Abstract
Background. Reported associations of condom use and human papillomavirus (HPV) infection have been inconsistent. We investigated self-reported frequency of condom use and detection of genital HPV among men.
Methods. A cross-sectional analysis was conducted in men aged 18–70 years from Brazil, Mexico, and the United States. Men completed questionnaires on sexual history, condom use, and sociodemographic characteristics. Among 2621 men reporting recent vaginal sex, prevalence of any HPV, any oncogenic type, and nononcogenic types only was estimated by frequency of condom use (“always” or “not always”). Multivariable models were used to estimate prevalence ratios (PRs) for HPV according to frequency of condom use.
Results. The prevalence of any HPV was 70.5%; any oncogenic type, 34%, and nononcogenic types only, 22.2%. The adjusted PR for always vs not always using condoms was 0.87 (95% confidence interval [CI], .77–.97) for all countries combined. The association was stronger in the United States (PR, 0.70; CI, .55–.90) than in Brazil (PR, 0.84; CI, .71–1.01) or Mexico (PR, 1.05; CI, .89–1.25) (P for interaction = .025).
Conclusions. HPV prevalence was high even among those who reported always using condoms, and its associations with always using condoms varied among countries.
Human papillomavirus (HPV) is highly infective and is the most commonly sexually transmitted pathogen [1]. In 2011 in the United States, 6.2 million new HPV infections were expected [2], and half of all Americans are expected to acquire a genital HPV infection in their lifetime [3]. The majority of HPV infections are asymptomatic and both sexes can be carriers. More than 100 types of HPV have been identified that can infect skin or mucosa, approximately 40 of which infect the genital tract mucosa. HPV causes complex infections associated with a range of diseases, from cervical dysplasia to anal and penile cancers [4]. Because there is no cure for HPV infection, the development of effective preventive measures such as condom use and vaccines is critical to reducing the HPV burden [5].
Factors previously reported to be associated with HPV infection in men include circumcision status, education, lifetime number of sexual partners, age, country of residence, and patterns of condom use [6]. Because HPV is transmitted by skin-to-skin contact, condom usage has been assumed to be less effective against this disease than it is against sexually transmitted diseases (STDs) transmitted by semen or vaginal secretions, such as gonorrhea or human immunodeficiency virus [7]. In 2000, the US National Institutes of Health concluded that there was insufficient epidemiologic evidence that condom use reduced the risk of HPV infection [7, 8]. However, several studies have demonstrated reduced risk of HPV infection with consistent and proper condom use [6, 9–14]. Most of these studies are small and involve only 1 geographic location. Thus, the association between condom use and HPV infection requires elucidation to determine effective prevention methods on the population level.
In the current analysis, genital HPV infection was evaluated at the baseline visit of the ongoing longitudinal HPV in Men (HIM) cohort study. This is a prospective study of penile HPV occurrence in male cohorts in Brazil, Mexico, and the United States that is designed to reveal the natural history of penile HPV infection. In addition to being tested for the presence of HPV DNA, participants completed a self-administered health and sexual behavior questionnaire. We used the results from both to determine the association between condom use during vaginal sex and presence of HPV types. The purpose of this study was to report the HPV prevalence in the largest international male cohort study to date and to quantify the association between condom use during vaginal sex and presence of HPV.
SUBJECTS, MATERIALS, AND METHODS
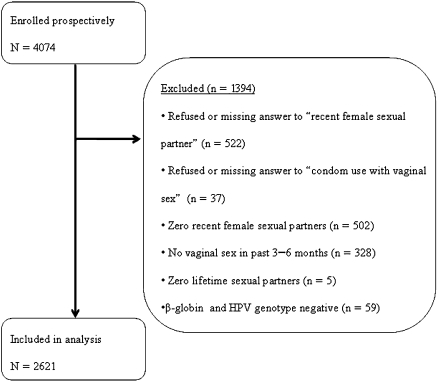
Study design, population, clinical sampling, and HPV testing procedures have been described in detail elsewhere [15]. A total of 4074 men (full cohort) completed an enrollment visit. HIM study participants were recruited from the general population, universities, and organized healthcare systems (Mexico only) in São Paulo, Brazil; Cuernavaca, Mexico; Tampa, Florida; and surrounding areas [15]. Participants were included in the HIM cohort if they (1) were aged 18–70 years, (2) were residents of 1 of the 3 sites, (3) had no previous diagnosis of genital warts or penile or anal cancer, (4) reported no current penile discharge or burning during urination, (5) had no current diagnosis of an STD, (6) were not participating in an HPV vaccine study, (7) had no history of imprisonment, homelessness, or drug treatment during the past 6 months, (8) had no plan to relocate in the next 4 years, and (9) were willing to comply with 10 scheduled visits every 6 months for 4 years. Participants were included in the present analysis if they met the above criteria and reported vaginal sexual intercourse with a woman during the previous 3–6 months. This left 2621 men (analytic cohort) included in this cross-sectional analysis (Figure 1). Written informed consent was obtained from all participants, and human subjects committees in the 3 sites reviewed all procedures (Human Subjects Committees of the University of South Florida; the Centro de Referencia e Treinamento de Doencas Sexualmente Transmissiveis e AIDS, São Paulo, Brazil; and the National Institute of Public Health of Mexico) [15].
