Background: Newly synthesized GDNF could be sorted into the regulatory secretion pathway; however the molecular mechanisms controlling this process are not known.
Results: SorLA could specifically interact with the GDNF prodomain and mediate its regulated secretion.
Conclusion: SorLA acts as a sorting receptor to regulate GDNF secretion.
Significance: This will advance our understanding of the molecular mechanism underlying GDNF-regulated secretion.
Keywords: Neurotrophic Factor, Protein Domains, Protein Secretion, Protein Sorting, Protein-Protein Interactions, SorLA, Glial Cell Line-derived Neurotrophic Factor (GDNF), Prodomain
Abstract
Glial cell line-derived neurotrophic factor (GDNF), after secreted from cells, plays a critical role in central and peripheral neuron survival and function. The secretion of GDNF can be either constitutive or regulated by physiological stimuli; however, the detailed mechanism driving GDNF secretion is still unknown. Here, we report that sorting protein-related receptor with A-type repeats (SorLA), a member of the mammal Vps10p domain receptor, interacts with GDNF and is localized to GDNF-containing vesicles. Overexpression of SorLA significantly increases, and knockdown of SorLA by siRNA decreases, the regulated secretion of GDNF in PC12 and MN9D cells but has no effect on GDNF constitutive secretion. In addition, overexpression of a truncated form of SorLA also impairs GDNF-regulated secretion. Finally, we found that the prodomain of GDNF mediates the interaction of GDNF with SorLA under acidic conditions. Moreover, overexpression of SorLA could enhance the regulated secretion of the GDNF prodomain-GFP fusion protein, suggesting that the prodomain of GDNF is responsible for its regulated secretion. Together, these findings will advance our understanding of the molecular mechanism underlying GDNF-regulated secretion.
Introduction
Glial cell line-derived neurotrophic factor (GDNF)3 promotes the survival, neurite outgrowth, and differentiation of distinct populations of central and peripheral neurons, especially the midbrain dopaminergic neurons and spinal motoneurons, and has been proposed as therapeutic agents for neurodegenerative diseases (1). Many studies in animal models of Parkinson disease (PD) and some studies in PD patients show that GDNF delivered to the striatum or the substantia nigra can rescue previously damaged dopaminergic neurons and promote recovery of the motor function (2–7). GDNF is widely distributed in the central nervous system including cortex, hippocampus, cerebellum, striatum, hypothalamus, midbrain, and spinal cord (8). GDNF immunoreactivity was observed mainly in the neuronal somata, dendrites, and axons with a punctate dot staining pattern (9). GDNF signals through a multicomponent receptor complex comprising the transmembrane RET tyrosine kinase and a member of the family of glycosylphosphatidylinositol (GPI)-anchored cell surface proteins, the GFRα (10). GDNF-mediated RET activation stimulates multiple intracellular signaling pathways including RAS/ERK1/2 and PI3K/Akt that promote neuronal survival, migration, and neurite outgrowth (10, 11).
Recently GDNF has been shown to play an important role in synapse formation and plasticity (12). GDNF-GFRα complexes may act as synaptogenic factors by inducing adhesion between pre- and postsynaptic specializations in developing hippocampal neurons (13). Baudet et al. (14) have shown that the GDNF-RET signal participates in the organization and maturation of neuromuscular synapses. A growing body of evidence suggests that GDNF can be released via the regulated secretory pathway in neuronal cells, possibly representing a mechanism for preferentially supplying GDNF to active synapses (15–18). Like other neurotrophic factors, GDNF is first synthesized as a precursor of 211 amino acids called pro-GDNF and then cleaved into a mature form of 134 amino acids, because of a proteolytic consensus sequence (19). We have previously shown that the GDNF short isoform (GDNFΔ78, also named as (β)pro-GDNF in the Lonka-Nevalaita et al. study, while the full-length GDNF is named as (α)pro-GDNF), which lacks 78 bp and results in a 26 amino acid deletion in the pro-region of GDNF, has a secretion deficit in neurosecretory cells (20). Though our previous study suggests that the prodomain of GDNF plays an important role in its secretion, the detailed molecular mechanism driving GDNF secretion is still unknown.
Sorting protein-related receptor with A-type repeats (SorLA) is a 250-kDa type-1 membrane glycoprotein mainly expressed in neurons in the brain (21, 22). It is a member of a family of five mammalian proteins that share structural similarity with the vacuolar protein-sorting 10 protein (Vps10p), a sorting receptor in yeast that transports carboxypeptidase Y from the Golgi to the vacuole (23). The major pool of SorLA was found in late Golgi compartments, suggesting mediating trans-Golgi network-to-endosome sorting of newly synthesized ligands (24). SorLA has been recognized as a novel sorting receptor that regulates trafficking and processing of the amyloid precursor protein (25–27). GDNF was previously identified as a ligand that specifically binds with SorLA but not its family member sortilin by biosensor measurement; however little is known about the functional significance of this interaction (28). Here, we tested the hypothesis that SorLA acts as a sorting receptor to regulate GDNF secretion.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Anti-HA (hemagglutinin) antibodies were obtained from Covance (Princeton, NJ) (monoclonal) and Sigma (polyclonal), Anti-Flag M2 monoclonal antibodies were obtained from Sigma-Aldrich, and rabbit c-Myc antibodies were purchased from Bethyl Laboratories Inc. (Montgomery, TX). The other antibodies were purchased as follows: mouse anti-GDNF, rabbit anti-SorLA, and goat anti-RET antibodies from Santa Cruz Biotechnology (Santa Cruz, CA); mouse anti-phosphotyrosine antibody 4G10 from Upstate Laboratories (Charlottesville, VA); anti-sortilin antibodies from BD Biosciences (Franklin Lakes, NJ); mouse anti-Akt, rabbit anti-phospho-AktS473, rabbit anti-p44/42 MAP kinase (ERK1/2), mouse anti-phospho-p44/42 (pERK1/2) (Thr202/Tyr204) antibodies from Cell Signaling Technology (Beverly, MA); Alexa Fluor 488 goat anti-mouse IgG (H+L), Alexa Fluor 594 goat anti-rabbit IgG (H+L) from Invitrogen (Carlsbad, CA), and Cy5-conjugated donkey anti-rabbit IgG (H+L) from Jackson ImmunoResearch Laboratories (West Grove, PA).
Plasmid Constructs and siRNA Oligos
The human full-length (FL) GDNF and GDNFΔ78 cDNA were amplified by RT-PCR from human brain tissue and subcloned into pcDNA3.1 Neo expression vector (Invitrogen) using HindIII and XhoI sites. The hemagglutinin (HA) epitope tag was added to the 3′-end of the GDNF cDNA by PCR method. The GFP sequence was fused to the 3′-end of HA tag in various GDNFHA-GFP mutants. Human SorLA cDNA was subcloned into pcDNA 3.1 Neo expression vector using NotI and XhoI sites. The N-terminal Flag epitope tag was added after the signal peptide of SorLA by PCR. To knockdown the expression of SorLA in PC12 cells, 19 nucleotides of the rat SorLA sequence (GACCTGGATCATGATTCAG) was targeted with small interfering RNA using pSuper mammalian RNA expression vector (OligoEngine, Seattle, WA) according to the manufacturer's instructions. The resulting SorLA siRNA and the scrambled siRNA constructs were transfected into PC12 cells by electroporation (Amaxa, Koln, Germany). All constructs were confirmed by DNA sequence to exclude potential PCR-introduced mutations. The GALT-YFP construct was kindly provided by Prof. Wei Liu from Zhejiang University.
