Background: Previously we have shown that PMCA4 interacts with nNOS.
Results: In PMCA4−/− mice, plasma membrane-associated nNOS protein was delocalized to the cytosol with no change in total nNOS protein.
Conclusion: The current study shows that PMCA4-nNOS complex modulates a spatially confined cyclic nucleotide microdomain at the plasma membrane.
Significance: Compartmentalization of the PMCA4-nNOS complex has a major role in regulating cardiac contractility.
Keywords: Adrenergic Receptor, Calcium, Cardiac Muscle, Nitric-oxide Synthase, Signal Transduction, Contractility, PMCA, Plasma Membrane Calcium ATPase, cAMP, cGMP
Abstract
Identification of the signaling pathways that regulate cyclic nucleotide microdomains is essential to our understanding of cardiac physiology and pathophysiology. Although there is growing evidence that the plasma membrane Ca2+/calmodulin-dependent ATPase 4 (PMCA4) is a regulator of neuronal nitric-oxide synthase, the physiological consequence of this regulation is unclear. We therefore tested the hypothesis that PMCA4 has a key structural role in tethering neuronal nitric-oxide synthase to a highly compartmentalized domain in the cardiac cell membrane. This structural role has functional consequences on cAMP and cGMP signaling in a PMCA4-governed microdomain, which ultimately regulates cardiac contractility. In vivo contractility and calcium amplitude were increased in PMCA4 knock-out animals (PMCA4−/−) with no change in diastolic relaxation or the rate of calcium decay, showing that PMCA4 has a function distinct from beat-to-beat calcium transport. Surprisingly, in PMCA4−/−, over 36% of membrane-associated neuronal nitric-oxide synthase (nNOS) protein and activity was delocalized to the cytosol with no change in total nNOS protein, resulting in a significant decrease in microdomain cGMP, which in turn led to a significant elevation in local cAMP levels through a decrease in PDE2 activity (measured by FRET-based sensors). This resulted in increased L-type calcium channel activity and ryanodine receptor phosphorylation and hence increased contractility. In the heart, in addition to subsarcolemmal calcium transport, PMCA4 acts as a structural molecule that maintains the spatial and functional integrity of the nNOS signaling complex in a defined microdomain. This has profound consequences for the regulation of local cyclic nucleotide and hence cardiac β-adrenergic signaling.
Introduction
The plasma membrane calcium pumps (PMCAs),6 which eject Ca2+ into the extracellular space (1, 2), have traditionally been assigned only minor roles in the heart when compared with proteins such as the sarcoplasmic reticulum calcium-ATPase (SERCA), the ryanodine receptor, the sodium calcium exchanger (3, 4), calsequestrin (5), and others (6). In sharp contrast, our studies have shown that the PMCA, in particular isoform 4, might serve functions not previously considered (reviewed in Ref. 7). Originally, we (8) and others (9) showed that PMCA4 is localized to caveolae, i.e. core sites of cellular signaling (10, 11). Furthermore, we have described that PMCA4 interacts with several signaling molecules such as Ras-associated factor 1A (RASSF1A) (12), calcineurin (13), α1-syntrophin (14), and nNOS (15). The findings of a recent genome-wide association study and other human studies have indicated that PMCA4 is highly relevant to cardiac disease (16–18) and thus underscore the necessity to fully elucidate the role of PMCA4 in the heart.
A complete understanding of the role of PMCA4 might help to provide a solution to a classical conundrum in cardiac physiology, i.e. how the cardiac cell uses calcium for signaling against the background of very large swings in calcium concentration during the excitation/contraction cycle (19). Essentially, two hypotheses have been brought forward. Firstly, signaling information is carried by “contractile” calcium. Alternatively, two pools of calcium can be distinguished, one for contraction and the other for signaling (19).
If it were possible to identify a calcium transporter that is involved exclusively in signaling, but not in contraction/relaxation, this finding would strongly favor a model in which the two functions of Ca2+ can clearly be separated. Such a model would also be key in facilitating the design of experiments to directly demonstrate the separate calcium pools, e.g. through cloning of calcium sensors attached to the transporter(s) responsible for signaling.
We hypothesized that PMCA4, similarly to transient receptor potential canonical channels (20) and inositol 1,4,5-triphosphate receptor (21), is such a calcium transporter, having an exclusive role in signaling. We further hypothesized that PMCA4 is essential for the tethering of nNOS to the cardiac membrane. It has previously been shown that nNOS is localized to both the sarcoplasmic reticulum and the cell membrane and can shuttle between these compartments with functional consequences in health and disease (22–24). However, the mechanisms underlying the localization of nNOS to the plasma membrane remained unknown. Here we show that PMCA4 is a key protein in this process and that tethering of nNOS by PMCA4 has profound functional consequences on local cyclic nucleotide signaling.
EXPERIMENTAL PROCEDURES
Animals
Our previously described PMCA4 germline null mutant mice were used in the study (25). All animal experiments were performed on adult mice (16–20 weeks old) in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986 and were approved by the University of Manchester Ethics Committee. Adenoviral constructs, hemodynamic analysis, confocal immunofluorescence, NOS activity assay, isolation of neonatal and adult cardiomyocytes, determination of whole cell calcium transients, electrophysiology, sarcoplasmic reticulum calcium contents, NCX current and fluorescence resonance emission transfer (FRET) imaging for phosphodiesterase (PDE) activity, assessment of S-nitrosylation, immunogold staining and electron microscopy, and PKA activity assay are detailed in the supplemental methods.
