Background: We have studied the properties of Ca2+ transport in Drosophila mitochondria.
Results: Drosophila mitochondria possess Ca2+ transport systems matching their mammalian equivalents but have a unique selective Ca2+ release channel that does not mediate swelling.
Conclusion: The Drosophila Ca2+ release channel is involved in Ca2+ homeostasis rather than cell death.
Significance: This channel may represent the missing link between the permeability transition pore of yeast and mammals.
Keywords: Calcium Transport, Drosophila, Membrane Transport, Mitochondria, Mitochondrial Permeability Transition
Abstract
We have studied the pathways for Ca2+ transport in mitochondria of the fruit fly Drosophila melanogaster. We demonstrate the presence of ruthenium red (RR)-sensitive Ca2+ uptake, of RR-insensitive Ca2+ release, and of Na+-stimulated Ca2+ release in energized mitochondria, which match well characterized Ca2+ transport pathways of mammalian mitochondria. Following larger matrix Ca2+ loading Drosophila mitochondria underwent spontaneous RR-insensitive Ca2+ release, an event that in mammals is due to opening of the permeability transition pore (PTP). Like the PTP of mammals, Drosophila Ca2+-induced Ca2+ release could be triggered by uncoupler, diamide, and N-ethylmaleimide, indicating the existence of regulatory voltage- and redox-sensitive sites and was inhibited by tetracaine. Unlike PTP-mediated Ca2+ release in mammals, however, it was (i) insensitive to cyclosporin A, ubiquinone 0, and ADP; (ii) inhibited by Pi, as is the PTP of yeast mitochondria; and (iii) not accompanied by matrix swelling and cytochrome c release even in KCl-based medium. We conclude that Drosophila mitochondria possess a selective Ca2+ release channel with features intermediate between the PTP of yeast and mammals.
Introduction
Mitochondria play a pivotal role in cellular Ca2+ homeostasis and thereby participate in the orchestration of a diverse range of cellular activities. Indeed, the mitochondrial proton electrochemical gradient is used not only to synthesize ATP but also to accumulate cations into the mitochondrial matrix (1–4). Consequently, when local cytoplasmic free Ca2+ levels rise, mitochondria rapidly accumulate cytoplasmic Ca2+ and then gradually release it as normal cytoplasmic levels are restored, amplifying and sustaining signals arising from elevation of cytoplasmic Ca2+, as well as protecting cells and neurons against transient elevation in intracellular Ca2+ during periods of hyperactivity (1, 5, 6). As a result, the mechanisms controlling cellular and mitochondrial Ca2+ homeostasis, metabolism, and bioenergetics must function as a tightly integrated system within the overall cellular Ca2+ homeostatic network (2, 7–9).
The pathways responsible for mitochondrial Ca2+ uptake and release have been intensely studied on a functional level for >50 years. In energized mitochondria, the Ca2+ uniporter mediates Ca2+ uptake across the inner mitochondrial membrane, whereas exchangers (Ca2+ for Na+ and/or H+) are responsible for Ca2+ efflux (9–13). However, when the mitochondrial Ca2+ load exceeds the capacity of inner membrane exchangers, an additional pathway for Ca2+ efflux from mitochondria may exist through opening of the permeability transition pore (PTP).3
The mitochondrial permeability transition (PT) describes a process of Ca2+-dependent, tightly regulated increase in the permeability of the inner mitochondrial membrane due to the opening of a high-conductance channel, the PTP (10). PTP opening causes collapse of the mitochondrial membrane potential (Δψ) and Ca2+ release through the pore itself, an event that for short “open” times may indeed be involved in physiological Ca2+ homeostasis (14, 15), as recently shown in mouse hearts (16) and adult neurons (17) consistent with a role of the PTP in cell signaling (18). Prolonged opening of the PTP, on the other hand, causes stable depolarization, loss of ionic homeostasis, depletion of pyridine nucleotides, respiratory inhibition, matrix swelling, release of cytochrome c, and cell death via apoptosis or necrosis depending on a variety of additional factors, among which cellular ATP and Ca2+ levels play a major role (19).
Together with matrix Ca2+, Pi is an essential inducer of PTP opening in mammals (19), whereas Pi exerts an inhibitory action on the yeast permeability pathways triggered by ATP and energization (20–24; see Ref. 25 for a recent review). In mammals, the PTP can be desensitized by submicromolar concentrations of the immunosuppressant drug cyclosporin A (26–28) via an interaction with its matrix receptor cyclophilin D (29). Our recent discovery that the inhibitory effect of cyclosporin A and of cyclophilin D ablation on the pore requires Pi (30) opens new scenarios. Indeed, this observation may bridge the gap between the pore of yeast and mammals, which we have hypothesized to be much closer than previously thought (31; see Ref. 32 for a review of earlier literature).
Despite its importance as a model organism, the characteristics of mitochondrial Ca2+ transport have been little studied in Drosophila melanogaster. The present study demonstrates that Drosophila mitochondria possess Ca2+ transport systems that are very close to those of mammals and that they can undergo a ruthenium red (RR)-insensitive Ca2+-induced Ca2+ release through a selective channel that is insensitive to cyclosporin A and inhibited by Pi, and whose general features may be intermediate between the properties of the PTP of yeast and that of mammals.
EXPERIMENTAL PROCEDURES
Cell Cultures
S2R+ cells (33) were cultured in Schneider's insect medium supplemented with 10% heat-inactivated FBS and kept in 75-cm2 T flasks or in tissue culture dishes (245 × 245 × 25 mm) at 25 °C.
Cell Permeabilization
Cells were detached with a sterile cell scraper, centrifuged at 200 × g for 10 min, and washed twice with Dulbecco's PBS without Ca2+ and Mg2+, pH 7.4 (Euroclone). The resulting pellet was resuspended in 130 mm KCl, 10 mm MOPS-Tris, pH 7.4 (KCl medium), containing 150 μm digitonin and 1 mm EGTA-Tris and incubated for 20 min on ice (6 × 107 cells × ml−1). Cells were then diluted 1:5 in KCl medium containing 10 μm EGTA-Tris and centrifuged at 200 × g in a refrigerated centrifuge (4 °C) for 6 min. The final pellet was resuspended in KCl medium containing 10 μm EGTA-Tris at 4 × 108 cells × ml−1 and kept on ice.
