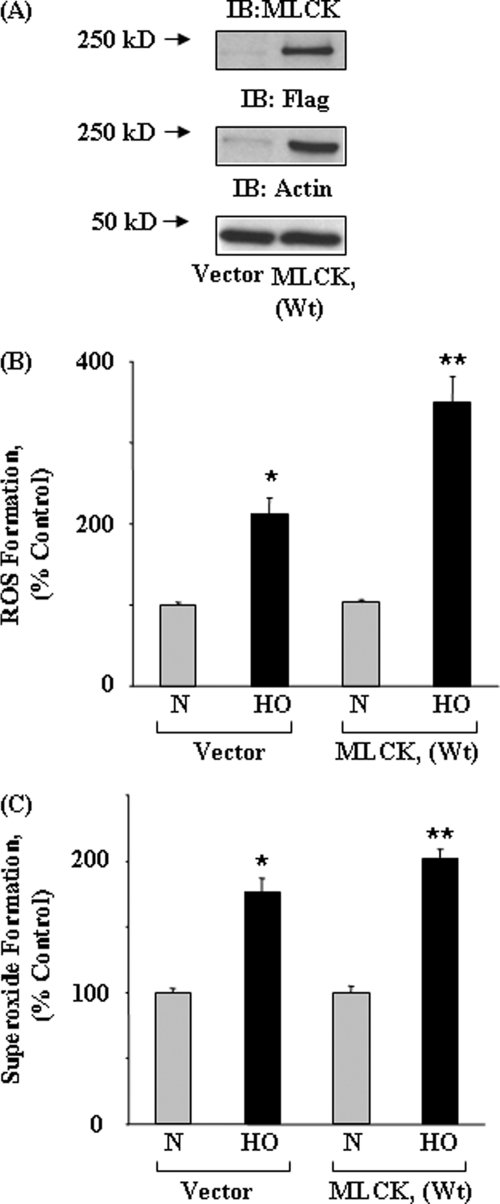
Recent studies have demonstrated a role for cytoskeletal proteins such as actin and cortactin in NADPH oxidase activation and ROS/O2˙̄ production in phagocytic and non-phagocytic cells (8, 29–31). Based on the ability of ML-7, an inhibitor of MLCK, to attenuate ROS generation in activated neutrophils (29, 30, 32), we investigated the role of nmMLCK in hyperoxia-induced ROS generation in human lung ECs. Pretreatment of HPAECs with ML-7 in a dose-dependent manner (0.1–5 μm) attenuated hyperoxia-induced ROS/O2˙̄ production as measured by DCFDA and hydroethidine fluorescence, respectively (Fig. 1, A and B). To further examine the role of nmMLCK in endothelial ROS generation by hyperoxia, nmMLCK expression was down-regulated in HPAECs using siRNA. The specificity of this down-regulation was indicated by the fact that nmMLCK siRNA, compared with scrambled siRNA, effectively down-regulated mRNA and protein expression of nmMLCK as well as phosphorylation of MLC without affecting expression of total MLC (Fig. 1, C and E). In cells exposed to hyperoxia, although the levels of MLCK remained unaltered, the phosphorylation status of its substrate MLC was enhanced, and inhibition of MLCK with ML-7 or down-regulation of nmMLCK with siRNA attenuated hyperoxia-induced MLC phosphorylation (Fig. 1, D and E) and ROS/O2˙̄ production (Fig. 1, F and G). Additional experiments with nmMLCK-specific GFP-tagged shRNA-transfected cells (48 h) resulted in down-regulation of nmMLCK expression and decreased O2˙̄ production (green fluorescence) compared with non-transfected cells in response to hyperoxia (supplemental Fig. 1). To further characterize the role of nmMLCK in hyperoxia-induced ROS/O2˙̄ production, HPAECs were transfected with FLAG-tagged nmMLCK1 wild type splice variant for 48 h, and cells were exposed to normoxia or hyperoxia for 3 h and analyzed for the expression of FLAG-tagged nmMLCK1 splice variant and ROS/O2˙̄ production by immunoblotting and DCFDA/hydroethidine oxidation, respectively. As shown in Fig. 2A, cells transfected with FLAG-tagged nmMLCK1 showed enhanced expression of the nmMLCK1 protein as determined by Western blotting with anti-FLAG and anti-nmMLCK antibodies. Furthermore, overexpression of FLAG-tagged nmMLCK1 wild type splice variant enhanced hyperoxia-mediated ROS/O2˙̄ production compared with non-transfected cells (Fig. 2, B and C). These results demonstrate a role for nmMLCK in hyperoxia-mediated ROS/O2.production in human lung endothelial cells.
FIGURE 1.
Role of nmMLCK in hyperoxia-mediated ROS and superoxide production in HPAECs. HPAECs grown on 35-mm dishes (∼90% confluence) were pretreated with increasing concentrations of ML-7 for 30 min and exposed to normoxia or hyperoxia for 3 h, and total ROS and superoxide accumulations were measured by DCFDA and hydroethidine immunofluorescence (A and B). In C, E, F, and G, HPAECs were transfected with scrambled (scRNA) or nmMLCK siRNA (50 nm) for 72 h as described under “Experimental Procedures.” Values for ROS and superoxide production are the mean ± S.D. from three independent experiments done in triplicate. *, significantly different from normoxia (p < 0.05); **, significantly different from untreated hyperoxia (p < 0.01). Total RNA was isolated from scrambled and nmMLCK siRNA-transfected cells, and real-time PCR was performed in a Light Cycler using SYBR Green QuantiTect (C). Data are from three independent experiments and are expressed as relative gene expression normalized to 18 S RNA. In D and E, cell lysates (20 μg of proteins) from ML-7, scrambled or nmMLCK siRNA-transfected cells were subjected to SDS-PAGE on 10% precast Tris-glycine gels and Western-blotted (IB) with anti-nmMLCK, phospho-MLC, and MLC antibodies. Shown is a representative blot of three independent experiments. In F and G, HPAECs were transfected with scrambled RNA or nmMLCK siRNA (50 nm) for 72 h, and cells were loaded with 10 μm DCFDA (F) or hydroethidine (G) for 30 min before exposure to normoxia or hyperoxia for 3 h. Formation of total ROS (F) and superoxide (G) was quantified as described above. Values are the mean ± S.D. of three independent experiments in triplicate. *, significantly different from normoxia (N) (p < 0.05); **, significantly different from hyperoxia exposure (HO) (p < 0.01).
FIGURE 2.
