Abstract
A low level of serum antibody to antigens expressed by Streptococcus pneumoniae has been proposed to explain the susceptibility of children to recurrent episodes of acute otitis media (hereafter, “otitis-prone children”). By use of enzyme-linked immunospot assays, the percentages of memory B cells to pneumococcal protein antigens PhtD, LytB, PcpA, PhtE, and Ply were compared between otitis-prone and non–otitis-prone children at the time of acute otitis media or nasopharyngeal colonization with S. pneumoniae. We found significantly lower percentages of memory B cells to 3 pneumococcal protein antigens (PhtD, PhtE, and Ply) and reduced antigen-specific immunoglobulin G concentrations in otitis-prone children, compared with non–otitis-prone children.
Acute otitis media (AOM) is a common infectious disease in children worldwide, leading to a substantial burden of temporary deafness and delayed speech development in developed countries and to more serious complications in developing countries [1]. About 60%–70% of children experience ≥1 episode during the first 3 years of their life, while a subpopulation of children representing 30% of the total have had ≥3 episodes of AOM within 6 months or 4 infections within a year and are considered otitis prone [2]. Children who experience repeat episodes of AOM have the greatest morbidity from this disease, sometimes resulting in permanent hearing loss [1, 2].
Streptococcus pneumoniae is one of the most common pathogens causing AOM [3]. Studies in animal models and, to some extent, in humans suggest that immune correlates of protection from infection by S. pneumoniae include memory CD4+ T cells, B cells, neutralizing serum, and mucosal antibody levels [4–6]. We have recently established that otitis-prone children have reduced frequencies of memory CD4+ T cells specific for S. pneumoniae antigen and for nontypeable Haemophilus influenzae antigen in their circulation at the time of AOM and following nasopharyngeal colonization [7]. After natural infection and vaccination, robust memory T-cell and B-cell responses should be generated, with memory lymphocytes populating lymphoid and nonlymphoid sites, to provide long-term protection from reinfection [8]. Once generated on subsequent exposure to a pathogen, memory B cells can proliferate into antibody-secreting cells and maintain serum antibody levels over time [9, 10].
Earlier reports describe that otitis-prone children produce lower amounts of antibodies specific to S. pneumoniae antigen and nontypeable H. influenzae antigen and/or do not produce functional bactericidal antibodies in response to AOM and/or nasopharyngeal colonization [11–13]. These findings, including ours, suggest that decreased concentrations of circulating antibodies to otopathogens may contribute to the otitis-prone condition. However, until this current work there has not been an evaluation of whether the observed reduction in the serum antibody level in otitis-prone children might be due to failure to generate robust antigen-specific memory B cells. To the best of our knowledge, this is the first report demonstrating that lower generation of pathogen-specific memory B cells may account for lower antibody levels to protein antigens displayed by S. pneumoniae among young children with recurrent episodes of AOM.
METHODS
Subjects
Subjects were participants from our 5-year prospective longitudinal AOM study funded by the National Institute on Deafness and Other Communication Disorders [13]. Enrolled children were from a middle-class, suburban population in Rochester, New York. Healthy children aged 6 months without prior AOM were enrolled and had blood, nasopharyngeal, and oropharyngeal specimens cultured 7 times, at ages 6, 9, 12, 15, 18, 24, and 30 months. Middle-ear fluid was obtained by tympanocentesis during AOM episodes. Colonization with S. pneumoniae and/or nontypeable H. influenzae in the nasopharyngeal and/or oropharyngeal regions was routinely determined by standard microbiologic culture. Otitis-prone children in the study population were defined as those who had tympanocentesis-confirmed infections and received antibiotic therapy directed to the otopathogen isolated from middle-ear fluid for each AOM event. Peripheral blood mononuclear cells (PBMCs) were isolated from the collected blood and frozen in liquid nitrogen until used. Children having 3 episodes of AOM within 6 months or 4 episodes within 1 year were considered otitis prone, while others who had fewer episodes were placed into the non–otitis-prone group. Written informal consent was obtained in association with a protocol approved by the Rochester General Hospital Investigational Review Board.
