Background: DGAT1 is a triglyceride biosynthetic enzyme with a possible role in metabolic disorders.
Results: T-863, a potent DGAT1 inhibitor acting on the acyl-CoA binding site of DGAT1, decreased body weight, improved insulin sensitivity, and alleviated hepatic steatosis in diet-induced obese mice.
Conclusion: These data support further exploration of DGAT1 inhibitors for metabolic disorders.
Significance: Our study reveals mechanisms of action for DGAT1 inhibitors.
Keywords: Diabetes, Diacylglycerol, Insulin Resistance, Lipid Absorption, Metabolic Diseases, Triacylglycerol, DGAT1, Acyltransferase, Inhibitor, Insulin Sensitivity
Abstract
Acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1) is one of two known DGAT enzymes that catalyze the final step in triglyceride synthesis. Findings from genetically modified mice as well as pharmacological studies suggest that inhibition of DGAT1 is a promising strategy for the treatment of obesity and type 2 diabetes. Here we characterize a tool DGAT1 inhibitor compound, T863. We found that T863 is a potent inhibitor for both human and mouse DGAT1 in vitro, which acts on the acyl-CoA binding site of DGAT1 and inhibits DGAT1-mediated triacylglycerol formation in cells. In an acute lipid challenge model, oral administration of T863 significantly delayed fat absorption and resulted in lipid accumulation in the distal small intestine of mice, mimicking the effects of genetic ablation of DGAT1. In diet-induced obese mice, oral administration of T863 for 2 weeks caused weight loss, reduction in serum and liver triglycerides, and improved insulin sensitivity. In addition to the expected triglyceride-lowering activity, T863 also lowered serum cholesterol. Hepatic IRS2 protein was dramatically up-regulated in mice treated with T863, possibly contributing to improved insulin sensitivity. In differentiated 3T3-L1 adipocytes, T863 enhanced insulin-stimulated glucose uptake, suggesting a possible role for adipocytes to improve insulin sensitivity upon DGAT1 inhibition. These results reveal novel mechanistic insights into the insulin-sensitizing effects of DGAT1 inhibition in mouse models. Taken together, our study provides a comprehensive evaluation of a small molecule inhibitor for DGAT1 and suggests that pharmacological inhibition of DGAT1 holds promise in treating diverse metabolic disorders.
Introduction
Synthesis of triacylglycerol (TAG)3 is a fundamental biochemical pathway important for nutrient utilization and energy storage. Excess storage of energy in the form of TAG or fatty acids, however, causes human diseases, such as obesity, insulin resistance, dyslipidemia, and hepatic steatosis. Inhibition of TAG biosynthetic enzymes has been suggested to be one of the potential strategies to treat these disorders (1). The enzyme catalyzing the final and committed step in the TAG biosynthetic pathway is acyl-CoA:diacylglycerol acyltransferase (DGAT). DGAT1 is one of the several enzymes with DGAT activity that were cloned and characterized at the molecular level. DGAT1−/− mice are viable and are protected from diet-induced or genetic obesity, and they exhibit improved insulin sensitivity and glucose homeostasis (2, 3). More importantly, DGAT1-heterozygous (DGAT1−/+) mice display a partial resistance to diet-induced obesity and have an intermediate level of adipose mass and adipocyte size as compared with inbred wild-type mice or mice with complete ablation of DGAT1 (1). Conversely, transgenic overexpression of DGAT1 in adipose tissues resulted in whole-body insulin resistance and other metabolic disturbances, such as liver steatosis (4). Fueled by these studies in genetically manipulated mice, interest in pursuing pharmacological inhibition of DGAT1 for the treatment of metabolic disorders has intensified. Potent and selective DGAT1 inhibitors have now been described by several groups (5–7). Limited in vivo studies published using these molecules suggest that pharmacological inhibition of DGAT1 in rodents can recapitulate many of the phenotypes observed in DGAT1 knock-out mice, including delayed fat absorption, protection from hepatic steatosis, and decreased body weight on a high fat diet (5–8). Published studies to date, however, still leave many questions unanswered. Specifically, the mechanisms by which DGAT1 inhibitors act have been poorly characterized. In addition, the effects of chronic DGAT1 inhibition on glucose and energy homeostasis have not been carefully examined.
We have used the DGAT1 inhibitor T863 (9) as a model compound to characterize the effects of pharmacological inhibition of DGAT1. Applying a novel, high throughput fluorescent assay for DGAT1 activity, as well as several complementary protein- and cell-based assays, we show that T863 is a potent and selective DGAT1 inhibitor that binds to the oleoyl-CoA binding pocket of DGAT1. We show that T863 has beneficial effects in a rodent model of diet-induced obesity and insulin resistance both in acute and in chronic settings, and we provide novel insights into the mechanism of action of DGAT1 inhibitors in vivo.
EXPERIMENTAL PROCEDURES
Materials
sn-2-Monooleoylglycerol, 1,2-dioleoyl-sn-glycerol (1,2-DOG), 1,3-dioleoyl-sn-glycerol, triolein, retinol, retinyl palmitate, and oleic acid were obtained from Sigma. Oleoyl-CoA and other phospholipids were purchased from Avanti Polar Lipids. T863 and its tritiated version were synthesized by our chemistry colleagues at Pfizer. The synthesis of T863 was performed according to the method described in a public disclosure (9). T863 is also commercially available from Syngene International (Iselin, NJ) (10). To obtain the radiolabeled T863, a relevant compound, ethyl 2-((1,4-trans)-4-(4-(4-amino-7,7-dimethyl-7H-pyrimido[4,5-b][1,4]oxazin-6-yl)-2-iodophenyl)cyclohexyl)acetate, was first prepared according to procedures described previously (9). The ester was subsequently hydrolyzed to 2-((1,4-trans)-4-(4-(4-amino-7,7-dimethyl-7H-pyrimido[4,5-b][1,4]oxazin-6-yl)-2-iodophenyl)cyclohexyl)acetic acid by an aqueous solution of sodium hydroxide. After reduction by sodium [3H]borohydride, the corresponding tritiated compound, 2-((1,4-trans)-4-(4-(4-amino-7,7-dimethyl-7H-pyrimido[4,5-b][1,4]oxazin-6-yl)-2-T-phenyl)cyclohexyl)acetic acid ([3H]T863), was obtained. The compound was further purified by HPLC prior to use.
Animals and Treatments
C57BL6 normal mice (8 weeks old) or diet-induced obese (DIO) mice (10 months old, fed a high fat diet for 8 months) were obtained from Taconic. The animals were maintained in a pathogen-free and temperature-controlled room (21–22 °C) on a 12-h light/dark cycle and supplied with food and water ad libitum. Compounds used for in vivo studies were dissolved in carboxymethy cellulose/Tween 80 (1:1) by grinding with a mortar and pestle. For acute lipid challenge study, C57BL6 normal or DIO mice (n = 5/group) were orally dosed with either vehicle or DGAT1 compounds (30 mg/kg). One hour after dosing, a corn oil bolus was administered via gavage, followed by collection of blood samples at different time points (0, 0.5, 1, 4, 8, and 24 h) for serum triglyceride measurement. For ex vivo intestinal DGAT1 activity studies, C57BL6 mice were orally dosed with either vehicle or compounds at 30 mg/kg. Sections of small intestines (jejunum) were harvested after 1 h of dosing and incubated with PBS containing 1 μCi of 14C-labeled oleic acid, 4.5 mg/ml glucose, and 5% BSA for 1 h at 37 °C, followed by homogenization and lipid extraction with hexane. Lipid-enriched hexane fraction was either mixed with Microscint O and counted by scintillation or subjected to TLC separation with hexane/ethyl ether/acetic acid (80:20:1, v/v/v). For chronic study, the DIO mice or normal chow-fed mice (n = 7/group) were orally administered T863 (30 mg/kg) or vehicle at a volume of 5 μl/g of body weight. The animals were dosed for 15 consecutive days (once a day for days 1–7 and twice a day for days 8–14, and a final single dose on the morning of day 15). Body weight and food intake were monitored twice a week. Blood samples were obtained on the morning of day 14 to measure fed glucose and insulin level. On day 15, 1 h after the final dose, all mice were subjected to an oral glucose tolerance test (OGTT). After completion of the OGTT, all animals were returned to their home cage, supplied with food and water ad libitum, and sacrificed 2 h later to acquire blood and tissue samples. To evaluate the effects of T863 on hepatic insulin signaling, we treated the DIO mice with vehicle or T863 for 14 consecutive days by following the same regime of chronic study as described above. On day 15, mice fasted overnight were given an intraperitoneal injection of saline or insulin (20 units/kg). Liver samples were collected 15 min after saline or insulin injection to measure Akt phosphorylation by standard Western analysis.