Western Blotting
Animals were killed by cervical dislocation, hearts were homogenized in radioimmune precipitation buffer (1× PBS, 1% IGEPAL, 0.5% sodium deoxycholate, 0.1% SDS, 20 μm PMSF, 500 ng/ml leupeptin, 1 μg/ml aprotinin, 500 ng/ml pepstatin), and protein content was determined using a BCA protein assay reagent kit (Pierce). For immunoprecipitation experiments, protein lysates (1 ml) were precleared by incubation with 200 μl of protein G-agarose beads (Roche Applied Science) and 5 μl of anti-luciferase polyclonal antibody (1 mg/ml, Promega) at 4 °C for 1 h. Beads were removed by centrifugation at 1000 × g, and 500 μl of precleared extracts were incubated overnight at 4 °C with either the anti-caveolin-3 monoclonal antibody (Abcam) or, as a control, an anti-luciferase polyclonal serum (Promega) and 40 μl of protein. Western blotting was conducted by separating equal amounts of protein on 8% polyacrylamide gels and electroblotting onto nitrocellulose membrane using the recommended transfer buffer. Blots were probed with antibodies selective for PMCA4 (JA9, Abcam), PMCA1 (anti-PMCA1, Abcam), nNOS (anti-NOS-1, Affinity BioReagents), eNOS (anti-eNOS, Abcam), L-type calcium channels (anti-DHPR α2 subunit, Abcam), SERCA2 (anti-SERCA2a, Affinity BioReagents), Na/K-ATPase (anti-Na/K-ATPase, Santa Cruz Biotechnology), GAPDH (anti-GAPDH, Abcam), RYR (anti-RYR, Abcam), Ser(P)-2808 RYR (Anti-RYR phospho-Ser2808, Badrilla), NCX (anti-NCX, SWANT Inc.) or α-tubulin (anti-α-tubulin, Calbiochem), and anti-mouse HRP (Dako) or anti-rabbit HRP (Jackson ImmunoResearch Laboratories) were used as secondary antibodies. Immunocomplexes were visualized using enhanced chemiluminescence according to the manufacturer's protocol (Amersham Biosciences) and exposed to autoradiographic film (Eastman Kodak Co.).
Subcellular Fractionation and nNOS Activity Assay
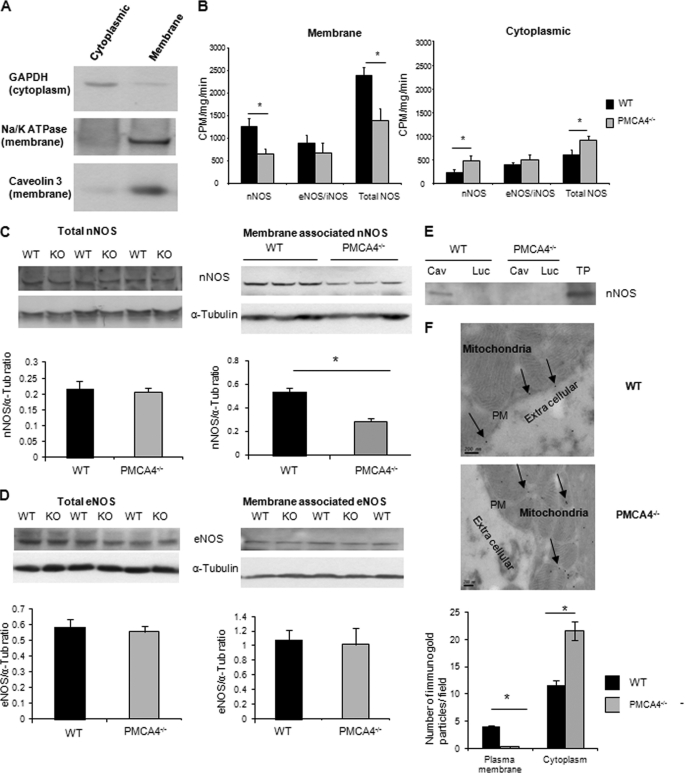
Heart tissue was fractionated into cytoplasmic and membrane fractions using the S-PEK subcellular fractionation kit (Calbiochem) following the manufacturer's protocol. The separation of compartments was validated (see Fig. 3A). Measurement of nNOS activity and of cGMP and cAMP levels was carried out as described previously (26).
FIGURE 3.
nNOS activity and expression are reduced in the membrane fraction of PMCA4−/− hearts. A, Western blot analysis of cytoplasmic and membrane fractions from WT cardiac tissue probed with antibodies against cytoplasmic (GAPDH) or membrane proteins (Na/K-ATPase and caveolin-3) to confirm the purity of the cardiac membrane and cytoplasmic fractions. B, membrane-associated nNOS and total NOS activities were significantly reduced in PMCA4−/− mice when compared with their WT littermates; however, activities of the other NOS isoforms (eNOS/ inducible NOS (iNOS)) were not changed. In contrast, cytoplasmic nNOS and total NOS activity were elevated in PMCA4−/− mice when compared with controls (*, p < 0.05, n = 11). C and D, Western blots showing nNOS (C) and eNOS (D) expression in the membrane fraction and total nNOS and eNOS expression in crude heart lysates from PMCA4−/− and WT controls. The quantification of these blots showed a significant reduction only in membrane-associated nNOS in PMCA4−/− when compared with WT littermates (n = 7, *, p < 0.05). α-Tub, α-tubulin. E, heart extracts were immunoprecipitated with anti-caveolin-3 (Cav) or anti-luciferase antibody (Luc) as a negative control. Western blot of precipitated protein using anti-nNOS antibody showed that caveolin-bound nNOS (plasma membrane nNOS) was abolished in PMCA4−/− hearts. Total protein (TP) from heart lysate was used as a positive control. F, representative electron micrographs of isolated adult cardiomyocytes stained with immunogold-labeled antibody for nNOS showing the different localization of nNOS in the PMCA4−/− myocytes when compared with WT. These micrographs showed the complete absence of nNOS from the plasma membrane (PM). Analysis of 60 fields (20 from each animal) in each group showed the virtual absence of nNOS from the membrane and ∼50% increase in cytoplasmic nNOS in PMCA4−/− when compared with their WT littermates (n = 3 animals in each group, *, p < 0.05).
Data Analysis and Statistics
Data are presented as mean ± S.E. Statistical analyses were carried out using the Student's t test or one-way analysis of variance, where appropriate, using the SPSS statistical software. Values were considered significantly different when p < 0.05.
