Abstract
Background. It has been reported that cytomegalovirus (CMV) pp65-specific T cells can protect hematopoietic cell transplant (HCT) recipients from CMV complications. Two candidate CMV peptide vaccines composed of the HLA A*0201 pp65495-503 cytotoxic CD8+ T-cell epitope fused to 2 different universal T-helper epitopes (either the synthetic Pan DR epitope [PADRE] or a natural Tetanus sequence) were clinically evaluated for safety and ability to elicit pp65 T cells in HLA A*0201 healthy volunteers.
Methods. Escalating doses (0.5, 2.5, 10 mg) of PADRE or Tetanus pp65495-503 vaccines with (30 adults) or without (28 adults) PF03512676 adjuvant were administered by subcutaneous injection every 3 weeks for a total of 4 injections.
Results. No serious adverse events were reported, although vaccines used in combination with PF03512676 had enhanced reactogenicity. Ex vivo responses were detected by flow cytometry exclusively in volunteers who received the vaccine coadministered with PF03512676. In addition, using a sensitive in vitro stimulation system, vaccine-elicited pp65495-503 T cells were expanded in 30% of volunteers injected solely with the CMV peptides and in all tested subjects receiving the vaccines coinjected with PF03512676.
Conclusions. Acceptable safety profiles and vaccine-driven expansion of pp65495-503 T cells in healthy adults support further evaluation of CMV peptide vaccines combined with PF03512676 in the HCT setting.
Clinical Trials Registration. NCT00722839.
Cytomegalovirus (CMV) infection causes significant complications in the recovery of immunocompromised recipients of hematopoietic cell transplant (HCT), a leading therapy for hematological malignancies [1]. Preemptive antiviral chemotherapy limits CMV infection, although its use can have significant toxicity and may delay immune reconstitution, in turn exposing HCT recipients to the risk of infections [2]. Additionally, when antivirals are stopped or if immunodeficiency persists, patients may still develop late-onset CMV disease [2]. A number of approaches to stimulate the host immune response to CMV with the purpose of reducing the usage of antiviral agents have been evaluated, although no vaccine/immunotherapeutic strategy to prevent or treat CMV infection or disease has been licensed [3, 4].
Control of CMV infection is primarily associated with cellular immune responses [5]. The abundant tegument protein pp65 is a major contributor to shaping the T-cell repertoire in CMV-exposed individuals [6–8] and is a principal target for HLA class I–restricted CD8+ cytotoxic T lymphocytes (CTLs) [9]. CMV-infected cells express pp65 both early and late after infection, making it an appropriate vaccine target [10–12]. Adoptive transfer of pp65-specific CTL reduced CMV viremia after HCT in pilot trials [13–16]. Additionally, studies showed that CMV reactivation and disease were associated with low levels of CMV-specific tetramer+ CD8+ T cells (including pp65-specific T cells) [17]. In contrast, protection from CMV reactivation and disease in HCT recipients was associated with CMV-specific CD8+ T-cell levels between 7 cells/μL and 10 cells/μL [1, 17].
A vaccine that induces protective levels of pp65 CTL has the potential to be of therapeutic benefit to HCT recipients by limiting CMV viremia or disease [17]. Our strategy is to provide sufficient CMV immunity to HCT recipients by vaccinating their immunocompetent donors [18–20]. Studies have shown that by immunizing the donor pre-HCT, it is possible to transfer vaccine-specific immunity to the recipient [20–23].
We and others have identified a repertoire of CTL epitopes within the pp65 protein that can expand human pp65-specific memory CTLs in vitro [6–8, 24]. The pp65495-503 epitope, which is restricted to the high-frequency HLA A*0201 allele, has been extensively characterized [6–8]. Use of the pp65495-503 CTL epitope is conserved with limited sequence variation among viral isolates [7, 24, 25]. Using HLA-restricted CTL epitopes to develop a noninfectious subunit CMV vaccine would eliminate the safety concerns for HCT recipients of live-attenuated CMV or recombinant live viral vaccines, while avoiding the many CMV-encoded products involved in immune evasion [10, 26, 27].
Covalently linking pp65 CTL and T-helper (TH) epitope by solid-phase synthesis dramatically enhanced the immunogenicity of candidate epitope vaccines [28]. In HLA A2 transgenic (Tg) mice, 2 candidate vaccine peptides containing the HLA A*0201 pp65495-503 CD8+ T-cell epitope fused to universal TH epitopes (either the synthetic Pan DR epitope [PADRE] or a natural Tetanus sequence) showed favorable immunogenicity profiles [29, 30]. CpGDNA further augmented their activity, providing a means to lower vaccine dosage [28, 31].
The phase 1b trial described here was designed to evaluate safety and immunogenicity of PADRE and Tetanus pp65495-503 candidate vaccines in healthy volunteers expressing the HLA A*0201 major histocompatibility complex class I allele sequence. Our results in healthy volunteers pave the way for future trials in HCT donors for the purpose of transferring CMV immunity to their recipients.
MATERIALS AND METHODS
Vaccines
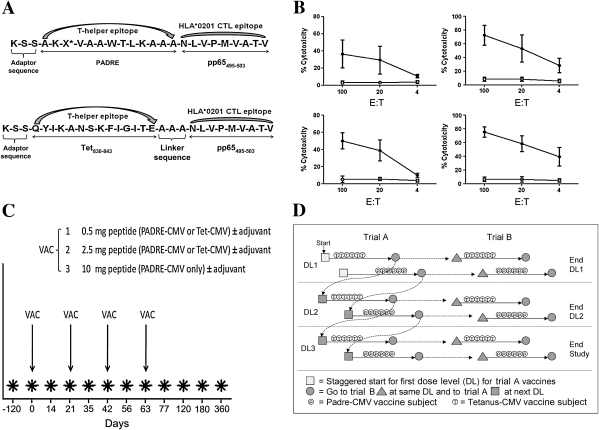
The National Cancer Institute (NCI)–sponsored Rapid Access to Interventional Development program contracted with Bachem for current good manufacturing practices (cGMP)–grade production of the peptide vaccines. Each peptide vaccine consists of the same HLA A*0201–specific CTL epitope (NLVPMVATV) [6] fused with a non-CMV-specific TH cell epitope that is recognized by many HLA-DR alleles. PADRE is the TH epitope (AKXVAAWTLKAAA, X = cyclohexylalanine [29]) in the PADRE-CMV (NSC-721433) and the Tetanus toxin P2 epitope (tt830-843:QYIKANSKFIGITE [30]) in the Tetanus (Tet)-CMV vaccine (NSC-721434) (Figure 1A). PF03512676 (10 mg/mL; CpGDNA adjuvant, known also as CpG 7909 and CpG 2006), a synthetic single-stranded phosphorothioate DNA-containing CpG motifs [32], was supplied by Pfizer and is classified as an investigational agent. A preclinical toxicology study was carried out in rats. The final report revealed little to no toxicity of the peptides at all investigated dosages (data not shown; from the Southern Research Institute, protocol no. 11200.11.01). The GMP vaccine products were also evaluated in HLA A*0201 HHDII Tg mice, and potency results were confirmed (Figure 1B) [28, 33].