Overexpression of nmMLCK potentiates hyperoxia-induced ROS and superoxide production in HPAECs. HPAECs grown on 35-mm dishes (∼60% confluence) were transfected with vector control or FLAG-tagged nmMLCK WT (1 μg/ml) using FuGENE HD (3 μg/ml) transfection reagent for 72 h as described under “Experimental Procedures.” A, cell lysates (20 μg of proteins) from vector control and FLAG-tagged nmMLCK transfected cells were subjected to SDS-PAGE and immunoblotted with anti-MLCK, anti-FLAG and anti-actin antibodies. A representative immunoblot from three independent experiments is shown. B and C, vector control and FLAG-tagged nmMLCK (WT)-transfected cells were loaded with 10 μm DCFDA (B) or hydroethidine (C) for 30 min before exposure to normoxia (N) or HO for 3 h. Formation of total ROS (B) and superoxide (C) were quantified as described under “Experimental Procedures.” Values are the mean ± S.D. of three independent experiments in triplicate. *, significantly different from normoxia (N) (p < 0.05); **, significantly different from vector control cells exposed to hyperoxia (HO) (p < 0.001).
nmMLCK Depletion Attenuates Hyperoxia-mediated Actin Rearrangement and Redistribution of Phosphorylated Cortactin and Myosin Light Chain to Cell Periphery in HPAECs
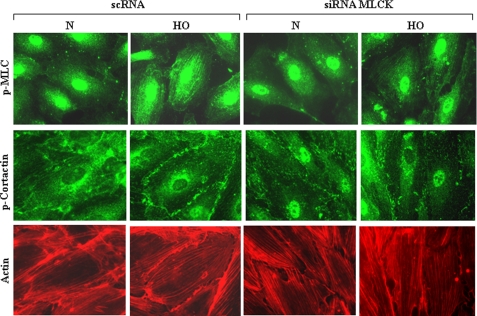
Cortactin, an actin-binding protein, is involved in the regulation of cortical actin rearrangement (33), and we have previously shown that hyperoxia-induced ROS production is regulated by actin and cortactin rearrangement in HPAECs (9). As nmMLCK is known to regulate actin polymerization and myosin phosphorylation in endothelial cells (34–41), we next examined the role of nmMLCK in hyperoxia-induced actin stress fiber formation, cortactin rearrangement to cell periphery, and phosphorylation of cortactin and MLC. In normoxia, cells revealed faint F-actin staining with few stress fibers in the central area of the cell and diffused phospho-cortactin distribution, with little localization near the cell periphery (Fig. 3). However, exposure of cells to hyperoxia (3 h) induced (i) F-actin reorganization and stress fiber formation in the center of the cell and thickening of the actin staining near the cell periphery and (ii) redistribution of phospho-cortactin and phospho-MLC to the cell periphery (Fig. 3). Down-regulation of nmMLCK with siRNA enhanced actin stress fibers under normoxia and hyperoxia; however, hyperoxia-induced phosphorylation as well as redistribution of phospho-cortactin and phospho-MLC to the cell periphery was attenuated (Fig. 3). These results suggest that nmMLCK regulates hyperoxia-dependent actin and cortactin rearrangement in HPAECs.
FIGURE 3.
nmMLCK siRNA attenuates hyperoxia-induced phosphorylation of MLC and cortactin and actin rearrangement in HPAECs. HPAECs grown on 8-well slide chambers were transfected with scrambled RNA or nmMLCK siRNA (50 nm) for 72 h before exposure to either normoxia (N) or HO for 3 h, washed, fixed, permeabilized, and probed with anti-phospho-cortactin, anti-phospho-MLC antibodies, or phalloidin for actin staining. nmMLCK siRNA blunted hyperoxia-induced phosphorylation of cortactin and MLC near the cell periphery and enhanced actin stress fibers under both normoxia and hyperoxia as determined by immunofluorescent microscopy. A representative immunofluorescence image from three independent experiments is shown.
Inhibition by nmMLCK siRNA of Hyperoxia-induced Association of Cortactin with p47phox in HPAECs
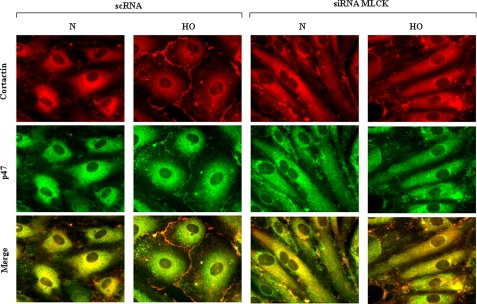
We previously demonstrated that hyperoxia enhances the association of cortactin with p47phox in human lung ECs (9). Based on our observation that nmMLCK modulates actin and cortactin redistribution, we investigated the possibility that nmMLCK regulates NADPH oxidase by modulating the interaction between cortactin and p47phox, an important component of the oxidase complex. For this purpose we used HPAECs that were transfected with scrambled or nmMLCK siRNA for 48 h. nmMLCK siRNA down-regulated nmMLCK protein expression by more than 80% in HPAECs (data not shown). As shown in Fig. 4, we observed diffused staining for both cortactin and p47phox throughout the cell (except in the nucleus). Exposure to hyperoxia, however, induced reorganization and co-localization of cortactin and p47phox to the cell periphery that were three-fold higher. In nmMLCK knockdown HPAECs (normoxia), the cellular distribution of cortactin and p47phox and co-localization were very similar to that of control cells; however, exposure to hyperoxia did not result in further enhancement of co-localization or relocation to the periphery (scrambled siRNA: normoxia, 100 ± 10%; hyperoxia, 312 ± 35; nmMLCK siRNA: normoxia, 100 ± 8; hyperoxia, 125 ± 26). Taken together these results suggest that nmMLCK is an important regulator of cortactin and p47phox reorganization to cell periphery under conditions of hyperoxia in HPAECs.
FIGURE 4.
Down-regulation of nmMLCK with nmMLCK siRNA blunted hyperoxia-induced translocation and co-localization of p47phox with cortactin. HPAECs grown on 8-well slide chambers were transfected with scrambled siRNA or MLCK siRNA for 72 h then exposed to normoxia or hyperoxia (3 h), washed, fixed, permeabilized, probed with anti-cortactin or anti-p47phox antibodies, and examined by immunofluorescence microscopy using a 60× oil objective. The cortactin (red) and p47phox (green) images show matched cell fields for each condition. Exposure of cells to hyperoxia resulted in redistribution of cortactin and p47phox to the cell periphery, whereas MLCK siRNA blunted cortactin and p47phox redistribution and co-localization (yellow in merged images). A representative image from three independent experiments is shown.
Hyperoxia Enhances Association of Cortactin and p47phox with nmMLCK in HPAECs
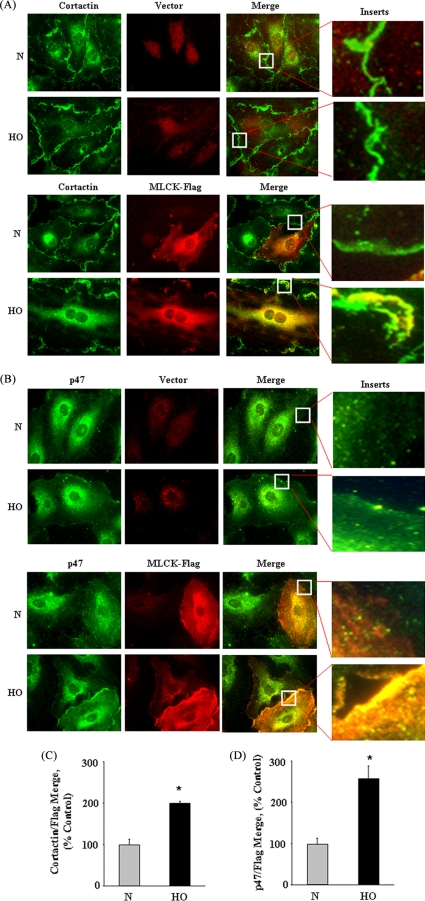
Having established a role for nmMLCK in hyperoxia-induced ROS/O2˙̄ production, cortactin and p47phox reorganization and co-localization, we next investigated the potential association of nmMLCK with cortactin and p47phox in response to hyperoxia. HPAECs were transfected with FLAG-tagged vector or FLAG-tagged-nmMLCK wild type plasmid (1 μg plasmid DNA/ml) for 48 h, cells were exposed to normoxia or hyperoxia (3 h), and localization of nmMLCK with cortactin and p47phox was analyzed using immunofluorescence microscopy. In normoxia, most of the FLAG-nmMLCK was localized in the cytoplasm and some in lamellipodia (Fig. 5A). Hyperoxia enhanced distribution of FLAG-nmMLCK to lamellipodia (red), cortactin (green), or p47phox (green) to cell periphery and lamellipodia structures (Fig. 5, A and B). Furthermore, hyperoxia enhanced co-localization of nmMLCK with cortactin and p47phox as shown in by the intensity of yellow color (Fig. 5, A and B). Semiquantitation of the co-localization using an image analyzer showed an ∼2.0-fold increase in co-localization (yellow) between nmMLCK (red) and cortactin (green) and a ∼2.5-fold increase in co-localization between nmMLCK and p47phox (Fig. 5, C and D). Similar results were obtained in cells transfected with FLAG-tagged nmMLCK1, GFP-cortactin, or GFP-p47phox plasmids (supplemental Fig. 2, A and B). These results indicate enhanced association of nmMLCK with cortactin and p47phox due to hyperoxia in HPAECs.