RESULTS
PMCA4 Does Not Contribute to Global Calcium Removal
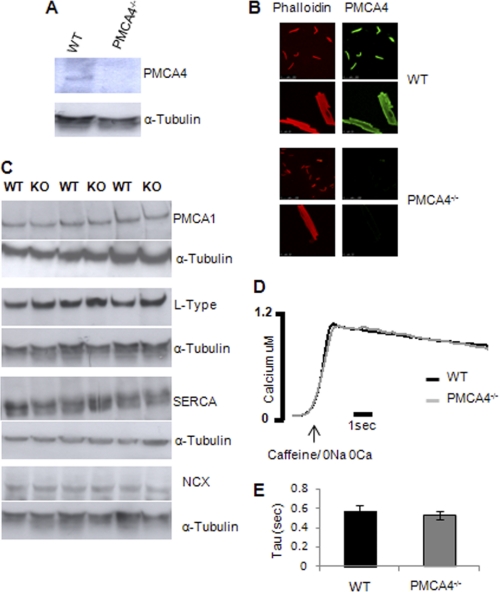
To study the role of PMCA4 in the heart, we used our mice with genetic ablation of the Pmca4 gene (PMCA4−/−) (25). The absence of PMCA4 in total heart homogenates and cardiomyocytes was confirmed by Western blot (Fig. 1A) and immunofluorescence (Fig. 1B), respectively. Ablation of PMCA4 did not result in changes in the expression of other major calcium-handling proteins such as SERCA2a, NCX, and L-type calcium channels or in the expression of the other isoform of PMCA expressed in the cardiomyocytes (PMCA1) (Fig. 1C). To determine the contribution of PMCA4 to the removal of Ca2+ during relaxation, PMCA4−/− cardiomyocytes were perfused with Na+/Ca2+-free solution containing 10 mm caffeine (Fig. 1D). Cardiomyocytes lacking PMCA4 showed a similar Ca2+ decay pattern to wild type (WT) cells (Fig. 1E; Ca2+ decay rate (relaxation time constant, τ) of 0.57 ± 0.04 ms in WT versus 0.52 ± 0.06 ms in PMCA4−/− cells), suggesting that ablation of PMCA4 did not modify the calcium decay rate during relaxation. These data confirmed that the contribution of PMCA4 to diastolic calcium removal is minor.
FIGURE 1.
The absence of PMCA4 in the heart had no effect on global calcium extrusion. A, Western blot showing PMCA4 deletion in cardiac tissue from PMCA4−/− mice. B, immunostaining of isolated single cardiomyocytes to verify efficiency of the deletion of PMCA4. Alexa Fluor 594 phalloidin was used to stain the F-actin to identify cardiomyocytes. C, representative Western blots to detect the expression of PMCA1, L-type calcium channel, SERCA2, and NCX in heart extracts from PMCA4−/− mice. D, representative traces of calcium decay following application of Na+/Ca2+ free solution (to completely inhibit the Na+/Ca2+ exchanger) in the presence of 10 mm caffeine (to empty the sarcoplasmic reticulum). E, quantification of the time constant of the Ca2+ decay (τ) suggested that Ca2+ efflux through PMCA is not impaired in the PMCA4−/− cardiomyocytes, which indicates the negligible contribution of PMCA4 to global calcium extrusion (n = 6).
PMCA4 Regulates Cardiac Contractility through an nNOS-dependent Mechanism
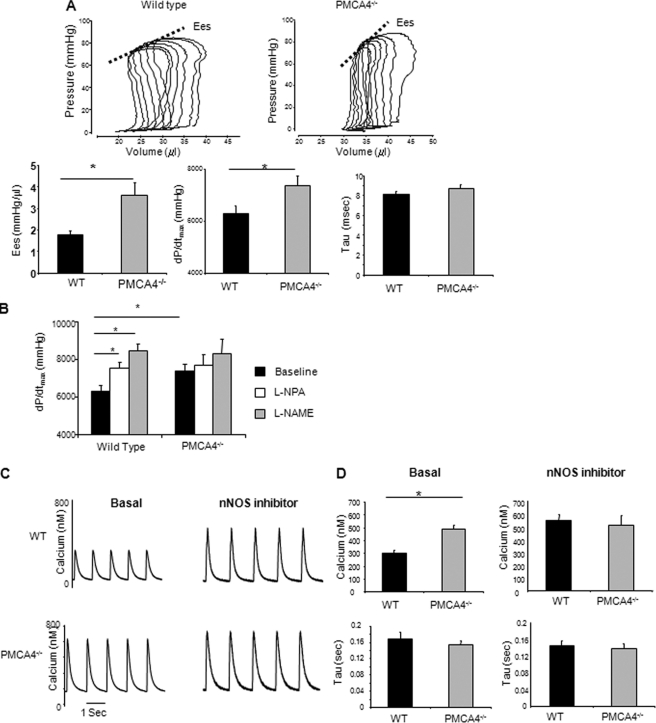
We used a pressure-volume system to investigate the role of PMCA4 in regulating cardiac contractility in vivo. Interestingly, ablation of PMCA4 resulted in enhancement of contractility as indicated by dP/dtmax and end systolic elastance (Ees), with no significant effect on relaxation as indicated by the τ values (Fig. 2A and supplemental Table 1, p < 0.05, n = 9). This suggests that contrary to the classical hypothesis that PMCA4 has a role in diastole, it regulates cardiac function through alternative means because its ablation enhances systolic function rather than impairing diastolic function. As PMCA4 forms a signaling complex with nNOS, a molecule previously described as a regulator of cardiac contractility (26–29), we hypothesized that the changes in contractile function might be due to the disruption of the PMCA4-nNOS complex and subsequent alteration in nNOS activity. In agreement with this idea, the selective nNOS inhibitor Nω-propyl-l-arginine (l-NPA, 1 mg/kg of body weight) caused a significant (p < 0.05) elevation in contractile function in WT mice, whereas in PMCA4−/− mice, l-NPA had no effect on contractility (Fig. 2B). These results indicate the absence of inherent regulatory activity of nNOS in PMCA4−/− mice. Subsequent injection of Nω-nitro L-arginine methyl ester (50 mg/kg of body weight) to inhibit other NOS isoforms produced no further changes in contractility (Fig. 2B).
FIGURE 2.
PMCA4−/− mice modulate contractility through an nNOS-dependent mechanism in vivo and in isolated adult cardiomyocytes. A, representative pressure-volume loops for invasive hemodynamic analysis of PMCA4−/− mice; end systolic elastance (Ees), dP/dtmax, and τ were used as measures for cardiac contractility and relaxation, respectively. PMCA4−/− mice showed a significant increase in basal contractility when compared with their WT littermates with no effect on the relaxation speed (n = 9, *, p < 0.05). B, changes in contractile function (dP/dtmax) following administration of the selective nNOS antagonist (l-NPA, 1 mg/kg of body weight). l-NPA caused a significant elevation in dP/dtmax in WT mice but not in PMCA4−/− mice (n = 10, *, p < 0.05). l-NPA also abolished the difference in contractile function between WT and PMCA4−/− mice. There was no further significant change upon subsequent administration of the total NOS inhibitor (Nω-nitro L-arginine methyl ester (l-NAME), 50 mg/kg of body weight) in either group of mice. C, representative calcium transient traces from isolated PMCA4−/− and WT myocytes loaded with Indo-1 dye showed higher calcium amplitude in PMCA4−/−; this phenotype was emulated in WT myocytes treated with an nNOS-specific inhibitor (100 μm N5-(1-imino-3-butenyl)-l-ornithine, monohydrochloride). D, quantification of calcium transient amplitude and time constant for calcium decay (τ) (*, p < 0.05 versus WT, n = 10–12 myocytes from 4–5 independent animals in each group).