Fluorescent Staining of S2R+ Cell Mitochondria
In the experiments of Fig. 1A energization of mitochondria in both intact and permeabilized S2R+ cells was analyzed based on accumulation of the potentiometric probe tetramethyl rhodamine methyl ester (TMRM, Molecular Probes). Three days before the experiments, cells were seeded onto sterilized 24-mm round glass coverslips at 2 × 106 cells per well in 2 ml of Schneider's medium supplemented with 10% FBS. On the day of experiment, cells were washed once with PBS and incubated for 20 min at room temperature with 1 ml of serum-free Schneider's medium supplemented with 1 μg/ml cyclosporin H and 10 nm TMRM. Cyclosporin H is an inhibitor of the plasma membrane multidrug resistance pumps and allows an appropriate loading with the probe by preventing its extrusion at the plasma membrane (34). Images were acquired with an Olympus IX71/IX51 inverted microscope equipped with a xenon light source (75 watts) for epifluorescence illumination and with a 12-bit digital cooled CCD camera (Micromax). For detection of TMRM fluorescence, 568 ± 25-nm bandpass excitation and 585-nm long pass emission filter settings were used.
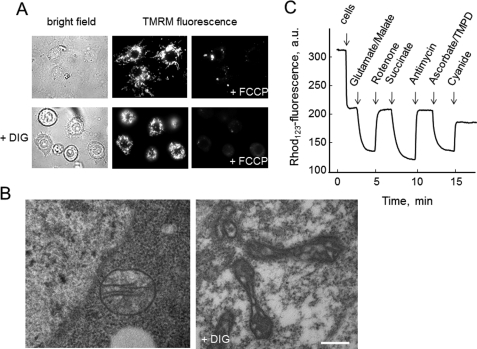
FIGURE 1.
Evaluation of mitochondrial energization and membrane potential in intact and permeabilized Drosophila S2R+ cells. A, cells were seeded on glass coverslips, loaded with 10 nm TMRM as described under “Experimental Procedures,” and observed under bright field conditions or for TMRM fluorescence before and after the addition of 4 μm FCCP either without further additions (upper row) or after permeabilization with 30 μm digitonin (+DIG, lower row). B, ultrastructural analysis of untreated (left panel) and digitonin-permeabilized (right panel, DIG) S2R+ cells; bar, 300 nm for both panels. C, cells were digitonized as described under “Experimental Procedures” and incubated in 130 mm KCl, 10 mm MOPS-Tris, 10 μm EGTA, and 0.15 μm Rhodamine 123 (Rhod123), pH 7.4. Further additions were 5 mm glutamate-Tris plus 2.5 mm malate-Tris, 2 μm rotenone, 5 mm succinate-Tris, 0.1 μg/ml antimycin A, 5 mm ascorbate-Tris plus 100 μm tetramethyl-p-phenylene diamine (TMPD) and 2 mm KCN. a.u., arbitrary units.
In the experiments of Fig. 1C, mitochondrial membrane potential was measured using a Perkin-Elmer LS50B spectrofluorometer and evaluated based on the fluorescence quenching of Rhodamine 123. Two milliliters of 130 mm KCl, 10 mm MOPS-Tris, 5 mm Pi-Tris, 10 μm EGTA, 0.15 μm Rhodamine 123, pH 7.4, were added to the cuvette. The fluorescence of Rhodamine 123 was monitored at the excitation and emission wavelengths of 503 and 523 nm, respectively, with the slit width set at 2.5 nm. After a short incubation to reach stabilization of the signal, 2 × 107 permeabilized S2R+ cells were added to the cuvette. Further additions were as indicated in the figure legends.
Electron Microscopy
S2R+ cells were washed with PBS and fixed in 2.5% glutaraldehyde in 0.1 m potassium phosphate buffer, pH 7.4, for 2 h at 4 °C. After washing with 0.15 m potassium phosphate buffer, pH 7.0, cells were finally embedded in 2% gelatin as described previously (35). Gelatin-embedded samples were post-fixed with 1% osmium tetroxide in cacodylate buffer 0.1 m, pH 7.4, and embedded in Epon812 resin, sectioned, and stained following standard procedures (36). Ultrathin sections were observed with a Philips EM400 transmission electron microscope operating at 100 kV.
Mitochondrial Respiration
Rates of mitochondrial respiration were measured using a Clark-type oxygen electrode equipped with magnetic stirring and thermostatic control maintained at 25 °C, and additions were made through a syringe port in the frosted glass stopper sealing the chamber. Intact S2R+ cells were incubated in Hank's balanced salt solution supplemented with 10 mm glucose and 5 mm Pi-Tris, pH 7.4, whereas digitonin-permeabilized cells (see above) were incubated in 130 mm KCl, 10 mm MOPS-Tris, 5 mm Pi-Tris, 5 mm succinate-Tris, 10 μm EGTA, pH 7.4. In both cases, 2 × 107 cells in 2 ml were used, and further additions are specified in the figure legends.
Light Scattering and Mitochondrial Ca2+ Fluxes
Light scattering at 90° was monitored with a PerkinElmer LS50B spectrofluorimeter at 540 nm with a 5.5-nm slit width. Extramitochondrial Ca2+ was measured with Calcium Green 5N (Molecular Probes) using either the PerkinElmer LS50B spectrofluorometer equipped with magnetic stirring (excitation and emission wavelengths of 505 and 535 nm, respectively) or a Fluoroskan Ascent FL (Thermo Electron Corp.) equipped with a plate shaker (excitation and emission wavelengths of 485 and 538 nm, respectively with a 10-nm band pass filter). The incubation medium contained 130 mm KCl, 10 mm MOPS-Tris, 5 mm succinate-Tris, 10 μm EGTA, 2 μm rotenone, pH 7.4, and Pi-Tris as indicated in the figure legends. In the Ca2+ measurements, 0.5 μm Calcium-Green 5N was also added. Permeabilized cells (2 × 107 in a final volume of 2 ml in the PerkinElmer spectrofluorometer and 2 × 106 in a final volume of 0.2 ml in the Fluoroskan) were used. Further additions were made as indicated in the figure legends.