FIGURE 5.
Overexpression of MLCK-FLAG wild type enhances hyperoxia-induced association of nmMLCK with cortactin and p47phox in cell periphery. HPAECs grown on 8-well slide chambers were transfected with vector control or FLAG-tagged MLCK wild type (1 μg/ml) and FuGENE HD (3 μg/ml) transfection reagent for 72 h and then exposed to either normoxia (N) or HO for 3 h. Cells were, washed, fixed, permeabilized, and probed with anti-cortactin (A), anti-p47phox (B), or anti-FLAG (A and B) antibodies and examined by immunofluorescence microscopy using a 60× oil objective. Exposure of cells to hyperoxia resulted in redistribution of cortactin and p47phox to the cell periphery with enhanced nmMLCK association and co-localization (yellow in merged images). Shown is a representative image from three independent experiments. C and D represent semiquantitation of the co-localization using an image analyzer showed an ∼2.0-fold increase in co-localization (yellow) between nmMLCK (red) and cortactin (green) and an ∼2.5-fold increase in co-localization between nmMLCK and p47phox.
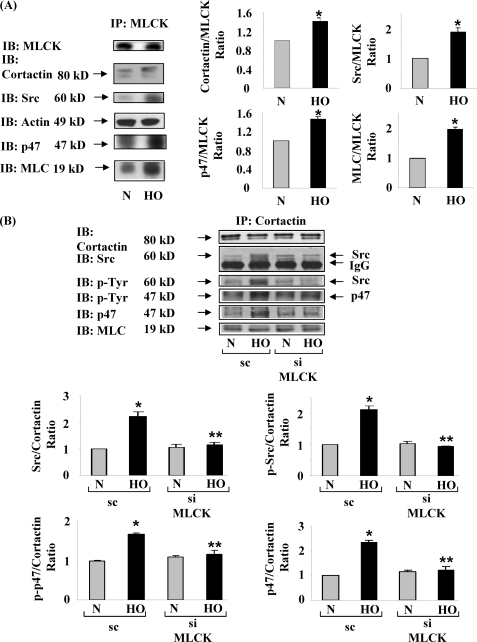
Interactions between nmMLCK, cortactin, Src, and p47phox were further investigated by co-immunoprecipitation studies. HPAECs were exposed to either normoxia or hyperoxia (3 h), and cell lysates were subjected to immunoprecipitation with anti-nmMLCK antibody and analyzed by Western blotting. Cortactin, Src, and p47phox were co-immunoprecipitated with nmMLCK under normoxic conditions, and exposure to hyperoxia further enhanced this association (Fig. 6A). Next we investigated the effect of down-regulation of nmMLCK with nmMLCK siRNA for 72 h on interactions between cortactin, Src, and p47phox by co-immunoprecipitation studies with cortactin. Transfection of HPAECs with nmMLCK siRNA for 72 h abolished the hyperoxia-induced association of cortactin with Src and p47phox as well as tyrosine phosphorylation of Src and p47phox as determined by Western blotting (Fig. 6B). Taken together these data show that hyperoxia enhances the interaction of nmMLCK with cortactin, Src, and p47phox, and depletion of nmMLCK attenuates the interactions between cortactin, Src, and p47phox in HPAECs.
FIGURE 6.
Down-regulation of nmMLCK with nmMLCK siRNA blunted the hyperoxia-induced association of cortactin with Src and p47phox and phosphorylation of cortactin, Src, and p47phox in HPAECs. A, HPAECs grown in 100-mm dishes to ∼90% confluence were exposed to either normoxia (N) or HO for 3 h. Cell lysates (500 μg proteins) from normoxia- or HO-exposed cells were immunoprecipitated (IP) with anti-nmMLCK antibody as described under “Experimental Procedures.” Immunoprecipitates were analyzed by 10% SDS-PAGE and probed with anti-nmMLCK, anti-cortactin, anti-Src, anti-p47phox, anti-actin, and anti-MLC antibodies. A representative blot from three independent experiments is shown. IB, immunoblot. B, HPAECs grown in 100-mm dishes to ∼50% confluence were transfected with scrambled (sc) RNA or nmMLCK siRNA (50 nm) for 72 h, and down-regulation of nmMLCK expression was verified by Western blotting as described under “Experimental Procedures.” Total cell lysates (500 μg of proteins) from scrambled and nmMLCK siRNA-treated cells were subjected to immunoprecipitation with anti-cortactin antibody, and immunoprecipitates were analyzed by 10% SDS-PAGE and probed with anti-cortactin (equal loading), anti-Src, anti-p47phox, anti-phosphotyrosine and anti-MLC antibodies. A representative blot from three independent experiments is shown.
nmMLCK Is Essential for NADPH Oxidase Assembly in Caveolin-enriched Microdomains
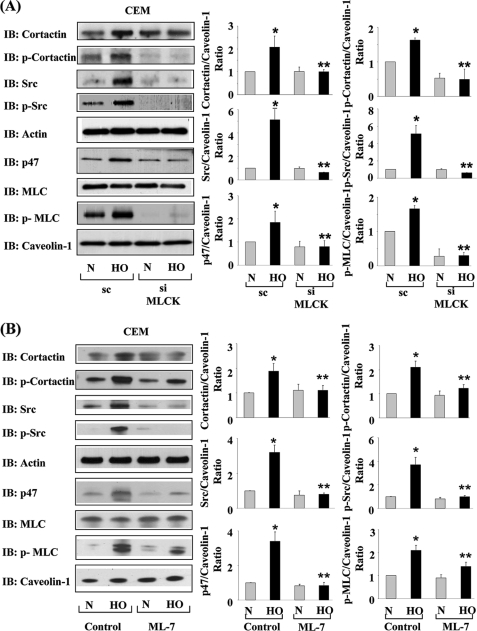
Cytoskeleton and actin-binding proteins are known to bind to CEMs (18, 20). Given our previous published data indicating recruitment of dynamin2, Rac1, cortactin, and NADPH oxidase subunits p47phox, p22phox and Nox2 (gp91phox) to CEMs (16), we examined whether nmMLCK regulates hyperoxia-mediated assembly of cytoskeletal proteins and NADPH oxidase components in CEM. As shown in Fig. 7, A and B, immunoblot analysis of CEMs isolated from normoxia- and hyperoxia-treated HPAECs revealed that hyperoxia-induced recruitment of cortactin, phospho-cortactin, Src, phospho-Src, phospho-MLC, p47phox, and Rac1 to CEMs was attenuated in nmMLCK knockdown and ML-7-treated cells. Interestingly, recruitment of Src to CEMs was drastically decreased even in normoxia-exposed nmMLCK knockdown cells. In addition, hyperoxia-induced recruitment of cortactin and p47phox was not apparent in nmMLCK abrogated cells. Furthermore, as expected, phosphorylations of MLC as well as Src were both compromised in nmMLCK knockdown cells. Furthermore, association of actin and MLC with CEMs whether the cells were exposed to normoxia or hyperoxia was not affected by nmMLCK expression. These results suggest that nmMLCK is essential for human lung EC NADPH oxidase assembly in CEMs even under normoxia conditions, and its requirement became more apparent under hyperoxia. The recruitment and activation of Src tyrosine kinase to CEMs also appeared to be completely dependent on nmMLCK.