Antigens
Five different pneumococcal protein antigens were used in this study: pneumococcal histidine triad proteins D (PhtD) and E (PhtE), LytB, PcpA, and Ply (a recombinantly expressed, genetically engineered variant of pneumolysin that is highly detoxified yet retains the appropriate antigenic/immunogenic properties. All antigens were procured from Sanofi Pasteur (Swiftwater, PA).
Humoral Responses
To measure immunoglobulin G (IgG) antibody levels in the samples, an enzyme-linked immunosorbent assay (ELISA) was performed as described previously [13]. Briefly, 96-well ELISA plates (Nunc-Immulon) were coated with 0.5 μg/mL of individual antigens (100 μL/well) in coating buffer (bicarbonate [pH 9.4]) and incubated overnight at 4°C. After washing, the plates were blocked with 3% skimmed milk at 37°C for 1 hour (200 μL/well). After 5 washes, 100 μL of serum at a starting dilution of 1:100 (in phosphate-buffered saline [PBS]–3% skim milk) was added to the wells and diluted serially 2-fold. The mixture was incubated at room temperature for 1 hour followed by the addition of affinity purified goat antihuman IgG, IgM, or IgA antibody conjugated to horseradish-peroxidase (Bethyl Laboratories, Montgomery, TX) as a secondary antibody. The reaction products were developed with TMB Microwell Peroxidase Substrate System (KPL, Gaithersburg, MD), stopped by the addition of 1.0 M phosphoric acid, and read by an automated ELISA reader, using a 450-nm filter. To provide quantitative results on antibody concentrations, the levels of the specific antibodies present in the unknown samples were determined by comparing them to reference serum (provided by Sanofi Pasteur). The total IgG concentration in the reference serum was calculated using an IgG estimation kit (Bethyl Laboratories). A 4-parameter logistic-log function was used to form the reference and sample curves.
Enzyme-Linked Immunospot Assays for Antibody-Secreting Cells
Antigen-specific cells and total IgG-secreting cells were quantified by an assay in which memory B cells were stimulated in vitro to differentiate into antibody-secreting cells, as standardized in our laboratory. Briefly, 1 million thawed PBMCs were placed in each well of a 24-well plate containing 1 mL complete media alone or complete media containing 1 μg/mL pokeweed mitogen. Cells were kept at 37°C for 3 days for differentiation, washed with complete media, counted, and distributed onto antigen-coated (10 μg/mL) 96-well enzyme-linked immunospot (ELISPOT) plates overnight (Millipore). For the detection of total IgG-secreting cells, wells were precoated with 10 μg/mL monoclonal antihuman IgG (MT91/145; Mabtech). As a negative control, wells were coated with the same amount of bovine serum albumin. Plates were then blocked with 10% fetal bovine serum in Roswell Park Memorial Institute (RPMI) 1640 media for 30 minutes at 37°C. Stimulated cells were counted and resuspended in fresh complete RPMI media before distribution onto control and antigen-coated wells (5 × 105 cells/well on a 96-well ELISPOT plate). Plates were then incubated at 37°C in a 5% CO2 incubator overnight and then washed with PBS at least 5 times. Next, 100 μL of 1 μg/mL biotinylated antihuman IgG antibodies (MT78/145; Mabtech) were added to the wells and incubated for 1 hour. After washing, streptavidin-alkaline phosphatase conjugate (1:1000) was added to the wells and incubated for 1 hour at 37°C. Plates were then washed 5 times with PBS before development with substrate (BCIP/NBT; Mabtech). Because of the low frequencies of antigen-specific antibody-secreting cells, developed spots were manually counted with the help of a dissection microscope. Antigen-specific data were expressed as a percentage of antigen-specific memory B cells and were calculated per million of PBMCs as follows: [(number of antigen-specific spots)/(number of total immunoglobulin spots)] × 100.
Statistical Analyses
All data were analyzed using Graph Pad Prism software. Two-tailed P values for the data were calculated using the Mann–Whitney U test.