Western Blotting
Cell suspensions were centrifuged at 3000 × g at 4 °C. Proteins from the supernatants were precipitated in acetone at −20 °C and centrifuged for 30 min at 18,000 × g at 4 °C. Pellets were washed twice in 20% methanol and finally solubilized in Laemmli gel sample buffer. Cell pellets were lysed in a buffer containing 150 mm NaCl, 20 mm Tris, pH 7.4, 5 mm EDTA-Tris, 10% glycerol, 1% Triton X-100, and supplemented with protease and phosphatase inhibitor cocktails (Sigma), and kept on ice for 20 min. Suspensions were then centrifuged at 18,000 × g for 25 min at 4 °C to remove insoluble materials. The supernatants were solubilized in Laemmli gel sample buffer. Samples were separated by 15% SDS-PAGE and transferred electrophoretically to nitrocellulose membranes using a Mini Trans-Blot system (Bio-Rad). Western blotting was performed in PBS containing 3% nonfat dry milk with monoclonal mouse anti-cytochrome c (BD Biosciences), monoclonal mouse anti-OxPhos complex IV subunit I (Invitrogen), or rabbit polyclonal anti-TOM20 (Santa Cruz Biotechnology) antibodies.
Reagents and Statistics
All chemicals were of the highest purity commercially available. Reported results are typical of at least three replicates for each condition, and error bars refer to the S.D.
RESULTS
We initially isolated mitochondria from Drosophila flight muscles after dissection of the thoraces to prevent contamination from the yeast on which Drosophila feeds and that may be present in the abdomen. Despite our great efforts mitochondria were of poor quality, as judged from the respiratory control ratios (results not shown). An additional problem we encountered was that the low yield of these preparations did not allow a reproducible analysis of the Ca2+ transport properties of mitochondria. Thus, we characterized mitochondrial function in intact S2R+ Drosophila cells and then used digitonin permeabilization to access mitochondria in situ, an approach that we have successfully applied to mammalian cells (37) and to cells from 6-h-old embryos from Danio rerio (zebrafish) (38).
Mitochondria in both intact and permeabilized S2R+ cells were energized, as shown by fluorescence images after the addition of the potentiometric probe TMRM (Fig. 1A). Mitochondria appeared as bright bodies, and fluorescence was lost upon addition of an uncoupler (Fig. 1A). Ultrastructural analysis of intact S2R+ cells revealed round-shaped mitochondria with thin cristae aligned in parallel rows (Fig. 1B, left panel illustrates a typical example), which is strikingly similar to the morphology of mammalian mitochondria in situ and to the “orthodox” configuration of Hackenbrock (39). After digitonin treatment, most cells showed evidence of permeabilization as reflected by a change in the electron density of the cytoplasm and loss of chromatin definition (results not shown), but the overall morphology of organelles was retained (Fig. 1B, right panel). Mitochondria, however, now displayed a “condensed” configuration very similar to that of isolated mammalian mitochondria (39), which is characterized by an electron-dense matrix and evident and well preserved cristae and outer membrane (Fig. 1B, right panel).
Digitonin-permeabilized cells are accessible to substrates, and this allows the study of their response to energization. Mitochondria readily developed a membrane potential (as judged on the basis of fluorescence quenching of Rhodamine 123) upon addition of the complex I substrates glutamate and malate (Fig. 1C). The sequential addition of rotenone, succinate, antimycin A, ascorbate plus tetramethyl-p-phenylene diamine, and finally cyanide caused the expected repolarization-depolarization cycles that indicate functioning of all respiratory complexes (Fig. 1C).
Intact S2R+ cells displayed a good respiratory activity that was largely inhibited by oligomycin, indicating that a prevalent fraction of oxygen uptake was devoted to ATP synthesis. Basal respiration could be stimulated >5-fold by the addition of the uncoupler carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), indicating a good reserve capacity of the respiratory chain (supplemental Fig. 1A). In addition, permeabilized cells displayed a good phosphorylation capacity after energization with succinate (supplemental Fig. 1B), and we used these conditions to study the properties of mitochondrial Ca2+ transport.
Energized mitochondria readily took up and retained a Ca2+ pulse of 25 μm (Fig. 2A, trace a), in a process that was fully inhibited by pretreatment with RR (Fig. 2A, trace b), the inhibitor of the mitochondrial Ca2+ uniporter (40, 41) in mammals (42–44). After accumulation of Ca2+ and addition of RR, Ca2+ efflux could be stimulated by Na+ (Fig. 2A, traces c–e) in the range 0.1–10 mm, with a concentration dependence (Fig. 2B) that is very similar to the Na+-Ca2+ antiporter of mammalian mitochondria (45–48), recently identified as NCLX (49).
FIGURE 2.
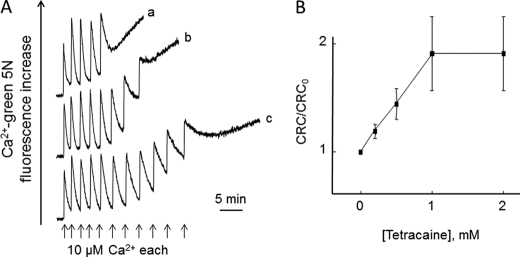
Mitochondrial Ca2+ transport in permeabilized Drosophila S2R+ cells. Digitonin-permeabilized S2R+ cells were incubated in 130 mm KCl, 10 mm MOPS-Tris, 5 mm Pi-Tris, 5 mm succinate-Tris, 10 μm EGTA, 2 μm rotenone, and 0.5 μm Calcium Green 5N, pH 7.4. A, in trace b only, the incubation medium was supplemented with 0.2 μm RR; where indicated, 25 μm Ca2+ with no further additions (trace a) or followed by 0.2 μm RR and by 0.1 mm (trace c), 1 mm (trace d), or 10 mm NaCl (trace e). B, rate of Na+-induced Ca2+ release obtained in protocols such as those depicted in A as a function of the added Na+ concentration; values were normalized to the rate observed after the addition of 10 mm NaCl (taken as maximal), and error bars report the S.D. of triplicate experiments. C, in trace e only, the medium was supplemented with 1 μg/ml cyclosporin A; where indicated, 25 μm Ca2+ was added followed by the addition of 0.2 μm RR and/or 0.5 μm FCCP where indicated by arrows as follows: no addition after the Ca2+ pulse (a), RR only (b), FCCP only (c), and RR and FCCP (d and e). D, where indicated, 25 μm Ca2+ pulse, 0.2 μm RR, 0.5 mm (b), 1 mm (c), or 2 mm (d) tetracaine and 0.5 μm FCCP. Trace a was obtained after the addition of RR and FCCP without tetracaine.