FIGURE 7.
nmMLCK regulates hyperoxia-induced enrichment of cortactin, Src, and p47phox in caveolin-enriched microdomains. HPAECs grown in 100-mm dishes were transfected with scrambled (sc) RNA or nmMLCK siRNA (50 nm) for 72 h (A) or pretreated with ML-7 (1 μm, 30 min) (B) before exposure to either normoxia (N) or HO for 3 h, and caveolin-enriched microdomains were isolated as described under “Experimental Procedures.” Samples were then analyzed by 4–20% SDS-PAGE and probed with anti-cortactin, anti-Src, anti-p47phox, anti-MLC, anti-actin, anti-phospho-cortactin, anti-phospho-Src, and anti-phospho-MLC antibodies. Immunoblots (IB) were quantified by ImageJ software and expressed as a ratio to total caveolin-1. Values are the mean ± S.D. from three independent experiments. *, significantly different from normoxia (p < 0.05); **, significantly different from scrambled siRNA transfected cells exposed to hyperoxia.
Knockdown of nmMLCK in Mice Minimizes Hyperoxia-mediated ROS Production and Pulmonary Leak
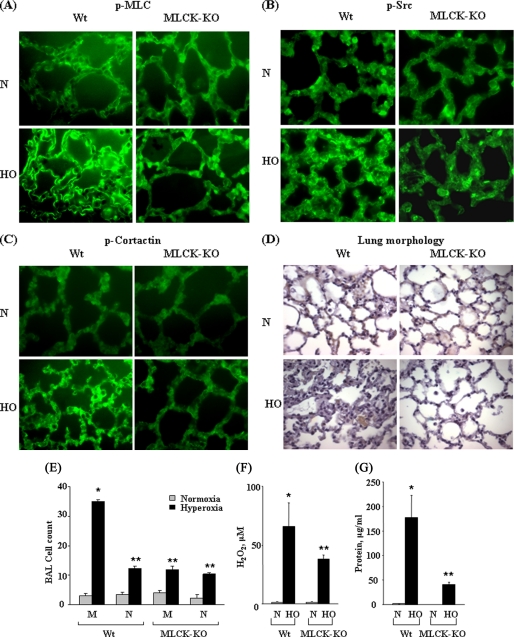
Having established a role for nmMLCK in hyperoxia-mediated NADPH oxidase assembly and activation in lung ECs, we next examined whether nmMLCK regulates ROS production and lung injury in vivo. Exposure of wild type mice to hyperoxia enhanced tyrosine phosphorylation of MLC, Src, and cortactin in mouse lungs that was attenuated in the nmMLCK knock-out mouse (Fig. 8, A–C). To rule out auto fluorescence, non-stained paraffin sections were analyzed by fluorescent microscopy under identical settings. As shown in supplemental Fig. 3, low auto fluorescence background was observed in paraffin sections compared with Alexa 488-stained sections. Histochemical analysis of lungs revealed nmMLCK1-dependent infiltration of cells into the alveolar space of mice exposed to hyperoxia (Fig. 8D). Furthermore, the increased levels of cells, hydrogen peroxide, and total protein in BAL fluids of hyperoxia-treated mice were significantly attenuated in the nmMLCK knock-out mice (Fig. 8, E–G). These results suggest that nmMLCK is an important regulator of hyperoxia-mediated ROS production, lung inflammation, and pulmonary leak in vivo.
FIGURE 8.
nmMLCK-KO mice exhibit reduced lung injury, leakage, and inflammation and ROS production in vivo. Male C57BL/6 WT or nmMLCK knock out (−/−) mice in the same background were exposed to either normoxia (N) or HO for 72 h. At the end of the experiment mice were anesthetized, BAL fluid was collected, lungs were perfused with fresh PBS several times, and whole lungs without trachea were paraffin-embedded. A–C, paraffin-embedded lung tissues from normoxia or HO animals were, sectioned, and immunostained with anti-phospho-MLC (A), anti-phospho-Src (B), or anti-phospho-cortactin (C) antibodies and examined under immunofluorescence microscopy using 60× oil objective. D, lung tissues from normoxia or HO-exposed animals were stored in formalin for 24 h before processing for staining with hematoxylin and eosin and lung morphology was evaluated using 40 X objective. E, BAL fluid collected from normoxia- or HO-exposed animals were subjected to cytospin, and differential cell counts were performed. Shown is a graphic representation of macrophages (M) and neutrophils (N) in BAL fluid from wild type and nmMLCK−/− mice exposed to normoxia or hyperoxia. Values are the mean ± S.D. from three independent experiments. *, significantly different from animals exposed to normoxia (p < 0.05); **, significantly different from animals exposed to normoxia (p < 0.01); ***, significantly different from wild type mice exposed to hyperoxia (p < 0.005). F and G, BAL fluid from mice exposed to normoxia or hyperoxia was analyzed for H2O2 (F) and total protein (G). Values are the mean ± S.D. from three independent experiments. *, significantly different from animals exposed to normoxia (p < 0.05); **, significantly different from wild type animals exposed to hyperoxia (p < 0.01).