RESULTS
Study Population
From a total study population of 387 children, samples from 10 otitis-prone children were selected. From the remainder, 12 children with zero to 2 AOM episodes who were of a similar age as the otitis-prone children were selected to serve as controls. Clinical characteristics of the children are shown in Table 1. For both the otitis-prone and non–otitis-prone populations, the study samples were obtained after each child had experienced ≥1 nasopharyngeal colonization and/or AOM event caused by S. pneumoniae. However, all 10 otitis-prone children had experienced at least 2 prior nasopharyngeal colonization and AOM events due to S. pneumoniae before the study sample. The total number of AOM episodes in the otitis-prone group (6 of 10 children had 4–5 episodes) was significantly greater than that for the non–otitis-prone group (0 of 12 children had 4–5 episodes; P = .003). Thus, the opportunity to establish immunologic memory to S. pneumoniae antigens was greater for the otitis-prone cohort, because 44% of all AOM cases in both groups were due to S. pneumoniae.
Table 1.
Characteristics of Study Subjects Who Were or Were Not Prone to Acute Otitis Media (AOM)
| Characteristic | Otitis Prone (n = 10) | Non–Otitis Prone (n = 12) | P Value |
| Sex | |||
| Male | 6 | 7 | 1.00 |
| Female | 4 | 5 | 1.00 |
| Mean age (months) | 13.3 | 12.1 | .50 |
| AOM episodes (no.) | |||
| >3 in 6 months | 5 | 0 | .01 |
| >4 in 12 months | 5 | 0 | .01 |
| Total AOM episodes (no.) | |||
| 1–3 | 3 | 4 | 1.00 |
| 4–5 | 6 | 0 | .003 |
| ≥6 | 1 | 0 | .45 |
| Pressure-equalizing tube insertion | 4 | 0 | .03 |
| Breastfeeding at age >6 months | 5 | 8 | .67 |
Data are no. of children, unless otherwise indicated.
Generation of Pneumococcal Antigen-Specific Memory B Cells Is Reduced in Otitis-Prone Children
The circulating frequencies of various S. pneumoniae antigen–specific memory B cells were compared between non–otitis-prone and otitis-prone children by stimulating their PBMCs with polyclonal antibody. Antigen-specific B-cell responses were normalized with the control ELISPOT plate wells left uncoated or coated with bovine serum albumin.
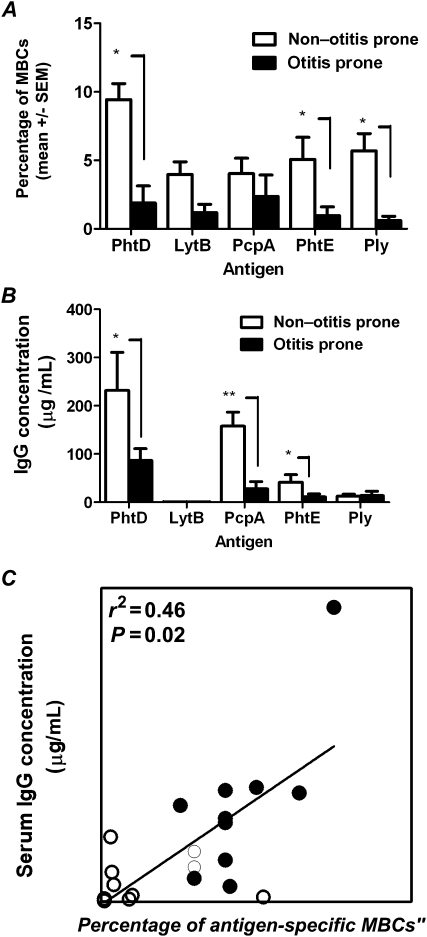
Figure 1A demonstrates percentages of memory B cells to 5 S. pneumoniae antigens in otitis-prone children and non–otitis-prone children with AOM caused by S. pneumoniae. In sharp contrast to the non–otitis-prone group, otitis-prone children had a marked reduction of circulating S. pneumoniae–specific memory B cells after an AOM episode or nasopharyngeal colonization (Figure 1). In particular, significantly lower percentages of memory B cells producing antigen-specific IgG were observed against antigens PhtD, PhtE, and Ply (P < .05). Otitis-prone children showed an overall lower percentage of memory B cells generated to LytB, although the difference was not statistically significant (P = .1). No statistically significant difference was found in the percentage of PcpA-specific memory B cells in otitis-prone and non–otitis-prone children (Figure 1A). Similarly, the total number of IgG-secreting cells present in the 2 groups did not differ (data not shown).
Figure 1.