Addition of RR alone after Ca2+ uptake was followed by a slow process of Ca2+ release (Fig. 2C, trace b), which suggests the existence of a Na+-insensitive Ca2+ release pathway as also found in mammalian mitochondria (10). Addition of FCCP after accumulation of Ca2+ caused a fast process of Ca2+ release (Fig. 2C, trace c), which was only partly inhibited by RR (Fig. 2C, trace d) without any additional inhibitory effect of cyclosporin A (Fig. 2C, trace e). These experiments suggest the presence of a voltage-dependent Ca2+ release pathway (the RR-insensitive fraction of FCCP-induced Ca2+ release) resembling the PTP of mammalian mitochondria except for its lack of sensitivity to cyclosporin A (50, 51). We screened additional compounds for potential inhibition of RR-insensitive, FCCP-induced Ca2+ release, and we found a concentration-dependent inhibition by tetracaine (Fig. 2D, traces b–d), which also inhibits the PTP of mammalian mitochondria (52, 53).
We next studied the Ca2+ retention capacity (CRC) of Drosophila mitochondria by adding a train of Ca2+ pulses to permeabilized cells (Fig. 3). Ca2+ uptake was followed by spontaneous Ca2+ release (Fig. 3A, trace a), which was accompanied by mitochondrial depolarization (results not shown) and delayed by tetracaine (Fig. 3A, traces b and c), which considerably increased the CRC (Fig. 3B). Note that the rate of Ca2+ uptake was not affected by tetracaine, indicating that the Ca2+ uniporter is not inhibited by this drug.
FIGURE 3.
Effect of tetracaine on CRC of permeabilized Drosophila S2R+ cells. Experimental conditions were as described in the legend to Fig. 2, except that the concentration of Pi was 1 mm. A, extramitochondrial Ca2+ was monitored, and CRC was determined by stepwise addition of 10 μm Ca2+ pulses (arrows) in the absence of further additions (trace a) or in the presence of 0.5 or 1 mm tetracaine (traces b and c, respectively). B, The amount of Ca2+ accumulated prior to onset of Ca2+-induced Ca2+ release in presence of the stated concentrations of tetracaine was normalized to that obtained in absence of tetracaine (CRC0).
The CRC was strikingly affected by Pi, in the sense that the threshold Ca2+ load required for onset of Ca2+ release increased at increasing concentrations of Pi (Fig. 4). The rate of spontaneous Ca2+ release decreased at increased Pi concentrations despite the larger matrix Ca2+ load (Fig. 4A). The half-maximal effect of Pi was seen at ∼1 mm, which is similar to that required for inhibition by Pi of the PTP of yeast (23, 54) and of the PTP of mammals in cyclophilin D null mitochondria and in wild-type mitochondria treated with cyclosporin A (30).
FIGURE 4.
Effect of Pi on CRC of permeabilized Drosophila S2R+ cells. Experimental conditions were as described in the legend to Fig. 2, except that the concentration of Pi in A was 0.1, 1, or 5 mm (traces a, b, and c, respectively) or as indicated on the abscissa in B, where the CRC was normalized to the one obtained in the presence of 1 mm Pi (CRC1 mm Pi).
We also tested the effect on the CRC of Ub0, a cyclophilin D-independent inhibitor of the mammalian pore (55, 56) and of the combination of ADP plus oligomycin, which is very effective at desensitizing the PTP to Ca2+ (57). No changes of CRC were observed with any of these PTP inhibitors, irrespective of whether the Pi concentration was 1 or 5 mm (supplemental Fig. 2).
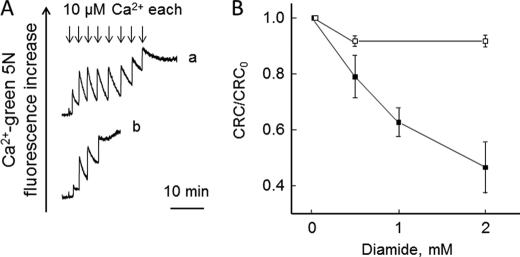
Mitochondrial Ca2+-induced Ca2+ release could be induced by the dithiol oxidant diamide (Fig. 5A) in a process that was prevented by dithiothreitol (Fig. 5B). Ca2+ release could also be induced by N-ethylmaleimide (NEM) (Fig. 6) after a lag phase that decreased as the concentration of NEM was increased (Fig. 6B) in the same range causing PTP opening in mammalian mitochondria (58).
FIGURE 5.
Effect of diamide on CRC of permeabilized Drosophila S2R+ cells. Experimental conditions were as in Fig. 2, except that the concentration of Pi was 1 mm. A,where indicated, 10 μm Ca2+ pulses (arrows) were added in the absence (trace a) or presence of 2 mm diamide (trace b). B, the CRC in presence of the stated concentrations of diamide alone (closed symbols) or after treatment with 1 mm dithiothreitol 1 min after diamide (open symbols) was normalized to that obtained in absence of diamide (CRC0).
FIGURE 6.
Effect of NEM on Ca2+ retention of permeabilized Drosophila S2R+ cells. Experimental conditions were as in Fig. 5. A, three 10 μm Ca2+ pulses were added followed by 0.05 (trace a), 0.1 (trace b), 0.25 (trace c), 0.5 (trace d), or 1 mm NEM (trace e). B, half-time required for Ca2+ release was calculated for experiments like those depicted in A.