Real-time PCR Assay
PC12 cells were transfected with SorLA siRNA or scrambled siRNA construct. After 48 h, PC12 cells were subjected to RNA extraction. Total RNA was isolated using TRIzol-A+ RNA isolation reagent (TIANGEN) according to the manufacturer's instructions. 0.5 μg aliquots of each sample were treated with DNase to avoid DNA contamination, and then reversely transcribed using the RevertAidTM First Strand cDNA Synthesis kit (Fermentas). The cDNA was used as template for real-time PCR. Primer sequences used were as following. Rat SorLA forward primer: 5′-TTA CAA GGA GTC TAC ATT GC-3′ and reverse primer 5′-AAG TTG GAG GTT GAG CAG TT-3′. Primers specific for β-actin were used as a control (forward: 5′-TCC ATC ATG AAG TGT GAC GT-3′ and reverse: 5′-GAG CAA TGA TCT TGA TCT TCA T-3′). Real-time PCR were performed in a Cycler (Light Cycler 2.0, Roche) with the use of SYBR-green (Takara). The threshold cycle, which correlated inversely with the mRNA levels of the target, was measured as the cycle number at which the reporter fluorescent emission increased above a threshold level. Each sample was assayed in duplicate, and the mRNA levels were normalized for each sample to the β-actin mRNA levels using the 2−ΔΔCT method.
Hippocampal Neuron Cultures and Immunofluorescence Staining
Cultured hippocampal neurons from timed-pregnant Sprague-Dawley rats were prepared as previously described (29). In brief, hippocampi were dissected from embryonic day 18 rats, dissociated with 0.25% trypsin in Hank's balanced salt solution without Ca2+ and Mg2+ at 37 °C for 15 min, triturated in Dulbecco's modified Eagle's medium plus 10% fetal bovine serum (Invitrogen). For transfection, the neurons were seeded onto coverslips coated with 0.1 mg/ml poly-d-lysine in 6-well plates at a cell density of 5 × 105 cells/ml. Neurons were grown in Neurobasal media (Invitrogen) containing 2% B27 supplement (Invitrogen), 0.5 mm l-glutamine (Invitrogen), and 100 units/ml penicillin-streptomycin (Sigma) under 37 °C, 5% CO2, and 95% humidity conditions. Cultures were grown for 8–12 days before being used for experiments, and the media were changed every 3 days. Neurons were transfected using Lipofectamine 2000 (Invitrogen) transfection reagent following the manufacturer's instructions. 24–48 h after transfection, experiments were performed.
To visualize the co-localization of GDNF and SorLA, GDNFHA and FlagSorLA were co-transfected into hippocampal neurons. After 24 h, cells were fixed, permeabilized with 0.4% Triton X-100, and blocked with 10% goat serum. Then, the specimens were incubated with primary antibodies and their respective subtype-specific fluorescent secondary antibodies. The immunostained cells were observed with a Zeiss LSM710 confocal microscope (Carl Zeiss, Thornwood, NY).
Cell Culture, Immunoprecipitation, and Western Blot
PC12 cells were maintained in DMEM (Invitrogen) containing 5% fetal bovine serum, 10% horse serum (Invitrogen), supplemented with 0.5 mm l-glutamine, and 100 units/ml penicillin-streptomycin. MN9D dopamine cells were grown in DMEM/F12 (Invitrogen) containing 10% fetal bovine serum, supplemented with 0.5 mm l-glutamine, and 100 units/ml penicillin-streptomycin. Plasmids were transfected into cells by electroporation. 48 h after transfection, lysates were collected and analyzed by immunoprecipitation and Western blot. For immunoprecipitation experiments, lysates were collected and incubated with polyclonal HA antibodies or rabbit anti-Flag antibodies followed by incubation with protein A-Sepharose beads (Sigma). For the precipitation experiments under acidic conditions, the cell lysates were collected in the lysis buffer (150 mm NaCl, 10 mm Tris, 1 mm EDTA, and 1% Nonidet P-40 with protease and phosphatase inhibitors) with a given pH value adjusted using 1 m HCl. Western blot was performed by separating proteins via SDS-PAGE, transferring to a polyvinylidene difluofide membrane (Bio-Rad), and immunoblotting with HA11 or anti-Flag M2 antibodies. The immunoreactive protein bands were detected by enhanced chemiluminescence (Millipore, Temecula, CA).
To observe the subcellular localization of GDNF and SorLA, GDNFHA, FlagSorLA, and GALT-YFP were co-transfected into PC12 cells. 24 h later, cells were treated as described in hippocampal neuron staining and observed with a Zeiss LSM710 confocal microscope.
Cell Surface Biotinylation Assay
Cells were carefully rinsed twice with ice-cold PBS containing 1 mm CaCl2 and 0.1 mm MgCl2 (pH 7.4) and incubated with Sulfo-NHS-biotin (0.3 mg/ml in cold PBS) for 40 min at 4 °C. Cells were then quenched twice with ice-cold Tris-buffered saline (TBS) containing 0.1 mm CaCl2 and lysed in TNE lysis buffer (150 mm NaCl, 10 mm Tris, 1 mm EDTA, and 1% Nonidet P-40) with protease and phosphatase inhibitors. Clarified lysates were immunoprecipitated with immobilized streptavidin at 4 °C overnight, and complexes were immunoblotted with anti-RET or anti-Flag antibodies.
GDNF Secretion Assay
Secretion of GDNF was analyzed as previously described (30). In brief, PC12 cells were seeded to 6-cm dishes. 1.5 μg of GDNF and 3 μg of SorLA construct or empty vector were co-transfected into PC12 cells per 6-cm dish. 48 h after transfection, cells were washed three times with Krebs-Ringers -Henseleit (KRH) buffer with the following composition (in mm): 125 NaCl, 4.8 KCl, 2.6 CaCl2, 25 HEPES, 1.2 MgSO4, 5.6 glucose, 1 sodium ascorbate, and 1.2 KH2PO4, adjusted to pH 7.4 with NaOH. The conditioned media were collected after 2 h of incubation at 37 °C and used as a measure of constitutive secretion. To determine regulated secretion, cells were washed three times with KRH buffer, followed by 10 min of incubation at 37 °C in stimulated media (KRH buffer with an increased KCl concentration (56 mm) and decreased NaCl concentration (75 mm)). The amount of constitutive secretion was normalized to 10 min. Cell lysates, constitutive and regulated secretion media were analyzed via immunoblotting with HA11 antibodies or an ELISA assay.
ELISA
The GDNF protein concentrations in the respective media samples were determined using the GDNF Emax ImmunoAssay system (Promega, Madison, WI) with recombinant GDNF as a standard. Standards and samples were performed in duplicates, and each group contains six independent samples. After collecting conditioned media, cells were fixed and immunostained to determine the transfection efficiency. Each sample had a consistent transfection efficiency of about 60%.