Overexpression of FLAG-tagged nmMLCK Wild Type in nmMLCK-deficient Mouse Lung ECs Restores Hyperoxia-induced ROS/O2˙̄ Production and Redistribution of p-Src and p-cortactin to Cell Periphery
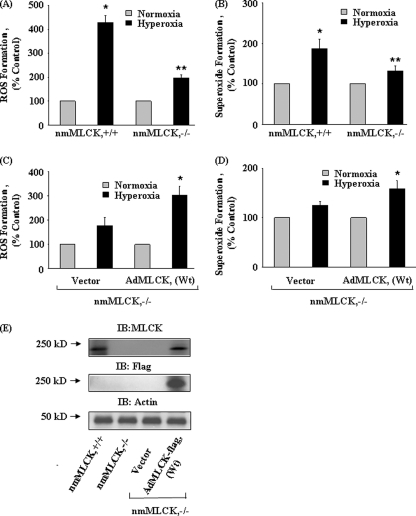
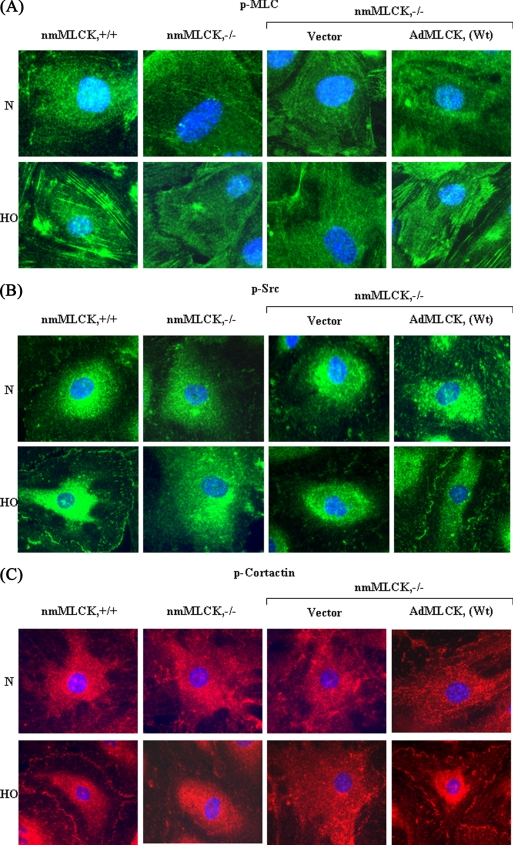
Given our in vivo results indicating nmMLCK regulation of hyperoxia-induced ROS production and pulmonary leak, we examined the effect of hyperoxia on ROS production and redistribution of p-MLC, p-Src, and p-cortactin to cell periphery in nmMLCK-deficient mouse lung ECs and nmMLCK-deficient cells overexpressing human wild type nmMLCK. Mouse lung ECs deficient in nmMLCK−/− generated minimal ROS/O2˙̄ after exposure to hyperoxia as compared with wild type cells (Fig. 9, A and B). However, expression of FLAG-tagged adenoviral human nmMLCK+/+ wild type in mouse nmMLCK−/− cells restored the ability of the nmMLCK-deficient cells to generate enhanced ROS/O2˙̄ in response to hyperoxia (Fig. 9, C and D). Similarly, expression of human nmMLCK+/+ wild type in nmMLCK−/− cells restored nmMLCK protein content (Fig. 9E) and hyperoxia-mediated redistribution of p-MLC, p-Src, and p-cortactin to cell periphery, respectively (Fig. 10, A–C). In the absence of overexpressed human nmMLCK+/+, hyperoxia exposure did not stimulate redistribution of p-Src and p-cortactin to cell periphery in nmMLCK−/− cells (Fig. 10, A–C). These results further confirm an important role for nmMLCK in hyperoxia-induced ROS production and redistribution of cytoskeletal proteins to cell periphery in mouse lung ECs.
FIGURE 9.
Overexpression of FLAG-tagged nmMLCK wild type in nmMLCK-deficient mouse lung ECs restores hyperoxia-induced ROS/O2˙̄ production. Mouse lung endothelial cells isolated from 4-5-week-old C57BL/6 and nmMLCK−/− null mice were isolated using collagenase A as described under “Experimental Procedures.” In A and B, lungs ECs grown to ∼80% confluence from wild type and nmMLCK−/− mice were exposed to normoxia or hyperoxia for 3 h, and total ROS and superoxide generated in cells were quantified as described under “Experimental Procedures.” Values for ROS and superoxide production are the mean ± S.D. from three independent experiments done in triplicate. *, significantly different from normoxia (p < 0.01); **, significantly different from untreated hyperoxia (p < 0.05). In C and D, lung ECs from nmMLCK−/− null mice were infected with vector control or adenoviral human nmMLCK (AdMLCK (WT)) (5 pfu/cell) in EBM-2 complete medium for 24 h before exposure to normoxia or hyperoxia for 3 h. Total ROS and superoxide accumulation in cells were measured by DCFDA and hydroethidine (HE) fluorescence. Values for ROS and superoxide production are the mean ± S.D. from three independent experiments done in triplicate. *, significantly different adenoviral nmMLCK wild type-infected cells exposed to normoxia (p < 0.01). In E, cell lysates (20 μg proteins) from wild type, nmMLCK−/−, and FLAG-tagged human nmMLCK-infected mouse lung ECs were subjected to SDS-PAGE and immunoblotted with anti-MLCK, anti-FLAG, and anti-actin antibodies. A representative immunoblot (IB) from three independent experiments is shown. Immunoblots were analyzed by densitometry and quantified.
FIGURE 10.
Overexpression of FLAG-tagged nmMLCK wild type in nmMLCK-deficient mouse lung ECs restores hyperoxia-induced redistribution of p-MLC, p-Src, and p-cortactin to cell periphery. Mouse lung ECs isolated from 4–5-week-old C57BL/6 wild type and nmMLCK−/− mice were grown on 8-well slide chambers to ∼80% confluence. In some experiments ECs isolated from nmMLCK−/− null mice were infected with vector control or adenoviral human nmMLCK wild type (AdMLCK, 5 pfu/cell) for 24 h. Cells were exposed to normoxia (N) or HO for 3 h, washed, fixed, permeabilized, and probed with anti-phospho-MLC (A), anti-phospho-Src (B), or anti-phospho-cortactin (C) antibodies and examined by immunofluorescence microscopy using a 60× oil objective. A representative image from three independent experiments is shown.
DISCUSSION
The experiments presented here demonstrate a novel role of EC nmMLCK in regulating hyperoxia-induced ROS generation by enhancing the recruitment of cortactin, p47phox, and MLC to CEM. Our data indicate that the association between nmMLCK and cortactin and between nmMLCK and p47phox at the cell periphery was enhanced in human lung endothelial cells. Moreover, our results on regulation of hyperoxia-mediated ROS production by nmMLCK in HPAECs are consistent with the adult in vivo model of hyperoxia-induced lung injury wherein nmMLCK−/− mice exhibited less inflammation, ROS production, and pulmonary leak compared with nmMLCK wild type mice. Together these studies indicated a critical role for nmMLCK in the assembly of NADPH oxidase components in CEMs and ROS production in the endothelium.