We assessed mitochondrial volume changes in mitochondria subjected to an appropriate Ca2+ load sufficient to cause spontaneous Ca2+ release at 0.1 mm Pi. Parallel readings of Ca2+ fluxes (Fig. 7A) and of light scattering at 540 nm (a sensitive measure of mitochondrial volume changes, Fig. 7B) revealed that after the small light scattering increase (matrix volume contraction) accompanying Ca2+ uptake no matrix swelling (which should manifest itself as a decreased light scattering) could be detected after the onset of Ca2+ release (Fig. 7B). It should be noted that mitochondria in permeabilized S2R+ cells can undergo swelling upon addition of the pore-forming peptide alamethicin, which also caused rapid release of residual matrix Ca2+ (Fig. 7), or of the selective K+ ionophore valinomycin (supplemental Fig. 3). Mitochondrial Ca2+-dependent Ca2+ release was not accompanied by cytochrome c release, which was instead readily elicited by the addition of alamethicin (Fig. 7C). This result is particularly striking because our experiments were carried out in KCl-based medium, which promotes ready cytochrome c removal if the outer membrane breaks following osmotic swelling of mammalian mitochondria (59). Electron microscopy fully confirmed that the condensed mitochondrial morphology was totally unaffected by a load of Ca2+ able to induce full Ca2+ release (compare the left and middle panels of Fig. 7D). This is a unique feature compared with the swelling response of mitochondria from all sources tested so far under similar conditions (19). On the other hand, mitochondrial swelling was readily detected after the addition of alamethicin (Fig. 7D, right panel).
FIGURE 7.
Effect of Ca2+ on light scattering, release of cytochrome c and mitochondrial ultrastructure in permeabilized Drosophila S2R+ cells. Experimental conditions were as described in the legend to Fig. 2, except that the concentration of Pi was 0.1 mm and calcium Green 5N was omitted in the experiments of B. A and B, where indicated, 40 μm Ca2+ and 3 μm alamethicin were added. C, permeabilized cells were centrifuged before the addition of Ca2+, after addition of 40 μm Ca2+ or after addition of 40 μm Ca2+ and 3 μm alamethicin (as indicated by + and − symbols); pellets (P) and supernatants (S) were subjected to SDS-PAGE, transfer, and subsequent Western blotting with specific antibodies against cytochrome (cyt) c, TOM 20, and cytochrome oxidase (COX) subunit I. D, permeabilized cells were fixed and processed for electron microscopy before the addition of Ca2+ (left panel), after addition of 40 μm Ca2+ (middle panel) or after addition of 40 μm Ca2+ and 3 μm alamethicin (right panel); bar, 200 nm.
DISCUSSION
In this work, we have characterized the pathways for Ca2+ transport in mitochondria from digitonin-permeabilized Drosophila S2R+ cells. These cells were originally derived from late embryonic stages (20–24 h), and selection was made based on the ability to adhere to tissue culture dishes (60). According to Schneider (60), they represent a variety of tissue precursors, and we assume that they are representative of Drosophila, although a full characterization of the Ca2+ release channel will have to await its molecular definition.
We have found that mitochondria of S2R+ cells possess the classical pathways found in mammalian mitochondria, i.e. (i) the RR-sensitive Ca2+ uniporter, which has been characterized by electrophysiology (12) and recently identified at the molecular level in mammals (40, 41). The existence in the Drosophila genome of close orthologs of the mitochondrial Ca2+ uniporter (40, 41) and of the previously identified MICU1 (61) (CG18769 and CG4495, respectively) predicts the existence of a mitochondrial Ca2+ uniporter in keeping with our findings. (ii) The Na+-Ca2+ antiporter recently identified as NCLX (49), whose ortholog also exists in Drosophila (CG14744) and is the likely mediator of the Na+-dependent Ca2+ release defined here. (iii) The putative H+-Ca2+ antiporter mediating Ca2+ release at high membrane potential, which can be unmasked by the addition of RR (10). Notably, it recently has been proposed that LETM1 (and its Drosophila ortholog CG4589) mediates H+-Ca2+ exchange by catalyzing RR-sensitive Ca2+ uptake in mitochondria (62). However, this contrasts with the well established role of LETM1 as a K+-H+ antiporter (63–66) and with the fact, confirmed here, that the putative H+-Ca2+ antiporter is insensitive to RR. (iv) A tetracaine-sensitive, RR-insensitive release pathway that opens in response to matrix Ca2+ loading or to depolarization and mediates Ca2+ release. The tetracaine-sensitive pathway, which displays unique features that appear to be intermediate between those of the PTP of yeast and mammals (31), is the main focus of the present manuscript.
Disequilibrium between Distribution of Ca2+ and Its Electrochemical Gradient
Ca2+ uptake is an electrophoretic process driven by the Ca2+ electrochemical gradient, ΔũCa.
In respiring mitochondria, the inside-negative Δψ favors uptake of Ca2+ (67, 68); and with a Δψ of −180 mV, the Ca2+ accumulation ratio at equilibrium (i.e. at ΔũCa = 0) should be 106 (69). This is never reached because at resting cytosolic Ca2+ levels, the rate of Ca2+ uptake is comparable with that of the efflux pathways, and Ca2+ distribution is governed by a kinetic steady state rather than by the thermodynamic equilibrium (69, 70). The activity of the mitochondrial Ca2+ uniporter and of the antiporters indeed creates a Ca2+ cycle across the inner membrane, whose energy requirement is very low (71) because the combined maximal rate of the efflux pathways is ∼20 nmol Ca2+ × mg−1 protein × min−1 (10). On the other hand, because the Vmax of the uniporter is ∼1400 nmol Ca2+ × mg−1 protein × min−1, and its activity increases sharply with the increase of extramitochondrial [Ca2+] (72), this arrangement exposes mitochondria to the hazards of Ca2+ overload when cytosolic [Ca2+] increases. We have argued that the PTP may serve the purpose of providing mitochondria with a fast Ca2+ release channel (10, 14). This hypothesis is consistent with the effects of cyclosporin A on Ca2+ distribution in rat ventricular cardiomyocytes (73), with a PTP activating response to the combined action of two physiological stimuli increasing cytosolic [Ca2+] without detrimental effects on cell survival (17), and with the demonstration that cyclophilin D ablation causes mitochondrial Ca2+ overload in vivo, which, in turn, increases the propensity to heart failure after transaortic constriction, overexpression of Ca2+/calmodulin-dependent protein kinase IIδc or swimming exercise (16; see Ref. 75 for discussion).