Statistical Analysis
Statistical significance was determined using a Student's t test. Differences were considered significant at p < 0.05.
RESULTS
Interaction of SorLA with GDNF
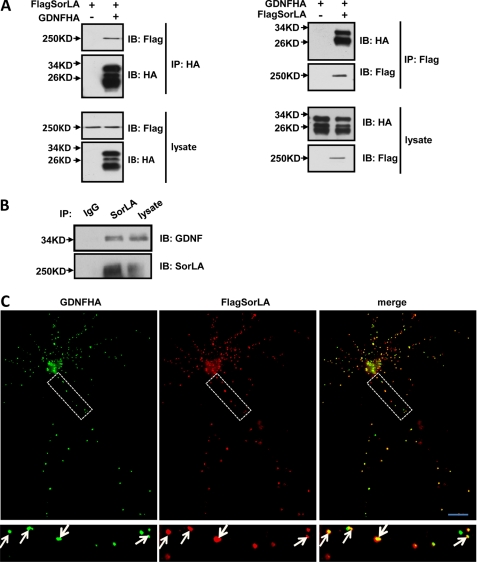
To explore the molecular mechanism of GDNF secretion, we sought to identify potential trafficking proteins that interact with GDNF. We focused on SorLA, a Vps10p domain protein, which has been reported to interact with GDNF by biosensor measurement (28). To confirm this result, we investigated the interaction between SorLA and GDNF by co-immunoprecipitation. We expressed both Flag-tagged SorLA and HA-tagged GDNF in PC12 cells. The two proteins were detected by specific antibodies against their respective epitope tags, and SorLA/GDNF association was assessed by immunoprecipitation. FlagSorLA was clearly detected in GDNFHA immunoprecipitates and GDNFHA in FlagSorLA immunoprecipitates (Fig. 1A). The HA tag antibodies recognized three bands between 17 and 34 kDa, likely representing glycosylated pro-GDNF, unglycosylated pro-GDNF, and mature GDNF, respectively (17). Only the upper two bands were detected in SorLA immunoprecipitates (Fig. 1A, right), which suggests that the prodomain of GDNF is required for the interaction. To exclude the possibility that the GDNF and SorLA interaction was because of protein overexpression, endogenous co-immunoprecipitation was performed. As shown in Fig. 1B, endogenous GDNF was detected in SorLA immunoprecipitates from rat brain lysates. Furthermore, when FlagSorLA and GDNFHA were co-transfected into cultured hippocampal neurons and immunostained with Flag antibodies and HA antibodies, punctate staining of SorLA was found, and co-localized well with GDNF (Fig. 1C). A previous study has shown that GDNF is localized primarily in secretogranin II-positive secretory granules (17). These data suggest that SorLA interacts with GDNF and localizes to GDNF-containing secretory granules. Next, we carried out additional experiments to investigate the possible functional significance of this interaction.
FIGURE 1.
Interaction of SorLA with GDNF. A, SorLA associates with GDNF. PC12 cells were co-transfected with FlagSorLA and/or GDNFHA. Cell lysates were immunoprecipitated with anti-HA antibodies or anti-Flag antibodies. Then Western blot analysis was performed to detect immunoprecipitated proteins. B, endogenous SorLA interacts with GDNF. The rat brain lysates were subjected to immunoprecipitation with anti-SorLA antibodies or normal rabbit lgG and immunoblotted with anti-GDNF and anti-SorLA antibodies. C, SorLA co-localizes with GDNF in hippocampal neurons. The cultured hippocampal neurons co-transfected with FlagSorLA and GDNFHA were immunostained with anti-Flag antibodies and anti-HA antibodies. The arrows indicate SorLA and GDNF co-localization. Scale bar, 20 μm.
SorLA Does Not Affect GDNF-induced Signaling on the Cell Surface
Previous studies revealed that sortilin, another member of the Vps10p domain protein, could act as co-receptor and molecular switch governing the p75NTR-mediated apoptosis induced by pro-neurotrophins (31, 32) and played an important role in regulating BDNF secretion as a sorting protein (29). We speculated whether SorLA might play similar roles. We first assessed whether SorLA could affect GDNF signaling on the cell surface as a co-receptor. PC12 cells were transfected with FlagSorLA or RET, and the cell surface SorLA level was measured by surface biotinylation assay. We found compared with RET receptor, SorLA exhibited significantly lower cell surface distribution (Fig. 2A), indicating its predominantly intracellular localization. To detect the interaction of SorLA and GDNF on the cell surface, PC12 cells co-transfected with RET and SorLA were treated with GDNFHA- and sGFRα1-containing media at 4 °C for 20 min to prevent GDNF internalization. The cell lysates were then immunoprecipitated with GDNF antibodies (anti-HA antibodies), and analyzed by Western blot to detect the SorLA and GDNF interaction on the cell surface because exogenous GDNF could not be endocytosed at 4 °C. We could not detect the association between SorLA and GDNF (Fig. 2B, lanes 3 and 4), while we did find a strong interaction between RET and GDNF on the cell surface (Fig. 2B, lane 2). Moreover, overexpression of SorLA had no significant effect on the association between RET and GDNF on the cell surface (Fig. 2B, lane 3), which could be due to their differences in surface distribution and binding affinity with GDNF. These results suggest that the interaction of SorLA and GDNF might mainly occur intracellularly. We then performed additional experiments to investigate the role of SorLA in GDNF-triggered intracellular signal transduction. We found that overexpression of SorLA had no effect on GDNF-induced activation of RET, ERK, and Akt (Fig. 2C), which further excluded the possibility of SorLA as a surface co-receptor for GDNF. The remaining question is the physiological role of SorLA in GDNF function. We investigated the subcellular distribution of SorLA and GDNF with β1,4-galactosyltransferase (GALT), a Golgi marker protein, and found SorLA and GDNF colocalized quite well in the Golgi apparatus (Fig. 2D). These data suggest that SorLA is not a cell surface co-receptor for GDNF but might serve as a sorting protein for GDNF and might be involved in GDNF secretion.
FIGURE 2.
SorLA does not affect GDNF-induced signaling on the cell surface. A, minor SorLA is expressed on the cell surface. PC12 cells were transfected with RET or FlagSorLA, followed by cell surface biotinylation and streptavidin pull-down of cell lysates, then analyzed by Western blot with anti-RET antibodies or anti-Flag antibodies. Surface levels of Ret and SorLA were quantified by surface/lysate ratios and normalized to that of RET. Results were represented as the mean ± S.E. from three independent experiments (*, p < 0.05; Student's t test). B, SorLA does not show obvious interaction with GDNF on the cell surface. PC12 cells were transfected with RET and/or FlagSorLA, treated with GDNFHA and/or sGFRα1-containing media for 20 min at 4 °C, and cell lysates were immunoprecipitated with anti-HA antibodies. Then Western blot analysis was performed to detect immunoprecipitated proteins. C, SorLA does not affect GDNF-induced signaling. PC12 cells were co-transfected with RET and FlagSorLA or empty vector as control, treated with GDNFHA and/or sGFRα1-containing media for 15 min at 37 °C. Cell lysates were immunoprecipitated with anti-RET antibodies followed by immunoblotting with 4G10 anti-phosphotyrosine and anti-RET antibodies, or were subjected to Western blot analysis directly with anti-pERK1/2, anti-ERK1/2, anti-pAkt, anti-Akt, or anti-Flag antibodies. D, SorLA co-localizes with GDNF in Golgi. PC12 cells co-transfected with FlagSorLA, GDNFHA and GALT-YFP were immunostained with anti-Flag and anti-HA antibodies. Scale bar, 20 μm.