Accumulating evidence supports a role for cytoskeletal proteins including actin and cortactin in the assembly and activation of NADPH oxidase in phagocytic and non-phagocytic cells (5, 9, 10, 14). We demonstrated previously that hyperoxia-induced activation of endothelial NADPH oxidase in part is mediated by MAPKs, Src, Src-mediated tyrosine phosphorylation of cortactin, and p47phox- and phospholipase D-dependent activation of IQGAP1 through Rac1 in human lung endothelial cells (7, 9, 10, 13). Earlier, studies showed that inhibition of MLCK by ML-7 or ML-9 blocked A23187-stimulated respiratory burst in human polymorphonuclear leukocytes (28) and decreased p47phox phosphorylation and its translocation to the Triton X-100-insoluble fraction (11) and phagocytosis (42). Compared with the role of smooth muscle MLCK (∼130–160 kDa) (22, 43, 44) in phagocytic NADPH oxidase activation, very little is known about the role of nmMLCK (∼214 kDa) in oxidative burst and ROS production in non-phagocytic cells. In this study we demonstrate for the first time a novel and essential role for EC MLCK in modulating ROS generation in response to hyperoxia. Both smooth muscle MLCK and nmMLCK are ATP- and Ca2+/calmodulin-dependent enzymes that catalyze the phosphorylation of the regulatory 20-kDa MLC that is essential for generating cellular contractile force through an ATP-dependent actomyosin motor activity (34–38, 44). MLCK has been mapped to human chromosome 3q2 and exists as high molecular mass (∼210 kDa) and low molecular mass (∼130–160 kDa) isoforms that are encoded in a single gene. ECs express the high molecular mass isoform with five splice variants; however, in human ECs only EC MLCK1 protein is expressed (35, 45). An emerging concept from our work and that of others is the requirement of a protein platform consisting of a number of cytoskeletal proteins including cortactin, Rac1, dynamin, and Src family kinases to assemble the NADPH oxidase components in response to a stimulus such as hyperoxia (9, 10, 13, 26). We earlier demonstrated that hyperoxia-induced ROS generation required c-Abl-mediated dynamin phosphorylation for p47phox recruitment to CEMs of the endothelium (16). In this study we have identified that nmMLCK-dependent MLC phosphorylation as part of the regulatory mechanism of hyperoxia-mediated assembly of cortactin, Src, and p47phox in CEMs of endothelial cells. Furthermore, our results also suggest that association of nmMLCK with cortactin and p47phox in response to hyperoxia is critical for ROS generation as down-regulation of nmMLCK by siRNA blocks hyperoxia-induced ROS production and enrichment of phospho-MLC, phospho-cortactin, cortactin, phospho-Src, Src, and p47phox in CEMs of human lung ECs. These results are in concurrence with a requirement of caveolin-1/lipid rafts in angiotensin type 1 receptor-mediated ROS production in vascular smooth muscle cells (47).
Although the precise domain interactions between nmMLCK, cortactin, and p47phox remain unknown, it has been demonstrated that the cortactin SH3 domain interaction with nmMLCK is essential for S1P-induced MLC phosphorylation in ECs (9). A similar interaction occurring via cortactin SH3 domain and p47phox for translocation of p47phox to cell periphery has been demonstrated for increased ROS production in human lung ECs following hyperoxia (9, 10, 13). Interestingly, nmMLCK is not only essential for hyperoxia-induced interaction between cortactin and p47phox (Fig. 4) but also for the recruitment and phosphorylation of Src and cortactin in CEMs (Fig. 7). Because Src phosphorylates cortactin, p47phox (13), and nmMLCK (46), the role of nmMLCK in recruitment and activation of Src, cortactin, and p47phox in CEMs is unclear and needs further investigation. In addition to Src, nmMLCK and cortactin are tyrosine-phosphorylated by c-Abl (9), and hyperoxia-induced c-Abl activation promotes dynamin-2-p47phox complex formation and ROS generation in human lung ECs (16). Given the complexity of NADPH oxidase activation in ECs by various cytoskeletal proteins and Src family of kinases, our results provide strong evidence that nmMLCK is an important intracellular target regulating the interactions between the cytoskeleton and NADPH oxidase components and ROS generation mediated by hyperoxia.
The potential role of nmMLCK and its interaction with cortactin and Src in NADPH oxidase assembly, activation, and ROS generation was investigated in an in vivo model of hyperoxia-induced lung injury. Utilizing nmMLCK knock-out mice, our data indicate that hyperoxia-mediated pulmonary ROS production and barrier dysfunction, as determined by influx of cells into alveolar space, BAL protein concentration, and lung inflammation as measured by proinflammatory cytokines in BAL fluid, are dependent on nmMLCK expression. Exposure of nmMLCK−/− null mice to hyperoxia resulted in less ROS, protein in BAL fluids, and inflammation in the lung. Furthermore, immunohistochemistry revealed decreased phosphorylation of MLC, Src, and cortactin in lung tissues from nmMLCK−/− null mice compared with the nmMLCK wild type mice, suggesting a potential link between nmMLCK, phosphorylation of cortactin, and Src and ROS generation in vivo. These results from a murine model of hyperoxia-induced lung injury indicate nmMLCK knock-out mice are protected from oxidant-induced lung injury due to a deficiency in the assembly of NADPH oxidase components that require phosphorylation of cortactin, Src, and MLC in the lung. As nmMLCK−/− mice lack expression of nmMLCK in various vascular cell types, the role of lung endothelial nmMLCK in hyperoxia-induced ROS production and cytoskeletal redistribution to cell periphery was investigated. Our results show that mouse lung ECs deficient in nmMLCK exhibited reduced ROS/O2˙̄ production and redistribution of p-Src and p-cortactin to the cell periphery in response to hyperoxia. However, expression of human nmMLCK+/+ in nmMLCK-deficient mouse lung ECs resulted in restoration of hyperoxia-induced ROS/O2˙̄ generation and translocation of p-Src and p-cortactin to the cell periphery, indicating a definite role for EC nmMLCK in hyperoxia response. However, these studies do not rule out the participation of nmMLCK in other vascular cell types and non-hemopoietic cells in hyperoxia-induced lung injury. Further investigation on nmMLCK involvement from other cell types is necessary to determine their role in hyperoxia-induced lung inflammation and injury.
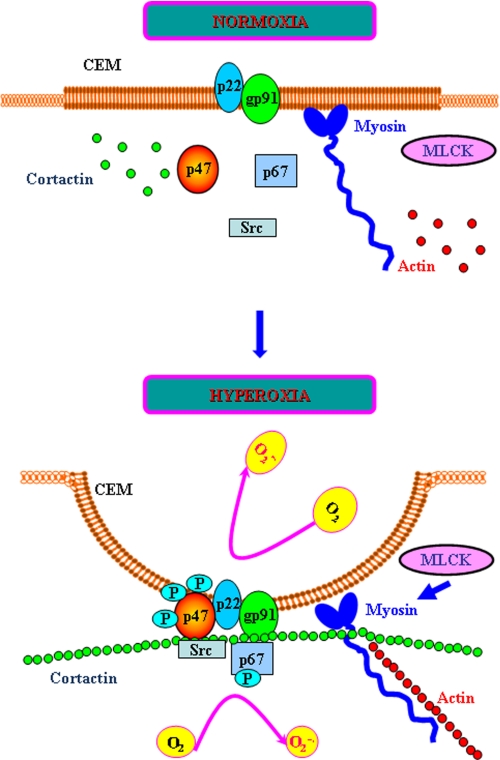
In summary, we have demonstrated a novel role for nmMLCK in hyperoxia-induced translocation of cortactin, Src, and p47phox to CEMs and generation of ROS. Furthermore, nmMLCK-mediated phosphorylation of MLC and interactions between nmMLCK, cortactin, Src, and p47phox suggest an essential requirement of a protein platform consisting of several cytoskeletal proteins including actin and myosin for the assembly and activation of NADPH oxidase in the endothelium (Fig. 11). Hyperoxia-induced lung injury is clinically relevant in the development of bronchopulmonary dysplasia in preterm babies exposed to high oxygen tension and in ventilator-induced lung injury of critically ill patients requiring ventilation in the intensive care unit. Thus, targeting of nmMLCK1 and other splice variants may provide a new therapeutic intervention in the management of acute lung injury in preterm babies and critically ill patients.
FIGURE 11.
Proposed model on involvement of ∼214-kDa nmMLCK in assembly and activation of non-phagocytic NADPH oxidase and ROS/superoxide production in lung endothelium. As depicted in the model, both cortactin and p47phox are diffused throughout the cell and mostly co-localized to the same intracellular compartment. Upon exposure to hyperoxia, nmMLCK facilitates the assembly and association of cortactin, p47phox, Src, and other components to the caveolin-enriched microdomains at the cell periphery for enhanced ROS/superoxide production.