Observations in isolated cardiomyocytes strongly supported the in vivo data. PMCA4−/− cardiomyocytes showed a significant increase in the amplitude of the Ca2+ transient, with no significant change in the rate of decay. Cardiomyocytes lacking PMCA4 did not respond to specific nNOS inhibition (100 μm N-5-(1-imino-3-butenyl)-l-ornithine, monohydrochloride) (Fig. 2, C and D). These results strongly indicate that PMCA4 regulates cardiomyocyte contractility via modulation of nNOS.
Mechanisms of nNOS Regulation by PMCA4 and the Structural Role of PMCA4
Using overexpression models, we have recently shown that PMCA4 binds to and negatively regulates nNOS activity in the heart (26, 27). It was therefore unexpected to also see reduced nNOS activity in PMCA4−/− mice. To explain this finding, we hypothesized that PMCA4 acts as a scaffolding molecule to tether nNOS to the plasma membrane and that in the absence of PMCA4, nNOS would be released from the sarcolemmal subcompartment. To address this hypothesis, we fractionated heart extracts from PMCA4−/− mice into membrane and cytoplasmic fractions. Detection of specific membrane and cytoplasmic proteins in the respective fractions confirmed successful fractionation (Fig. 3A). A significant reduction in nNOS activity in the membrane fraction of PMCA4−/− cardiac tissue was observed (Fig. 3B). In the cytoplasmic fraction, nNOS activity was higher in PMCA4−/− when compared with WT. There were no significant differences in the activity of other NOS isoforms at the membrane and cytoplasm. In agreement with these findings, we also found that the level of nNOS but not eNOS expression in the membrane fraction was markedly reduced in PMCA4−/− hearts when compared with WT controls (Fig. 3C), but with no overall reduction in total nNOS expression. To confirm the loss of PMCA4 specifically from the plasma membrane, we conducted a co-immunoprecipitation experiment. We precipitated heart tissue lysate using anti-caveolin-3 (plasma membrane marker) and then performed Western blot analysis to detect the nNOS level. In knock-out mice, nNOS was not detected in the lysates precipitated with anti caveolin-3 (Fig. 3D). Electron microscopy with immunogold staining also supported the findings that nNOS was translocated from the plasma membrane to the cytoplasm in PMCA4−/− myocytes (Fig. 3E). We also analyzed the localization of eNOS, the other NOS isoform, which is normally localized at the plasma membrane (22), in PMCA4−/− mice. Contrary to nNOS, the plasma membrane localization of eNOS was not affected by PMCA4 genetic deletion (supplemental Fig. 1B). Altogether our data demonstrate that PMCA4 has a structural role that involves tethering nNOS and not eNOS to the plasma membrane of cardiomyocytes.
Downstream Effectors of the PMCA4-nNOS Complex Are Activated in PMCA4−/− Mice
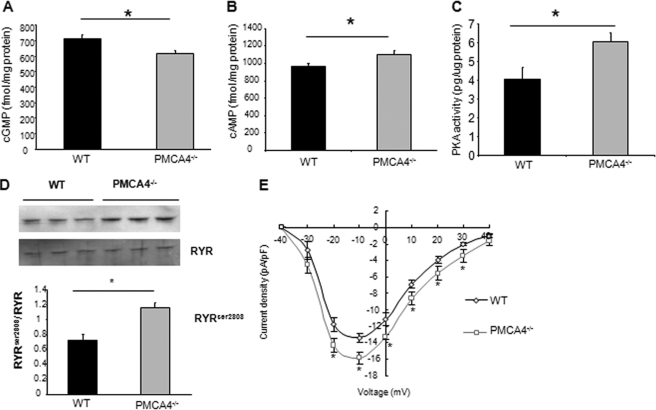
We further investigated the regulatory actions of PMCA4 downstream of nNOS. We found that the reduction of nNOS activity in the PMCA4−/− membranes led to a significant decrease in cGMP levels, accompanied by an elevation of cAMP levels. PKA activity and phosphorylation of ryanodine receptors at Ser-2808 were also elevated (Fig. 4, A–D). Patch clamp experiments also showed that PMCA4−/− myocytes displayed a significant elevation in the L-type channel activity when compared with their WT controls (Fig. 4E). However, there were no significant changes in the major parameters determining relaxation such as NCX current, sarcoplasmic reticulum calcium content, and phospholamban phosphorylation or in the nitrosylation status of RYR receptors (supplemental Figs. 2 and 3). In addition, there were no significant changes in the levels of guanylyl cyclase and protein kinase A expression (supplemental Fig. 1A). The increases in ryanodine receptor phosphorylation and L-type calcium channel activity are likely the factors responsible for the elevated calcium amplitude and basal contractility in PMCA4−/− mice. It is interesting to note that the other PMCA isoform expressed in the heart (PMCA1) did not show any functional effect on nNOS activity (supplemental Fig. 5).
FIGURE 4.
Genetic ablation of PMCA4 modulates cyclic nucleotide levels and RYR and L-type channel activity. A, cGMP levels were significantly decreased in total heart homogenates from PMCA4−/− mice when compared with their respective controls (n = 7, *, p < 0. 05). In B and C, however, cAMP levels (B) and PKA activity (C) were significantly elevated in PMCA4−/− when compared with their WT littermates (n = 7, *, p < 0.05). D, RYR phosphorylation at Ser-2808 was significantly elevated (n = 7, *, p < 0.05) in PMCA4−/−. E, to determine the L-type channel activity, patch-clamped cardiomyocytes were depolarized from a holding potential of −40 mV to a family of 200-ms test potential pulses from −30 to +40 mV. A significant increase in L-type calcium current was noticed in PMCA4−/− when compared with the WT cardiomyocytes, which is maximal at −10 mV (n = 12–15 cells in each group, *, p < 0.05). pF, picofarads.
PMCA4-nNOS Complex Mediates Local Signaling
The changes in cAMP and cGMP levels in total heart homogenate were relatively small, which did not reflect the exact compartmentalized changes at the membrane. Therefore, we hypothesized that PMCA4 is involved in the regulation of local nNOS signaling at the membrane and hence regulates only local cGMP and PDE2 (cGMP-activated cAMP phosphodiesterase) (30) specifically in the vicinity of the plasma membrane. To test this idea, we used FRET-based cAMP sensors (Epac1camps) (31) that are targeted either to the cytoplasm (cyt-Epac1camps) or to the plasma membrane (mp-Epac1camps). We generated adenoviruses expressing both types of Epac1camps and used them to infect neonatal rat cardiomyocytes (NRCM) (Fig. 5A). The FRET signal was then examined following treatment of Epac1camps-expressing NRCM with either isoform-specific or pan-PDE inhibitors, thus reflecting the PDE activity (supplemental Fig. 4).