Figure 1.
Peptide vaccines and vaccination schedule. A, Details of the primary structure of the peptide immunogens are shown for PADRE-cytomegalovirus (CMV) (top row) and Tet-CMV (bottom row) fusion peptide vaccines. The synthetic peptides were manufactured using good manufacturing practices (GMP)–grade material; the final products contained l-amino acids. X* indicates cyclohexylalanine. A GMP-grade solution of 10 mM sodium acetate, pH 4.2, was used as a diluent for both peptides. CTL, cytotoxic T lymphocyte. B, Potency tests in mice of the GMP-grade peptide vaccines to confirm their biologic activity in HHDII transgenic mice, immunized with 100 nmol of each CMV peptide vaccine with or without PF03512676. The plots show percentages of cytotoxicity at different effector-to-target ratios (E:T) detected in a 4-h chromium release assay against either relevant (pp65495-503, solid circles) or control (p53149-157, open circles) target cells [28]. Upper panels show results from mice immunized with Tet-CMV (left) and Tet-CMV + PF03512676 (right). Lower panels show results from mice immunized with PADRE-CMV (left) and PADRE-CMV + PF03512676 (right). Data in each plot are representative of at least 6 immunized mice. C, Each vaccine (VAC, either PADRE-CMV or Tet-CMV vaccines ± adjuvant) was administered subcutaneously in 4 injections at each dose level (1–3 as shown) for both trial A (CMV peptide vaccines without adjuvant) and trial B (CMV peptide vaccines with adjuvant). Days of vaccination (x-axis) are indicated by the arrows. The type of CMV peptide vaccines used at each dose level is shown. *Timing of blood draws for lab evaluations and immune assessments. D, Eligible subjects were assigned to trial A or trial B based on the time of their enrollment. Trial A dose level (DL) 1 started the study and was staggered such that Tet-CMV subjects were treated prior to PADRE-CMV subjects (solid arrows). At the completion of a DL in trial A, there was progression to the DL + 1 in trial A (dotted arrows) and to the DL in trial B. As mandated by the US Food and Drug Administration, no dose level began in trial B until after that level was safely completed in trial A.
Study Design and Vaccine Regimen
This is a nonrandomized, open-label, dose-escalating phase 1b safety and immunogenicity clinical trial. It was conducted at the City of Hope National Medical Center (COH), Duarte, California, and was approved by the local institutional review board (IRB 03121) and by the US Food and Drug Administration (FDA) as an investigational new drug (BB-13124). Healthy male and female volunteers, aged 18–55 years, CMV-seropositive or CMV-seronegative (Virgo CMV IgG/IgM kits, Hemagen Diagnostics) and molecularly subtyped as HLA A*0201-positive (PerkinElmer Gene Amp PCR System 9600), were enrolled after providing written informed consent. Participants were eligible unless they had ≥1 of the following exclusion criteria: abnormal serum chemistry and blood count; hepatitis B or C positive; immunodeficiency, including human immunodeficiency virus (HIV); taking daily medications for chronic illness; surgery in past 6 months requiring general anesthesia; known cardiac disease including patients being treated for hypertension and high cholesterol; positive urine pregnancy test/planning to become pregnant within the next 6 months; immunization with other vaccines within 1 month of the study period; participation in a CMV immunotherapy trial in the last 6 months; and history of cancer, depression, allergic diatheses, or frequent migraines.
The safety (primary endpoint) and immunogenicity (secondary endpoint) assessment of PADRE-CMV and Tet-CMV peptide vaccines was performed separately in 2 concomitant stages: trial A evaluated CMV peptide vaccines alone and trial B also included coinjected PF03512676 adjuvant, used at a constant dosage of 1 mg for all research subjects at all dose levels of CMV peptide vaccines [34]. The vaccine dose escalation (0.5, 2.5, 10 mg vaccine) was based on previous peptide vaccine studies [35]. A GMP-grade sodium acetate solution (pH 4.2; NCI-Frederick) was used as peptide diluent. The trial A formulation comprised 1 mL of peptide solution and 0.1 mL sodium bicarbonate USP to adjust pH (NEUT, Abbott Laboratories); trial B formulation comprised 1.0 mL of peptide solution and 0.1 mL PF03512676 (1 mg). The final 1.1-mL injection volume was administered subcutaneously in the upper arm. Booster doses of vaccine plus PF03512676 (1 mg) were given at days 21, 42, and 63 (Figure 1C). This regimen was chosen to assess safety of multiple injections because we anticipated immunizing both HCT donors and recipients. Six volunteers (4 CMV-seropositive and 2 CMV-seronegative) were allotted to each dose level, and the vaccine was administered by subcutaneous injection in the upper arm. The FDA mandated that each dose level of peptide vaccine be safely completed in at least 4 subjects of trial A before that same dose level could be evaluated in trial B (Figure 1D).
Changes to Trial Design
The COH IRB approved the following protocol amendments: (1) suspension of CMV-negative participation in January 2010 owing to unavailablility of HLA A*0201 CMV-seronegative volunteers (actual numbers of CMV-seronegative subjects are shown in Table 1); (2) trial A was closed due to lack of vaccine-related ex vivo immune responses, assessed during an interim analysis before enrollment for Tet-CMV vaccine at the highest dose (10 mg). Consequently, the following cohorts were completed: 0.5 and 2.5 mg PADRE-CMV and Tet-CMV in trials A and B and 10 mg PADRE-CMV in trials A and B (Figure 1C).
Table 1.
Demographic Characteristics of Evaluable Volunteers
| Trial B |
Trial A |
|||
| Characteristic | PADRE-CMV + PF03512676 | Tet-CMV + PF03512676 | PADRE-CMV | Tet-CMV |
| No. of subjects | 18 | 12 | 18 | 10 |
| Median age, years (range, 18–55) | 48 | 35 | 45 | 36 |
| CMV serology | ||||
| Positive | 14 | 12 | 12 | 9 |
| Negative | 4 | 0 | 6 | 1 |
| Sex | ||||
| Female | 11 | 11 | 16 | 7 |
| Male | 7 | 1 | 2 | 3 |
| Race | ||||
| White | 14 | 7 | 16 | 9 |
| African American | 1 | 0 | 0 | 0 |
| Asian | 1 | 0 | 0 | 0 |
| Hispanic | 2 | 4 | 2 | 0 |
| Other | 0 | 1 | 1 | 1 |
Abbreviation: CMV, cytomegalovirus; PADRE, Pan DR epitope.