For pharmacokinetic studies, an oral dose of T863 at 10 mg/kg was administered to mice via gavage. Blood samples (∼0.5 ml from each mouse) were withdrawn via cardiac puncture at 0, 0.167, 0.5, 1, 2, 3, 4, 7, and 24 h. Plasma concentrations of T863 were determined by LC-MS/MS. Briefly, a 50-μl plasma sample was used for the assay. The protein precipitation was carried out by adding 400 μl of acetonitrile containing a T863 analog at 250 ng/ml as the internal standard. The mixture was vortexed for 2 min. After centrifugation at 3400 rpm for 5 min, 350 μl of supernatant was transferred onto a fresh vial and dried under N2 using a Turbovap96 (40 °C). The sample was reconstituted in 100 μl of 20% acetonitrile in water, and 10 μl of supernatant was injected onto the LC-MS/MS system, which consisted of Agilent 1200 HPLC and Sciex API 4000 mass spectrometer. The separation was achieved using a Supelco Ascentis Phenyl analytical column (50 × 2.1 mm, 5 μm). The mobile phase consisted of Solvent A (0.1% formic acid in water) and Solvent B (100% acetonitrile). The flow rate was 0.4 ml/min with a gradient starting at 95% Solvent A to 95% Solvent B in 4 min. The MS/MS transition of 393.256 → 134.8 for T863 was monitored, and the lower limit of quantitation was 10 ng/ml.
Histological Analyses
Tissue samples were either flash-frozen (liver) and embedded in frozen medium (optimal cutting temperature compound, Ted Pella) or fixed in PBS-buffered 10% formalin (small intestine) at room temperature for 24 h prior to embedding in optimal cutting temperature compound. The 10-μm sections were cut and fixed to microscope slides, allowed to air-dry overnight (liver) or 2 h (small intestine) at room temperature, and stained with fresh Oil Red O for at least 7 min, rinsed in water, and then visualized under the microscope.
Protein Expression and Membrane Preparations for in Vitro Biochemical Assays
The Bac-to-Bac® baculovirus expression system (Invitrogen) was used to express recombinant human DGAT1, DGAT2, monoacylglycerol acyltransferase 2 (MGAT2), and MGAT3 in Sf-9 cells according to the manufacturer's manual and methods described previously (11). Sf-9 cells infected with recombinant baculovirus at an optimal multiplicity of infection for 64 h were harvested in ice-cold PBS and lysed by using nitrogen decompression (Parr Instrument Co., Moline, IL). The microsomal membranes were prepared by a differential centrifugation procedure. Lysates were first centrifuged at 8,000 × g to remove nuclei debris and mitochondrial fractions, and the resulting supernatants were further centrifuged at 100,000 × g to collect microsomal fractions. We have used the same batch of microsomal preparation throughout the entire study. Using the radioligand assay developed in this study and assuming a 1:1 stoichiometry of binding, we found that the amount of hDGAT1 enzyme in our hDGAT1-overexpressing microsomal preparations is estimated to be ∼0.043 pmol/μg of total microsomal protein. For preparation of microsomal membranes from primary tissues, freshly excised small intestine fragments (flushed with cold PBS to remove debris) and white adipose tissue were homogenized in a FastPrep-24 system (MP Biomedicals, Irvine, CA), followed by differential centrifugation as described above to obtain microsomal fractions. Protein concentration was determined by a Bio-Rad protein assay with BSA as a standard.
TLC-based in Vitro Assays for Acyltransferase Activities
The acyltransferase activity was determined by measuring the incorporation of [14C]oleoyl moiety from [14C]oleoyl-CoA into different acyl acceptors. Unless otherwise mentioned, the reaction mixture contained 100 mm Tris/HCl, pH 7.4, 5 mm MgCl2, 1 mg/ml fatty acid-free bovine serum albumin, 200 mm sucrose, 25 μm [14C]oleoyl-CoA (50 mCi/mmol), 200 μm acyl acceptors delivered in ethanol (final concentration less than 1%) and 50 μg of total lysates or microsomal protein in a final volume of 50 μl. The acyl acceptors used in the present study are as follows: 2-monooleoylglycerol (for MGAT activity) and 1,2-DOG for DGAT activity). The reactions were typically carried out at room temperature for 15 min and were terminated by adding 1 ml of chloroform/methanol (2:1, v/v). Lipids were extracted to organic solvent by vortexing for 30 s. After centrifugation to remove debris, aliquots of the organic phase containing lipids were separated by the Linear-K Preadsorbent TLC plate (Waterman Inc., Clifton, NJ) with hexane/ethyl ether/acetic acid (80:20:1, v/v/v). The TLC plates were exposed to a PhosphorImager screen to assess the incorporation of 14C-labeled acyl moieties into respective lipid products. Bands corresponding to each lipid species were verified by standards with exposure to I2 vapor. Phosphorimaging signals derived from radiolabeled lipid products were visualized using a Bio-Rad PhosphorImager and quantitated using QuantityOne software.
Fluorescence-based DGAT1 Assay
In this assay, DGAT activity is measured by monitoring the released CoASH from a DGAT1-mediated reaction. 7-Diethylamino-3-(4-maleimidophenyl)-4-methylcoumarin (CPM), a coumarin derivative, reacts with the sulfhydryl (-SH) group and forms a highly fluorescent product that is readily detected with excitation and emission wavelengths of 355 and 460 nm, respectively. The assay was originally developed in the 96-well format and has been miniaturized to a 384-well format. In the 96-well format, the assay was carried out in a total volume of 100 μl for up to 30 min under the following conditions: 100 mm Tris/HCl, pH 7.4, 200 μm 1, 2-DOG, 100 μm oleoyl-CoA, 1% Triton X-100, and 0.1–2 μg of microsomal proteins. A similar assay condition was also applied to the 384-well format carried out in a total volume of 25 μl. During the development of an assay for HTS and compound characterization, the assay conditions have been further modified to consist of 0.25 μg of total microsomal protein, 50 mm HEPES, pH 7.5, 1% Triton X-100, 10% DMSO, 312.5 μm oleoyl-CoA, and 625 μm 1,2-DOG. The reaction was initiated by the addition of DGAT1 microsomes and carried out for 30 min at room temperature (22 ± 2 °C). The reaction was stopped by SDS at a final concentration of 0.1% and incubated for an additional 30 min. Then one-tenth volume of CPM at 1 mm was added to the reaction, and the plate was incubated at room temperature for another 30 min, followed by detection of fluorescent signal by a PerkinElmer Wallac Victor3V 1420 multiple counter (excitation, 355 nm; emission, 460 nm). A standard curve of CoASH (ranging from 0 to 100 μm CoASH) was generated together with each assay to calculate the amount of CoASH produced in the DGAT1 reaction.
Stable Expression of DGAT1 in Mammalian Cells
To evaluate the compound activities in a cell-based assay, we established a stable cell line overexpressing hDGAT1. We have chosen to use HEK293A because of low endogenous DGAT activity in this cell line. Cells were maintained in Eagle's minimum essential medium supplemented with 10% fetal bovine serum, 2 mm l-glutamine, and appropriate antibiotics. A full-length hDGAT1 cDNA with an in-frame N-terminal FLAG tag was cloned into the mammalian expression vector pcDNA3.1(−)/Hygro as described (12). The plasmid was transfected into the HEK293A cells with Fugene 6 (Roche Applied Science) according to the manufacturer's protocol. The cells were placed in selection medium containing 400 μg/ml hygromycin 24 h after transfection. Cells were grown in selection medium until colony formation was observed and all cells had died in the dish of the naive cells. Then surviving cell colonies were transferred by trypsinization to 96-well plates at ∼3 cells/plate for continued culture. Cells in wells containing a monoclonal colony were selected and continued to expand in a 24-well plate. DGAT1 expression level and DGAT activity were checked by Western analysis (with anti-FLAG antibody) and TLC assay, respectively. For the cell-based assay, naive or hDGAT1-overexpressing HEK293A cells were pretreated with DGAT1 inhibitors at various concentrations for 1 h, followed by incubation with 10 μm [14C]oleic acid (50 Ci/mmol) supplemented with 0.1% fatty acid-free BSA for 4 h. Cells were then washed twice with PBS, and total lipids were extracted as described above.
Radioligand Filtration Assay
To assess the specific binding of a given compound to hDGAT1, we have developed a radioligand filtration assay using 3H-labeled DGAT1 inhibitor T863 as a probe. Briefly, aliquots of Sf-9 cell microsomal membranes were incubated with a binding reaction mixture that contained [3H]T863, at a predetermined concentration, various concentrations of DGAT1 inhibitor, and buffers that consisted of 50 mm Tris/HCl, pH 7.5, 5 mm MgCl2, 2.5 mm EDTA, 0.5 mg/ml BSA, and protease inhibitors for 90 min. The total reaction volume was 100 μl. Nonspecific binding was defined by incubating the same amount of probe with naive Sf-9 microsomal membrane without overexpression of hDGAT1 or by a performing parallel binding assay in the presence of 10 μm cold T863. After incubation, bound radioligand to microsomal membrane was separated from free radioligand by rapid filtration through a Unifilter-96 GF/B glass fiber FilterMate precoated with 0.5% polyethyleneimine. The membrane retained in the filter containing bound radioligand was then washed with cold PBS buffer nine times. Captured radioligand was detected with the MicroBeta liquid scintillation counter (PerkinElmer Life Sciences) by adding 50 μl of Microscint 20.