Role of SorLA in Regulating GDNF Secretion
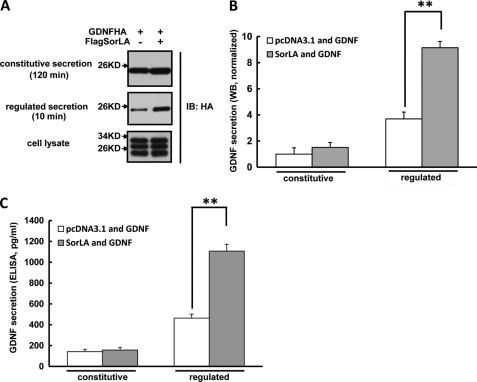
Considerable evidence supports that SorLA has multifunctional roles in Golgi-endosomal transport (21, 24, 33). The interaction of SorLA with GDNF, the localization of SorLA to GDNF-containing vesicles, and the assemblage in Golgi of both proteins prompts the hypothesis that SorLA might regulate the secretion of GDNF. To test this, we co-expressed GDNFHA and FlagSorLA or empty vector in PC12 cells, a cell line that has both constitutive and regulated secretion pathways. After 48 h, the cell lysates, constitutive and regulated secretion media, collected as described under “Experimental Procedures,” were analyzed by Western blot and ELISA. We detected GDNF protein in both 2-h constitutive secretion and 10-min regulated secretion media (Fig. 3A). By normalization, we found the amount of GDNF-regulated secretion was about 3.5-fold compared with its constitutive secretion (Fig. 3B), which suggested that GDNF was primarily secreted via the regulated pathway and this was consistent with our previous report (20). Only the mature form of GDNF was detected in the secretion media as compared with the lysates (Fig. 3A), suggesting GDNF was immediately processed to its mature form after secretion. Interestingly, a considerable increase (about 2-fold) was found in the amount of GDNF-regulated secretion from the PC12 cells overexpressing SorLA compared with that in the empty vector-expressing cells. However, GDNF constitutive secretion was not significantly different between SorLA-overexpressing and empty vector-expressing cultures (Fig. 3, A and B). The GDNF ELISA assay further confirmed that SorLA overexpression could enhance GDNF-regulated secretion but not its constitutive secretion (Fig. 3C).
FIGURE 3.
Overexpression of SorLA enhances GDNF-regulated secretion. A, overexpression of SorLA enhances GDNF-regulated secretion. PC12 cells were co-transfected with GDNFHA and FlagSorLA or empty vector. 48 h later, GDNF constitutive and regulated secretion media and cell lysates were collected, as described under “Experimental Procedures,” and analyzed by Western blot with anti-HA antibodies. B, quantitation of GDNF constitutive and regulated secretions measured by Western blot in A. The value of GDNF constitutive secretion in the absence of FlagSorLA was arbitrarily set to 1, and the values of GDNF secretions under various conditions were normalized to it. Results were represented as the mean ± S.E. from three independent experiments (**, p < 0.01; Student's t test). C, GDNF constitutive and regulated secretions were collected from cells treated as in A and measured by ELISA assay. Results were represented as the mean ± S.E. from three independent experiments (**, p < 0.01; Student's t test).
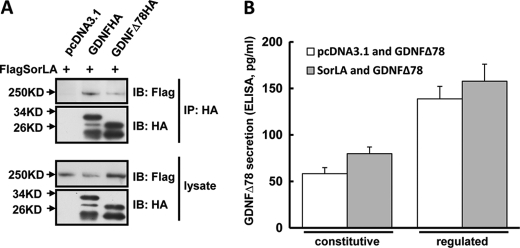
GDNF has an alternative spliced isoform GDNFΔ78 with a 26 amino acid deletion in the prodomain, which has been previously reported to lead to impaired GDNF secretion (20). We determined the effect of SorLA on GDNFΔ78 secretion. Co-immunoprecipitation analysis between SorLA and GDNFΔ78 showed that SorLA could interact with GDNFΔ78, but their association was significantly weaker compared with that between SorLA and full-length GDNF (Fig. 4A). We then co-expressed GDNFΔ78HA and FlagSorLA or empty vector in PC12 cells, and the constitutive and regulated secretion of GDNFΔ78 were measured by ELISA assay. As shown in Fig. 4B, overexpression of SorLA tended to increase GDNFΔ78 secretion but could not reach a significant level, which was in contrast to the effect of SorLA on full-length GDNF secretion (Fig. 3, B and C). The differential effects of SorLA on the secretion of the two isoforms of GDNF suggest the importance of the GDNF prodomain in SorLA-regulated GDNF secretion.
FIGURE 4.
Overexpression of SorLA does not affect GDNFΔ78 secretion. A, weaker association of GDNFΔ78 with SorLA. PC12 cells were co-transfected with FlagSorLA and GDNFHA, GDNFΔ78HA, or empty vector. Cell lysates were immunoprecipitated with anti-HA antibodies. Then Western blot analysis was performed to detect the immunoprecipitated proteins. B, overexpression of SorLA has no effect on GDNFΔ78 secretion. PC12 cells were co-transfected with GDNFΔ78HA and FlagSorLA or empty vector. The constitutive and regulated secretions of GDNFΔ78 were analyzed by ELISA assay. Results were represented as the mean ± S.E. from three independent experiments.
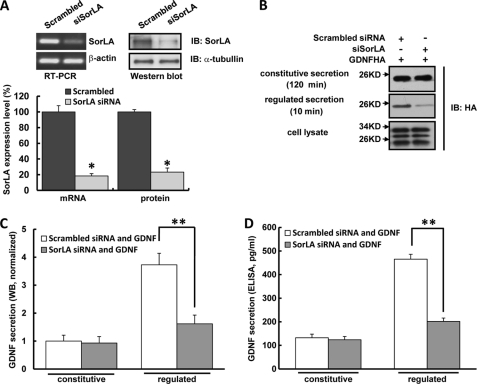
To further determine the specificity of the role of SorLA in full-length GDNF-regulated secretion, we assessed the effect of knocking down endogenous SorLA levels by small interfering RNA (siRNA). Real-time PCR and Western blot confirmed that SorLA siRNA transfection could successfully knock down endogenous SorLA level by ∼80% (Fig. 5A). In PC12 cells transfected with SorLA siRNA, the amount of GDNF-regulated secretion was reduced by ∼60% compared with scrambled siRNA transfection, which was further confirmed by the GDNF ELISA assay (Fig. 5, B–D). In contrast, GDNF constitutive secretion was not affected by SorLA siRNA transfection (Fig. 5, B–D). These data suggest that SorLA acts to positively affect GDNF-regulated secretion whereas it has no influence on GDNF constitutive secretion.