Figure 1.
Analytic cohort for HPV in Men Study. HPV, human papillomavirus.
Risk Factor Questionnaire
All men completed an extensive risk factor questionnaire that solicited a detailed sexual history and information about their sexual practices, sociodemographic characteristics, condom use patterns, alcohol and tobacco use, partners’ sexual histories, and partners’ history of abnormal Pap smears. Participants were given the option of refusing to answer each question. All men were also asked to specify their frequency of condom use during vaginal sex with any partner in the past 3–6 months by selecting 1 of the following responses: “always,” “more than half the time,” “half the time,” “less than half the time,” or “never” [6]. Men were excluded from the present analysis if they did not answer this question or the question about whether they had vaginal sex in the previous 3–6 months or reported zero lifetime vaginal sex partners (including men who only had sex with men).
HPV Penile and Scrotal Sampling
Sampling techniques have been explained in detail elsewhere [15]. Briefly, all participants’ external genitalia were swabbed with 3 prewetted Dacron applicators. The areas swabbed were the coronal sulcus, glans penis, and entire surface of the shaft of the penis and scrotum. Before DNA extraction, the 3 swab samples were combined to produce 1 DNA sample per participant per clinic [15].
HPV DNA Detection and Genotyping
The detailed protocol for HPV analysis has been published elsewhere [15, 16]. Briefly, HPV testing of swabbed cellular material was conducted using polymerase chain reaction (PCR) for amplification of a fragment of the HPV L1 gene [17]. Specimens were tested for presence of HPV using the linear array HPV genotyping test [16], and HPV genotyping was conducted on all samples regardless of HPV PCR result. Samples that were human β-globin negative with no HPV genotype were excluded from all analyses. The oncogenic HPV types detected in this assay included 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 66 [18]. The nononcogenic types detected with the line blot method were 6, 11, 26, 40, 42, 53–55, 61, 62, 64, 67–73, 81–84, IS39, and CP6108.
Key Variables
A participant was considered positive for “any HPV” if he tested HPV-positive by PCR or genotyping. A positive β-globin test without detection of HPV DNA by PCR or genotyping was defined as “HPV negative.” The category of “any oncogenic type” included those who were positive for ≥1 oncogenic type. Single or multiple infections with only nononcogenic HPV types were classified as “nononcogenic type only.” Specimens testing HPV positive by PCR but negative for any HPV genotype were categorized as “unclassified.” A list of outcomes and covariates used in the analysis is presented in Table 1. All independent variables listed in Table 1 were evaluated for inclusion in the multivariable model.
Table 1.
Variables Used in Regression Analysis
| Outcome Variable | Primary Independent Variable | Covariate |
| Any HPV | Condom use | Age |
| Any oncogenic HPV | Ethnicity | |
| Only nononcogenic HPV | Race | |
| Marital status | ||
| Has a steady partner | ||
| Amount of education | ||
| Current cigarette smoker | ||
| Log smoking pack-years | ||
| Monthly alcohol intake | ||
| Circumcised | ||
| Age at first sexual intercourse | ||
| Lifetime number of partners | ||
| Number of female partners in the past 3–6 mo | ||
| History of any sexually transmitted disease | ||
| Partner history of sexually transmitted disease | ||
| Partner with abnormal Pap smear in the past 6 mo | ||
| Country of residence | ||
| Positive for herpes simplex virus, syphilis, gonorrhea, and/or chlamydia | ||
| Frequency of sexual intercourse |
Abbreviation: HPV, human papillomavirus
Statistical Analysis
Frequency and mean values were calculated for all variables used in this analysis to allow for qualitative comparison of the full cohort (N = 4074) with the analytic cohort (N = 2621). Variables of the analytic cohort were compared across the 5 levels of condom use using Pearson χ2 test for categorical variables. Differences in the distribution of HPV prevalence were explored by country and associations were tested with Pearson χ2 test.