3T3-L1 Adipocyte Differentiation and Glucose Uptake Assay
3T3-L1 fibroblasts were maintained in DMEM supplemented with 10% (v/v) FBS, 100 units/ml penicillin, 100 mg/ml streptomycin, and 2 mm l-glutamine. Two days after the confluence, the cells grown in a 6-well plate were refreshed with DMEM containing 10% FBS, 2 μg/ml insulin, 0.25 μm dexamethasone, and 0.5 mm isobutylmethylxanthine. The cells were cultured in this medium for 4 days, and then the medium was switched to DMEM containing 10% FBS and 2 μg/ml insulin for 2 days, followed by another 2-day incubation with medium containing only 10% FBS. The cells were used for glucose uptake assay after this stage (at least 90% of the cell population showed the adipocyte phenotype with the accumulation of lipid droplets). For the glucose uptake assay, cells were first incubated in the presence or absence of T863 for 3 days (we have chosen 3 days of incubation to achieve enough exposure of adipocytes to the compound). On the day of the assay, cells were incubated for 2 h in Krebs-Ringer phosphate buffer supplemented with 30 mm HEPES, pH 7.4, 0.5% BSA, and 2.5 mm glucose with or without T863. The cells were washed once with PBS and incubated for another 15 min in the buffer without glucose in the presence or absence of T863. Insulin (100 nm final) was added, and the cells were incubated for 15 min. Then [14C]deoxyglucose (1 μCi/sample) in 10 μm cold deoxyglucose (final) was added to initiate the assay. The assay was terminated after 15 min by washing cells three times in ice-cold PBS with 10 mm glucose. Cells were solubilized in 0.5 ml of 0.5 m NaOH, 0.1% SDS. The extracts were neutralized by the addition of glacial acetic acid, and cell-associated radioactivity was determined by scintillation counting.
Data Analysis
Unless otherwise indicated, all results are expressed as mean ± S.E. All assay results were evaluated using GraphPad Prism software, and statistical differences among groups were assessed by analysis of variance or t test.
RESULTS
Development of a Novel, Fluorescence-based Assay for Assessing DGAT1 Activity
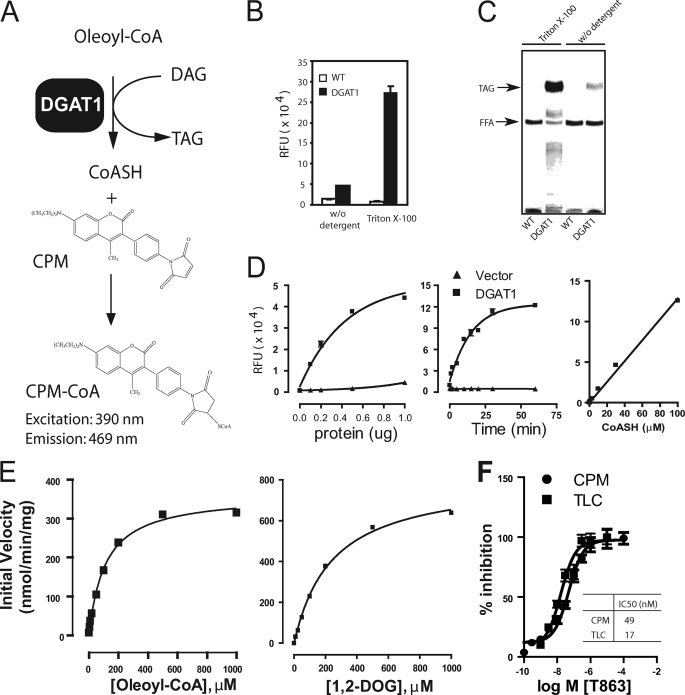
Conventionally, DGAT1 activity is assessed by measuring the incorporation of a radiolabeled acyl moiety from acyl-CoA to diacylglycerol with the formation of TAG; the radiolabeled TAG is separated and detected by thin layer chromatography (TLC). This method, however, is not practical for applications that require large sample sizes, such as high throughput screening or routine compound assessment. Efforts to develop more high throughput assays for DGAT1 activity have centered on alternative separation and detection methods, such as organic solvent-mediated extraction of TAG and a scintillation proximity assay for selective detection of radiolabeled TAG; in addition, mass spectrometry-based assays that detect the substrates and products of the DGAT reaction, DAG and TAG, have also been described (13–15). However, all of these assays involve either handling of radioisotopes associated with high cost or specialized equipment with limited throughput. To overcome these limitations, we developed a fluorescent assay that measures the free CoASH released during the DGAT1-mediated reaction using a thio-reactive probe, CPM (Fig. 1A). A similar assay format has been used in other CoASH-releasing reactions (16, 17). Using microsomal membranes prepared from insect cells overexpressing N-terminally FLAG-tagged human DGAT1 as an enzyme source, we initially found a relatively small assay window (Fig. 1B, left). Because the detergent Triton X-100 can enhance the specific activity of DGAT1, while inactivating many other acyltransferases (12), we examined its effect on the fluorescent assay. Triton X-100 increased the assay window in the fluorescent assay more than 10-fold (Fig. 1B, right), consistent with a large increase in DGAT1-dependent TAG formation, as assessed by a TLC assay (Fig. 1C), making this a viable assay for high throughput evaluation of DGAT1 activity. CoASH generation from DGAT1-containing microsomes increased with time and the amount of microsomal proteins (Fig. 1D).
FIGURE 1.
Development of a fluorescence-based assay for high throughput screening of DGAT1 inhibitors. A, schematic of the fluorescent CPM assay for DGAT1. The assay is designed to detect CoASH released from acyl-CoA in a DGAT1-mediated reaction with a thio-reactive probe, CPM. The yielding product CoA-CPM emits strong fluorescence at 460 nm. B, Triton X-100 increases the assay window for the DGAT1 CPM assay. C, Triton X-100 markedly increases TAG formation, as assessed by the TLC-based DGAT1 assay. D, time and protein dependence of DGAT1 activity and a standard curve of CoASH in the CPM assay. The assay was performed in a 96-well plate as described under “Experimental Procedures.” E, substrate kinetics of DGAT1 activity in the CPM assay. Assays were conducted in a 96-well plate by using 0.5 μg of microsomes with the indicated concentrations of oleoyl-CoA or 1,2-DOG in the presence of 200 μm 1,2-DOG or 100 μm oleoyl-CoA, respectively. F, a reference DGAT1 inhibitor, T863, shows comparable IC50 values in the CPM and the TLC assay. The percentage inhibition is calculated by the formula, % inhibition = (RUhigh − RUcpd)/(RUhigh − RUlow) × 100, where RUhigh, RUcpd, and RUlow are readings (fluorescence or radioactivity) obtained from reaction without the addition of T863, with T863 at different concentration, or with T863 at 10 μm, respectively. Data shown in B, D, and F represent mean ± S.E. (error bars) obtained from three independent experiments; data in C and E are representative of three independent experiments with similar results.
We next examined the kinetics of the DGAT1 reaction with respect to the two substrates, oleoyl-CoA and 1,2-DOG. These studies were conducted within the initial linear phase of the reaction (5 min). As shown in Fig. 1E, DGAT1-mediated formation of CoASH increased with increasing concentrations of oleoyl-CoA or 1,2-DOG, reaching maximal values of 362 and 812 nmol/min/mg protein, respectively. The data also confirmed the substrate specificity of the CPM-based fluorescent assay for DGAT1-mediated reaction. It should be noted that our study was conducted with impure enzyme preparations at 1% Triton X-100, a concentration well above the critical micelle concentration of Triton X-100 (0.02%, w/v) (18). Therefore, our results reflect the activity of DGAT1 in a micellar system. Preliminary analysis suggests that the DGAT1 reaction shows hallmarks of interfacial catalysis, including sensitivity to varying surface and bulk concentration of the substrates, with both substrates, oleoyl-CoA and 1,2-DOG, probably incorporated into the micelles (data not shown). Additional careful enzymatic analysis will be required to better understand the mechanism of DGAT1-mediated catalysis.
T863 is a potent and specific DGAT1 inhibitor that was originally disclosed in a patent application (9) (Example 2 in the patent; see Fig. 2A for molecular structure). The IC50 value for DGAT1 inhibition by T863 was similar in the CPM fluorescent assay (49 nm) and the TLC assay (17 nm) (Fig. 1F). A recently published DGAT1 inhibitor (7) (compound 4a) also had similar inhibitory activity in both assays (data not shown). These data suggest that the CPM assay can be used to evaluate small molecule inhibitors of DGAT1.
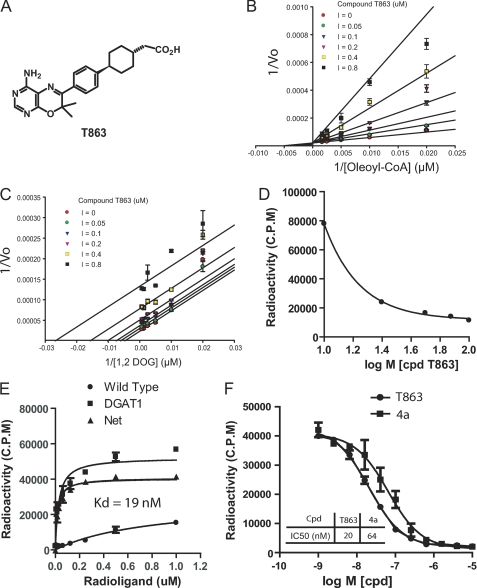
FIGURE 2.