FIGURE 5.
Knockdown of SorLA expression by siRNA decreases GDNF-regulated secretion. A, PC12 cells were transfected with scrambled siRNA or SorLA siRNA, 48 h later, endogenous SorLA mRNA and protein levels were assessed by real-time PCR and Western blot (n = 3, *, p < 0.05, Student's t test). B, knock-down of endogenous SorLA decreases GDNF-regulated secretion. PC12 cells were co-transfected with GDNFHA and SorLA siRNA or scrambled siRNA, and GDNF constitutive and regulated secretions were analyzed as in Fig. 2A. C, quantitation of GDNF secretion measured by Western blot in B. The value of GDNF constitutive secretion from cells co-transfected with empty vector was arbitrarily set to 1, and the values of GDNF secretions under various conditions were normalized to it. Results were represented as the mean ± S.E. from four independent experiments (**, p < 0.01; Student's t test). D, GDNF constitutive and regulated secretions were collected from cells treated as in B and measured by ELISA assay. Results were represented as the mean ± S.E. from three independent experiments (**, p < 0.01; Student's t test).
To examine whether SorLA regulates GDNF secretion in dopamine cells, we carried additional experiments to investigate the effect of SorLA on GDNF secretion in MN9D dopamine cells. MN9D cell lines derived from the fusion of mice embryonic ventral mesencephalic and neuroblastoma cells has gained popularity as a model of CNS dopaminergic neurons because it expresses tyrosine hydroxylase and synthesizes and releases dopamine (34). We co-expressed GDNFHA and FlagSorLA in MN9D cells and found overexpression of SorLA significantly increased GDNF-regulated secretion while it had no influence on its constitutive secretion (supplemental Fig. S1A). Moreover, introducing SorLA siRNA into MN9D cells could decrease GDNF-regulated secretion (supplemental Fig. S1B). These results are consistent with the data we got from PC12 cells, which further confirms that SorLA controls GDNF-regulated secretion.
Truncated SorLA Impairs GDNF-regulated Secretion
Because the Vps10p domain of sortilin has been recognized as the major ligand binding region (30, 35), we speculated that the Vps10p domain in SorLA might also mediate its interaction with GDNF. We generated a truncated SorLA construct (named Vps10p), which only contained the Vps10p domain of SorLA (Fig. 6A). The immunoprecipitation experiment demonstrated that the SorLA Vps10p domain was able to interact with GDNF (Fig. 6B). The cell surface biotinylation experiment showed that this truncated mutant did not appear on the plasma membrane, indicating it could only interact with GDNF inside of cells (Fig. 6C). We postulated that the Vps10p domain of SorLA, which interacts with GDNF, may alter GDNF trafficking and secretion, because it lacks the appropriate post-Golgi trafficking signals located in the cytoplasmic domain (27, 36). PC12 cells were co-transfected with GDNFHA and FlagVps10p or empty vector as control; regulated and constitutive GDNF secretion were assessed 48 h later by Western blot analysis (Fig. 6, D and E) as well as ELISA assay (Fig. 6F). There was a significant decrease in GDNF-regulated secretion in the presence of Vps10p. In contrast, no significant change was detected in GDNF constitutive secretion (Fig. 6, D--F). This result suggests that the truncated form of SorLA interacts with GDNF and impairs GDNF-regulated secretion, which may be caused by disrupting the interaction of endogenous SorLA with GDNF.
FIGURE 6.
Truncated SorLA impairs GDNF-regulated secretion. A, schematic presentation of the truncated SorLA used in this study. B, the Vps10p domain of SorLA associates with GDNF. PC12 cells were co-transfected with GDNFHA and FlagVps10p, FlagSorLA, or empty vector. Cell lysates were immunoprecipitated with anti-Flag antibodies. Then Western blot analysis was performed to detect the immunoprecipitated proteins. C, the truncated mutant FlagVps10p does not appear on the cell surface. PC12 cells were transfected with FlagVps10p or FlagSorLA, followed by cell surface biotinylation and streptavidin pull-down of cell lysates, then analyzed by Western blot with anti-Flag antibodies. D, overexpression of SorLA Vps10p domain decreases GDNF-regulated secretion. PC12 cells were co-transfected with GDNFHA and FlagVps10p or empty vector. GDNF constitutive and regulated secretions were analyzed as in Fig. 3A. E, quantitation of GDNF secretion measured by Western blot in C. The value of GDNF constitutive secretion from cells co-transfected with empty vector was arbitrarily set to 1 and the values of GDNF secretions under various conditions were normalized to it. Results were represented as the mean ± S.E. from three independent experiments (**, p < 0.01; Student's t test). F, GDNF constitutive and regulated secretions were collected from cells treated as in D and measured by ELISA assay. Results were represented as the mean ± S.E. from three independent experiments (**, p < 0.01; Student's t test).
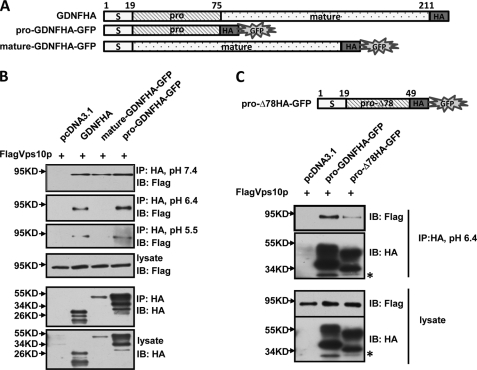
Identification of the Prodomain of GDNF as the Key Interaction Domain with SorLA
GDNF protein is first synthesized in the form of pro-GDNF and then converted to its mature form during the intracellular trafficking and secretion process. Previous studies have shown the prodomains in neurotrophic factors play important roles in their trafficking and secretion (18, 30). We have tried to determine which part of GDNF, prodomain or mature domain, could mediate its interaction with SorLA. Given that the 58 amino acid GDNF prodomain is too small to be detected, we generated GFP fusion protein-expressing constructs for both the GDNF pro and mature domain (Fig. 7A). Co-immunoprecipitation experiments were performed between the pro or mature domain of GDNF and the Vps10p domain of SorLA. Under neutral pH conditions, both pro and mature GDNF domain interacted with the SorLA Vps10p domain (Fig. 7B). Because the process of sorting secretion proteins into distinct exocytotic carriers occurs in the trans-Golgi network and most SorLA is located in the late Golgi compartment (24), we expect that protein sorting mediated by SorLA may occur in this location. So we performed additional experiments to determine whether it was feasible for such an interaction to occur under pH 6.4 conditions; the pH value in the lumen of the trans-Golgi network (37). We found that the prodomain retained, while the mature domain lost, its binding capacity with the SorLA Vps10p domain (Fig. 7B), which suggests that the prodomain of GDNF is the key domain mediating its interaction with SorLA in the trans-Golgi network where GDNF sorting occurs. Next, we performed the co-immunoprecipitation study under more acidic conditions, pH 5.5; the intraluminal pH of mature secretory granules (38). The prodomain of GDNF still bound with the Vps10p domain of SorLA under pH 5.5 conditions (Fig. 7B), which suggests that GDNF may not only be targeted to but also retained in the secretory granules with binding to the SorLA lumen domain in this compartment. That GDNF prodomain could interact with SorLA under acidic conditions is consistent with our previous immunocytochemistry staining results showing that GDNF and SorLA are colocalized in the puncate vesicular structures.