Safety Assessment
After each vaccination, volunteers were kept under observation for 30 minutes to assess reactogenicity to vaccine formulations and provided with diaries to document local or systemic reactions. At each visit (Figure 1C), adverse events (AEs) were monitored and laboratory tests and symptom-directed clinical evaluations were performed. For grading severity of AEs, the National Institutes of Health HIV Vaccine Trials Network/Division of Acquired Immunodeficiency Syndrome toxicity table was used (Table 2).
Table 2.
Reactogenicity to Synthetic Immunogens in All Vaccinated Volunteers
| Trial B |
Trial A |
||||||||||||
| AE | PADRE-CMV + PF03512676 (n = 19) |
Tet-CMV + PF03512676 (n = 13) |
PADRE-CMV (n = 19) |
Tet-CMV (n = 12) |
Total Vaccinated (N = 63) | ||||||||
| AE gradea | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | % Total |
| Local | |||||||||||||
| Pain | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 8 |
| Pruritus | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Erythema | 0 | 1 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 8 |
| Allergic reaction | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 17 |
| Systemic | |||||||||||||
| Malaise | 7 | 3 | 1 | 2 | 0 | 1 | 3 | 1 | 0 | 3 | 1 | 0 | 35 |
| Fever | 2 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 13 |
| Chills | 4 | 1 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 |
| Myalgia | 2 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 14 |
| Arthralgia | 3 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 10 |
| Nausea | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Vomiting | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Headache | 0 | 2 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 11 |
Abbreviations: AE, adverse event; CMV, cytomegalovirus.
AEs are listed according to the HIV Vaccine Trials Network [HVTN]/Division of AIDS intensity grading scale: grade 1, mild; grade 2, moderate; grade 3, severe; grade 4, life-threatening. The table shows the number of volunteers who experienced grade 1, 2, or 3 local or systemic AEs (listed according to the HTVN intensity grading scale) for each vaccine cohort. No toxicity grade 4 AEs were reported. The rightmost column shows the percentages of the postvaccination-related AEs in the total vaccinated population (including 5 withdrawn volunteers). AEs that were considered unrelated or unlikely to be related to the investigational agent are not provided.
CMV-Specific T-Cell Monitoring by Flow Cytometric Analysis
Specimens were collected according to the US Public Health Service guidelines and the Helsinki declaration (Figure 1B) [36]. To evaluate ex vivo antigen-specific T cells, the CMV pp65495-503 and HIVgag77-85 (control) tetramers were prepared and titrated against reference peripheral blood mononuclear cells (PBMCs) at the COH Translational Vaccine Research Laboratory, as previously described [7, 37]. PBMCs for each blood draw were labeled and analyzed for tetramer binding by fluorescence-activated cell sorting (FACS; FACSCanto with FACSDiva software; BD Biosciences) [28]. To detect interferon γ (IFN-γ)–producing T cells, intracellular cytokine staining (ICS) was performed [33]. For each volunteer/time point, PBMC aliquots were stimulated with CMV pp65495-503, HIVgag77-85 (negative control), and CEF [38] (positive control) peptides.
In Vitro Stimulation
PBMCs from a subset of volunteers whose pp65495-503 tetramer binding remained minimal to low (<0.2%) throughout the study period were further analyzed at multiple time points using a simplification of a previously published protocol for in vitro stimulation (IVS) [7]. In brief, 5 million PBMCs were pulsed for 2 hours with 5 μg/mL of pp65495-503 and then plated in a 24-well plate [7]. Cells were fed with medium containing 10 IU/mL recombinant interleukin 2 (Novartis). On day 10, the bulk culture was evaluated for tetramer binding and IFN-γ production [28, 33].
Statistical Analysis
AEs were compared with immune responses at baseline and during and after vaccination using Wilcoxon rank-sum test. Due to the marked differences in recognition of pp65495-503 tetramers among CMV-seropositive subjects, these volunteers were stratified into 2 groups based on the pp65495-503 tetramer levels prevaccination: subjects with minimal to low (<0.2%) and those with ≥0.2% pp65495-503 tetramer levels. Results from trial A were compared with those from trial B separately within each stratum, using Wilcoxon rank-sum test, with ex vivo measurements ≤0.1% regarded as 0. Two-sided Fisher exact test was used to compare post-IVS results between trial A and trial B. Wilcoxon rank-sum test was applied to compare postinjection pp65495-503 and HIVgag77-85 tetramer level means for each individual.
RESULTS
Enrolled Volunteer Population
Between May 2007 and October 2010, 225 volunteers were screened and 63 eligible volunteers were enrolled, vaccinated, and monitored for safety (Table 2) and immunogenicity. However, postvaccination evaluation of CMV immune responses was not performed for 5 volunteers (1 CMV-seronegative and 4 CMV-seropositive) who received only the first vaccine dose and withdrew prematurely from the study. Among the 58 evaluable subjects (Table 1), 3 vaccinated volunteers were lost to follow-up after the day 180 visit.
Reactogenicity to the Vaccine Compounds
The peptide vaccines were safe and tolerated in most subjects, although addition of the PF03512676 (1 mg) adjuvant increased reactogenicity (Table 2). Most common AEs included mild to moderate cutaneous reactions at the injection site and systemic flulike symptoms. The duration of related grade 1 and 2 AEs ranged from 1 to 2 days. Grade 3 AEs were reported for 4 volunteers: 3 from trial B and 1 from trial A (vaccinated with Tet-CMV peptide). These grade 3 AEs included marked malaise, 39.4°C –40.5°C fever, and urticaria, which were resolved with nonprescription analgesics (acetaminophen and antihistamine, respectively) within 8 days. In trial A, grade 2 AEs were reported in 2 subjects using both PADRE-CMV (10.5%) and Tet-CMV (16.7%) peptide vaccines. In trial B, 6 (31.6%) subjects experienced grade 2 AEs and 2 (10.5%) subjects experienced grade 3 AEs, following vaccination with PADRE-CMV peptide vaccine plus PF03512676. Tet-CMV peptide vaccine plus PF03512676 was less reactogenic: 2 (15.3%) subjects experienced grade 2 AEs and 1 (7.7%) experienced grade 3 AEs. No association was found between severity or grade of AEs and pp65-specific immunity.