, Biophysical characterization of DGAT1 inhibitors. A, structure of T863. B and C, Lineweaver-Burk plots for oleoyl-CoA or 1,2-DOG in the presence of T863 at various concentrations. The data were obtained from the CPM fluorescent assay performed in the 384-well format as described under “Experimental Procedures.” The pattern of fitting curves suggests that T863 is competitive against oleoyl-CoA (B) and uncompetitive against 1,2-DOG (C). D, T863 competes with the binding of acyl-CoA to hDGAT1 microsomes in a dose-dependent manner. Increasing concentrations of T863 were incubated with 50 μm [14C]oleoyl-CoA and DGAT1 microsomes containing 0.5 μg of total protein in 100 μl of reaction mixture. Filtration was performed as described under “Experimental Procedures.” E, the radiolabeled DGAT1 inhibitor T863 shows specific binding to hDGAT1 microsomes. 3H-Labeled T863 at various concentrations was incubated with wild-type or DGAT1-overexpressing Sf-9 cell microsomes for 90 min, followed by filtration to remove unbound radioligand. Retained radioligand was detected with a liquid scintillation counter. F, DGAT1 inhibitors T863 and compound 4a compete with the radiolabeled [3H]T863 for binding to hDGAT1. Increasing concentrations of compound T863 or 4a were incubated with DGAT1-microsomes and [3H]T863 for 90 min before filtration. See “Experimental Procedures” for a detailed description of the radioligand filtration assay. Data represent mean ± S.E. (error bars) obtained from three independent experiments, each performed in triplicate.
T863 Is a Competitive Inhibitor of DGAT1 and Binds Directly to DGAT1
To further understand the mechanism by which T863 inhibits DGAT1, we measured the DGAT activity of DGAT1-expressing microsomal membranes at varying concentrations of each of the two substrates in the presence of different concentrations of compounds. The data were then fit to different inhibition models with the lowest AICc (Akaike's criterion with a correction for low sample size) value as the criterion to assess the best fit. T863 appeared to behave as a competitive inhibitor with respect to oleoyl-CoA (Fig. 2B). Consistent with this notion, T863 showed a dose-dependent inhibition of the binding of radiolabeled oleoyl-CoA to DGAT1 (Fig. 2D). The best fit analysis of 1,2-DOG-mediated kinetics implies that T863 may act as an uncompetitive inhibitor with regard to 1,2-DOG (Fig. 2C). However, we have not been able to perform direct binding and competition studies with 1,2-DOG to confirm such an inhibitory pattern with respect to this substrate due to its poor solubility under the non-detergent conditions required for a binding assay. Our data can best be explained by a model in which T863 binds to the oleoyl-CoA binding site of DGAT1 bound with 1,2-DOG, although an allosteric mechanism cannot be completely ruled out. In order to assess whether T863 inhibits DGAT1 activity by binding directly to the enzyme, we synthesized 3H-labeled T863 and utilized it as a radioligand tracer to measure its binding to DGAT1-expressed microsomal membranes. As shown in Fig. 2E, DGAT1-containing microsomal membranes exhibited a typical saturable binding curve when exposed to 3H-labeled T863, whereas control microsomes showed only low, non-saturable binding to [3H]T863. The Kd for binding of 3H-labeled T863 was 19 nm, agreeing well with the IC50 value of 15–50 nm as determined above. Competition studies were conducted next, using 40 nm of 3H-labeled T863 (equal to ∼2 × Kd). As expected, unlabeled T863 fully competed with the radiolabeled probe with an IC50 value similar to the Kd for binding (20 nm; Fig. 2F), Interestingly, a structurally unrelated DGAT1 inhibitor, compound 4a (7), also competed with 3H-labeled T863 with an IC50 value (64 nm) that is comparable with the IC50 determined by enzymatic activity (data not shown) (7), suggesting that compound 4a binds to the same site as T863 (Fig. 2F). We propose that the acyl-CoA binding site of DGAT1 may serve as a binding site for structurally distinct DGAT1 inhibitors.
T863 is a Selective, Cell-permeable DGAT1 Inhibitor That Inhibits DGAT Activity in Mouse Tissues in Vitro
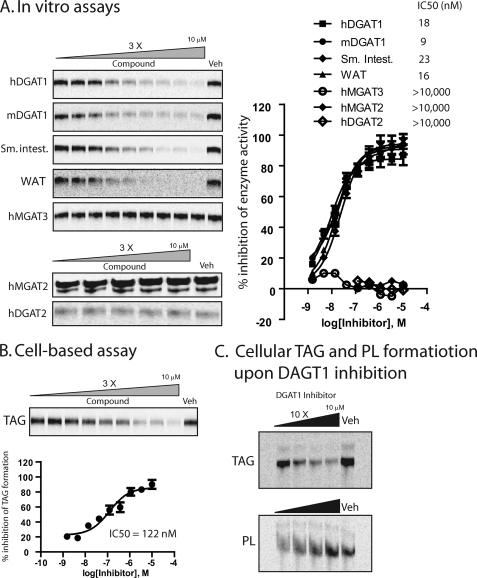
Our previous data confirmed that T863 is a potent inhibitor of recombinant human DGAT1. To determine the selectivity of this compound, we examined its effects on the activity of various related enzymes expressed in insect cells. As shown in Fig. 3A, T863 inhibited mouse DGAT1 as potently as human DGAT1. In contrast, T-863 had no inhibitory activity against human MGAT3, human DGAT2, or human MGAT2 (Fig. 3A) at concentrations as high as 10 μm.
FIGURE 3.
T863 inhibited DGAT activity in primary tissues and TAG formation in a cell-based model. A, inhibition of in vitro enzymatic activity by T863. The acyltransferase activity was measured by TLC-based assays in which membrane fractions prepared from Sf-9 cells overexpressing hDGAT1, mouse DGAT1, human MGAT3, human MGAT2, or human DGAT2, mouse small intestine (Sm. intest.), or white adipose tissue (WAT) were used as enzymatic sources. See “Experimental Procedures” for a detailed description of the TLC-based acyltransferase activity assays. Images shown on the left are the radiolabeled TAG or DAG (human MGAT2 only) bands captured from the TLC plate. The radioactivity of TAG or DAG bands was quantitated and used to calculate the percentage inhibition of enzymatic activity by T863 (with vehicle-treated sample as a control). Data shown on the right represent mean ± S.E. obtained from three independent experiments. The values of IC50 calculated for each enzymatic source were also listed. Veh, vehicle (1% DMSO). B, inhibition of TAG formation by T863 in a cell-based assay. HEK293 cells overexpressing hDGAT1 were incubated with 10 μm [14C]oleic acid (50 Ci/mmol) in the absence (Veh) or presence of T863 at various concentrations for 4 h. Cellular lipids were extracted and analyzed by TLC as described under “Experimental Procedures.” The top panel is a representative TLC image showing formation of radiolabeled TAG. The quantitative values of TAG bands were used to calculate the percentage inhibition of DGAT1 activity in the presence of T863 and plotted against the concentrations of T863 (bottom); data shown here represent mean ± S.E. (error bars) obtained from three independent experiments. C, increased cellular phospholipid (PL) formation upon DGAT1 inhibition. Metabolic labeling experiments were performed as described above. Cellular lipids were extracted and analyzed by TLC to monitor formation of TAG (top) or phospholipid (bottom).
To determine whether T863 can inhibit endogenous DGAT1 activity, we conducted enzymatic assays under conditions favoring detection of endogenous DGAT1 activity (19), using microsomes prepared from mouse small intestine or adipose tissue. T863 potently inhibited DGAT activity in both enzyme sources with an IC50 similar to what was observed in recombinant systems (16 and 23 nm for adipose tissue and small intestine, respectively) (Fig. 3A).
We next assessed the ability of T863 to inhibit DGAT activity in intact cells using HEK293 cells overexpressing human DGAT1 (HEK293-DGAT1). When incubated with 14C-labeled oleic acid, cells overexpressing DGAT1 are expected to show increased generation of 14C-labeled TAG, which is abolished by inhibition of DGAT1 (supplemental Fig. S1A). Compared with control HEK293 cells, HEK293-DGAT1 cells have increased hDGAT1 protein (supplemental Fig. S1B), increased in vitro DGAT activity (supplemental Fig. S1C), and increased incorporation of 14C-labeled oleate into TAG (supplemental Fig. S1D); all of these phenotypes can be reversed by siRNA-mediated knockdown of hDGAT1 in these cells, demonstrating dependence on hDGAT1 (supplemental Fig. S1, E and F). T863 caused a dose-dependent inhibition of cellular TAG formation in HEK293-DGAT1 cells, with an IC50 of 122 nm (Fig. 3B). Interestingly, in contrast to the decreased TAG formation (Fig. 3C, top), formation of phosphatidylcholine was increased by T863 (Fig. 3C, bottom). This increase in phosphatidylcholine synthesis is probably due to increased flux of fatty acyl-CoA and DAG to the phospholipid biosynthesis pathway in the face of DGAT1 inhibition, as has been observed previously in the reverse direction in fibroblasts overexpressing DGAT1 (20); in addition, this finding demonstrates that the effects of T863 on cells are not due to toxicity. Collectively, the above studies demonstrated that T863 is a potent, selective, and cell-permeable DGAT1 inhibitor.