Supplementary Material
Acknowledgment
We thank Dr. Prasad Kanteti for excellent assistance in the manuscript preparation.
This work was supported, in whole or in part, by National Institutes of Health Grants HL08553 and HL58064 (to V. N.).

This article contains supplemental Figs. 1–3.
- ROS
- reactive oxygen species
- MLC
- myosin light chain
- MLCK
- MLC kinase
- nmMLCK
- non-muscle MLCK
- DCFDA
- 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate
- HPAEC
- human pulmonary artery endothelial cell
- EBM
- endothelial basal medium
- EGM
- endothelial growth medium
- CEM
- caveolin-enriched microdomain
- HO
- hyperoxia
- BAL
- bronchoalveolar
- EC
- endothelial cell.
REFERENCES
- 1. Frey R. S., Ushio-Fukai M., Malik A. B. (2009) NADPH oxidase-dependent signaling in endothelial cells. Role in physiology and pathophysiology. Antioxid. Redox Signal. 11, 791–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pendyala S., Usatyuk P. V., Gorshkova I. A., Garcia J. G., Natarajan V. (2009) Regulation of NADPH oxidase in vascular endothelium. The role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid. Redox Signal. 11, 841–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Griffith B., Pendyala S., Hecker L., Lee P. J., Natarajan V., Thannickal V. J. (2009) NOX enzymes and pulmonary disease. Antioxid. Redox Signal. 11, 2505–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li J. M., Shah A. M. (2002) Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J. Biol. Chem. 277, 19952–19960 [DOI] [PubMed] [Google Scholar]
- 5. Touyz R. M., Yao G., Quinn M. T., Pagano P. J., Schiffrin E. L. (2005) p47phox associates with the cytoskeleton through cortactin in human vascular smooth muscle cells. Role in NAD(P)H oxidase regulation by angiotensin II. Arterioscler. Thromb. Vasc. Biol. 25, 512–518 [DOI] [PubMed] [Google Scholar]
- 6. Sumimoto H., Hata K., Mizuki K., Ito T., Kage Y., Sakaki Y., Fukumaki Y., Nakamura M., Takeshige K. (1996) Assembly and activation of the phagocyte NADPH oxidase. Specific interaction of the N-terminal Src homology 3 domain of p47phox with p22phox is required for activation of the NADPH oxidase. J. Biol. Chem. 271, 22152–22158 [DOI] [PubMed] [Google Scholar]
- 7. Parinandi N. L., Kleinberg M. A., Usatyuk P. V., Cummings R. J., Pennathur A., Cardounel A. J., Zweier J. L., Garcia J. G., Natarajan V. (2003) Hyperoxia-induced NAD(P)H oxidase activation and regulation by MAP kinases in human lung endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 284, L26–L38 [DOI] [PubMed] [Google Scholar]
- 8. Moldovan L., Moldovan N. I., Sohn R. H., Parikh S. A., Goldschmidt-Clermont P. J. (2000) Redox changes of cultured endothelial cells and actin dynamics. Circ. Res. 86, 549–557 [DOI] [PubMed] [Google Scholar]
- 9. Usatyuk P. V., Romer L. H., He D., Parinandi N. L., Kleinberg M. E., Zhan S., Jacobson J. R., Dudek S. M., Pendyala S., Garcia J. G., Natarajan V. (2007) Regulation of hyperoxia-induced NADPH oxidase activation in human lung endothelial cells by the actin cytoskeleton and cortactin. J. Biol. Chem. 282, 23284–23295 [DOI] [PubMed] [Google Scholar]
- 10. Usatyuk P. V., Gorshkova I. A., He D., Zhao Y., Kalari S. K., Garcia J. G., Natarajan V. (2009) Phospholipase D-mediated activation of IQGAP1 through Rac1 regulates hyperoxia-induced p47phox translocation and reactive oxygen species generation in lung endothelial cells. J. Biol. Chem. 284, 15339–15352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. El Benna J., Dang P. M., Andrieu V., Vergnaud S., Dewas C., Cachia O., Fay M., Morel F., Chollet-Martin S., Hakim J., Gougerot-Pocidalo M. A. (1999) p40phox associates with the neutrophil Triton X-100-insoluble cytoskeletal fraction and PMA-activated membrane skeleton. A comparative study with p67phox and p47phox. J. Leukoc. Biol. 66, 1014–1020 [DOI] [PubMed] [Google Scholar]
- 12. Zhan Y., He D., Newburger P. E., Zhou G. W. (2004) p47phox PX domain of NADPH oxidase targets cell membrane via moesin-mediated association with the actin cytoskeleton. J. Cell. Biochem. 92, 795–809 [DOI] [PubMed] [Google Scholar]
- 13. Chowdhury A. K., Watkins T., Parinandi N. L., Saatian B., Kleinberg M. E., Usatyuk P. V., Natarajan V. (2005) Src-mediated tyrosine phosphorylation of p47phox in hyperoxia-induced activation of NADPH oxidase and generation of reactive oxygen species in lung endothelial cells. J. Biol. Chem. 280, 20700–20711 [DOI] [PubMed] [Google Scholar]
- 14. Touyz R. M., Yao G., Schiffrin E. L. (2003) c-Src induces phosphorylation and translocation of p47phox. Role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 23, 981–987 [DOI] [PubMed] [Google Scholar]
- 15. Wu R. F., Gu Y., Xu Y. C., Nwariaku F. E., Terada L. S. (2003) Vascular endothelial growth factor causes translocation of p47phox to membrane ruffles through WAVE1. J. Biol. Chem. 278, 36830–36840 [DOI] [PubMed] [Google Scholar]
- 16. Singleton P. A., Pendyala S., Gorshkova I. A., Mambetsariev N., Moitra J., Garcia J. G., Natarajan V. (2009) Dynamin 2 and c-Abl are novel regulators of hyperoxia-mediated NADPH oxidase activation and reactive oxygen species production in caveolin-enriched microdomains of the endothelium. J. Biol. Chem. 284, 34964–34975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Callera G. E., Montezano A. C., Yogi A., Tostes R. C., Touyz R. M. (2007) Vascular signaling through cholesterol-rich domains. Implications in hypertension. Curr. Opin. Nephrol. Hypertens. 16, 90–104 [DOI] [PubMed] [Google Scholar]
- 18. Chichili G. R., Rodgers W. (2007) Clustering of membrane raft proteins by the actin cytoskeleton. J. Biol. Chem. 282, 36682–36691 [DOI] [PubMed] [Google Scholar]
- 19. Patel H. H., Insel P. A. (2009) Lipid rafts and caveolae and their role in compartmentation of redox signaling. Antioxid. Redox Signal. 11, 1357–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chichili G. R., Rodgers W. (2009) Cytoskeleton-membrane interactions in membrane raft structure. Cell. Mol. Life Sci. 66, 2319–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ushio-Fukai M. (2006) Localizing NADPH oxidase-derived ROS. Sci. STKE 2006, re8. [DOI] [PubMed] [Google Scholar]
- 22. Gallagher P. J., Herring B. P., Stull J. T. (1997) Myosin light chain kinases. J. Muscle Res. Cell Motil. 18, 1–16 [DOI] [PubMed] [Google Scholar]
- 23. Li Y., Liu J., Zhan X. (2000) Tyrosine phosphorylation of cortactin is required for H2O2-mediated injury of human endothelial cells. J. Biol. Chem. 275, 37187–37193 [DOI] [PubMed] [Google Scholar]