Owing to the high prevalence of HPV, the association of HPV detection and condom use was characterized using prevalence ratios (PRs). A Poisson regression model with robust estimates for standard error was used [19–22]. The associations between dichotomous “always” vs “not always” condom use and each HPV outcome (any HPV, any oncogenic, and only nononcogenic) were evaluated. Confounding was controlled by retaining any variable in the multivariable model that altered the unadjusted PR by >10%. Effect modification of condom use by recent number of sexual partners in the past 3–6 months [6] and by country was hypothesized and tested. All tests were considered statistically significant if P <.05. Analyses were conducted using Stata IC 11.1 software for Macintosh (StataCorp).
RESULTS
Cohorts
The distribution of country, alcohol and tobacco use, sexual history, and condom use patterns is shown for both full and analytic cohorts in Table 2. Men included in this analytic cohort were similar to those in the full cohort. Behavioral and other attributes of the present analytic cohort are presented in Table 3 by frequency of condom use. Men who always used a condom were more likely to be from Brazil or the United States and more likely to have had ≥2 partners in the past 3–6 months. Men who did not always use a condom were more likely to be from Mexico and to report only 1 sexual partner in the past 3–6 months.
Table 2.
Comparison Between Full Cohort and Analytic Cohort
| Participants, No. (%) |
||
| Variable | Full Cohort (N = 4074) | Analytic Cohort (N = 2621) |
| Smoking pack-years (quartiles) | ||
| 0.1–0.70 | 408 (24.6) | 277 (25.9) |
| 0.71–2.50 | 415 (25.0) | 273 (25.5) |
| 2.51–8.10 | 407 (24.5) | 253 (23.6) |
| ≥8.2 | 429 (25.9) | 267 (25.0) |
| Monthly alcohol intake, drinks | ||
| 0 | 1106 (25.7) | 562 (21.9) |
| 1–30 | 1848 (46.7) | 1187 (46.3) |
| 31–60 | 444 (11.2) | 327 (12.8) |
| ≥61 | 649 (16.4) | 486 (19.0) |
| Female partners in past 3–6 mo | ||
| 0 | 847 (22.0) | |
| 1 | 1654 (45.0) | 1159 (60.7) |
| 2 | 519 (14.1) | 509 (19.4) |
| 3 | 262 (7.1) | 255 (9.7) |
| ≥4 | 270 (7.3) | 267 (10.2) |
| Country of residence at enrollment | ||
| United States | 1343 (33.0) | 923 (35.2) |
| Brazil | 1401 (34.9) | 936 (35.7) |
| Mexico | 1330 (32.7) | 762 (29.1) |
| Condom use | ||
| Always | 723 (22.0) | 599 (22.9) |
| More than half the time | 504 (15.1) | 431 (16.4) |
| Half the time | 245 (7.3) | 214 (8.2) |
| Less than half the time | 490 (14.7) | 423 (16.1) |
| Never | 1321 (39.6) | 954 (36.4) |
Table 3.