T863 Has a Favorable Pharmacokinetic Profile in Mice
To examine whether T863 is suitable for in vivo studies, we assessed the pharmacokinetic profile of T863, using normal C57Bl/6J mice. Following a single oral dose of 10 mg/kg, the plasma concentration of the compound reached a peak at ∼8.5 μg/ml (equal to 22 μm) within 0.5 h and remained above the in vitro IC50 values for >12 h. The half-life and oral bioavailability of the compound was 3 h and 42%, respectively; clearance was relatively low (clearance value less than 5 ml/min/kg). These data indicate that T863 is suitable for in vivo studies in mice.
T863 Inhibits Acute Lipid Absorption
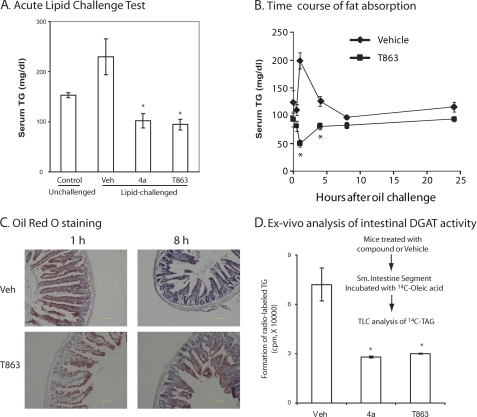
We first evaluated the in vivo efficacy of T863 in an acute lipid challenge model. C57/BL6 mice, fasted overnight, were given an oral dose of compound or vehicle followed by a bolus of corn oil 1 h later; serum triglycerides were measured 1 h after the lipid challenge. Compound 4a, which had been previously reported to inhibit triglyceride absorption (7), was included as a positive control. As shown in Fig. 4A, administration of the corn oil bolus resulted in a significant, 60% increase in serum triglyceride levels in vehicle-treated animals, whereas no increase was observed in mice treated with 10 mg/kg either T863 or compound 4a. A time course study showed that, in contrast to a robust increase and dynamic drop of serum triglyceride level in vehicle-treated mice challenged with corn oil, the serum TAG level in T863-treated animals remained constantly blunted up to 24 h after the lipid challenge (Fig. 4B). Thus, consistent with data reported for other DGAT1 inhibitors, T863 blocks or delays the absorption of triglyceride after an acute lipid challenge.
FIGURE 4.
Effects of T863 on intestinal fat absorption. A, oral administration of T863 significantly decreased serum TAG level in an acute lipid challenge test. In this experiment, C57/BL6 mice were first treated with an oral dose of compounds or vehicle. One hour later, a bolus of corn oil was administered via gavage, followed by measurement of serum triglyceride at 1 h after the lipid challenge. B, time course study of fat absorption in mice treated with vehicle or T863. Compound administration and lipid challenge followed the same procedure as described in A. Blood samples were withdrawn at 0, 0.5, 1, 4, 8, and 24 h after lipid challenge for measurement of serum triglyceride level. C, DGAT1 inhibition delayed the intestinal absorption of fat. Shown are sections of jejunum stained with Oil Red O. Bar, 100 μm. D, impaired formation of triglyceride in intestinal segment from compound-treated mice. C57/BL6 mice were first treated with an oral dose of compounds or vehicle. One hour later, the small intestine segments were collected and incubated with 14C-labeled oleic acid for 0.5 h. Total lipids were extracted and subjected for TLC analysis to measure TAG formation. Quantitative data are mean ± S.E. (error bars) (n = 5). *, p < 0.05 versus vehicle (Veh).
To gain further insight into how DGAT1 inhibition affects intestinal absorption of triglyceride, we performed histological studies examining lipid accumulation in enterocytes with Oil Red O, a dye that binds neutral lipid, especially triglycerides. As shown in Fig. 4C, at 1 h after the intragastric administration of a bolus of corn oil, accumulation of lipids in the jejunum was similar in both vehicle- and T863-treated mice. However, at 8 h after the lipid challenge, in contrast to a nearly complete disappearance of lipid staining in vehicle-treated animals, the accumulation of lipid in the jejunal enterocytes in T863-treated animals was still prominent (Fig. 4C). Thus, similar to what has been observed in DGAT1 knock-out mice, pharmacological inhibition of DGAT1 resulted in an impairment of lipid clearance within enterocytes, probably reflecting decreased chylomicron secretion. Interestingly, ex vivo studies using freshly isolated intestinal segments from mice treated with T863 or compound 4a also showed a significant decrease of TAG formation from exogenously added oleate (Fig. 4D). Thus, we conclude that the blunted appearance of serum triglycerides after DGAT1 inhibition is due to the impairment in both TAG biosynthesis and lipid clearance within enterocytes.
T863 Causes Weight Loss and Improves Insulin Sensitivity in Diet-induced Obese Mice
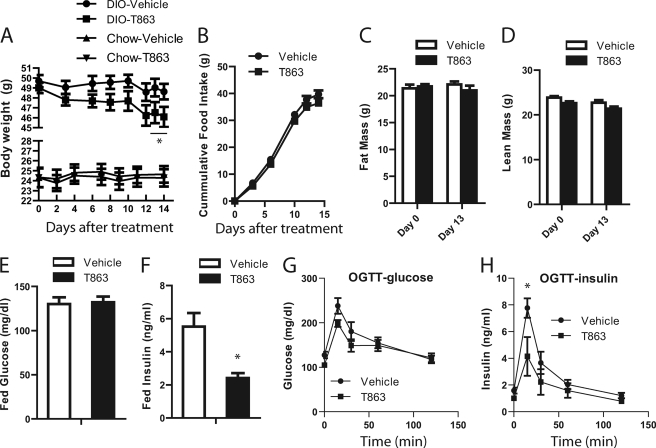
Having observed a favorable in vitro profile, sustained oral exposure, and potent efficacy in the acute lipid challenge model, we next investigated the effects of chronic administration of T863 on energy and glucose homeostasis. Diet-induced obese (DIO) mice fed a high fat diet ad libitum for 10 months or normal chow-fed mice were treated with vehicle or T863 at a dose of 30 mg/kg for 15 days. As shown in Fig. 5A, administration of T863 to DIO mice caused a reduction in body weight, which reached statistical significance by the 13th day of treatment. At the conclusion of the experiment, the average body weight of T863-treated DIO mice was 6.7% less than that of vehicle-treated DIO mice (46.1 ± 1.1 versus 49.2 ± 1.6 g). In contrast, there was no change in body weight and food intake in chow-fed mice treated with T863 (Fig. 5A) (data not shown). Although there was a trend toward a decrease in food intake in T863-treated DIO mice, the change was not statistically significant (Fig. 5B). As recorded by the echo MRI, T863 treatment in DIO mice resulted in a non-statistically significant decrease in both fat mass and lean mass (Fig. 5, C and D) on the 13th day of treatment. Fed plasma glucose and insulin levels were measured in DIO mice on day 14. Although blood glucose levels in both groups of mice were similar (Fig. 5E), fed plasma insulin levels in the T863-treated mice were significantly lower than those of vehicle-treated animals (p < 0.05) (Fig. 5F). In response to an oral glucose challenge (performed on day 15 of the study), T863-treated DIO mice showed a modest improvement in clearing glucose from peripheral circulation (Fig. 5G). Consistent with the decreased fed insulin levels, mice treated with T863 had lower serum insulin levels during the OGTT (Fig. 5H). These data suggest that treatment with T863 improved insulin sensitivity in DIO mice.
FIGURE 5.
Chronic treatment of DIO mice with T863 caused weight loss and improved insulin sensitivity. A, body weight of chow-fed or DIO mice treated with vehicle or T863. B, cumulative food intake in DIO mice treated with vehicle or T863. C and D, body composition of DIO mice treated with vehicle or T863. The fat mass (C) and lean mass (D) were analyzed by echo MRI on the 13th day of the treatment. E and F, fed state blood glucose (E) and plasma insulin (F) in DIO mice treated with vehicle or T863. G and H, blood glucose excursion curve (G) and serum insulin levels (H) in an OGTT test performed in DIO mice on the 15th day of the treatment. Mice were treated with vehicle or 30 mg/kg T863 as described under “Experimental Procedures.” Data are expressed as mean ± S.E. (error bars) (n = 7). *, p < 0.05 versus vehicle (Veh).
T863 Improved Serum Lipid Profile in Diet-induced Obese Mice
Table 1 lists serum lipid and other parameters measured under fed conditions at the end of the 15-day study. Serum triglyceride, LDL cholesterol, HDL cholesterol, and total cholesterol levels were all significantly lower in T863-treated animals. The non-esterified fatty acids levels tended to be lower in T863-treated animals, although the difference was not statistically significant. There were no changes in ALT and AST levels in mice receiving T863 treatment, suggesting a normal liver function. Taken together, these data indicate that DGAT1 inhibition in DIO mice not only decreased serum triglyceride but also improved cholesterol metabolism.