FIGURE 5.
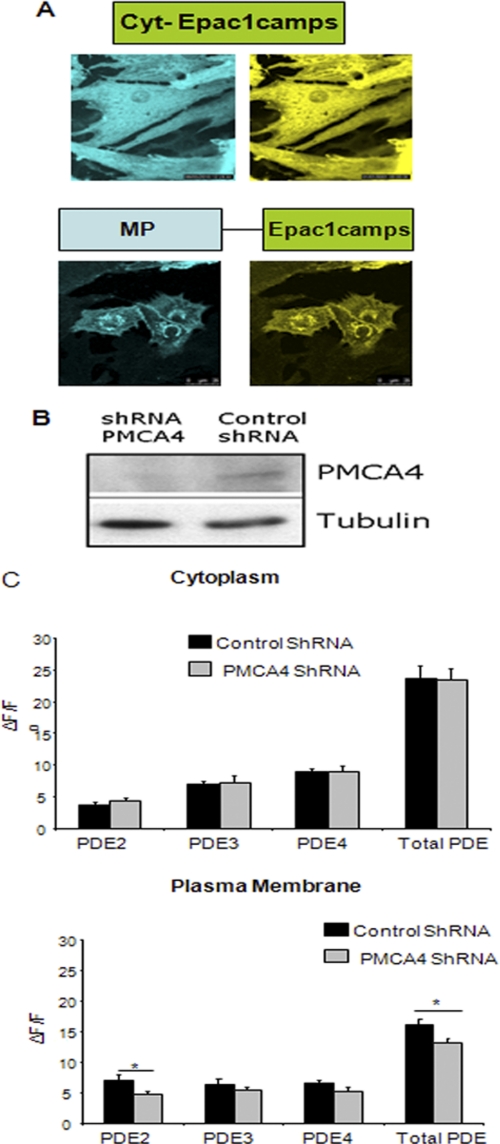
PMCA4 modulates local signaling at the membrane. A, representative images of NRCM infected with adenovirus expressing cytoplasmic cAMP FRET sensor (cyt-Epac1camps) or a membrane-targeted version of Epac1camps (MP-Epac1camps). B, Western blot showing the reduction of PMCA4 level in NRCM infected with adenovirus shRNA to knock down PMCA4. C, FRET technology was used to assess the spatial activity of each PDE isoform in NRCM at the membrane (using mp-Epac1camps FRET sensor) as well as in the cytoplasm (using cyt-Epac1camps FRET sensor). We found both PDE2 and total PDE activities to be significantly reduced at the membrane following PMCA4 knockdown, whereas cytoplasmic activity remained unchanged (n = 8, *, p < 0.05). To assess PDE isoform activities, specific PDE inhibitors were used as follows: 10 mm BAY-60-7550 for PDE2, 1 mm Cilostamide for PDE3, 10 mm Rolipram for PDE4, and 500 mm isobutylmethylxanthine for total PDE activity.
In NRCM lacking PMCA4 (using an shRNA system; Fig. 5B), only membrane PDE2 activity was significantly reduced by 38% when compared with control cells (n = 8, p < 0.05) (Fig. 5C). Cytoplasmic PDE2 was not changed in PMCA4-deficient myocytes, suggesting that PMCA4 regulates the local signal only in the vicinity of the membrane. As expected, total PDE activity was only decreased in the membrane compartment and not in the cytoplasmic compartment of PMCA4-deficient cells. This localized decrease in PDE2 activity at the membrane compartment led to the localized increase in cAMP and hence PKA activity. The membrane-compartmentalized elevation in PKA activity explains the increased phosphorylation of L-type calcium channels and RYR. It is evident that RYR is located in close proximity to the L-type calcium channel (32). This compartmentalized membrane increase in PKA activity did not diffuse through the cytoplasm as it has no effect on PLB phosphorylation (supplemental Fig. 3C).
β-Adrenergic Contractile Response Was Reduced in PMCA4 Knock-out Mice
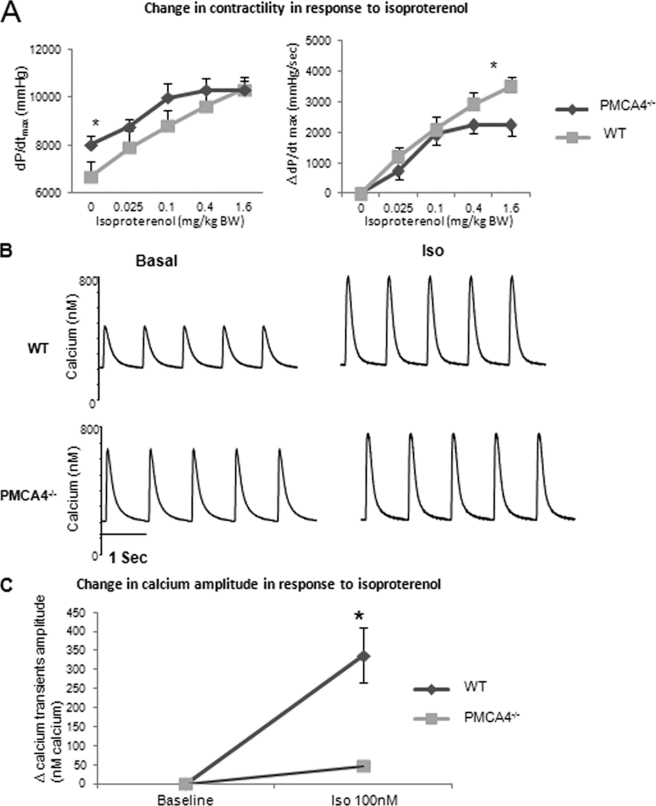
The β-adrenergic signaling pathway has been shown to be regulated by overexpression of the PMCA4 (27) as well as by deletion of nNOS (29). To investigate whether PMCA4 gene ablation would result in modification of the β-adrenergic pathway, we assessed the effect of isoproterenol stimulation in PMCA4−/− cardiomyocytes as well as in whole animals. Although exhibiting increased basal contractility (Fig. 2A), PMCA4−/− mice also displayed a significantly attenuated response to isoproterenol stimulation at a dose of 1.6 mg/kg of body weight, as indicated by the change in dP/dtmax values (n = 8–10, p < 0.05) (Fig. 6A, right panel), resulting in both PMCA4−/− and wild type mice reaching the same maximal contractile response (Fig. 6A, left panel). In keeping with the in vivo data, as a result of the high basal calcium transient amplitude in the PMCA4−/− when compared with their WT littermates, the change in calcium transient amplitude following isoproterenol administration (100 nm) was significantly reduced in PMCA4−/− cardiomyocytes (Fig. 6, B and C). These data suggest that the PMCA4-nNOS complex modifies the β-adrenergic induced-contractile response in cardiomyocytes and in vivo as a result of higher basal contractility.