Characteristics of Men in the Human Papillomavirus Study by Condom Use (N = 2621)
| Participants by Frequency of Condom Use, No. (%) |
|||
| Variable | Always (n = 599) | Not Always (n = 2022) | P for χ2 |
| Smoking pack-years (quartiles) | .662 | ||
| 0.1–0.70 | 51 (24.5) | 226 (26.2) | |
| 0.71–2.50 | 52 (25.0) | 221 (25.6) | |
| 2.51–8.10 | 56 (26.9) | 197 (22.9) | |
| ≥8.2 | 49 (23.6) | 218 (25.3) | |
| Monthly alcohol intake, drinks | .656 | ||
| 0 | 126 (21.8) | 436 (22.0) | |
| 1–30 | 271 (46.9) | 916 (46.2) | |
| 31–60 | 80 (13.8) | 247 (12.5) | |
| ≥61 | 101 (17.5) | 385 (19.4) | |
| Female partners in past 3–6 mo | .084 | ||
| 1 | 337 (56.3) | 1253 (62.0) | |
| 2 | 126 (21.0) | 383 (18.9) | |
| 3 | 65 (10.9) | 190 (9.4) | |
| ≥4 | 71 (11.9) | 196 (9.7) | |
| Country of residence at enrollment | <.001 | ||
| United States | 248 (41.1) | 675 (33.4) | |
| Brazil | 231 (38.6) | 705 (35.9) | |
| Mexico | 120 (20.0) | 642 (31.8) | |
Condom Use
The proportion of HPV detected by frequency of condom use is displayed in Table 4. For any type of HPV, the proportion of HPV-positive samples was lowest for men who always used condoms (65.9%) vs 71.9% for men who reported not always using condoms (P = .005). Similar differences were observed for oncogenic HPV and multiple-type infections, although no statistically significant differences in the proportion of HPV-positive men were observed for nononcogenic or unclassified HPV types.
Table 4.
Human Papillomavirus (HPV) Detection, by Frequency of Condom Use (N = 2621)
| Participants by Frequency of Condom Use, No. (%) |
|||
| Always | Not Always | ||
| HPV Detected | (n = 599) | (n = 2022) | P for χ2 |
| Any HPV type | 395 (65.9) | 1454 (71.9) | .005 |
| Any oncogenic type | 177 (29.6) | 715 (35.4) | .008 |
| Oncogenic types | |||
| 16 | 37 (6.2) | 184 (9.1) | .024 |
| 18 | 7 (1.2) | 53 (2.6) | .037 |
| Nononcogenic type(s) only | 123 (20.5) | 460 (22.8) | .252 |
| Nononcogenic types | |||
| 6 | 35 (5.8) | 142 (7.0) | .312 |
| 11 | 9 (1.5) | 26 (1.3) | .685 |
| Unclassified type(s) only | 95 (15.9) | 279 (13.8) | .205 |
| Multiple types | 170 (28.4) | 677 (33.0) | .034 |
Association With Condom Use by Country
The crude association between HPV type and always using condoms differed by country (Table 5). Always using condoms was significantly associated with reduced detection of any HPV in the US crude model (crude PR, 0.79; 95% confidence interval [CI], .70–.89). Also in the US crude model, condom use was associated with lower prevalence of any oncogenic HPV (crude PR, 0.66; 95% CI, .51–.84) and only nononcogenic HPV (crude PR, 0.62; 95% CI, .44–.89).
Table 5.
Prevalence Ratios (PRs) for Associations Between Human Papillomavirus (HPV) and Frequency of Condom Use
| Overall Model | Crude PR (95% CI)a | Adjusted PR (95% CI)b |
| Any HPV | ||
| Always | 0.92 (.86–.98) | 0.87 (.77–.97) |
| Not always | Reference | Reference |
| Any oncogenic HPV | ||
| Always | 0.84 (.73–.96) | 0.81 (.65–1.01) |
| Not always | Reference | Reference |
| Only nononcogenic HPV | ||
| Always | 0.90 (.76–1.08) | 0.93 (.69–1.25) |
| Not always | Reference | Reference |
| Any HPV by frequency of condom use Interaction of country × condom use, crude model (P = .025) | ||
| United States | ||
| Always | 0.79 (.70–.89) | 0.70 (.55–.90) |
| Not always | Reference | Reference |
| Brazil | ||
| Always | 0.96 (.88–1.05) | 0.84 (.71–1.01) |
| Not always | Reference | Reference |
| Mexico | ||
| Always | 1.04 (.92–1.19) | 1.05 (.89–1.25) |
| Not always | Reference | Reference |
| Any oncogenic HPV by frequency of condom use Interaction of country × condom use, crude model (P = .78) | ||
| United States | ||
| Always | 0.66 (.51–.84) | 0.72 (.47–1.10) |
| Not always | Reference | Reference |
| Brazil | ||
| Always | 0.91 (.74–1.10) | 0.82 (.59–1.16) |
| Not always | Reference | Reference |
| Mexico | ||
| Always | 1.01 (.76–1.34) | 0.88 (.60–1.29) |
| Not always | Reference | Reference |
| Only nononcogenic HPV by frequency of condom use Interaction of country × condom use, crude model (P = .91) | ||
| United States | ||
| Always | 0.62 (.44–.89) | 0.88 (.51–1.50) |
| Not always | Reference | Reference |
| Brazil | ||
| Always | 1.13 (.88–1.44) | 1.00 (.63–1.62) |
| Not always | Reference | Reference |
| Mexico | ||
| Always | 0.97 (.67–1.41) | 0.89 (.52–1.53) |
| Not always | Reference | Reference |
Unadjusted model.