FIGURE 7.
Identification of the prodomain of GDNF as the key interaction domain with SorLA. A, schematic presentation of various GDNF mutants with a GFP sequence fused to the 3′-end of HA tag. B, the association of SorLA Vps10p domain with GDNF mutants under different pH conditions. PC12 cells were transiently transfected with SorLA FlagVps10p domain and full-length GDNFHA or prodomain GDNFHA-GFP or mature domain GDNFHA-GFP or empty vector. Lysis buffer was prepared under the indicated pH condition. Cell lysates were immunoprecipitated with anti-HA antibodies and immunoblotted with anti-Flag antibodies or anti-HA antibodies. C, the weaker association of GDNFΔ78 prodomain with SorLA Vps10p domain under pH 6.4 compared with GDNF full-length prodomain. PC12 cells were co-transfected with FlagVps10p and pro-GDNFHA-GFP or pro-Δ78HA-GFP or empty vector. Cells were lysed at pH 6.4, immunoprecipitated with anti-HA antibodies, and immunoblotted with anti-Flag antibodies or anti-HA antibodies. The asterisk indicates GFP.
Next, we performed the co-immunoprecipitation experiment for the prodomain of GDNFΔ78 and SorLA Vps10p domain under pH 6.4 acidic conditions. Compared with pro-GDNF, a significant reduced association between pro-GDNFΔ78 and SorLA Vps10p domain was observed (Fig. 7C). The weaker association between GDNFΔ78 and SorLA may explain in part the secretion deficit in the GDNFΔ78 isoform. Together, all these findings demonstrate that the prodomain may play key roles in GDNF trafficking and secretion.
Interestingly, we observed three bands in pro-GDNF and full-length GDNF lysates; however there was only one band detected in the mature GDNF lysates. A previous study reported that the mature GDNF polypeptide contained two putative N-glycosylation sites (19), and recently Mart Saarma and co-workers (17) have demonstrated that the different bands of full-length GDNF may represent glycosylated and unglycosylated forms of GDNF, respectively. We speculated that pro-GDNF could traffic in the same pathway as full-length GDNF and remain to be glycosylated, while the GDNF mature domain entered an aberrant trafficking pathway and as a result was defective in glycosylation.
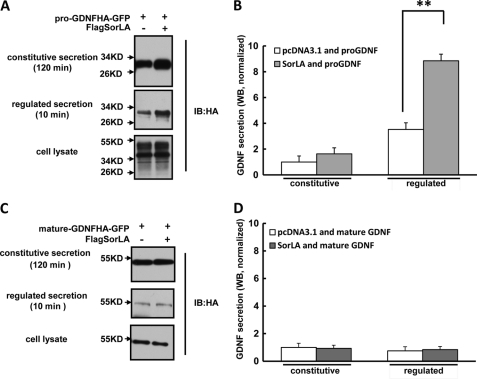
SorLA Enhances the Regulated Secretion of the GDNF Prodomain
Because the pro and mature domain of GDNF shows differential binding abilities with SorLA under acidic conditions, we compared the effect of SorLA on their secretion. We co-expressed GFP-fused GDNF pro or mature domain and FlagSorLA in PC12 cells, and their constitutive and regulated secretion were analyzed by Western blot. As shown in Fig. 8, A and B, pro-GDNFHA-GFP fusion protein could be secreted in a regulated manner and overexpression of SorLA significantly increased its regulated secretion, which supports our hypothesis that SorLA binds with GDNF via its prodomain and could drive GDNF into the regulated secretion pathway. In contrast, mature-GDNFHA-GFP fusion protein was not secreted via the regulated secretion pathway, and overexpression of SorLA had no effect on its secretion (Fig. 8, C and D), which could be explained by the observation that the GDNF mature domain could not interact with SorLA under physiological conditions such as pH 6.4 in the Golgi apparatus. These findings further demonstrate that SorLA mediates GDNF-regulated secretion by interacting with the prodomain of GDNF.
FIGURE 8.
Overexpression of SorLA enhances GDNF prodomain-mediated regulated secretion. A, SorLA enhances GDNF prodomain-mediated regulated secretion. PC12 cells were co-transfected with pro-GDNFHA-GFP and FlagSorLA or empty vector. The constitutive secretion media, regulated secretion media, and cell lysates were detected by Western blot. B, quantitation of secreted protein measured by Western blot in A. The value of constitutive secretion from cells co-transfected with empty vector was arbitrarily set to 1. Results were represented as the mean ± S.E. from three independent experiments (**, p < 0.01; Student's t test). C, overexpression of SorLA has no effect on GDNF mature domain-mediated secretion. PC12 cells were co-transfected with mature-GDNFHA-GFP and FlagSorLA or empty vector. The constitutive secretion media, regulated secretion media, and cell lysates were detected by Western blot. D, quantitation of secreted protein measured by Western blot in C. The value of constitutive secretion from cells co-transfected with empty vector was arbitrarily set to 1. Results were represented as the mean ± S.E. from three independent experiments.
DISCUSSION
Newly synthesized GDNF may be sorted into the regulatory secretion pathway; however, the molecular mechanisms controlling this process are not known. The GDNF short isoform with 26 amino acids deletion in the prodomain was recently found to lead to impaired regulatory secretion, which suggests that a trafficking signal in the prodomain is involved in optimal GDNF targeting to the regulated secretion pathway (20). From our studies, we conclude that in neurosecretory cells, efficient GDNF sorting to regulated secretory pathway is controlled by a protein interaction of a GDNF prodomain with a Vps10p domain protein, SorLA, which has been established previously to have multifunctional roles in Golgi-endosomal transport as well as endocytosis (25–27, 33, 36).
Our studies have provided three new insights into the regulation of GDNF trafficking to secretory pathways. First, to our knowledge, this is the first time that a sorting molecule, SorLA, has been shown to control GDNF-regulated secretion. We demonstrated the endogenous interaction of SorLA and GDNF in brain lysates as well as their co-localization in the same secretory granule compartment in neurons. Overexpression of SorLA significantly enhances and the loss of function study with SorLA siRNA dramatically reduces GDNF-regulated secretion. Our co-immunoprecipitation experiment found the Vps10 luminal domain of SorLA binds with GDNF, which is consistent with previous BIAcore experiment (28). Overexpressing the Vps10 domain of SorLA lacking its cytoplasmic tail, which mediates efficient Golgi body-endosome transport, disrupts GDNF-regulated secretion (27). This provides evidence that SorLA is involved in controlling GDNF-regulated secretion. Interestingly, we also detected the association of GDNF with another Vps10p domain protein, sortilin, which has been shown to mediate BDNF-regulated secretion (30). Compared with BDNF, the association between GDNF and sortilin was much weaker (supplemental Fig. S2A). Endogenous interaction of GDNF and sortilin was also observed in rat brain lysates (supplemental Fig. S2B). Under neutral pH conditions, the GDNF mature domain but not its prodomain interacted with sortilin; however, their interaction was abolished under pH 6.4 conditions, the pH value in the lumen of the trans-Golgi network (supplemental Fig. S2C). This result suggested that sortilin might not participate in GDNF trafficking. Indeed, overexpression of sortilin had no effect on GDNF-regulated and constitutive secretion (supplemental Fig. S2, D and E). These results suggest the specific regulation of the trafficking and secretion of different neurotrophic factors by Vps10p domain family members.