- 24. Tiruppathi C., Freichel M., Vogel S. M., Paria B. C., Mehta D., Flockerzi V., Malik A. B. (2002) Impairment of store-operated Ca2+ entry in TRPC4−/− mice interferes with increase in lung microvascular permeability. Circ. Res. 91, 70–76 [DOI] [PubMed] [Google Scholar]
- 25. Moitra J., Evenoski C., Sammani S., Wadgaonkar R., Turner J. R., Ma S. F., Garcia J. G. (2008) A transgenic mouse with vascular endothelial overexpression of the non-muscle myosin light chain kinase-2 isoform is susceptible to inflammatory lung injury. Role of sexual dimorphism and age. Transl. Res. 151, 141–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brown M., Adyshev D., Bindokas V., Moitra J., Garcia J. G., Dudek S. M. (2010) Quantitative distribution and colocalization of non-muscle myosin light chain kinase isoforms and cortactin in human lung endothelium. Microvasc. Res. 80, 75–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wainwright M. S., Rossi J., Schavocky J., Crawford S., Steinhorn D., Velentza A. V., Zasadzki M., Shirinsky V., Jia Y., Haiech J., Van Eldik L. J., Watterson D. M. (2003) Protein kinase involved in lung injury susceptibility. Evidence from enzyme isoform genetic knockout and in vivo inhibitor treatment. Proc. Natl. Acad. Sci. U.S.A. 100, 6233–6238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mirzapoiazova T., Moitra J., Moreno-Vinasco L., Sammani S., Turner J. R., Chiang E. T., Evenoski C., Wang T., Singleton P. A., Huang Y., Lussier Y. A., Watterson D. M., Dudek S. M., Garcia J. G. (2011) Non-muscle myosin light chain kinase isoform is a viable molecular target in acute inflammatory lung injury. Am. J. Respir. Cell Mol. Biol. 44, 40–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heyworth P. G., Erickson R. W., Ding J., Curnutte J. T., Badwey J. A. (1995) Naphthalene sulfonamides block neutrophil superoxide production by intact cells and in a cell-free system. Is myosin light chain kinase responsible for these effects? Biochem. J. 311, 81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Odani K., Kobayashi T., Ogawa Y., Yoshida S., Seguchi H. (2003) ML-7 inhibits exocytosis of superoxide-producing intracellular compartments in human neutrophils stimulated with phorbol myristate acetate in a myosin light chain kinase-independent manner. Histochem. Cell Biol. 119, 363–370 [DOI] [PubMed] [Google Scholar]
- 31. Yuan S. Y., Wu M. H., Ustinova E. E., Guo M., Tinsley J. H., De Lanerolle P., Xu W. (2002) Myosin light chain phosphorylation in neutrophil-stimulated coronary microvascular leakage. Circ. Res. 90, 1214–1221 [DOI] [PubMed] [Google Scholar]
- 32. Bergstrand H., Eriksson T., Hallberg A., Johansson B., Karabelas K., Michelsen P., Nybom A. (1992) Modulation of neutrophil superoxide generation by inhibitors of protein kinase C, calmodulin, diacylglycerol, and myosin light chain kinases and peptidyl prolyl cis-trans isomerase. J. Pharmacol. Exp. Ther. 263, 1334–1346 [PubMed] [Google Scholar]
- 33. Dudek S. M., Garcia J. G. (2001) Cytoskeletal regulation of pulmonary vascular permeability. J. Appl. Physiol. 91, 1487–1500 [DOI] [PubMed] [Google Scholar]
- 34. Garcia J. G., Davis H. W., Patterson C. E. (1995) Regulation of endothelial cell gap formation and barrier dysfunction. Role of myosin light chain phosphorylation. J. Cell. Physiol. 163, 510–522 [DOI] [PubMed] [Google Scholar]
- 35. Garcia J. G., Lazar V., Gilbert-McClain L. I., Gallagher P. J., Verin A. D. (1997) Myosin light chain kinase in endothelium. Molecular cloning and regulation. Am. J. Respir. Cell Mol. Biol. 16, 489–494 [DOI] [PubMed] [Google Scholar]
- 36. Garcia J. G., Verin A. D., Schaphorst K., Siddiqui R., Patterson C. E., Csortos C., Natarajan V. (1999) Regulation of endothelial cell myosin light chain kinase by Rho, cortactin, and p60Src. Am. J. Physiol. 276, L989–L998 [DOI] [PubMed] [Google Scholar]
- 37. Goeckeler Z. M., Wysolmerski R. B. (1995) Myosin light chain kinase-regulated endothelial cell contraction. The relationship between isometric tension, actin polymerization, and myosin phosphorylation. J. Cell Biol. 130, 613–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goeckeler Z. M., Bridgman P. C., Wysolmerski R. B. (2008) Nonmuscle myosin II is responsible for maintaining endothelial cell basal tone and stress fiber integrity. Am. J. Physiol. Cell Physiol. 295, C994–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Verin A. D., Gilbert-McClain L. I., Patterson C. E., Garcia J. G. (1998) Biochemical regulation of the nonmuscle myosin light chain kinase isoform in bovine endothelium. Am. J. Respir. Cell Mol. Biol. 19, 767–776 [DOI] [PubMed] [Google Scholar]
- 40. Verin A. D., Lazar V., Torry R. J., Labarrere C. A., Patterson C. E., Garcia J. G. (1998) Expression of a novel high molecular weight myosin light chain kinase in endothelium. Am. J. Respir. Cell Mol. Biol. 19, 758–766 [DOI] [PubMed] [Google Scholar]
- 41. Vicente-Manzanares M., Ma X., Adelstein R. S., Horwitz A. R. (2009) Non-muscle myosin II takes center stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 10, 778–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mansfield P. J., Shayman J. A., Boxer L. A. (2000) Regulation of polymorphonuclear leukocyte phagocytosis by myosin light chain kinase after activation of mitogen-activated protein kinase. Blood 95, 2407–2412 [PubMed] [Google Scholar]
- 43. Gallagher P. J., Stull J. T. (1997) Localization of an actin binding domain in smooth muscle myosin light chain kinase. Mol. Cell. Biochem. 173, 51–57 [DOI] [PubMed] [Google Scholar]
- 44. Gallagher P. J., Garcia J. G., Herring B. P. (1995) Expression of a novel myosin light chain kinase in embryonic tissues and cultured cells. J. Biol. Chem. 270, 29090–29095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lazar V., Garcia J. G. (1999) A single human myosin light chain kinase gene (MLCK; MYLK). Genomics 57, 256–267 [DOI] [PubMed] [Google Scholar]
- 46. Birukov K. G., Csortos C., Marzilli L., Dudek S., Ma S. F., Bresnick A. R., Verin A. D., Cotter R. J., Garcia J. G. (2001) Differential regulation of alternatively spliced endothelial cell myosin light chain kinase isoforms by p60Src. J. Biol. Chem. 276, 8567–8573 [DOI] [PubMed] [Google Scholar]
- 47. Parton R. G., Simons K. (2007) The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 8, 185–194 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.