Multivariable models are adjusted for monthly alcohol intake, log pack-years of smoking, interaction of country and condom use, and number of female sexual partners in the past 3–6 mo.
For adjusted models, our evaluation of potential confounders led us to include log smoking pack-years, monthly alcohol intake, and recent number of sexual partners. No other variables that may affect HPV prevalence, including age, race, and relationship status, acted as confounders. Only the United States demonstrated an association of condom use with reduced detection of any HPV type (adjusted PR, 0.70; 95% CI, .55–.90). There was no significant association of condom use and HPV detection for any country in the adjusted models for any oncogenic or only nononcogenic HPV (Table 5).
The adjusted association between any HPV type and always using condoms differed by country (P for interaction = .025). The interaction terms for condom use and country were not significant for any oncogenic (P = .78) or only nononcogenic types (P = .91). When multivariable models were fit without the interaction term and adjusted for country and other independent variables, the main association of condom use was significant for any HPV but not any oncogenic or only nononcogenic HPV (Table 5). There was no interaction detected for recent number of sexual partners for any of the 3 HPV outcomes.
DISCUSSION
Our results demonstrate that always using condoms was significantly associated with the lowest proportion of HPV detection for any HPV type, any oncogenic type, and multiple types (Table 4). There was no significant association found for only nononcogenic types and unclassified HPV types. This finding is consistent with the previous literature [6, 12].
Country was strongly associated with condom use and the detection of HPV. Consistent with our previous findings, we observed statistically significant differences in the distribution of all study characteristics evaluated by country [15]. The United States demonstrated the strongest association of always using condoms and reduced detection of HPV. In adjusted models for the United States, always using condoms was significantly associated with lower prevalence of any HPV type. In the adjusted Brazilian model, always using condoms was borderline protective for any HPV type but not for any oncogenic or only nononcogenic. Interestingly, in Mexico there was no protective association of always using condoms for any model of any HPV type. These results suggest that actual condom use behaviors differ by country. Although we did not find differences in condom use by racial or ethnic group within each country, others have reported that black and Hispanic adults in the United States were more likely to use condoms than white participants [23]. Future research focusing on specific condom practices and failure rates by country and by cultural groups within countries may be necessary to understand these differences.
Many factors affect condoms’ efficacy at preventing STD transmission: user experience, STD infectivity, cumulative risk, user failure, method failure, and STD mode of transmission [8]. Possible reasons for the high HPV prevalence, even among consistent condom users, could be due to ≥1 of these factors. Condom breakage, slippage, use of inappropriate lubricants, and application errors are disturbingly common [24–29]. Experience seems to facilitate successful condom usage, because repeated use is a predictor of lowered failure rate for both male and female condoms [25, 30]. Combining these behavioral factors with the existence of HPV on skin areas not covered by a condom, high HPV prevalence, even with consistent condom use, is not surprising.
Inaccurate self-reporting of condom use may also explain differences in the protective effect of condoms by country. A randomized crossover trial comparing male condom failure rates in the United States and Brazil found that there was a significant difference in “any problem” reported, with Brazilian men reporting significantly fewer condom use problems than Americans [31]. Men from both countries reported similar condom breakage and slippage upon withdrawal, but the Brazilian participants reported significantly fewer incidents of partial slippage, total slippage, and semen leakage than men in the United States [31]. However, the prostate-specific antigen detected from postcoital samples of vaginal fluid indicated the prostate-specific antigen detection rate was similar between participants from the United States and Brazil. Thus, exposure to semen and vaginal secretions despite condom use was similar for participants in both countries, but self-reporting differed, with Brazilian men systematically underreporting condom use problems. Inaccurate reporting of condom use may have occurred in all 3 study countries and could reduce the possibility of detecting a true reduction of infection risk from using condoms by 25%–30% [32].