FIGURE 6.
Effect of isoproterenol stimulation in PMCA4−/− mice. A, dose response to isoproterenol following administration of 0. 025, 0.1, 0.4, and 1.6 mg/kg of isoproterenol in PMCA4−/− and their WT littermates. The left panel shows absolute values for dP/dtmax, and the right panel shows changes in contractile function (ΔdP/dtmax) in response to various doses of isoproterenol. The response to 1.6 mg/kg of isoproterenol injection was significantly attenuated in PMCA4−/− mice when compared with their WT littermates (n = 10, *, p < 0.05). mg/kg BW, mg/kg of body weight. B, representative calcium transient traces from isolated PMCA4−/− and WT myocytes at baseline and after stimulation with isoproterenol (Iso, 100 nm). C, quantification of the change in calcium transient amplitude after isoproterenol (100 nm) perfusion indicated that PMCA4−/− cardiomyocytes showed a significantly reduced response to isoproterenol stimulation (*, p < 0.05 versus WT, n = 10–12 myocytes from 4–5 independent animals in each group).
DISCUSSION
The key novel findings in this study are as follows. (i) PMCA4 plays a pivotal structural role in myocardial signaling by tethering nNOS to the subsarcolemmal space. (ii) Functionally, the displacement of the sarcolemmal fraction of nNOS in PMCA4−/− mice leads to an increase in contractility via alteration of cyclic nucleotide levels and phosphodiesterase 2 activity in a microdomain defined by PMCA4. (iii) These alterations in cyclic nucleotides lead to subsequent phosphorylation of the ryanodine receptor and a significant increase in L-type calcium channel activity. (iv) PMCA4 does not measurably contribute to beat-to-beat regulation of diastolic calcium and hence relaxation.
We had previously shown, in HEK293 cells (15) as well as in transgenic animals overexpressing PMCA4 (26, 27), that PMCA4 interacts with and regulates the calcium/calmodulin-dependent enzyme nNOS. These proteins are linked together via a PDZ domain in nNOS and a cognate ligand at the C-terminal end of PMCA4. As PMCA4 depletes calcium in the vicinity of nNOS, the latter produces less NO. Nitric oxide from nNOS has been shown to be a key regulator of cardiac contractility (28, 29). By using a PMCA4 knock-out model in this study, we revealed a novel and unexpected scaffolding role of PMCA4 in regulating nNOS function. In addition, it became clear that the underlying mechanism involved compartmentalization of cyclic nucleotide signaling around PMCA4 as well as regulation of the L-type calcium channel and the ryanodine receptor; this was information that could not be gleaned by studying the PMCA4 overexpression model (26, 27). Western blot and electron microscopic analyses clearly showed that in the absence of PMCA4 protein, nNOS was translocated from the plasma membrane to the cytoplasm. The reduction of nNOS enzymatic activity in the plasma membrane led to a decrease in cGMP and a subsequent increase in cAMP, likely through reduced activity of PDE2, a well known mechanism of mutual cyclic nucleotide regulation (26, 30). Using a novel FRET-based sensor, we were able to demonstrate that this signaling event only occurred in the local plasma membrane microdomain. This compartmentalized decrease in PDE2 activity led to a membrane-localized increase in cAMP and PKA (33) and ultimately to an increase in L-type calcium channel activity and in ryanodine receptor phosphorylation: two key mechanisms of positive inotropy at and very close to the membrane compartment (32). This explains the phenotype of increased basal contractility in PMCA4−/− mice.
Our PMCA4−/− model is only comparable in part with the total nNOS−/− model (28). The elevated calcium amplitude in PMCA4−/− was similar to that observed in isolated cardiomyocytes from nNOS−/− mice (28, 34). However, nNOS−/− mice showed a reduction in relaxation speed as well as in the rate of Ca2+ decay, which is not observed in our PMCA4−/− mice. Cardiac nNOS is known to be localized to the plasma membrane (35), sarcoplasmic reticulum (36, 37), and mitochondria (38). The effects of NO have been shown to be dependent upon the cellular localization of the generating enzyme (reviewed by Hare and Stamler (39)). It is therefore likely that the difference in relaxation and calcium decay between PMCA4−/− and nNOS−/− mice is due to a difference in the cellular compartments in which nNOS is depleted. In PMCA4−/−, only plasma membrane-bound nNOS is decreased when compared with its total ablation in nNOS−/− mice. In addition, it should be noted that nNOS−/− is not a complete knock-out, with nNOSα still being expressed in the mouse line (29).
Therefore, based on present and previous data from our group and others, a picture emerges in which nNOS serves radically different functions depending on its localization. Indeed, based on our finding that PMCA4 forms a functional, structural complex with nNOS, it becomes possible to speculate that an individual nNOS binding partner, such as PMCA4, determines the function of the individual nNOS molecule, even within one membrane compartment. The role of PMCA4 to regulate the function of nNOS is not limited to the heart and has also been reported in vasculature (40, 41). However, it appears that PMCA4-nNOS modulation is specific to the cardiovascular system, where PMCA4 is mainly localized to caveolae (9, 42).
In addition to our present work on the physiological function and the molecular mechanisms of PMCA4 action, there is a growing body of evidence regarding involvement of the nNOS-PMCA4 structural complex at the plasma membrane in cardiac disease. The complex is associated with other proteins such as carboxyl-terminal PDZ ligand of nNOS (CAPON) (23) and α1-syntrophin (43). Beigi et al. (23) have shown that during myocardial infarction, the PMCA4-CAPON-nNOS complex is enhanced, resulting in the enrichment of nNOS at the plasma membrane. Crucially, recent human genome-wide association studies found an association between an SNP in CAPON with prolonged QT syndrome in humans and sudden death (17, 18). The subsarcolemmal localization of nNOS is also vital in the development of heart failure, as shown both in patients (44) and in animal models (45). Whether nNOS translocation in disease is associated with or caused by changes in PMCA4 expression needs further clarification, but these findings clearly illustrate the relevance of this structural complex in health and disease and the need to unravel its exact role.