Properties of Drosophila Ca2+-induced Ca2+ Release
The properties of the Drosophila Ca2+ release system described here appear to be intermediate between those of the PTP of mammals and yeast. Like the mammalian pore, Drosophila Ca2+ release is inhibited by tetracaine (52) and opens in response to matrix Ca2+ loading (76), inner membrane depolarization (77), thiol oxidation (78), and treatment with relatively high concentrations of NEM (58); like the yeast PTP (and at variance from the mammalian pore), it is inhibited by Pi (22, 23) and insensitive to cyclosporin A (23). The latter observations may be strictly related. Pi is a classical inducer of the mammalian PTP, yet Pi is essential for PTP inhibition by cyclosporin A and cyclophilin D ablation (30), suggesting that cyclophilin D masks an inhibitory site for Pi (79). It is interesting to note that a Drosophila mitochondrial cyclophilin has not been found and that even Drosophila Cyp1-PA, which according to the primary sequence, has a high probability of import into mitochondria, could not be found in the organelle after tagging with GFP and expression in S2R+ and KC cells.4 It is tempting to speculate that lack of mitochondrial cyclophilin leaves the Pi inhibitory site unhindered and that the PTP-stimulating ability of Pi has developed after the evolutionary divergence of Drosophila and vertebrates.
At the onset of Ca2+-dependent Ca2+ release, Drosophila mitochondria undergo depolarization, suggesting that the putative channel is also permeable to H+. On the other hand, no matrix swelling is observed in KCl-based medium, indicating that the channel is not permeable to K+ (and Cl−), despite the fact that the hydrated radius of Ca2+ is larger than that of K+. Lack of swelling, which was confirmed by lack of cytochrome c release and by ultrastructural analysis, is not due to peculiar features of Drosophila mitochondria because matrix swelling and cytochrome c release readily followed the addition of the K+ ionophore valinomycin or of the pore-forming peptide alamethicin. We conclude that the putative Ca2+ release channel of Drosophila mitochondria is also permeable to H+. This is an essential feature because the Ca2+ diffusion potential created by efflux through a Ca2+-selective channel would otherwise oppose Ca2+ release (10).
Mitochondrial Ca2+-dependent Ca2+ Release as Mediator of Cell Death in Drosophila?
Available evidence points to persistent activation of the PTP as a prime mediator of apoptotic or necrotic cell death in a variety of situations (19). Indeed, unregulated opening of the PTP and ensuing mitochondrial and cellular dysfunction may be responsible for the pathology that characterizes a variety of human diseases (19). Although many of the proteins important for apoptosis in mammalian cells are conserved in Drosophila, the role that mitochondria play in cell death in this organism remains controversial (74, 80). The apparent absence of a regulatory role for a mitochondrial cyclophilin in the function of the “Drosophila PTP” prevents an investigation based on the effects of cyclosporin A in cells. However, our functional studies pave the way for the application of the sophisticated genetic strategies available in Drosophila to define the molecular nature of the channel and its role in pathophysiology of Ca2+ homeostasis.
This work was supported, in whole or in part, by National Institutes of Health Grant GM069883. This work was also supported in part by grants from the Fondazione Cariparo and the University of Padova Progetti di Eccellenza Models of Mitochondrial Diseases.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
K. R. Jones and M. A. Forte, unpublished results.
- PTP
- permeability transition pore
- CRC
- Ca2+ retention capacity
- Δψ
- inner membrane potential difference
- FCCP
- carbonylcyanide-p-trifluoromethoxyphenylhydrazone
- NEM
- N-ethylmaleimide
- OMM
- outer mitochondrial membrane
- RR
- ruthenium red
- TMRM
- tetramethylrhodamine methyl ester.
REFERENCES
- 1. Friel D. D. (2000) Cell Calcium 28, 307–316 [DOI] [PubMed] [Google Scholar]
- 2. Giacomello M., Drago I., Pizzo P., Pozzan T. (2007) Cell Death Differ. 14, 1267–1274 [DOI] [PubMed] [Google Scholar]
- 3. Csordás G., Hajnóczky G. (2009) Biochim. Biophys. Acta 1787, 1352–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rizzuto R., Marchi S., Bonora M., Aguiari P., Bononi A., De Stefani D., Giorgi C., Leo S., Rimessi A., Siviero R., Zecchini E., Pinton P. (2009) Biochim. Biophys. Acta 1787, 1342–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kann O., Kovács R. (2007) Am. J. Physiol. Cell Physiol. 292, C641–657 [DOI] [PubMed] [Google Scholar]
- 6. Nicholls D. G. (2009) Biochim. Biophys. Acta 1787, 1416–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rizzuto R., Bernardi P., Pozzan T. (2000) J. Physiol. Lond. 529, 37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rizzuto R., Pozzan T. (2003) Nat. Genet. 34, 135–141 [DOI] [PubMed] [Google Scholar]