Baseline Levels of pp65495-503 CD8+ T Cells
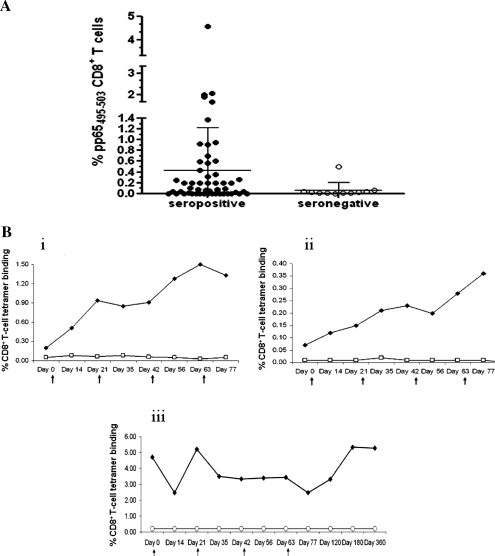
Two pretreatment measurements of pp65495-503 CD8+ T cells were performed in vaccinees: 1 on the day of the first injection and 1 within 120 days of immunization. As expected, levels of pp65 CD8+ T cells, measured using tetramers folded with the pp65495-503 HLA A*0201 epitope, showed modest variation between samples obtained prior to vaccination from the same volunteer [39]. All CMV-seronegative volunteers had pp65495-503 tetramer binding below 0.1%, with the exception of unique patient number 190, whose pp65495-503 tetramer levels were approximately 0.6%; further testing at day 180 proved this volunteer to be CMV-seropositive (Figure 2A) [17]. In contrast, among CMV-seropositive subjects, levels of pp65495-503 tetramer binding were highly variable, ranging from 0% to 4.57% (mean, 0.42%) [40]. Of interest, approximately 60% of CMV-seropositive subjects had minimal to low pp65495-503 tetramer levels, suggesting that use of the HLA A*0201 allele for the pp65-restricted response was not uniform [17]. CMV-seropositive subjects whose baseline pp65495-503 tetramer levels were minimal to low (<0.2%) are hereafter referred to as CMV+ <0.2%.
Figure 2.
Tetramer binding before and after vaccination. A, Levels of pp65495-503 tetramer binding (expressed in percentages) among cytomegalovirus (CMV)–seropositive (solid circles) and CMV-seronegative (open circles) volunteers (x-axes) before vaccination. The bars indicate mean (horizontal bar) and standard deviation. B, Individual plots showing the change in CD8+ T-cell tetramer binding following immunizations for peripheral blood mononuclear cells of (i) CMV-seropositive unique patient number (UPN) 212 vaccinated with 0.5 mg Tet-CMV + PF03512676 adjuvant; (ii) CMV-seropositive UPN 195 vaccinated with 2.5 mg PADRE-CMV + PF03512676 adjuvant; (iii) CMV-seropositive UPN 159 vaccinated with 0.5 mg CMV PADRE + PF03512676 adjuvant. Solid circles show pp65495-503–specific tetramer binding; open circles indicate binding to HIVgag77-85 (control) tetramer. Arrows indicate the day of vaccination.
Usage of PF03512676 Adjuvant Induces Ex Vivo CMV Vaccine Responses
The induction of postvaccination ex vivo immune responses was monitored by flow cytometry (Figure 2B). Positive postvaccination responses were defined as a >3-fold increase in either pp65495-503 tetramer binding or IFN-γ expression by CD8+ T cells compared with baseline [11]. Responders had at least 2 positive, >0.1% pp65495-503 tetramer binding/IFN-γ responses before day 120. By measuring percentages of pp65495-503 tetramer+ CD8+ T cells, postvaccination ex vivo responses were detected exclusively in trial B CMV+<0.2% volunteers (Table 3). In particular, 3 of 10 (30%) CMV+<0.2% volunteers injected with PADRE-CMV plus PF03512676 and 5 of 7 (71%) injected with Tet-CMV plus PF03512676 had vaccine-induced responses (Table 3; Figure 2Bi and ii). In contrast, in all trial A CMV+<0.2% subjects, pp65495-503 tetramer levels remained minimal after vaccination. In addition, CMV-seropositive subjects who had ≥0.2% pp65495-503 tetramer binding from both trial A and trial B at baseline showed decreases in pp65495-503 tetramer+ CD8+ T-cell levels after immunization (Figure 2Biii) [41]. None of the CMV-seronegative subjects had ex vivo increases in pp65495-503 tetramer binding after vaccination (Table 3). Using ICS, we investigated whether the peptide vaccine augmented the production of IFN-γ, measured ex vivo in CD8+ T cells. Postvaccination increases in pp65495-503–specific IFN-γ production in CD8+ T cells were all <3-fold in volunteers for both trials A and B.
Table 3.
Trial B Vaccine Responders
| CMV Peptide Vaccine/Dose | CMV-Seropositive | CMV-Seropositive<0.2% | CMV-Seronegative |
| PADRE-CMV, 0.5 mg | 0/2 | 1/2 | 0/2 |
| PADRE-CMV, 2.5 mg | None | 1/4 | 0/2 |
| PADRE-CMV, 10 mg | 0/2 | 1/4 | None |
| Tet-CMV, 0.5 mg | 0/3 | 2/3 | None |
| Tet-CMV, 2.5 mg | 0/2 | 3/4 | None |
For each cohort of 6 volunteers: numerators represent vaccine responders (denominators indicate total vaccinated).
Abbreviation: CMV, cytomegalovirus; CMV-seropositive<0.2%, CMV-seropositive subjects whose baseline pp65495-503 tetramer levels were minimal to low (<0.2%).
Longitudinal Profiles of Ex Vivo Immune Responses
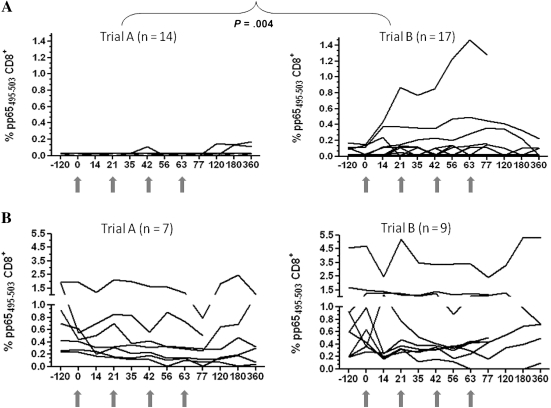
In the absence of an apparent dose effect, all CMV-seropositive immunized subjects from both trial A and trial B (n = 47) were stratified by pp65495-503 tetramer levels at baseline. Comparing the longitudinal profiles of ex vivo pp65495-503 tetramer+ CD8+ T-cell levels, a striking difference was noted between trial A and trial B CMV+<0.2% immunized volunteers (Figure 3A; P = .004, Wilcoxon rank-sum test comparing postvaccination averages between day 14 and day 77). Although tetramer levels remained mostly flat during the entire observation period among trial A CMV+<0.2% volunteers (Figure 3A), increases in pp65495-503 CD8+ T cells after each vaccination were frequent in trial B CMV+<0.2% subjects. In all responders, ex vivo CMV responses were still detectable at day 77 and persisted until day 180 in 2 subjects injected with PADRE-CMV plus PF03512676 (Figure 3A). These results suggest that PF03512676 adjuvant contributed to the stimulation of CMV vaccine responses, although its effect varied widely in the vaccinated CMV+<0.2% population. Limited variation in pp65495-503 tetramer levels were observed postvaccination in the CMV-seropositive population, with baseline ≥0.2% pp65495-503 tetramer binding from both trials (Figure 3B).