Overall, our current study demonstrates the novel and crucial role of PMCA4 as a structural molecule in maintaining the spatial and functional integrity of the nNOS signaling complex in the plasma membrane in the heart. It also provides mechanistic evidence for a central role for this complex in determining cardiac contractility, both in the basal and in the β-stimulated state. On the other hand, PMCA4 clearly has no role in beat-to-beat cardiac relaxation based on observations from our knock-out mouse model. Hence, PMCA4 acts both as a scaffold for nNOS at the membrane and as a regulator that maintains the local calcium level at optimal concentrations for nNOS functionality. Therefore, PMCA4 is one of the calcium transport proteins in the heart, in addition to transient receptor potential canonical channels (20) and inositol 1,4,5-triphosphate receptor (21), that serves a role in signaling. It is a classical, but still open, question in cardiac physiology whether the calcium pools that mediate contraction also mediate signaling or whether these functions are separate. Although direct high-resolution determination of local calcium pools in the heart is not yet reliably possible, the existence of a protein, PMCA4, that carries a calcium signaling function strongly suggests a model of separate and spatially confined calcium pools that subserve the two functions of calcium in the heart. Based on this unique protein, it may be possible in the future to clone calcium sensors that are able to directly demonstrate the existence of separate pools for contractile and signaling calcium.
Acknowledgments
We acknowledge the bioimaging and electron microscopy facilities in the University of Manchester for help and advice. We thank Dr. Yatong Li, University of Manchester for help during the manuscript preparation. We thank Dr. Martin Lohse for providing the Epac1 FRET plasmid. The L. Neyses laboratory is supported by the National Institute for Health Research, Manchester Biomedical Research Centre Funding Scheme.
This work was supported by the Medical Research Council (MRC) International Appointee Grant (G0200020), MRC Programme/Research Grants (G0500025 and G0802004) (to L. N.), and a British Heart Foundation (BHF) Project Grant (PG/05/082) (to D. O. and E. J. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Experimental Procedures, Table 1, and Figs. 1–5.
- PMCA
- plasma membrane Ca2+/calmodulin-dependent ATPase
- SERCA
- sarcoplasmic reticulum calcium-ATPase
- NCX
- sodium calcium exchanger
- RYR
- ryanodine receptor
- NOS
- nitric-oxide synthase
- nNOS
- neuronal nitric-oxide synthase
- eNOS
- endothelial nitric-oxide synthase
- l-NPA
- N-propyl-l-arginine
- PDE
- phosphodiesterase
- Epac
- cAMP-regulated guanine nucleotide exchange factor
- NRCM
- neonatal rat cardiomyocytes
- CAPON
- carboxyl-terminal PDZ ligand of nNOS.
REFERENCES
- 1. Carafoli E., James P., Strehler E. E. (1990) Prog. Clin. Biol. Res. 332, 181–193 [PubMed] [Google Scholar]
- 2. Cartwright E. J., Schuh K., Neyses L. (2005) J. Mol. Cell Cardiol. 39, 403–406 [DOI] [PubMed] [Google Scholar]
- 3. Bers D. M., Bassani J. W., Bassani R. A. (1993) Cardiovasc. Res. 27, 1772–1777 [DOI] [PubMed] [Google Scholar]
- 4. Bers D. M. (2008) Annu. Rev. Physiol. 70, 23–49 [DOI] [PubMed] [Google Scholar]
- 5. Hayakawa K., Swenson L., Baksh S., Wei Y., Michalak M., Derewenda Z. S. (1994) J. Mol. Biol. 235, 357–360 [DOI] [PubMed] [Google Scholar]
- 6. Lee D., Michalak M. (2010) BMB Rep. 43, 151–157 [DOI] [PubMed] [Google Scholar]
- 7. Cartwright E. J., Oceandy D., Neyses L. (2007) Ann. N.Y. Acad. Sci. 1099, 247–253 [DOI] [PubMed] [Google Scholar]
- 8. Hammes A., Oberdorf-Maass S., Rother T., Nething K., Gollnick F., Linz K. W., Meyer R., Hu K., Han H., Gaudron P., Ertl G., Hoffmann S., Ganten U., Vetter R., Schuh K., Benkwitz C., Zimmer H. G., Neyses L. (1998) Circ. Res. 83, 877–888 [DOI] [PubMed] [Google Scholar]
- 9. Fujimoto T. (1993) J. Cell Biol. 120, 1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohen A. W., Hnasko R., Schubert W., Lisanti M. P. (2004) Physiol. Rev. 84, 1341–1379 [DOI] [PubMed] [Google Scholar]
- 11. Parton R. G., Simons K. (2007) Nat. Rev. Mol. Cell Biol. 8, 185–194 [DOI] [PubMed] [Google Scholar]
- 12. Armesilla A. L., Williams J. C., Buch M. H., Pickard A., Emerson M., Cartwright E. J., Oceandy D., Vos M. D., Gillies S., Clark G. J., Neyses L. (2004) J. Biol. Chem. 279, 31318–31328 [DOI] [PubMed] [Google Scholar]
- 13. Buch M. H., Pickard A., Rodriguez A., Gillies S., Maass A. H., Emerson M., Cartwright E. J., Williams J. C., Oceandy D., Redondo J. M., Neyses L., Armesilla A. L. (2005) J. Biol. Chem. 280, 29479–29487 [DOI] [PubMed] [Google Scholar]
- 14. Williams J. C., Armesilla A. L., Mohamed T. M., Hagarty C. L., McIntyre F. H., Schomburg S., Zaki A. O., Oceandy D., Cartwright E. J., Buch M. H., Emerson M., Neyses L. (2006) J. Biol. Chem. 281, 23341–23348 [DOI] [PubMed] [Google Scholar]
- 15. Schuh K., Uldrijan S., Telkamp M., Rothlein N., Neyses L. (2001) J. Cell Biol. 155, 201–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ueda K., Valdivia C., Medeiros-Domingo A., Tester D. J., Vatta M., Farrugia G., Ackerman M. J., Makielski J. C. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 9355–9360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arking D. E., Pfeufer A., Post W., Kao W. H., Newton-Cheh C., Ikeda M., West K., Kashuk C., Akyol M., Perz S., Jalilzadeh S., Illig T., Gieger C., Guo C. Y., Larson M. G., Wichmann H. E., Marbán E., O'Donnell C. J., Hirschhorn J. N., Kääb S., Spooner P. M., Meitinger T., Chakravarti A. (2006) Nat. Genet. 38, 644–651 [DOI] [PubMed] [Google Scholar]