- 9. Szabadkai G., Duchen M. R. (2008) Physiology 23, 84–94 [DOI] [PubMed] [Google Scholar]
- 10. Bernardi P. (1999) Physiol. Rev. 79, 1127–1155 [DOI] [PubMed] [Google Scholar]
- 11. Gunter T. E., Gunter K. K. (2001) IUBMB Life 52, 197–204 [DOI] [PubMed] [Google Scholar]
- 12. Kirichok Y., Krapivinsky G., Clapham D. E. (2004) Nature 427, 360–364 [DOI] [PubMed] [Google Scholar]
- 13. Gunter T. E., Sheu S. S. (2009) Biochim. Biophys. Acta 1787, 1291–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bernardi P., Petronilli V. (1996) J. Bioenerg. Biomembr. 28, 131–138 [DOI] [PubMed] [Google Scholar]
- 15. Ichas F., Jouaville L. S., Mazat J. P. (1997) Cell 89, 1145–1153 [DOI] [PubMed] [Google Scholar]
- 16. Elrod J. W., Wong R., Mishra S., Vagnozzi R. J., Sakthievel B., Goonasekera S. A., Karch J., Gabel S., Farber J., Force T., Brown J. H., Murphy E., Molkentin J. D. (2010) J. Clin. Invest. 120, 3680–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barsukova A., Komarov A., Hajnóczky G., Bernardi P., Bourdette D., Forte M. (2011) Eur. J. Neurosci. 33, 831–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rasola A., Sciacovelli M., Pantic B., Bernardi P. (2010) FEBS Lett. 584, 1989–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bernardi P., Krauskopf A., Basso E., Petronilli V., Blachly-Dyson E., Di Lisa F., Forte M. A. (2006) FEBS J. 273, 2077–2099 [DOI] [PubMed] [Google Scholar]
- 20. Prieto S., Bouillaud F., Ricquier D., Rial E. (1992) Eur. J. Biochem. 208, 487–491 [DOI] [PubMed] [Google Scholar]
- 21. Guérin B., Bunoust O., Rouqueys V., Rigoulet M. (1994) J. Biol. Chem. 269, 25406–25410 [PubMed] [Google Scholar]
- 22. Prieto S., Bouillaud F., Rial E. (1996) Arch. Biochem. Biophys. 334, 43–49 [DOI] [PubMed] [Google Scholar]
- 23. Jung D. W., Bradshaw P. C., Pfeiffer D. R. (1997) J. Biol. Chem. 272, 21104–21112 [DOI] [PubMed] [Google Scholar]
- 24. Roucou X., Manon S., Guérin M. (1997) Biochim. Biophys. Acta 1324, 120–132 [DOI] [PubMed] [Google Scholar]
- 25. Uribe-Carvajal S., Luévano-Martínez L. A., Guerrero-Castillo S., Cabrera-Orefice A., Corona-de-la-Peña N. A., Gutiérrez-Aguilar M. (2011) Mitochondrion 11, 382–390 [DOI] [PubMed] [Google Scholar]
- 26. Fournier N., Ducet G., Crevat A. (1987) J. Bioenerg. Biomembr. 19, 297–303 [DOI] [PubMed] [Google Scholar]
- 27. Crompton M., Ellinger H., Costi A. (1988) Biochem. J. 255, 357–360 [PMC free article] [PubMed] [Google Scholar]
- 28. Broekemeier K. M., Klocek C. K., Pfeiffer D. R. (1998) Biochemistry 37, 13059–13065 [DOI] [PubMed] [Google Scholar]
- 29. Halestrap A. P., Davidson A. M. (1990) Biochem. J. 268, 153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Basso E., Petronilli V., Forte M. A., Bernardi P. (2008) J. Biol. Chem. 283, 26307–26311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Azzolin L., von Stockum S., Basso E., Petronilli V., Forte M. A., Bernardi P. (2010) FEBS Lett. 584, 2504–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Manon S., Roucou X., Guérin M., Rigoulet M., Guérin B. (1998) J. Bioenerg. Biomembr. 30, 419–429 [DOI] [PubMed] [Google Scholar]
- 33. Yanagawa S., Lee J. S., Ishimoto A. (1998) J. Biol. Chem. 273, 32353–32359 [DOI] [PubMed] [Google Scholar]
- 34. Bernardi P., Scorrano L., Colonna R., Petronilli V., Di Lisa F. (1999) Eur. J. Biochem. 264, 687–701 [DOI] [PubMed] [Google Scholar]
- 35. Taupin P. (2008) Eur. J. Histochem. 52, 135–139 [DOI] [PubMed] [Google Scholar]
- 36. Angelin A., Tiepolo T., Sabatelli P., Grumati P., Bergamin N., Golfieri C., Mattioli E., Gualandi F., Ferlini A., Merlini L., Maraldi N. M., Bonaldo P., Bernardi P. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 991–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chiara F., Castellaro D., Marin O., Petronilli V., Brusilow W. S., Juhaszova M., Sollott S. J., Forte M., Bernardi P., Rasola A. (2008) PLoS One 3, e1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Azzolin L., Basso E., Argenton F., Bernardi P. (2010) Biochim. Biophys. Acta 1797, 1775–1779 [DOI] [PubMed] [Google Scholar]
- 39. Hackenbrock C. R. (1966) J. Cell Biol. 30, 269–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Stefani D., Raffaello A., Teardo E., Szabó I., Rizzuto R. (2011) Nature 476, 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baughman J. M., Perocchi F., Girgis H. S., Plovanich M., Belcher-Timme C. A., Sancak Y., Bao X. R., Strittmatter L., Goldberger O., Bogorad R. L., Koteliansky V., Mootha V. K. (2011) Nature 476, 341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moore C. L. (1971) Biochem. Biophys. Res. Commun. 42, 298–305 [DOI] [PubMed] [Google Scholar]
- 43. Carafoli E., Sacktor B. (1972) Biochem. Biophys. Res. Commun. 49, 1498–1503 [DOI] [PubMed] [Google Scholar]
- 44. Vasington F. D., Gazzotti P., Tiozzo R., Carafoli E. (1972) Biochim. Biophys. Acta 256, 43–54 [DOI] [PubMed] [Google Scholar]