TABLE 1.
Serum biochemical profiles in control and T863-treated mice
Note that serum samples were collected on the day of the OGTT study. After the completion of OGTT, all animals were returned to their home cage, supplied with food and water ad libitum, and sacrificed 2 h later to obtain blood samples. The average serum concentration of T863 in compound-treated mice at the time of sacrifice was approximately 50 μm. BUN, blood urea nitrogen; ALT, alanine aminotransferase; AST, aspartate aminotransferase; NS, not significant.
| Treatment group | TAG | LDL cholesterol | HDL cholesterol | Total cholesterol | Free fatty acid | BUN | Total protein | ALT | AST |
|---|---|---|---|---|---|---|---|---|---|
| Vehicle | 172.7 ± 64.5 | 16.1 ± 2.8 | 85 ± 3.2 | 233.6 ± 16 | 1.2 ± 0.2 | 40.8 ± 5.5 | 5.8 ± 0.2 | 357 ± 81 | 423 ± 120 |
| T863 | 86.1 ± 64.5 | 6.8 ± 2.2 | 63.6 ± 5.9 | 138.8 ± 23 | 1.0 ± 0.1 | 36.8 ± 5.2 | 5.3 ± 0.5 | 287 ± 122 | 394 ± 182 |
| p value | <0.05 | <0.05 | <0.05 | <0.05 | NS | NS | <0.05 | NS | NS |
T863 Alleviated Hepatic Steatosis and Up-regulated Expression Level of Liver IRS-2 in DIO Mice
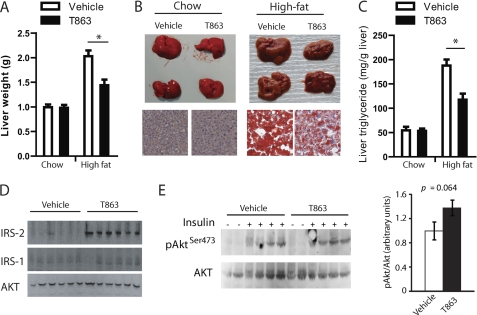
The average liver weight in DIO mice treated with T863 was reduced by 30% (Fig. 6, A and B), which was associated with a 40% decrease in hepatic triglyceride content as revealed by Oil Red O staining (Fig. 6B, bottom right) and a quantitative analysis of liver triglyceride content (Fig. 6C). In contrast, no change in liver weight, morphology, and triglyceride level was observed in chow-fed normal mice treated with T863 for 2 weeks (Fig. 6, A–C). To explore the mechanism(s) by which DGAT1 inhibition improves insulin sensitivity, we analyzed the expression level of several important proteins involved in insulin signaling in the liver. Notably, insulin receptor substrate 2 (IRS2) was markedly induced by T863 treatment (Fig. 6D). In contrast, the expression level of insulin receptor substrate 1 (IRS1) and the downstream kinase Akt2 remained unchanged (Fig. 6D). To examine whether the increased IRS2 levels in mice treated with T863 were associated with a better hepatic insulin response, we treated DIO mice with T863 or vehicle for 2 weeks at a dose of 30 mg/kg and then injected these mice with insulin and measured the phosphorylation status of hepatic Akt, an important downstream kinase in insulin signaling cascades. As shown in Fig. 6E, the ratio of insulin-induced phosphorylation of Akt at residue Ser-473 and total Akt protein was increased by ∼40% in the liver of T863-treated mice compared with vehicle-treated animals, although the change did not reach a statistical difference (p = 0.064).
FIGURE 6.
Chronic treatment of DIO mice with T863 improved hepatic steatosis, induced liver IRS-2 expression, and caused a modest increase in insulin-stimulated hepatic Akt phosphorylation. A–C, liver weight (A), representative size and morphology (B, top), Oil Red O-stained sections (B, bottom), and liver triglyceride content (C) in normal chow-fed or DIO (High-fat) mice treated with vehicle or T863 for 15 days. Data are expressed as mean ± S.E. (n = 7). *, p < 0.05 versus vehicle. D and E, Western blot analysis of hepatic IRS-2, IRS-1, and total Akt (D) and phosphorylated Akt protein at residue Ser-473 and total Akt (E) in DIO mice treated with vehicle or T863 for 2 weeks. Liver homogenates containing 50 μg of total proteins were subjected to Western analysis probed by antibodies as indicated. In E, mice were fasted overnight and given an intraperitoneal injection of insulin (20 units/kg) or saline at 15 min prior to liver sample collection. Phosphorylation status is also presented as the ratio between phosphorylated and total Akt protein. Data are mean ± S.E. (error bars) (n = 4).
T863 Enhanced Insulin-stimulated Glucose Uptake
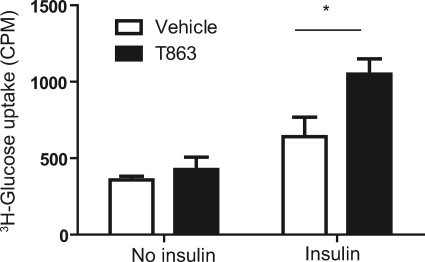
It was previously shown that white adipose tissue plays an important role in promoting insulin sensitivity in DGAT1-deficient mice (21). To investigate the effects of small molecule DGAT1 inhibition on insulin sensitivity in adipocytes, we measured basal and insulin-stimulated 2-deoxyglucose uptake in differentiated 3T3-L1 adipocytes, which had been incubated for 3 days in the presence or absence of T863. The adipocytes treated with or without T863 had similar levels of glucose uptake at base line (Fig. 7). In response to insulin (100 nm), the glucose uptake was significantly greater in T863-treated compared with vehicle-treated adipocytes (Fig. 7).
FIGURE 7.
T863 enhanced insulin-stimulated glucose uptake in differentiated 3T3-L1 adipocytes. Differentiation of 3T3-L1 cells to adipocytes, compound treatment, and insulin-stimulated glucose uptake test are described under “Experimental Procedures.” Data represent mean ± S.E. (error bars) obtained from three independent experiments, each performed in triplicate. *, p < 0.05 versus vehicle.
DISCUSSION
Here we provide a comprehensive assessment of a DGAT1 inhibitor by exploring both in vitro and in vivo properties of the model compound T863 in detail. We have developed a number of biochemical, biophysical, and cell-based assays to characterize T863 in vitro. To overcome limitations of previously described enzymatic assays for DGAT1, we developed a novel, homogenous fluorescent assay that uses the thio-reactive probe CPM to measure the formation of CoASH. This assay is robust enough to be used for routine compound characterization as well as high throughput screening and results in similar IC50 values compared with the traditional TLC-based assay. Using classical enzyme kinetics as well as binding studies, we found that T863 inhibits DGAT1 activity in a manner consistent with binding to the substrate oleoyl-CoA site of DGAT1. Using T863 as a radioligand, we were able to show that structurally distinct DGAT1 inhibitors, including the compound 4a (Fig. 2F) (7), compete with T863 for binding to DGAT1; several of these molecules also showed competitive inhibition with respect to oleoyl-CoA.4 Thus, there may be a prominent binding site for small molecule inhibitors in DGAT1 that overlaps with the oleoyl-CoA binding site. It is interesting to note that none of the inhibitors identified for the closest homolog of DGAT1, ACAT1, are competitive with respect to oleoyl-CoA (22, 23). This points to a lack of conservation of this binding pocket in ACAT1 and may explain the selectivity of DGAT1 inhibitors to date with respect to ACAT inhibition (5–7).
Having characterized the activity of T863 with respect to human DGAT1, we proceeded to show that T863 inhibits mouse DGAT1 activity in assays employing either recombinant or endogenous enzyme with similar potency and did not cross-react with other acyltransferases, including MGAT3, MGAT2, and DGAT2. T863 also showed potent activity in a cell-based assay, effectively blocking cellular formation of TAG. Interestingly, inhibition of DGAT1 in cells increased the formation of phospholipids, suggesting that suppression of the TAG biosynthetic pathway redirects the flux of fatty acids into phospholipid synthesis. This is consistent with a recent publication (20) that shows an increased flux of fatty acid to TAG from phospholipid synthesis pathway in fibroblasts overexpressing DGAT1.
One goal of our studies was to determine whether pharmacological inhibition of DGAT1 can recapitulate the phenotypes of the DGAT1 knock-out mice. In an acute lipid challenge mouse model, administration of T863 decreased the appearance of serum TAG, consistent with altered kinetics of fat absorption in the DGAT1 knock-out mice and with previous publications on acute effects of DGAT1 inhibitors (5–7, 24). Oil Red O staining revealed significant accumulation of lipids in enterocytes of T863-treated animals; this accumulation was most prominent at later time points and in the distal part of the small intestine after corn oil challenge, suggesting a delayed or retarded fat absorption. Such a delay may be caused by a deficiency in delivery of triglyceride from enterocytes to circulation upon DGAT1 inhibition, similar to what has been described for DGAT1 knock-out mice (24). These results suggest that DGAT1 is required for the generation of a pool of TAG that can efficiently be secreted from the enterocyte (probably in forms of chylomicrons and VLDL). However, given the large absorptive surface of the small intestine, it is likely that the delayed fat absorption induced by pharmacological inhibition of DGAT1 will not induce overt fat malabsorption under chronic conditions, similar to what has been described in DGAT1 knock-out mice.