A, Frequencies of antigen-specific memory B cells (MBCs). B, Serum immunoglobulin G (IgG) titers to 5 pneumococcal protein antigens. C, Correlation between PhtD-specific serum antibody titers and PhtD-specific percentages of antigen-specific MBCs. Data are for 10 otitis-prone children and 12 non–otitis-prone children. Open circles denote otitis prone and closed circles denote non–otitis prone. P values were calculated using the Mann–Whitney U test. *P < .05; ** P < .005.
Otitis-Prone Children Have Reduced IgG Titers to Pneumococcal Protein Antigens
We evaluated antigen-specific IgG titers in the serum of otitis-prone and non–otitis-prone children who were matched by age. Serum IgG levels to S. pneumoniae antigens in the respective groups are shown in Figure 1B. In the cohort of non–otitis-prone children, IgG titers to PhtD, PcpA, and PhtE were significantly higher than those in otitis-prone children (P < .05, P < .005, and P < .05, respectively); Ply levels were lower and did not differ in a statistically significant manner between the groups (Figure 1B). LytB antibody titers were the lowest among all antigens tested in both of the cohorts (Figure 1B).
DISCUSSION
We found a reduced percentage of memory B cells circulating in the blood of otitis-prone children following AOM and/or nasopharyngeal colonization, despite prior priming by preceding AOM and nasopharyngeal colonization events (Figure 1A). After encounter of antigen with naive B cells, antigen-specific memory B cells and antibody-secreting cells are generated in the secondary lymphoid structures that transit through the blood to bone marrow, spleen, or target tissues such as the respiratory tract [10]. Since serum antibody levels are maintained by memory B cells [14], by analyzing the percentages of antigen-specific memory B cells generated, we provide a more precise immunological explanation for lower antibody levels in otitis-prone children. To confirm the association of lower frequencies of memory B cells with serum antibody levels, we measured S. pneumoniae–specific antibody titers and found that they were significantly lower in otitis-prone children (Figure 1B).
Recently, in a different subset of children in our prospective, longitudinal study, we demonstrated that otitis-prone children have suboptimal pneumococcal antigen–specific memory CD4+ T-cell responses [7]. Findings from this study indicate that otitis-prone children may develop some antibody responses, since antibodies and memory B cells were detectable among these children after AOM and nasopharyngeal colonization with otopathogens (Figure 1A and 1B). However, in the absence of antigen-specific memory B-cell and/or memory CD4+ T-cell generation, the antibody levels wane, and otitis-prone children are unable to maintain adequate serum antibody levels and become susceptible to frequent repeat AOM episodes.
Pneumococcal polysaccharide conjugate vaccine is helpful in boosting protective levels of antipolysaccharide antibodies[15]; however, serotype variation limits the protective efficacy of strain-specific antipolysaccharide antibodies [3]. Moreover, although otitis-prone children can induce serotype-specific antibodies to conjugate vaccines, repeat infections are common among this vulnerable group [15], indicating that serotype-specific immunity is brief and incomplete.
Interestingly, we found that the percentage of circulating PhtD-specific memory B cells correlated with serum PhtD levels (Figure 1C). A difference in the percentages of antigen-specific B cells and serum antibodies levels to PcpA and Ply was observed (Figure 1A and 1B). We speculate that (1) by binding to the circulating IgG, an active state of nasopharyngeal colonization or AOM infection may affect the detection of serum antibody levels as opposed to memory B cells, and that (2) during infection in the uncontrolled inflammatory environment of the nasopharynx, a different dose of pathogen antigen and stimulation of pathogen-associated molecular patterns may elicit variable frequencies of B-cell differentiation into antibody-secreting cells and thus affect serum IgG levels, even in the presence of memory B cells.
In conclusion, with respect to the antigens evaluated here, otitis-prone children generate significantly lower percentages of memory B cells that can differentiate into antibody-secreting cells. The clinical relevance of the finding is clear. Antigen-specific memory B cells act as reservoirs for serum antibody maintenance that, on antigen reencounter and with adequate help with CD4+ T cells, can proliferate into antibody-secreting cells and lead to an increase in the serum antibody levels. We found that otitis-prone children do not lack total IgG-secreting cells. Furthermore, our flow cytometry results showed that in response to polyclonal stimulation, otitis-prone children do not have mechanistic dysfunction in the transformation of memory B cells (CD19+IgD−) to antibody secreting plasma-cells (CD27+CD38+CD138+; data not shown). Whether naive B cells in the secondary lymphoid organs of otitis-prone children are unable to get optimal CD4+ T cells or T-follicular cell help for differentiation into memory B cells and/or antibody-secreting cells for eventually maintaining higher serum IgG levels is now an area of investigation in our laboratory.