- 18. Chang K. C., Barth A. S., Sasano T., Kizana E., Kashiwakura Y., Zhang Y., Foster D. B., Marbán E. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 4477–4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Molkentin J. D. (2006) J. Clin. Invest. 116, 623–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuwahara K., Wang Y., McAnally J., Richardson J. A., Bassel-Duby R., Hill J. A., Olson E. N. (2006) J. Clin. Invest. 116, 3114–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakayama H., Bodi I., Maillet M., DeSantiago J., Domeier T. L., Mikoshiba K., Lorenz J. N., Blatter L. A., Bers D. M., Molkentin J. D. (2010) Circ. Res. 107, 659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sears C. E., Ashley E. A., Casadei B. (2004) Philos. Trans. R. Soc. Lond. B Biol. Sci. 359, 1021–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beigi F., Oskouei B. N., Zheng M., Cooke C. A., Lamirault G., Hare J. M. (2009) Nitric Oxide 21, 226–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saraiva R. M., Hare J. M. (2006) Curr. Opin. Cardiol. 21, 221–228 [DOI] [PubMed] [Google Scholar]
- 25. Schuh K., Cartwright E. J., Jankevics E., Bundschu K., Liebermann J., Williams J. C., Armesilla A. L., Emerson M., Oceandy D., Knobeloch K. P., Neyses L. (2004) J. Biol. Chem. 279, 28220–28226 [DOI] [PubMed] [Google Scholar]
- 26. Mohamed T. M., Oceandy D., Prehar S., Alatwi N., Hegab Z., Baudoin F. M., Pickard A., Zaki A. O., Nadif R., Cartwright E. J., Neyses L. (2009) J. Biol. Chem. 284, 12091–12098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oceandy D., Cartwright E. J., Emerson M., Prehar S., Baudoin F. M., Zi M., Alatwi N., Venetucci L., Schuh K., Williams J. C., Armesilla A. L., Neyses L. (2007) Circulation 115, 483–492 [DOI] [PubMed] [Google Scholar]
- 28. Sears C. E., Bryant S. M., Ashley E. A., Lygate C. A., Rakovic S., Wallis H. L., Neubauer S., Terrar D. A., Casadei B. (2003) Circ. Res. 92, e52–e59 [DOI] [PubMed] [Google Scholar]
- 29. Barouch L. A., Harrison R. W., Skaf M. W., Rosas G. O., Cappola T. P., Kobeissi Z. A., Hobai I. A., Lemmon C. A., Burnett A. L., O'Rourke B., Rodriguez E. R., Huang P. L., Lima J. A., Berkowitz D. E., Hare J. M. (2002) Nature 416, 337–339 [DOI] [PubMed] [Google Scholar]
- 30. Zaccolo M., Movsesian M. A. (2007) Circ. Res. 100, 1569–1578 [DOI] [PubMed] [Google Scholar]
- 31. Nikolaev V. O., Bünemann M., Hein L., Hannawacker A., Lohse M. J. (2004) J. Biol. Chem. 279, 37215–37218 [DOI] [PubMed] [Google Scholar]
- 32. Bito V., Heinzel F. R., Biesmans L., Antoons G., Sipido K. R. (2008) Cardiovasc. Res. 77, 315–324 [DOI] [PubMed] [Google Scholar]
- 33. Stangherlin A., Gesellchen F., Zoccarato A., Terrin A., Fields L. A., Berrera M., Surdo N. C., Craig M. A., Smith G., Hamilton G., Zaccolo M. (2011) Circ. Res. 108, 929–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Y. H., Zhang M. H., Sears C. E., Emanuel K., Redwood C., El-Armouche A., Kranias E. G., Casadei B. (2008) Circ. Res. 102, 242–249 [DOI] [PubMed] [Google Scholar]
- 35. Xu K. Y., Kuppusamy S. P., Wang J. Q., Li H., Cui H., Dawson T. M., Huang P. L., Burnett A. L., Kuppusamy P., Becker L. C. (2003) J. Biol. Chem. 278, 41798–41803 [DOI] [PubMed] [Google Scholar]
- 36. Burkard N., Rokita A. G., Kaufmann S. G., Hallhuber M., Wu R., Hu K., Hofmann U., Bonz A., Frantz S., Cartwright E. J., Neyses L., Maier L. S., Maier S. K., Renné T., Schuh K., Ritter O. (2007) Circ. Res. 100, e32–e44 [DOI] [PubMed] [Google Scholar]
- 37. Xu K. Y., Huso D. L., Dawson T. M., Bredt D. S., Becker L. C. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 657–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kanai A. J., Pearce L. L., Clemens P. R., Birder L. A., VanBibber M. M., Choi S. Y., de Groat W. C., Peterson J. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 14126–14131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hare J. M., Stamler J. S. (1999) Nat. Med. 5, 273–274 [DOI] [PubMed] [Google Scholar]
- 40. Gros R., Afroze T., You X. M., Kabir G., Van Wert R., Kalair W., Hoque A. E., Mungrue I. N., Husain M. (2003) Circ. Res. 93, 614–621 [DOI] [PubMed] [Google Scholar]
- 41. Schuh K., Quaschning T., Knauer S., Hu K., Kocak S., Roethlein N., Neyses L. (2003) J. Biol. Chem. 278, 41246–41252 [DOI] [PubMed] [Google Scholar]
- 42. Hammes A., Oberdorf-Maass S., Jenatschke S., Pelzer T., Maass A., Gollnick F., Meyer R., Afflerbach J., Neyses L. (1996) J. Biol. Chem. 271, 30816–30822 [DOI] [PubMed] [Google Scholar]
- 43. Brenman J. E., Chao D. S., Gee S. H., McGee A. W., Craven S. E., Santillano D. R., Wu Z., Huang F., Xia H., Peters M. F., Froehner S. C., Bredt D. S. (1996) Cell 84, 757–767 [DOI] [PubMed] [Google Scholar]
- 44. Damy T., Ratajczak P., Shah A. M., Camors E., Marty I., Hasenfuss G., Marotte F., Samuel J. L., Heymes C. (2004) Lancet 363, 1365–1367 [DOI] [PubMed] [Google Scholar]
- 45. Bendall J. K., Damy T., Ratajczak P., Loyer X., Monceau V., Marty I., Milliez P., Robidel E., Marotte F., Samuel J. L., Heymes C. (2004) Circulation 110, 2368–2375 [DOI] [PubMed] [Google Scholar]