Chronic treatment of diet-induced obese mice with T863 for a 2-week period caused weight loss by ∼6.7%. This weight loss was similar in magnitude to what has been seen in previous studies where other DGAT1 inhibitors were administered chronically (5, 7). Consistent with these studies, we did not observe a statistically significant decrease in food consumption, although T863-treated animals had a tendency to eat less. The weight loss of T863-treated mice appears to be due to a collective loss in both fat and lean mass as well as a decrease of liver mass in T863-treated mice. Our acute lipid challenge findings suggest that the dietary fat in T863-treated animals enter into the circulation at a reduced rate, similar to DGAT1-deficient mice or mice lacking MGAT2 (24, 25). Presumably, the altered kinetics of lipid absorption affects the intestinal homeostasis, thus regulating energy intake. Interestingly, DGAT1 inhibition did not cause weight loss in chow-fed mice, pointing to an important role for the high fat diet in the mechanism of DGAT1-induced weight loss. It was recently reported that perturbation in lipid absorption caused by DGAT1 or MGAT2 deficiency increases secretion of gut-derived satiety hormones, such as GLP-1 and PYY (25, 26). It is tempting to speculate that pharmacological inhibition of intestinal DGAT1 might cause similar changes in these gut hormones. However, in our 2-week chronic study, we found no increase in serum total GLP-1 levels at 2 h postprandial in compound-treated mice (data not shown). We have not yet had a chance to examine energy expenditure and locomotor activity in our T863-treated mice. T863 did not cause any apparent illness of the animals, and no abnormalities in skin and fur similar to what has been reported in DGAT1-deficient mice (27, 28) were observed in our studies (data not shown).
Chronic inhibition of DGAT1 with T863 in mice fed a Western diet resulted in a striking improvement in serum lipids ranging from triglyceride to cholesterol. Consistent with our results, King et al. (29) recently reported that small molecule inhibition of DGAT1 in the Zucker fatty rats and diet-induced hyperlipidemic hamsters resulted in a similar improvement in serum lipid profile. Conceivably, such an improvement in lipid metabolism observed in this study and previous studies might be associated with altered lipid absorption upon DGAT1 inhibition. In addition, inhibition of DGAT1 with T863 significantly decreased hepatic TAG levels and decreased liver weight. In a recent study, Villanueva et al. (30) revealed a specific role for DGAT1 in liver triglyceride biosynthesis using exogenous fatty acids (i.e. circulating fatty acids from intestinal absorption or mobilization of adipose tissue). Our current study is consistent with an important role for DGAT1 in hepatic TAG biosynthesis; in addition, it clearly demonstrates that small molecule inhibition of DGAT1 within a relatively short period of time (2 weeks) was sufficient to reverse hepatic steatosis caused by a high fat diet, without apparent liver toxicity. Because DGAT1 is highly expressed in human liver, and its expression level is reported to be increased in human non-alcoholic fatty liver diseases (31, 32), small molecule inhibitors of DGAT1 hold a promise to treat these disorders.
A significant decrease in fed serum insulin and a trend toward a decrease in fasting serum insulin was observed in mice treated with T863. Because glucose levels were unchanged or tended to be decreased by T863 treatment, this points toward an improvement in insulin resistance in DIO mice. In a glucose tolerance test, insulin excursion was significantly decreased compared with vehicle-treated mice, whereas glucose excursion showed a trend toward a decrease, again consistent with improved insulin sensitivity. Accumulation of lipids in the liver has been suggested to cause insulin resistance (33). Conversely, alleviation of lipid accumulation in fatty liver might be expected to improve insulin sensitivity. However, the causal relationship between hepatic lipid accumulation and insulin signaling remains controversial. Although some studies suggested that a lipid-derived metabolic intermediate, such as diacylglycerol or acyl-CoA, is the primary mediator of hepatic insulin resistance (33), others showed that overexpression of either DGAT1 or DGAT2 in liver was not sufficient to induce glucose or insulin intolerance, despite the increased triglyceride, diacylglycerol, and fatty acyl-CoA in liver (34). In an attempt to explore the insulin-sensitizing mechanisms in mice treated with DGAT1 inhibitor, we unexpectedly found that the expression levels of IRS2 in liver were dramatically up-regulated in these mice. A body of evidence from genetically modified mice and cell-based studies shows that IRS2 is one of the major mediators for metabolic actions of insulin in multiple tissues, including liver, pancreas, and brain (35, 36). Therefore, up-regulation of IRS2 would be predicted to facilitate insulin signaling, alleviating insulin resistance caused by a high fat diet. We assessed hepatic insulin signaling in T863-treated mice by measuring liver phospho-Akt levels after an acute insulin injection. Although we observed a trend toward increased Akt phosphorylation in T863-treated mouse liver (Fig. 6E), our data did not reach statistical significance. Additional studies using larger cohorts of mice will need to be conducted to conclusively demonstrate an effect of DGAT1 inhibition on hepatic insulin signaling. Another mechanism by which T863 may improve insulin sensitivity is through improving peripheral glucose uptake. Increased insulin-stimulated glucose uptake into myocytes and adipocytes has been observed in DGAT1-deficient mice (21). We found that in 3T3-L1 adipocytes, T863 enhanced insulin-stimulated glucose uptake, and it is conceivable that increased glucose disposal contributed to the improved insulin resistance observed in our study. It will be important to examine the possible effects of DGAT1 inhibition on peripheral glucose uptake in vivo and to more precisely define the tissues through which DGAT1 inhibition exerts its beneficial metabolic effects. It is also important to note that we currently do not know whether the improved glucose homeostasis and lipid profile as well as altered hepatic IRS2 levels observed in our study are secondary to the decrease in body weight or reflect an independent effect of DGAT1 inhibition.
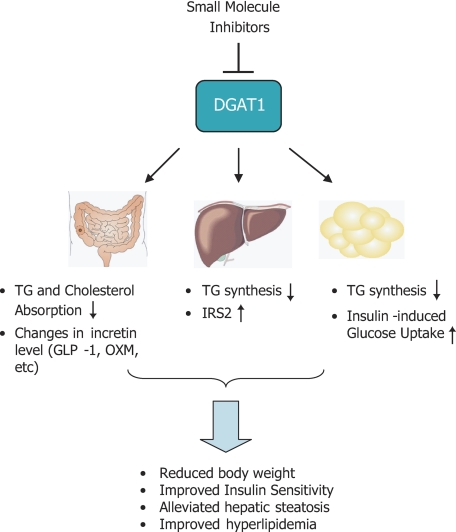
The proposed mechanisms of action of pharmacological inhibitors of DGAT1 are illustrated in Fig. 8. Inhibition of DGAT1 caused dynamic changes in TAG and cholesterol excursion in intestine, which might be associated with alteration of gut microenvironment and hormone secretion. In liver, DGAT1 inhibition caused a decrease in TG formation and increased expression level of IRS2, possibly contributing to improved insulin sensitivity. In adipose tissues, inhibition of DGAT1 may facilitate insulin-induced glucose uptake, as supported by our results in 3T3-L1 cells.
FIGURE 8.
Proposed mechanisms of action upon pharmacological inhibition of DGAT1 in the context of treatment of metabolic disorders. In small intestine, inhibition of DGAT1 resulted in dynamic changes in triglyceride and cholesterol absorption, which might in turn lead to changes in gut microenvironments and incretin release. Inhibition of hepatic DGAT1 improved steatosis caused by an excess of exogenous fatty acids (i.e. under high fat feeding) and up-regulated the expression level of IRS2, an important protein mediating insulin signaling. In adipose tissues, DGAT1 inhibition led to diminished fat biosynthesis and stimulated insulin-induced glucose uptake. The combined effects from these actions are believed to contribute to the beneficial outcomes of DGAT1 inhibition in vivo, including reduced body weight, improved insulin resistance, and hyperlipidemia, and alleviated hepatic steatosis.
In summary, our findings provide pharmacological validation in mice for the development of a small DGAT1 inhibitor to treat a number of metabolic disorders, including obesity, insulin resistance, dyslipidemia, and hepatic steatosis. It should be cautioned, however, that there is relatively little information available on the role of DGAT1 in humans, and no studies of DGAT1 inhibitors in either normal human volunteers or patients have been described in the literature to date, although several trials are ongoing (8). Furthermore, potential negative consequences of DGAT1 inhibition have not yet been fully explored. Overall, our data suggest that additional studies toward evaluating DGAT1 inhibitors as a therapeutic option for metabolic disorders are warranted.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
J. Cao, H. Peng, X. Huang, S. Stahler, J. McKew, M. Shi, J. F. Tobin, and R. E. Gimeno, unpublished data.
- TAG
- triacylglycerol
- DGAT
- acyl-CoA:diacylglycerol acyltransferase
- MGAT
- acyl-CoA:monoacylglycerol acyltransferase
- DIO
- diet-induced obese
- hDGAT1
- human DGAT1
- DAG
- diacylglycerol
- 1,2-DOG
- 1,2-dioleoylglycerol
- OGTT
- oral glucose tolerance test
- CPM
- 7-diethylamino-3-(4-maleimidophenyl)-4-methylcoumarin
- IRS
- insulin receptor substrate.