Figure 3.
Kinetics of vaccination responses. Longitudinal profiles of ex vivo pp65495-503–specific tetramer binding (expressed in percentages) for trial A (left plots) and trial B (right plots). A, Cytomegalovirus (CMV)<0.2% subjects. P value indicates the difference between trial A and trial B, calculated by Wilcoxon rank-sum test. B, CMV-seropositive subjects who had at baseline ≥0.2% pp65495-503 tetramer binding.
Postvaccination Expansion of pp65495-503 CD8+ T Cells
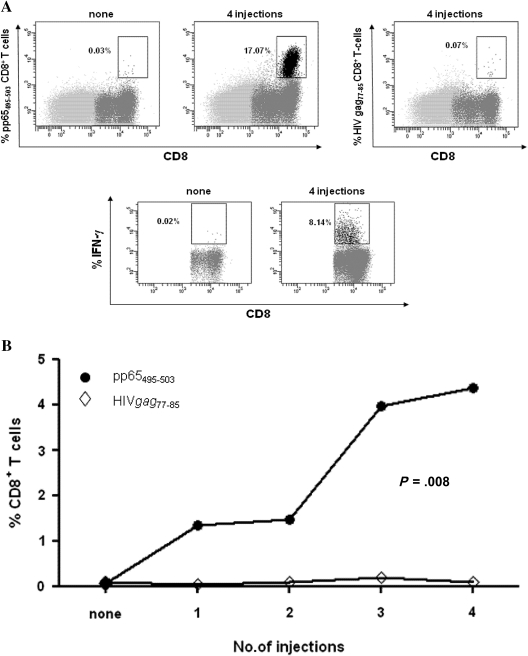
To evaluate whether low frequencies of pp65495-503–specific CD8+ T cells elicited by CMV peptide vaccines could be expanded, a sensitive in vitro stimulation system was used [7]. Minimal expansion of pp65495-503 CD8+ T cells was detected at baseline time points after IVS (Figure 4A, upper and lower left plots). Additionally, bulk T-cell cultures did not bind control-HIV tetramer or produce IFN-γ in response to control-HIV peptides (Figure 4A, upper right plot). In contrast, specific and functional postvaccination responses, as measured by pp65495-503 tetramer binding and IFN-γ expression, were markedly higher than baseline time points (Figure 4A) and progressively increased after each vaccination (Figure 4B). Although none of the trial A volunteers showed ex vivo postvaccination increases in pp65495-503 CD8+ T cells, by using IVS we were able to detect priming of memory T-cell responses (>10-fold expansions) in approximately 30% (4 of 14) of CMV+<0.2% and approximately 40% (3 of 7) of CMV-seronegative subjects from trial A. In addition, pp65495-503 CD8+ T cells could be consistently expanded in all 4 CMV-seronegative subjects and in 4 CMV-seropositive volunteers from trial B who failed to demonstrate ex vivo responses (Table 3 and Figure 4). Confirming the ex vivo data, the IVS results showed that use of PF03512676 adjuvant induced significantly higher (P = .002; 2-sided Fisher exact test) postvaccination responses in trial B compared with trial A.
Figure 4.
Vaccine memory responses. For each subject, in vitro stimulation (IVS) was performed at 3 or more time points and always included baseline blood draws. A, Individual fluorescence-activated cell sorting plots showing the levels of pp65495-503 CD8+ T cells after IVS, using peripheral blood mononuclear cells (PBMCs) of volunteers who did not have an ex vivo response to the cytomegalovirus (CMV) peptide vaccines. The upper plots refer to IVS performed with PBMC of representative CMV-seropositive unique patient number (UPN) 196 vaccinated with 2.5 mg PADRE-CMV + PF03512676 adjuvant. The tetramer used and the number of injections performed are reported on the y-axes and top plot, respectively. The lower plots refer to IVS performed with PBMC of CMV-seronegative UPN 172 vaccinated with 0.5 mg PADRE-CMV + PF03512676 adjuvant and report the levels of interferon γ (IFN-γ) produced by CD8+ T cells using pp65495-503 peptide as stimulant during intracellular cytokine staining. The number of injections is reported on the top plot. B, The graph shows the median levels of CD8+ T cells specific for either the pp65495-503 (solid symbols) or the HIVgag77-85 (control, open symbols) tetramers in function of vaccine injections in 4 CMV-seronegatives and 4 CMV+<0.2% subjects from trial B, who failed to show an ex vivo CMV peptide vaccine response (Table 3). P value was calculated by Wilcoxon rank-sum test to assess the significance of postvaccination binding difference between pp65495-503 and control (HIVgag77-85) tetramer in the IVS-expanded CD8+ T cells.
DISCUSSION
Improved clinical outcomes in HCT recipients, achieved by adoptive transfer of pp65-CTL from CMV-seropositive donors, provides a strategic premise for controlling CMV by immunization of the donors [13–16]. During HCT, the stem cell product contains large numbers of mature donor T cells [42]. Increasing the CMV-specific proportion of these cells by immunizing the donor could result in CMV control in the recipient [17]. The current study evaluated the CMV-subunit vaccines in healthy adults as a surrogate model of immunizing HCT donors of at-risk HCT recipients. A vaccine containing the HLA A*0201 pp65495-503 epitope will cover 30%−40% of the at-risk population in the United States. In fact, the HLA A*0201 allele is most frequently represented in white individuals (∼46%) and is much less common in African Americans and Asian Americans (∼16%) [24].