Second, we identified that the prodomain of GDNF is the key interaction region with SorLA. Previous BIAcore experiment found the modest interaction of mature GDNF with Vps10 domain of SorLA, and we also detected mature GDNF and SorLA in the same immunoprecipitation; both the experiments were performed under pH 7.4 conditions. When the co-immunoprecipitation experiment was performed at pH 6.4, mature GDNF lost its binding with SorLA; however the prodomain of GDNF still bound with SorLA to the same level compared with pH 7.4. This result suggests that the interaction of SorLA and GDNF prodomain is quite resistant to pH changes. As the pH in trans-Golgi was reported to be around 6.4 and the majority pool of SorLA was found in the late Golgi compartment (24, 39, 40), the interaction between the GDNF prodomain and SorLA may be uniquely required for its efficient regulated secretion. The hypothesis was further supported by the result that the prodomain of GDNF could efficiently drive GFP to the regulated secretion pathway; however the mature GDNF could not. The GDNF prodomain still binds with SorLA at pH 5.5, which suggests that GDNF may not only be targeted to the secretory granules but remains bound to the SorLA luminal domain in this compartment. Thus, the prodomain of GDNF is responsible for its intracellular transport and regulated secretion.
We found the GDNF short isoform that lacks 26 amino acids in the GDNF prodomain showed reduced binding affinity with SorLA and led to dramatically decreased regulated secretion, which further demonstrates the importance of the prodomain in regulating GDNF secretion. This result is in agreement with a recent publication showing that partial deletion in the prodomain dramatically reduced GDNF secretion (18). However, another study characterizing the intracellular trafficking and secretion of the GDNFΔ78 isoform has recently been published (17). This study demonstrates a significantly increased regulated secretion for GDNFΔ78 compared with full-length GDNF, which is in contrast to our results. The main difference between these studies is that we have used 56 mm KCl for 10 min to measure GDNF-regulated secretion, whereas Lonka-Nevalaita et al. used 50 mm KCl for 2 h to stimulate the regulated secretion of GDNF. To clarify this discrepancy, we measured the amount of the two GDNF isoforms released after 10 min versus 2 h of KCl stimulation. We found that after 10 min of KCl stimulation, the amount of full-length GDNF secretion was significantly more than that of GDNFΔ78, which was consistent with our previous study (20) (supplemental Fig. S3). Interestingly, after 2 h of KCl stimulation, the amount of full-length GDNF release was significantly less compared to that of GDNFΔ78, which was in contrast to the 10 min of KCl stimulation result and similar to the conclusion in the Lonka-Nevalaita et al. report (17) (supplemental Fig. S3). We speculate that additional factors might affect GDNF secretion when the high potassium solution incubation time was extended to 2 h. It is not clear how long GDNF-regulated secretion lasted, and therefore GDNF constitutive secretion might be predominant during the 2-h period. In addition, we could not exclude the possibility of synthesis of new GDNF isoform proteins during the 2 h of incubation with high potassium.
Third, our data have general implications for the concept that the prodomain of neurotrophic factors plays important roles in their intracellular trafficking and secretion. A previous study has shown that sortilin bound with a distinct sequence element in the BDNF prodomain and mediated BDNF to the regulated secretion pathway (30). Our data and previous reports have shown that Vps10 family members such as SorLA and sortilin could bind differentially with different neurotrophic factors such as pro-BDNF, pro-NGF, or pro-GDNF (28, 30, 31, 41). Our findings suggest that differential presynaptic sorting of GDNF and BDNF to secretory pathways may provide a more divergent point of regulation that may explain the differences in activity-dependent biological functions of the two neurotrophic factors. Proper localization and activity of SorLA are dependent on functional interaction with GGA, AP-1, and PACS-1, adaptor proteins involved in protein transport in the trans-Golgi network (26, 27, 36). Thus, in the future, identification of the adaptor proteins involved in GDNF-SorLA trafficking will allow for further understanding of the molecular mechanism in regulating GDNF sorting to secretory pathways.
In all, GDNF is primarily secreted in an activity-dependent manner, after which it can have a variety of biological effects such as modulating synaptic transmission and synaptic plasticity. Our studies have identified a sorting molecule, SorLA, that could specifically interact with the GDNF prodomain and mediate its regulated secretion. Our studies suggest a general implication for the important roles of prodomains of neurotrophic factors in their intracellular trafficking and secretion and diverse molecular mechanisms underlying these processes.
This study was supported by the National Natural Science Foundation of China (No. 30725020, 30900717, 31071254, 31130026), the National 973 Basic Research Program of China (No. 2012CB911004, 2009CB941403), the State Program of National Natural Science Foundation of China for Innovative Research Group (No. 81021001), the Foundation for Excellent Young Scientists of Shandong Province (BS2010SW022), Research Fund for the Doctoral Program of Higher Education of China (200804221070), and Independent Innovation Foundation of Shandong University (2011DX001).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- GDNF
- glial cell line-derived neurotrophic factor
- SorLA
- sorting protein-related receptor with A-type repeats
- GPI
- glycosylphosphatidylinositol
- GALT
- β1,4-galactosyltransferase
- Vps10p
- vacuolar protein-sorting 10 protein.