- 45. Carafoli E., Tiozzo R., Lugli G., Crovetti F., Kratzing C. (1974) J. Mol. Cell Cardiol. 6, 361–371 [DOI] [PubMed] [Google Scholar]
- 46. Crompton M., Capano M., Carafoli E. (1976) Eur. J. Biochem. 69, 453–462 [Google Scholar]
- 47. Crompton M., Künzi M., Carafoli E. (1977) Eur. J. Biochem. 79, 549–558 [DOI] [PubMed] [Google Scholar]
- 48. Crompton M., Moser R., Lüdi H., Carafoli E. (1978) Eur. J. Biochem. 82, 25–31 [DOI] [PubMed] [Google Scholar]
- 49. Palty R., Silverman W. F., Hershfinkel M., Caporale T., Sensi S. L., Parnis J., Nolte C., Fishman D., Shoshan-Barmatz V., Herrmann S., Khananshvili D., Sekler I. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 436–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Broekemeier K. M., Dempsey M. E., Pfeiffer D. R. (1989) J. Biol. Chem. 264, 7826–7830 [PubMed] [Google Scholar]
- 51. Petronilli V., Cola C., Bernardi P. (1993) J. Biol. Chem. 268, 1011–1016 [PubMed] [Google Scholar]
- 52. Dawson A. P., Selwyn M. J., Fulton D. V. (1979) Nature 277, 484–486 [DOI] [PubMed] [Google Scholar]
- 53. Broekemeier K. M., Schmid P. C., Schmid H. H., Pfeiffer D. R. (1985) J. Biol. Chem. 260, 105–113 [PubMed] [Google Scholar]
- 54. Yamada A., Yamamoto T., Yoshimura Y., Gouda S., Kawashima S., Yamazaki N., Yamashita K., Kataoka M., Nagata T., Terada H., Pfeiffer D. R., Shinohara Y. (2009) Biochim. Biophys. Acta 1787, 1486–1491 [DOI] [PubMed] [Google Scholar]
- 55. Cesura A. M., Pinard E., Schubenel R., Goetschy V., Friedlein A., Langen H., Polcic P., Forte M. A., Bernardi P., Kemp J. A. (2003) J. Biol. Chem. 278, 49812–49818 [DOI] [PubMed] [Google Scholar]
- 56. Krauskopf A., Eriksson O., Craigen W. J., Forte M. A., Bernardi P. (2006) Biochim. Biophys. Acta 1757, 590–595 [DOI] [PubMed] [Google Scholar]
- 57. Novgorodov S. A., Gudz T. I., Milgrom Y. M., Brierley G. P. (1992) J. Biol. Chem. 267, 16274–16282 [PubMed] [Google Scholar]
- 58. Pfeiffer D. R., Schmid P. C., Beatrice M. C., Schmid H. H. (1979) J. Biol. Chem. 254, 11485–11494 [PubMed] [Google Scholar]
- 59. Jacobs E. E., Sanadi D. R. (1960) J. Biol. Chem. 235, 531–534 [PubMed] [Google Scholar]
- 60. Schneider I. (1972) J. Embryol. Exp. Morphol. 27, 353–365 [PubMed] [Google Scholar]
- 61. Perocchi F., Gohil V. M., Girgis H. S., Bao X. R., McCombs J. E., Palmer A. E., Mootha V. K. (2010) Nature 467, 291–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jiang D., Zhao L., Clapham D. E. (2009) Science 326, 144–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nowikovsky K., Froschauer E. M., Zsurka G., Samaj J., Reipert S., Kolisek M., Wiesenberger G., Schweyen R. J. (2004) J. Biol. Chem. 279, 30307–30315 [DOI] [PubMed] [Google Scholar]
- 64. Nowikovsky K., Reipert S., Devenish R. J., Schweyen R. J. (2007) Cell Death Differ. 14, 1647–1656 [DOI] [PubMed] [Google Scholar]
- 65. Dimmer K. S., Navoni F., Casarin A., Trevisson E., Endele S., Winterpacht A., Salviati L., Scorrano L. (2008) Hum. Mol. Genet. 17, 201–214 [DOI] [PubMed] [Google Scholar]
- 66. McQuibban A. G., Joza N., Megighian A., Scorzeto M., Zanini D., Reipert S., Richter C., Schweyen R. J., Nowikovsky K. (2010) Hum. Mol. Genet. 19, 987–1000 [DOI] [PubMed] [Google Scholar]
- 67. Scarpa A., Azzone G. F. (1970) Eur. J. Biochem. 12, 328–335 [DOI] [PubMed] [Google Scholar]
- 68. Wingrove D. E., Amatruda J. M., Gunter T. E. (1984) J. Biol. Chem. 259, 9390–9394 [PubMed] [Google Scholar]
- 69. Azzone G. F., Pozzan T., Massari S., Bragadin M., Dell'Antone P. (1977) FEBS Lett. 78, 21–24 [DOI] [PubMed] [Google Scholar]
- 70. Nicholls D. G. (1978) Biochem. J. 176, 463–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stucki J. W., Ineichen E. A. (1974) Eur. J. Biochem. 48, 365–375 [DOI] [PubMed] [Google Scholar]
- 72. Bragadin M., Pozzan T., Azzone G. F. (1979) Biochemistry 18, 5972–5978 [DOI] [PubMed] [Google Scholar]
- 73. Altschuld R. A., Hohl C. M., Castillo L. C., Garleb A. A., Starling R. C., Brierley G. P. (1992) Am. J. Physiol. 262, H1699–1704 [DOI] [PubMed] [Google Scholar]
- 74. Wang C., Youle R. J. (2009) Annu. Rev. Genet. 43, 95–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Di Lisa F., Carpi A., Giorgio V., Bernardi P. (2011) Biochim. Biophys. Acta 1813, 1316–1322 [DOI] [PubMed] [Google Scholar]
- 76. Hunter D. R., Haworth R. A., Southard J. H. (1976) J. Biol. Chem. 251, 5069–5077 [PubMed] [Google Scholar]
- 77. Bernardi P. (1992) J. Biol. Chem. 267, 8834–8839 [PubMed] [Google Scholar]
- 78. Petronilli V., Costantini P., Scorrano L., Colonna R., Passamonti S., Bernardi P. (1994) J. Biol. Chem. 269, 16638–16642 [PubMed] [Google Scholar]
- 79. Giorgio V., Soriano M. E., Basso E., Bisetto E., Lippe G., Forte M. A., Bernardi P. (2010) Biochim. Biophys. Acta 1797, 1113–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Krieser R. J., White K. (2009) Apoptosis 14, 961–968 [DOI] [PMC free article] [PubMed] [Google Scholar]