As reported by others and confirmed by our data (Figure 1A), pp65495-503 epitope recognition was broad, but not uniform, among HLA A*0201 CMV-seropositive individuals [17, 40]. Although a recent study has confirmed the immunodominance of the pp65495-503 epitope in HLA A*0201 Tg mice immunized with pp65-encoding DNA, the divergence with the animal model data indicates that immunodominance hierarchies in CMV-infected humans exposed to multiple CMV antigens are complex and difficult to predict [7, 9, 28, 43].
The CMV immunogens coadministered with or without PF03512676 adjuvant were safe and generally well tolerated, although the addition of PF03512676 (1 mg) substantially raised their reactogenicity (Table 2) [6]. Evidence of AEs associated with the use of PF03512676 has been documented in clinical trials [44]; hence, the elevated number of local AEs and systemic flulike symptoms observed in trial B (Table 2) are consistent with previous reports.
PF03512676 is an immunomodulating synthetic oligonucleotide designed to specifically agonize Toll-like receptor 9 (TLR9) [45]. It is being evaluated as a single agent for several conditions and as an adjuvant for vaccines against infectious diseases [46]. In the current study, pp65-specific ex vivo vaccine-induced immune responses were detected exclusively when PF03512676 was coadministered with the CMV vaccine peptides containing either PADRE or Tetanus epitopes (Table 3). In contrast to the preclinical results in mouse models using CMV peptide vaccines (Figure 1B) [28], there was no appreciable ex vivo postvaccination increase in pp65495-503–specific CD8+ T-cell levels from subjects immunized with the CMV peptides without adjuvant. These data indicate that coadministration of PF03512676 was critical to significantly improving vaccine-induced immune responses.
In this preliminary evaluation of CMV peptide vaccines, levels of CD4+ T cells specific for the vaccine TH epitope were not assessed, which is a limitation of our study. In our judgment, the portion of the immune response attributable to either vaccine TH epitope will be inextricably linked and confounded by use of the PF03512676 adjuvant, which independently induces potent T-helper 1 responses and alters immune cell trafficking when administered with a wide range of antigens [34].
Vaccine-driven CMV immune responses were measured among HLA A*0201 CMV-seronegative subjects and in CMV-seropositive subjects in whom pp65495-503 tetramer+ CD8+ T cells were minimal to low before vaccination (Table 3; Figure 2Bi and ii; Figure 3A). Additionally, as previously reported using ALVAC-pp65 vaccine [12], a decrease in pp65495-503 CD8+ T-cell levels was detected in CMV-seropositive subjects who had substantial baseline pp65495-503 tetramer binding (Figures 2Biii and 3B). In a nonviremic CMV-seropositive, a noninfectious CMV vaccine is likely to target the T-central memory (TCM) compartment. However, a recent study showed that the TCM pool is tightly regulated during secondary expansion and is under homeostatic and physiologic control [47]. In particular, alteration of a robust antigen-specific TCM compartment by immunization is likely difficult to achieve and may compromise preexisting T-cell memory [48]. Difficulty in expanding preexisting natural immunity by using subunit vaccines has been reported not only for pp65 vaccines [11, 12] but also for a blood-stage malaria vaccine plus PF03512676, which induced vaccine-specific response in malaria-naíve subjects but failed to enhance it in malaria-exposed adults [49]. Understanding the molecular basis of this apparent refractoriness to vaccine boosting in healthy immune individuals is of significant interest in vaccine design [48, 49].
Similar to previous pp65-vaccine clinical studies, evidence of CMV peptide vaccine–induced responses increased substantially in trial A and trial B subjects when IVS methodologies were used [11, 12]. Stimulation strategies to evaluate vaccine immunogenicity in clinical trials have limitations due to the length and conditions of the in vitro culture [11, 12]. However, breadth and consistency of post-IVS responses detected in trial B subjects (Figure 4), after a brief exposure of PBMC to the pp65495-503 peptide, are promising. In fact, the vaccine-elicited pp65495-503 T cells could be significantly expanded upon exposure to CMV antigens in a viremic individual. In particular, the CMV peptide vaccines may have the potential for greater potency when injected into HCT recipients because further massive expansion has been reported to occur under the natural stimulation of CMV infection [16]. The resulting pp65495-503 T-cell increase in the HCT recipients can be critical to the control of CMV viremia [10–12].
In summary, CMV peptide vaccines plus PF03512676 (1 mg) are safe and effective in priming CMV immune responses. However, the high percentage of flulike AEs reported in this study when using PF03512676 (Table 2) indicates a need for caution in further development of the product for use in immunocompetent donors. Efficacy of recipient vaccination both before and early after transplantation has been described [20, 50]. Hence, the CMV peptide vaccines plus PF03512676 could be used to directly immunize HCT recipients. In these patients with nascent hematopoietic reconstitution and prolonged CD4+ T-cell deficit, stimulation of TLR 9 by PF03512676 could effectively substitute for the normal requirement of TH, as shown in HIV subjects with compromised TH function [34]. Because PF03512676 was found to be safe when administered to HCT recipients in a pilot trial (J. S. Miller, University of Minnesota, oral communication, May 2011), there is a precedent for our proposed use in HCT recipients. Conducting efficacy trials of CMV peptide vaccines in HCT recipients represents the next objective of this vaccine program.
Notes
Acknowledgments.
We acknowledge the assistance of the COH General Clinical Research Center personnel in vaccinating volunteers, including Sherri Stinson and Mary Lee for blood specimen processing. We thank the volunteers for kindly participating in the study; Aparna Krishnan, Joy Medina, Maria Lalimarmo, Gideon Blumstein, and Pooja Manchanda for excellent technical assistance; and Dr James Merson, senior vice president and head of Pfizer Vaccine Research West and global investigator-initiated research managers Chineze Enekwechi and Manali Talathi for kind assistance and continuous support in providing PF03512676. We acknowledge the support and strategic contributions of Dr Stephen Creekmore and collaborators to assist us throughout the manufacturing and FDA registration phases (Biopharmaceutical Development Program at NCI-Frederick, Maryland). We thank Dr Karen Schweikart of the Toxicology and Pharmacology Department, DTP DCTD, NCI for her design of the Toxicology protocol and supervision of the provider, SRI. Constructs expressing HLA A*0201 heavy chain and β2 microglobulin were obtained from Patricia Roth (Beckman-Coulter, Fullerton, California). The administrative assistance of Donna Packer is gratefully acknowledged.
Financial support.
This work was supported in part by NIH grants from the following institutes: National Cancer Institute (R01-CA77544 and P01-CA30206, Project III to D. J. D. and contract 24XS044); and also supported in part through the NCI-RAID Program of the Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute; National Institute of Allergy and Infectious Diseases (R21 AI084019 to C. L. R.); National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); R21 DK077374 to S. F. L.); NCI (CA33572 to the COH Comprehensive Cancer Center); National Center for Research Resources (MO1-RR0043-38 to COH General Clinical Research Center [GCRC; satellite of University of Southern California GCRC]); and the Edwin and Bea Wolfe Charitable Foundation to the Division of Translational Vaccine Research.