REFERENCES
- 1. Chen H. C., Farese R. V., Jr. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 482–486 [DOI] [PubMed] [Google Scholar]
- 2. Chen H. C., Smith S. J., Ladha Z., Jensen D. R., Ferreira L. D., Pulawa L. K., McGuire J. G., Pitas R. E., Eckel R. H., Farese R. V., Jr. (2002) J. Clin. Invest. 109, 1049–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smith S. J., Cases S., Jensen D. R., Chen H. C., Sande E., Tow B., Sanan D. A., Raber J., Eckel R. H., Farese R. V., Jr. (2000) Nat. Genet. 25, 87–90 [DOI] [PubMed] [Google Scholar]
- 4. Chen N., Liu L., Zhang Y., Ginsberg H. N., Yu Y. H. (2005) Diabetes 54, 3379–3386 [DOI] [PubMed] [Google Scholar]
- 5. Birch A. M., Birtles S., Buckett L. K., Kemmitt P. D., Smith G. J., Smith T. J., Turnbull A. V., Wang S. J. (2009) J. Med. Chem. 52, 1558–1568 [DOI] [PubMed] [Google Scholar]
- 6. Cheng D., Iqbal J., Devenny J., Chu C. H., Chen L., Dong J., Seethala R., Keim W. J., Azzara A. V., Lawrence R. M., Pelleymounter M. A., Hussain M. M. (2008) J. Biol. Chem. 283, 29802–29811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao G., Souers A. J., Voorbach M., Falls H. D., Droz B., Brodjian S., Lau Y. Y., Iyengar R. R., Gao J., Judd A. S., Wagaw S. H., Ravn M. M., Engstrom K. M., Lynch J. K., Mulhern M. M., Freeman J., Dayton B. D., Wang X., Grihalde N., Fry D., Beno D. W., Marsh K. C., Su Z., Diaz G. J., Collins C. A., Sham H., Reilly R. M., Brune M. E., Kym P. R. (2008) J. Med. Chem. 51, 380–383 [DOI] [PubMed] [Google Scholar]
- 8. Birch A. M., Buckett L. K., Turnbull A. V. (2010) Curr. Opin. Drug Discov. Devel. 13, 489–496 [PubMed] [Google Scholar]
- 9. Tularik Inc./Japan Tobacco Inc. Fox B. M., Furukawa N., Hao X., Iio K., Inaba T., Jackson S. M., Kayser F., Labelle M., Li K., Matsui T., McMinn D. L., Ogawa N., Rubenstein S. M., Sagawa S., Sugimoto K., Suzuki M., Tanaka M., Ye G., Yoshida A., Zhang J. (2004) U. S. Patent Application US 2004/0209871 A1
- 10. Herker E., Harris C., Hernandez C., Carpentier A., Kaehlcke K., Rosenberg A. R., Farese R. V., Jr., Ott M. (2010) Nat. Med. 16, 1295–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao J., Li J. L., Li D., Tobin J. F., Gimeno R. E. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 19695–19700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cao J., Cheng L., Shi Y. (2007) J. Lipid Res. 48, 583–591 [DOI] [PubMed] [Google Scholar]
- 13. Ramharack R. R., Spahr M. A. (2002) U. S. Patent Application US 2002/0127627 A1
- 14. Seethala R., Peterson T., Dong J., Chu C. H., Chen L., Golla R., Ma Z., Panemangalore R., Lawrence R. M., Cheng D. (2008) Anal. Biochem. 383, 144–150 [DOI] [PubMed] [Google Scholar]
- 15. Zhang J. H., Roddy T. P., Ho P. I., Horvath C. R., Vickers C., Stout S., Hubbard B., Wang Y. K., Hill W. A., Bojanic D. (2010) J. Biomol. Screen. 15, 695–702 [DOI] [PubMed] [Google Scholar]
- 16. Chung C. C., Ohwaki K., Schneeweis J. E., Stec E., Varnerin J. P., Goudreau P. N., Chang A., Cassaday J., Yang L., Yamakawa T., Kornienko O., Hodder P., Inglese J., Ferrer M., Strulovici B., Kusunoki J., Tota M. R., Takagi T. (2008) Assay Drug Dev. Technol. 6, 361-374 [DOI] [PubMed] [Google Scholar]
- 17. Trievel R. C., Li F. Y., Marmorstein R. (2000) Anal. Biochem. 287, 319–328 [DOI] [PubMed] [Google Scholar]
- 18. Tan C. H., Huang Z. J., Huang X. G. (2010) Anal. Biochem. 401, 144–147 [DOI] [PubMed] [Google Scholar]
- 19. Cases S., Stone S. J., Zhou P., Yen E., Tow B., Lardizabal K. D., Voelker T., Farese R. V., Jr. (2001) J. Biol. Chem. 276, 38870–38876 [DOI] [PubMed] [Google Scholar]
- 20. Bagnato C., Igal R. A. (2003) J. Biol. Chem. 278, 52203–52211 [DOI] [PubMed] [Google Scholar]
- 21. Chen H. C., Rao M., Sajan M. P., Standaert M., Kanoh Y., Miura A., Farese R. V., Jr., Farese R. V. (2004) Diabetes 53, 1445–1451 [DOI] [PubMed] [Google Scholar]
- 22. Bello A. A., Bright C., Burton B. J., Bush R. C., Casey J. H., Dron D. I., Facchini V., Joannou P. P., Parrott D. P., Riddell D., Roberts S. A., Williams R. J. (1996) Biochem. Pharmacol. 51, 413–421 [DOI] [PubMed] [Google Scholar]
- 23. Chiari A., Lovisolo P., Radice A., Giorgini L., Fancelli D., Severino D., Ghiselli G. (1995) Pharmacol. Res. 32, 189–199 [DOI] [PubMed] [Google Scholar]
- 24. Buhman K. K., Smith S. J., Stone S. J., Repa J. J., Wong J. S., Knapp F. F., Jr., Burri B. J., Hamilton R. L., Abumrad N. A., Farese R. V., Jr. (2002) J. Biol. Chem. 277, 25474–25479 [DOI] [PubMed] [Google Scholar]
- 25. Yen C. L., Cheong M. L., Grueter C., Zhou P., Moriwaki J., Wong J. S., Hubbard B., Marmor S., Farese R. V., Jr. (2009) Nat. Med. 15, 442–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Okawa M., Fujii K., Ohbuchi K., Okumoto M., Aragane K., Sato H., Tamai Y., Seo T., Itoh Y., Yoshimoto R. (2009) Biochem. Biophys. Res. Commun. 390, 377–381 [DOI] [PubMed] [Google Scholar]
- 27. Chen H. C., Smith S. J., Tow B., Elias P. M., Farese R. V., Jr. (2002) J. Clin. Invest. 109, 175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shih M. Y., Kane M. A., Zhou P., Yen C. L., Streeper R. S., Napoli J. L., Farese R. V., Jr. (2009) J. Biol. Chem. 284, 4292–4299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. King A. J., Segreti J. A., Larson K. J., Souers A. J., Kym P. R., Reilly R. M., Zhao G., Mittelstadt S. W., Cox B. F. (2009) J. Pharmacol. Exp. Ther. 330, 526–531 [DOI] [PubMed] [Google Scholar]
- 30. Villanueva C. J., Monetti M., Shih M., Zhou P., Watkins S. M., Bhanot S., Farese R. V., Jr. (2009) Hepatology 50, 434–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cases S., Smith S. J., Zheng Y. W., Myers H. M., Lear S. R., Sande E., Novak S., Collins C., Welch C. B., Lusis A. J., Erickson S. K., Farese R. V., Jr. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 13018–13023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kohjima M., Enjoji M., Higuchi N., Kato M., Kotoh K., Yoshimoto T., Fujino T., Yada M., Yada R., Harada N., Takayanagi R., Nakamuta M. (2007) Int. J. Mol. Med. 20, 351–358 [PubMed] [Google Scholar]
- 33. Samuel V. T., Liu Z. X., Qu X., Elder B. D., Bilz S., Befroy D., Romanelli A. J., Shulman G. I. (2004) J. Biol. Chem. 279, 32345–32353 [DOI] [PubMed] [Google Scholar]
- 34. Monetti M., Levin M. C., Watt M. J., Sajan M. P., Marmor S., Hubbard B. K., Stevens R. D., Bain J. R., Newgard C. B., Farese R. V., Sr., Hevener A. L., Farese R. V., Jr. (2007) Cell Metab. 6, 69–78 [DOI] [PubMed] [Google Scholar]
- 35. Dong X., Park S., Lin X., Copps K., Yi X., White M. F. (2006) J. Clin. Invest. 116, 101–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. White M. F. (2006) Can. J. Physiol. Pharmacol. 84, 725–737 [DOI] [PubMed] [Google Scholar]