Ordinal self-report of condom use has limitations. Research on ordinal condom use measurements indicates that there is considerable interpersonal variability assigned to categories of condom use frequency across populations [33, 34]. For example, a proportion of people may classify using condoms in 19 of 20 encounters as “always” using condoms, which introduces bias into this condom use category, potentially diluting any observed protective association of condoms. However, the greatest variation in condom label assignment is seen in the middle categories of condom use [34], which were combined in our analysis. The validity of self-reported condom use is greatest when the recall period is short and sexual activity is low [33]. Our recall period for condom use was for encounters in the past 3–6 months; the majority of men reported only 1 sexual partner during that time.
To our knowledge, our study sample is the largest male cohort reporting the association between condom use and HPV detection in Brazil, Mexico, and the United States. The limitations of our study include its cross-sectional design, self-report of sexual behavior and condom use, combined HPV samples that include sites not covered by a condom, and lack of assessment of correct condom usage. Given the high prevalence of HPV, estimating the associations with PRs gives less biased estimates of risk than odd ratios (ORs), which have been reported in previous studies [15, 35]. Because PRs are more conservative than ORs, this is likely to explain some of the difference in reported strength of association from other studies. For example, our adjusted PR of 0.70 in the United States corresponds to an adjusted OR of 0.51 for the same model. Therefore, our findings are likely similar to previous reports that used ORs [6, 36, 37]. Taking a cross-sectional snapshot of the largest international male HPV cohort, our study demonstrates that always using condoms is associated with lower HPV detection in men. This protection differs by country, with American men experiencing the most protective effect of always using condoms.
Notes
Acknowledgments.
The authors thank the following staff for their dedication in recruiting, examining, and maintaining cohort participants, as well as conducting HPV DNA laboratory analyses: Kathy Eyring, CCRP; Christine Gage, ARNP; Nadia Lambermont, ARNP; Kim Isaacs, BA; Andrea M. Leto, BA; Emily Jolles, MPH; Kayoko Kennedy, BA; Amanda Sivia; Pauline Schwalm-Andel, BS; Rana Zaki, MPH; Sireesha Banduvula, MS; Kyle Wolf; Steven McAnany; and Shannon McCarthy. The authors thank the Tissue Core staff of the Moffitt Cancer Center for their help managing biological samples from the US site: M. Luiza Baggio, Roberto Silva, Lenice Galan, Elimar Gomes, Ricardo Cintra, Viviane Relvas, Filomena Cernicchiaro, Raquel Hessel, Sandra Araujo, Graça Ribeiro, Rosária Otero, Roberta Bocalon, Juliana Antunes, Rossana Terreri, Fernanda Silva, Rubens Matsuo, Ricardo Cunha, Vera Souza, Elisa Brito, and Birgit Fietzek from the Brazil site; A. Cruz, P. Hernández, A. Rodríguez-Cid, G. Alvarez, O. Rojas, D. A. Salazar, N. Herrera, A. Rodríguez, and P. Román from the Mexico site. The authors also thank the Digene Corporation for kindly providing Specimen Transport Medium for the collection and storage of samples at no charge to the study.
Financial support.
This work was supported by a grant from the National Cancer Institute, National Institutes of Health (CA RO1CA098803), and by a Union for International Cancer Control (UICC) American Cancer Society Beginning Investigators Fellowship (funded by the American Cancer Society).
Potential conflicts of interest.
L. L. V. is a consultant for Merck Sharp & Dohme for the HPV quadrivalent vaccine. A. R. G. interacts with companies involved in HPV vaccines, but these activities are unrelated to the content of the this article. A. R. G. is a consultant to Merck and Co and on its speakers’ bureau. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of this article have been disclosed.