Potential conflicts of interest.
A. M. K. is an inventor on patents relating to PF03512676 adjuvant (also known as CpG 7909) and was employed by Pfizer during the period of the study. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Aubert G, Hassan-Walker AF, Madrigal JA, et al. Cytomegalovirus-specific cellular immune responses and viremia in recipients of allogeneic stem cell transplants. J Infect Dis. 2001;184:955–63. doi: 10.1086/323354. [DOI] [PubMed] [Google Scholar]
- 2.Hakki M, Riddell SR, Storek J, et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood. 2003;102:3060–7. doi: 10.1182/blood-2002-11-3472. [DOI] [PubMed] [Google Scholar]
- 3.Khanna R, Diamond DJ. Human cytomegalovirus vaccine: time to look for alternative options. Trends Mol Med. 2006;12:26–33. doi: 10.1016/j.molmed.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Einsele H, Hebart H. CMV-specific immunotherapy. Hum Immunol. 2004;65:558–64. doi: 10.1016/j.humimm.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Gamadia LE, Remmerswaal EB, Weel JF, Bemelman F, van Lier RA, ten Berge IJ. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood. 2003;101:2686–92. doi: 10.1182/blood-2002-08-2502. [DOI] [PubMed] [Google Scholar]
- 6.Diamond DJ, York J, Sun J, Wright CL, Forman SJ. Development of a candidate HLA A*0201 restricted peptide-based vaccine against human cytomegalovirus infection. Blood. 1997;90:1751–67. [PubMed] [Google Scholar]
- 7.La Rosa C, Krishnan R, Markel S, et al. Enhanced immune activity of cytotoxic T-lymphocyte epitope analogs derived from positional scanning synthetic combinatorial libraries. Blood. 2001;97:1776–86. doi: 10.1182/blood.v97.6.1776. [DOI] [PubMed] [Google Scholar]
- 8.Wills MR, Carmichael AJ, Mynard K, et al. The human CTL response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T cell receptor usage of pp65-specific CTL. J Virol. 1996;70:7569–79. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sylwester AW, Mitchell BL, Edgar JB, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–85. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernstein DI, Reap EA, Katen K, et al. Randomized, double-blind, phase 1 trial of an alphavirus replicon vaccine for cytomegalovirus in CMV seronegative adult volunteers. Vaccine. 2009;28:484–93. doi: 10.1016/j.vaccine.2009.09.135. [DOI] [PubMed] [Google Scholar]
- 11.Wloch MK, Smith LR, Boutsaboualoy S, et al. Safety and immunogenicity of a bivalent cytomegalovirus DNA vaccine in healthy adult subjects. J Infect Dis. 2008;197:1634–42. doi: 10.1086/588385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berencsi K, Gyulai Z, Gonczol E, et al. A canarypox vector-expressing cytomegalovirus (CMV) phosphoprotein 65 induces long-lasting cytotoxic T cell responses in human CMV-seronegative subjects. J Infect Dis. 2001;183:1171–9. doi: 10.1086/319680. [DOI] [PubMed] [Google Scholar]
- 13.Cobbold M, Khan N, Pourgheysari B, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005;202:379–86. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feuchtinger T, Opherk K, Bethge WA, et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010;116:4360–7. doi: 10.1182/blood-2010-01-262089. [DOI] [PubMed] [Google Scholar]
- 15.Einsele H. Immunotherapy for CMV infection. Cytotherapy. 2002;4:435–6. doi: 10.1080/146532402320776080. [DOI] [PubMed] [Google Scholar]
- 16.Peggs KS, Verfuerth S, Pizzey A, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362:1375–7. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- 17.Gratama JW, Boeckh M, Nakamura R, et al. Immune monitoring with iTAg MHC tetramers for prediction of recurrent or persistent cytomegalovirus infection or disease in allogeneic hematopoietic stem cell transplant recipients: a prospective multicenter study. Blood. 2010;116:1655–62. doi: 10.1182/blood-2010-03-273508. [DOI] [PubMed] [Google Scholar]
- 18.Horn B, Bao L, Dunham K, et al. Infusion of cytomegalovirus specific cytotoxic T lymphocytes from a sero-negative donor can facilitate resolution of infection and immune reconstitution. Pediatr Infect Dis J. 2009;28:65–7. doi: 10.1097/INF.0b013e318182026f. [DOI] [PubMed] [Google Scholar]
- 19.Zhou W, Longmate J, Lacey SF, et al. Impact of donor CMV status on viral infection and reconstitution of multifunction CMV-specific T cells in CMV-positive transplant recipients. Blood. 2009;113:6465–76. doi: 10.1182/blood-2009-02-203307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Storek J, Dawson MA, Lim LC, et al. Efficacy of donor vaccination before hematopoietic cell transplantation and recipient vaccination both before and early after transplantation. Bone Marrow Transplant. 2004;33:337–46. doi: 10.1038/sj.bmt.1704336. [DOI] [PubMed] [Google Scholar]
- 21.Shenoy AG, Solomon SR, Pichon S, Cadoz M, Hensel N, Barrett AJ. Protecting stem cell transplant recipients against CMV reactivation by vaccinating their donors with a canarypox pp65 vaccine (ALVAC). [abstract] Blood. 2006;11:108. [Google Scholar]
- 22.Lindemann M, Barsegian V, Runde V, et al. Transfer of humoral and cellular hepatitis B immunity by allogeneic hematopoietic cell transplantation. Transplantation. 2003;75:833–8. doi: 10.1097/01.TP.0000054841.42796.68. [DOI] [PubMed] [Google Scholar]
- 23.Leung AY, Chow HC, Kwok JS, et al. Safety of vaccinating sibling donors with live-attenuated varicella zoster vaccine before hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;39:661–5. doi: 10.1038/sj.bmt.1705673. [DOI] [PubMed] [Google Scholar]
- 24.Longmate J, York J, La Rosa C, et al. Population coverage by HLA class-I restricted cytotoxic T-lymphocyte epitopes. Immunogenetics. 2001;52:165–73. doi: 10.1007/s002510000271. [DOI] [PubMed] [Google Scholar]