Notes
Acknowledgments.
We acknowledge the help of Kathy Dermody, her team, for IgG measurements involving the studied proteins, and Sally Thomas, LPN, CCRC, for her clinical support.
Financial support.
This work was supported by the National Institutes of Health (NIH) (National Institute on Deafness and Other Communication Disorders (NIDCD; grant RO1 08671) and Sanofi Pasteur.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Vergison A, Dagan R, Arguedas A, et al. Otitis media and its consequences: beyond the earache. Lancet Infect Dis. 2010;10:195–203. doi: 10.1016/S1473-3099(10)70012-8. [DOI] [PubMed] [Google Scholar]
- 2.Poehling KA, Szilagyi PG, Grijalva CG, et al. Reduction of frequent otitis media and pressure-equalizing tube insertions in children after introduction of pneumococcal conjugate vaccine. Pediatrics. 2007;119:707–15. doi: 10.1542/peds.2006-2138. [DOI] [PubMed] [Google Scholar]
- 3.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29:304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Q, Bagrade L, Bernatoniene J, et al. Low CD4 T cell immunity to pneumolysin is associated with nasopharyngeal carriage of pneumococci in children. J Infect Dis. 2007;195:1194–202. doi: 10.1086/512617. [DOI] [PubMed] [Google Scholar]
- 5.Snapper CM, Shen Y, Khan AQ, et al. Distinct types of T-cell help for the induction of a humoral immune response to Streptococcus pneumoniae. Trends Immunol. 2001;22:308–11. doi: 10.1016/s1471-4906(01)01926-3. [DOI] [PubMed] [Google Scholar]
- 6.Weiser JN, Bae D, Fasching C, Scamurra RW, Ratner AJ, Janoff EN. Antibody-enhanced pneumococcal adherence requires IgA1 protease. Proc Natl Acad Sci USA. 2003;100:4215–20. doi: 10.1073/pnas.0637469100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma SK, Casey JR, Pichichero ME. Reduced memory CD4+ T-cell generation in the circulation of young children may contribute to the otitis-prone condition. J Infect Dis. 2011;204:645–53. doi: 10.1093/infdis/jir340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pichichero ME. Booster vaccinations: can immunologic memory outpace disease pathogenesis? Pediatrics. 2009;124:1633–41. doi: 10.1542/peds.2008-3645. [DOI] [PubMed] [Google Scholar]
- 9.Lanzavecchia A, Sallusto F. Human B cell memory. Curr Opin Immunol. 2009;21:298–304. doi: 10.1016/j.coi.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly DF, Pollard AJ, Moxon ER. Immunological memory: the role of B cells in long-term protection against invasive bacterial pathogens. JAMA. 2005;294:3019–23. doi: 10.1001/jama.294.23.3019. [DOI] [PubMed] [Google Scholar]
- 11.Faden H. The microbiologic and immunologic basis for recurrent otitis media in children. Eur J Pediatr. 2001;160:407–13. doi: 10.1007/s004310100754. [DOI] [PubMed] [Google Scholar]
- 12.Murphy TF, Yi K. Mechanisms of recurrent otitis media: importance of the immune response to bacterial surface antigens. Ann N Y Acad Sci. 1997;830:353–60. doi: 10.1111/j.1749-6632.1997.tb51907.x. [DOI] [PubMed] [Google Scholar]
- 13.Kaur R, Casey JR, Pichichero ME. Serum antibody response to three non-typeable Haemophilus influenzae outer membrane proteins during acute otitis media and nasopharyngeal colonization in otitis prone and non–otitis prone children. Vaccine. 2011;29:1023–8. doi: 10.1016/j.vaccine.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 15.Barnett ED, Pelton SI, Cabral HJ, et al. Immune response to pneumococcal conjugate and polysaccharide vaccines in otitis-prone and otitis-free children. Clin Infect Dis. 1999;29:191–2. doi: 10.1086/520151. [DOI] [PubMed] [Google Scholar]