References
- 1.Burchell AN, Richardson H, Mahmud SM, et al. Modeling the sexual transmissibility of human papillomavirus infection using stochastic computer simulation and empirical data from a cohort study of young women in Montreal, Canada. Am J Epidemiol. 2006;163:534–43. doi: 10.1093/aje/kwj077. [DOI] [PubMed] [Google Scholar]
- 2.Weinstock H, Berman S, Cates W., Jr Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36:6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Fact sheet: genital HPV. http://www.cdc.gov/std/hpv/HPV-Factsheet-Nov-2011.pdf. Accessed 26 February 2011. [Google Scholar]
- 4.Giuliano AR, Salmon D. The case for a gender-neutral (universal) human papillomavirus vaccination policy in the United States. Cancer Epidemiol Biomarkers Prev. 2008;17:805–8. doi: 10.1158/1055-9965.EPI-07-0741. [DOI] [PubMed] [Google Scholar]
- 5.Giuliano A, Palefsky J, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364:401–11. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielson CM, Harris RB, Nyitray AG, Dunne EF, Stone KM, Giuliano AR. Consistent condom use is associated with lower prevalence of human papillomavirus infection in men. J Infect Dis. 2010;202:445–51. doi: 10.1086/653708. [DOI] [PubMed] [Google Scholar]
- 7.Holmes KK, Levine R, Weaver M. Effectiveness of condoms in preventing sexually transmitted infections. Bull World Health Organ. 2004;82:454–61. [PMC free article] [PubMed] [Google Scholar]
- 8.Fitch JT, Stine C, Hager D, Mann J, Adam MB, McIlhaney J. Condom effectiveness: factors that influence risk reduction. Sex Transm Dis. 2002;29:811–17. doi: 10.1097/00007435-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Bleeker MC, Hogewoning CJ, Voorhorst FJ, et al. Condom use promotes regression of human papillomavirus-associated penile lesions in male sexual partners of women with cervical intraepithelial neoplasia. Int J Cancer. 2003;107:804–10. doi: 10.1002/ijc.11473. [DOI] [PubMed] [Google Scholar]
- 10.Winer R, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354:2645–54. doi: 10.1056/NEJMoa053284. [DOI] [PubMed] [Google Scholar]
- 11.Baldwin SB, Wallace DR, Papenfuss MR, Abrahamsen M, Vaught LC, Giuliano AR. Condom use and other factors affecting penile human papillomavirus detection in men attending a sexually transmitted disease clinic. Sex Transm Dis. 2004:601–7. doi: 10.1097/01.olq.0000140012.02703.10. [DOI] [PubMed] [Google Scholar]
- 12.Shew ML, Fortenberry JD, Wanzhu T, et al. Association of condom use, sexual behaviors, and sexually transmitted infections with duration of genital human papillomavirus infection among adolescent women. Arch Pediatr Adolesc Med. 2006;160:151–6. doi: 10.1001/archpedi.160.2.151. [DOI] [PubMed] [Google Scholar]
- 13.Smith JS, Moses S, Hudgens MG, et al. Human papillomavirus detection by penile site in young men from Kenya. Sex Transm Dis. 2007;34:928–34. doi: 10.1097/OLQ.0b013e318065b8ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Partridge JM, Koutsky LA. Genital human papillomavirus infection in men. Lancet Infect Dis. 2006;6:21–31. doi: 10.1016/S1473-3099(05)70323-6. [DOI] [PubMed] [Google Scholar]
- 15.Giuliano AR, Lazcano-Ponce E, Villa LL, et al. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev. 2008;17:2036–43. doi: 10.1158/1055-9965.EPI-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gravitt P, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol. 1998;36:3020–7. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gravitt P, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, Ghissassi F. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6:204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 19.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 20.Zocchetti C, Consonni D, Bertazzi PA. Relationship between prevalence rate ratios and odds ratios in cross-sectional studies. Int J Epidemiol. 1997;26:220–3. doi: 10.1093/ije/26.1.220. [DOI] [PubMed] [Google Scholar]