- 25.Zaia JA, Gallez-Hawkins G, Li X, et al. Infrequent occurrence of natural mutations in the pp65(495-503) epitope sequence presented by the HLA A*0201 allele among human cytomegalovirus isolates. J Virol. 2001;75:2472–4. doi: 10.1128/JVI.75.5.2472-2474.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mocarski ES., Jr Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol. 2002;10:332–9. doi: 10.1016/s0966-842x(02)02393-4. [DOI] [PubMed] [Google Scholar]
- 27.Adler SP, Hempfling SH, Starr SE, Plotkin SA, Riddell S. Safety and immunogenicity of the Towne strain cytomegalovirus vaccine. Pediatr Infect Dis J. 1998;17:200–6. doi: 10.1097/00006454-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 28.La Rosa C, Wang Z, Brewer JC, et al. Preclinical development of an adjuvant-free peptide vaccine with activity against CMV pp65 in HLA transgenic mice. Blood. 2002;100:3681–9. doi: 10.1182/blood-2002-03-0926. [DOI] [PubMed] [Google Scholar]
- 29.Alexander J, Sidney J, Southwood S, et al. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. 1994;1:751–61. doi: 10.1016/s1074-7613(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 30.Panina-Bordignon P, Tan A, Termijtelen A, Demotz S, Corradin G, Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989;19:2237–42. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- 31.Rothenfusser S, Hornung V, Ayyoub M, et al. CpG-A and CpG-B oligonucleotides differentially enhance human peptide-specific primary and memory CD8+ T-cell responses in vitro. Blood. 2004;103:2162–9. doi: 10.1182/blood-2003-04-1091. [DOI] [PubMed] [Google Scholar]
- 32.Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J Immunol. 2000;164:944–53. doi: 10.4049/jimmunol.164.2.944. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, La Rosa C, Mekhoubad S, et al. Attenuated poxviruses generate clinically relevant frequencies of CMV-specific T cells. Blood. 2004;104:847–56. doi: 10.1182/blood-2003-10-3469. [DOI] [PubMed] [Google Scholar]
- 34.Cooper CL, Davis HL, Angel JB, et al. CPG 7909 adjuvant improves hepatitis B virus vaccine seroprotection in antiretroviral-treated HIV-infected adults. AIDS. 2005;19:1473–9. doi: 10.1097/01.aids.0000183514.37513.d2. [DOI] [PubMed] [Google Scholar]
- 35.Vitiello A, Ishioka G, Grey HM, et al. Development of a lipopeptide-based therapeutic vaccine to treat chronic HBV infection. I. Induction of a primary cytotoxic T lymphocyte response in humans. J Clin Invest. 1995;95:341–9. doi: 10.1172/JCI117662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.La Rosa C, Limaye AP, Krishnan A, Longmate J, Diamond DJ. Longitudinal assessment of cytomegalovirus (CMV)-specific immune responses in liver transplant recipients at high risk for late CMV disease. J Infect Dis. 2007;195:633–44. doi: 10.1086/511307. [DOI] [PubMed] [Google Scholar]
- 37.Lacey SF, Villacres MC, La Rosa C, et al. Relative dominance of HLA-B*07 restricted CD8(+) T-lymphocyte immune responses to human cytomegalovirus pp65 in persons sharing HLA-A*02 and HLA-B*07 alleles. Hum Immunol. 2003;64:440–52. doi: 10.1016/s0198-8859(03)00028-4. [DOI] [PubMed] [Google Scholar]
- 38.Currier JR, Kuta EG, Turk E, et al. A panel of MHC class I restricted viral peptides for use as a quality control for vaccine trial ELISPOT assays. J Immunol Methods. 2002;260:157–72. doi: 10.1016/s0022-1759(01)00535-x. [DOI] [PubMed] [Google Scholar]
- 39.Gillespie GM, Wills MR, Appay V, et al. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8(+) T lymphocytes in healthy seropositive donors. J Virol. 2000;74:8140–50. doi: 10.1128/jvi.74.17.8140-8150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunn HS, Haney DJ, Ghanekar SA, Stepick-Biek P, Lewis DB, Maecker HT. Dynamics of CD4 and CD8 T cell responses to cytomegalovirus in healthy human donors. J Infect Dis. 2002;186:15–22. doi: 10.1086/341079. [DOI] [PubMed] [Google Scholar]
- 41.Shenoy AG, Solomon S, Pichon S, Cadoz M, Barrett AJ. Vaccination with canarypox pp65 CMV vaccine induces reliable CD4+ and CD8+ T cell responses only in individuals who lack baseline responses. Implications for donor vaccination to boost CMV immunity in stem cell transplant recipients. Biol Blood Marrow Transplant. 2008;14:394. [Google Scholar]
- 42.Cwynarski K, Ainsworth J, Cobbold M, et al. Direct visualization of cytomegalovirus-specific T-cell reconstitution after allogeneic stem cell transplantation. Blood. 2001;97:1232–40. doi: 10.1182/blood.v97.5.1232. [DOI] [PubMed] [Google Scholar]
- 43.Reiser M, Wieland A, Plachter B, Mertens T, Greiner J, Schirmbeck R. The immunodominant CD8 T cell response to the human cytomegalovirus tegument phosphoprotein pp65495-503 epitope critically depends on CD4 T cell help in vaccinated HLA-A*0201 transgenic mice. J Immunol. 2011;187:2172–80. doi: 10.4049/jimmunol.1002512. [DOI] [PubMed] [Google Scholar]
- 44.Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011;10:499–511. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krieg AM, Hartmann G, Yi AK. Mechanism of action of CpG DNA. Curr Top Microbiol Immunol. 2000;247:1–21. doi: 10.1007/978-3-642-59672-8_1. [DOI] [PubMed] [Google Scholar]
- 46.Krieg AM. Toll-free vaccines? Nat Biotechnol. 2007;25:303–5. doi: 10.1038/nbt0307-303. [DOI] [PubMed] [Google Scholar]
- 47.Ahlers JD, Belyakov IM. Memories that last forever: strategies for optimizing vaccine T-cell memory. Blood. 2010;115:1678–89. doi: 10.1182/blood-2009-06-227546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vezys V, Yates A, Casey KA, et al. Memory CD8 T-cell compartment grows in size with immunological experience. Nature. 2009;457:196–9. doi: 10.1038/nature07486. [DOI] [PubMed] [Google Scholar]
- 49.Traore B, Kone Y, Doumbo S, et al. The TLR9 agonist CpG fails to enhance the acquisition of Plasmodium falciparum-specific memory B cells in semi-immune adults in Mali. Vaccine. 2009;27:7299–303. doi: 10.1016/j.vaccine.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kharfan-Dabaja MA, Boeckh M, Wilck MB, et al. Lancet Infect Dis. 2012. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial. doi:10.1016/S1473-3099(11)70344-9. [DOI] [PubMed] [Google Scholar]