REFERENCES
- 1. Airaksinen M. S., Saarma M. (2002) Nat. Rev. Neurosci. 3, 383–394 [DOI] [PubMed] [Google Scholar]
- 2. Soderstrom K., O'Malley J., Steece-Collier K., Kordower J. H. (2006) Cell Transplant. 15, 251–265 [DOI] [PubMed] [Google Scholar]
- 3. Gill S. S., Patel N. K., Hotton G. R., O'Sullivan K., McCarter R., Bunnage M., Brooks D. J., Svendsen C. N., Heywood P. (2003) Nat. Med. 9, 589–595 [DOI] [PubMed] [Google Scholar]
- 4. Slevin J. T., Gerhardt G. A., Smith C. D., Gash D. M., Kryscio R., Young B. (2005) J. Neurosurg 102, 216–222 [DOI] [PubMed] [Google Scholar]
- 5. Beck K. D., Valverde J., Alexi T., Poulsen K., Moffat B., Vandlen R. A., Rosenthal A., Hefti F. (1995) Nature 373, 339–341 [DOI] [PubMed] [Google Scholar]
- 6. Gash D. M., Zhang Z., Ovadia A., Cass W. A., Yi A., Simmerman L., Russell D., Martin D., Lapchak P. A., Collins F., Hoffer B. J., Gerhardt G. A. (1996) Nature 380, 252–255 [DOI] [PubMed] [Google Scholar]
- 7. Tomac A., Lindqvist E., Lin L. F., Ogren S. O., Young D., Hoffer B. J., Olson L. (1995) Nature 373, 335–339 [DOI] [PubMed] [Google Scholar]
- 8. Saavedra A., Baltazar G., Duarte E. P. (2008) Prog Neurobiol. 86, 186–215 [DOI] [PubMed] [Google Scholar]
- 9. Kawamoto Y., Nakamura S., Matsuo A., Akiguchi I., Shibasaki H. (2000) Neuroscience 100, 701–712 [DOI] [PubMed] [Google Scholar]
- 10. Sariola H., Saarma M. (2003) J. Cell Sci. 116, 3855–3862 [DOI] [PubMed] [Google Scholar]
- 11. Pachnis V., Mankoo B., Costantini F. (1993) Development 119, 1005–1017 [DOI] [PubMed] [Google Scholar]
- 12. Paratcha G., Ledda F. (2008) Trends Neurosci. 31, 384–391 [DOI] [PubMed] [Google Scholar]
- 13. Ledda F., Paratcha G., Sandoval-Guzmán T., Ibáñez C. F. (2007) Nat. Neurosci. 10, 293–300 [DOI] [PubMed] [Google Scholar]
- 14. Baudet C., Pozas E., Adameyko I., Andersson E., Ericson J., Ernfors P. (2008) J. Neurosci. 28, 963–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McAllister A. K., Katz L. C., Lo D. C. (1999) Annu. Rev. Neurosci. 22, 295–318 [DOI] [PubMed] [Google Scholar]
- 16. Verity A. N., Wyatt T. L., Hajos B., Eglen R. M., Baecker P. A., Johnson R. M. (1998) J. Neurochem. 70, 531–539 [DOI] [PubMed] [Google Scholar]
- 17. Lonka-Nevalaita L., Lume M., Leppänen S., Jokitalo E., Peränen J., Saarma M. (2010) J. Neurosci. 30, 11403–11413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oh-hashi K., Ito M., Tanaka T., Hirata Y., Kiuchi K. (2009) Mol. Cell Biochem. 323, 1–7 [DOI] [PubMed] [Google Scholar]
- 19. Lin L. F., Doherty D. H., Lile J. D., Bektesh S., Collins F. (1993) Science 260, 1130–1132 [DOI] [PubMed] [Google Scholar]
- 20. Wang Y., Geng Z., Zhao L., Huang S. H., Sheng A. L., Chen Z. Y. (2008) Brain Res. 1226, 1–7 [DOI] [PubMed] [Google Scholar]
- 21. Jacobsen L., Madsen P., Moestrup S. K., Lund A. H., Tommerup N., Nykjaer A., Sottrup-Jensen L., Gliemann J., Petersen C. M. (1996) J. Biol. Chem. 271, 31379–31383 [DOI] [PubMed] [Google Scholar]
- 22. Yamazaki H., Bujo H., Kusunoki J., Seimiya K., Kanaki T., Morisaki N., Schneider W. J., Saito Y. (1996) J. Biol. Chem. 271, 24761–24768 [DOI] [PubMed] [Google Scholar]
- 23. Marcusson E. G., Horazdovsky B. F., Cereghino J. L., Gharakhanian E., Emr S. D. (1994) Cell 77, 579–586 [DOI] [PubMed] [Google Scholar]
- 24. Jacobsen L., Madsen P., Jacobsen C., Nielsen M. S., Gliemann J., Petersen C. M. (2001) J. Biol. Chem. 276, 22788–22796 [DOI] [PubMed] [Google Scholar]
- 25. Andersen O. M., Reiche J., Schmidt V., Gotthardt M., Spoelgen R., Behlke J., von Arnim C. A., Breiderhoff T., Jansen P., Wu X., Bales K. R., Cappai R., Masters C. L., Gliemann J., Mufson E. J., Hyman B. T., Paul S. M., Nykjaer A., Willnow T. E. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 13461–13466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schmidt V., Sporbert A., Rohe M., Reimer T., Rehm A., Andersen O. M., Willnow T. E. (2007) J. Biol. Chem. 282, 32956–32964 [DOI] [PubMed] [Google Scholar]
- 27. Nielsen M. S., Gustafsen C., Madsen P., Nyengaard J. R., Hermey G., Bakke O., Mari M., Schu P., Pohlmann R., Dennes A., Petersen C. M. (2007) Mol. Cell. Biol. 27, 6842–6851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Westergaard U. B., Sørensen E. S., Hermey G., Nielsen M. S., Nykjaer A., Kirkegaard K., Jacobsen C., Gliemann J., Madsen P., Petersen C. M. (2004) J. Biol. Chem. 279, 50221–50229 [DOI] [PubMed] [Google Scholar]
- 29. Chen Z. Y., Patel P. D., Sant G., Meng C. X., Teng K. K., Hempstead B. L., Lee F. S. (2004) J. Neurosci. 24, 4401–4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen Z. Y., Ieraci A., Teng H., Dall H., Meng C. X., Herrera D. G., Nykjaer A., Hempstead B. L., Lee F. S. (2005) J. Neurosci. 25, 6156–6166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nykjaer A., Lee R., Teng K. K., Jansen P., Madsen P., Nielsen M. S., Jacobsen C., Kliemannel M., Schwarz E., Willnow T. E., Hempstead B. L., Petersen C. M. (2004) Nature 427, 843–848 [DOI] [PubMed] [Google Scholar]
- 32. Kim T., Hempstead B. L. (2009) EMBO J. 28, 1612–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nilsson S. K., Christensen S., Raarup M. K., Ryan R. O., Nielsen M. S., Olivecrona G. (2008) J. Biol. Chem. 283, 25920–25927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Choi H. K., Won L., Roback J. D., Wainer B. H., Heller A. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 8943–8947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mazella J., Zsürger N., Navarro V., Chabry J., Kaghad M., Caput D., Ferrara P., Vita N., Gully D., Maffrand J. P., Vincent J. P. (1998) J. Biol. Chem. 273, 26273–26276 [DOI] [PubMed] [Google Scholar]
- 36. Jacobsen L., Madsen P., Nielsen M. S., Geraerts W. P., Gliemann J., Smit A. B., Petersen C. M. (2002) FEBS Lett. 511, 155–158 [DOI] [PubMed] [Google Scholar]
- 37. Wu M. M., Llopis J., Adams S., McCaffery J. M., Kulomaa M. S., Machen T. E., Moore H. P., Tsien R. Y. (2000) Chem. Biol. 7, 197–209 [DOI] [PubMed] [Google Scholar]
- 38. Wu M. M., Grabe M., Adams S., Tsien R. Y., Moore H. P., Machen T. E. (2001) J. Biol. Chem. 276, 33027–33035 [DOI] [PubMed] [Google Scholar]
- 39. Llopis J., McCaffery J. M., Miyawaki A., Farquhar M. G., Tsien R. Y. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 6803–6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seksek O., Biwersi J., Verkman A. S. (1995) J. Biol. Chem. 270, 4967–4970 [DOI] [PubMed] [Google Scholar]
- 41. Willnow T. E., Petersen C. M., Nykjaer A. (2008) Nat. Rev. Neurosci. 9, 899–909 [DOI] [PubMed] [Google Scholar]