- 21.Pearce N. Effect measures in prevalence studies. Environ Health Perspect. 2004;112:1047–50. doi: 10.1289/ehp.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson ML, Meyers JE, Kriebel D. Prevalence odds ratio or prevalence ratio in the analysis of cross sectional data: what is to be done? Occup Environ Med. 1998;55:272–7. doi: 10.1136/oem.55.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanders SA, Reece M, Herbenick D, Schick V, Dodge B, Fortenberry JD. Condom use during most recent vaginal intercourse event among a probability sample of adults in the United States. J Sex Med. 2010;7(Suppl 5):S362–73. doi: 10.1111/j.1743-6109.2010.02011.x. [DOI] [PubMed] [Google Scholar]
- 24.Crosby R, DiClemente RJ, Yarber WL, Snow G, Troutman A. An event-specific analysis of condom breakage among African American men at risk of HIV acquisition. Sex Transm Dis. 2008;35:174–7. doi: 10.1097/OLQ.0b013e3181585bf5. [DOI] [PubMed] [Google Scholar]
- 25.Valappil T, Kelaghan J, Macaluso M, et al. Female condom and male condom failure among women at high risk of sexually transmitted diseases. Sex Transm Dis. 2005;32:35–43. doi: 10.1097/01.olq.0000148295.60514.0b. [DOI] [PubMed] [Google Scholar]
- 26.Duerr A, Gallo MF, Warner L, Jamieson DJ, Kulczycki A, Macaluso M. Assessing male condom failure and incorrect use. Sex Transm Dis. 2011;38:1–7. doi: 10.1097/OLQ.0b013e3182096b62. [DOI] [PubMed] [Google Scholar]
- 27.Crosby R, Milhausen R, Sanders SA, Graham CA, Yarber WL. Two heads are better than one: the association between condom decision-making and condom use errors and problems. Sex Transm Infect. 2008;84:198–201. doi: 10.1136/sti.2007.027755. [DOI] [PubMed] [Google Scholar]
- 28.Crosby RA, Yarber WL, Sanders SA, et al. Men with broken condoms: who and why? Sex Transm Infect. 2006;83:71–5. doi: 10.1136/sti.2006.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shlay JC, McClung MW, Patnaik JL, Douglas JM. Comparison of sexually transmitted disease prevalence by reported condom use: errors among consistent condom users seen at an urban sexually transmitted disease clinic. Sex Transm Dis. 2004;31:526–32. doi: 10.1097/01.olq.0000137897.17919.d1. [DOI] [PubMed] [Google Scholar]
- 30.Crosby R, Sanders S, Yarber WL, Graham CA. Condom-use errors and problems: a neglected aspect of studies assessing condom effectiveness. Am J Prev Med. 2003;24:367–70. doi: 10.1016/s0749-3797(03)00015-1. [DOI] [PubMed] [Google Scholar]
- 31.Chen MP, Macaluso M, Blackwell R, et al. Self-reported mechanical problems during condom use and semen exposure: comparison of two randomized trials in the United States of America and Brazil. Sex Transm Dis. 2007;24:557–62. doi: 10.1097/01.olq.0000258487.38309.b9. [DOI] [PubMed] [Google Scholar]
- 32.Devine OJ, Aral SO. The impact of inaccurate reporting of condom use and imperfect diagnosis of sexually transmitted disease infection in studies of condom effectiveness. Sex Transm Dis. 2004;31:588–95. doi: 10.1097/01.olq.0000140010.25191.b3. [DOI] [PubMed] [Google Scholar]
- 33.Cecil H, Pinkerton SD, Bogart LM, Pavlovic J, Kimball AM. An empirical study of ordinal condom use measures. J Sex Res. 2005;42:353–8. doi: 10.1080/00224490509552291. [DOI] [PubMed] [Google Scholar]
- 34.Cecil H, Zimet D. Meanings assigned by undergraduates to frequency statements of condom use. Arch Sex Behav. 1998;27:493–505. doi: 10.1023/a:1018756614107. [DOI] [PubMed] [Google Scholar]
- 35.Manhart LE, Koutsky LA. Do condoms prevent genital HPV infection, external genital warts, or cervical neoplasia? Sex Transm Dis. 2002;29:725–35. doi: 10.1097/00007435-200211000-00018. [DOI] [PubMed] [Google Scholar]
- 36.Lajous M, Mueller N, Cruz-Valdez A, et al. Determinants of prevalence, acquisition, and persistence of human papillomavirus in healthy Mexican military men. Cancer Epidemiol Biomarkers Prev. 2005;14:1710–16. doi: 10.1158/1055-9965.EPI-04-0926. [DOI] [PubMed] [Google Scholar]
- 37.Giuliano AR, Lazcano E, Villa LL, et al. Circumcision and sexual behavior: factors independently associated with human papillomavirus detection among men in the HIM study. Int J Cancer. 2009;124:1251–7. doi: 10.1002/ijc.24097. [DOI] [PMC free article] [PubMed] [Google Scholar]