Xenopus maternal Norrin, which activates Wnt signaling but inhibits TGF-β family molecules, is essential for neuroectoderm formation. Loss of TGF-β inhibition in Norrin may contribute to the development of Norrie disease.
Abstract
Dorsal–ventral specification in the amphibian embryo is controlled by β-catenin, whose activation in all dorsal cells is dependent on maternal Wnt11. However, it remains unknown whether other maternally secreted factors contribute to β-catenin activation in the dorsal ectoderm. Here, we show that maternal Xenopus Norrin (xNorrin) promotes anterior neural tissue formation in ventralized embryos. Conversely, when xNorrin function is inhibited, early canonical Wnt signaling in the dorsal ectoderm and the early expression of the zygotic neural inducers Chordin, Noggin, and Xnr3 are severely suppressed, causing the loss of anterior structures. In addition, xNorrin potently inhibits BMP- and Nodal/Activin-related functions through direct binding to the ligands. Moreover, a subset of Norrin mutants identified in humans with Norrie disease retain Wnt activation but show defective inhibition of Nodal/Activin-related signaling in mesoderm induction, suggesting that this disinhibition causes Norrie disease. Thus, xNorrin is an unusual molecule that acts on two major signaling pathways, Wnt and TGF-β, in opposite ways and is essential for early neuroectoderm specification.
Author Summary
A key step during early embryogenesis is the generation of neural precursors, which later form the central nervous system. In vertebrates, this process requires proper dorsal–ventral axis specification, and we know that the canonical Wnt and BMP signaling pathways help pattern the dorsal ectoderm. In this study, we examine other factors that are involved in neuroectoderm development in the frog species Xenopus laevis. We find that maternal Xenopus Norrin (xNorrin) is required for canonical Wnt signaling in the dorsal ectoderm, functions upstream of neural inducers, and is required for neural formation. We also find that xNorrin not only activates Wnt signaling, but also inhibits BMP/Nodal-related signaling. In humans, mutations in Norrin cause Norrie disease. Using Norrin mutants identified in patients with Norrie disease, we find that some Norrin mutants fail to inhibit BMP/Nodal-related signaling (specifically, TGF-β) but retain the ability to activate the Wnt pathway, suggesting that loss of TGF-β inhibition may contribute to Norrie disease development.
Introduction
Dorsal–ventral axis specification is one of the earliest patterning events in the embryo. In vertebrates, early dorsal ectoderm gives rise to the neural plate, which in turn develops into the central nervous system (CNS). Previous studies have found that dorsal axis formation in amphibians is initiated during cortical rotation after fertilization. Current evidence strongly suggests that the canonical Wnt signaling pathway, operating at blastula stages, plays a critical role in dorsal specification [1]. For example, Wnt signaling was discovered to induce secondary axes when ectopically activated in the ventral cells of early embryos. Loss-of-function studies indicate that the Wnt/β-catenin signaling pathway is also essential for dorsal specification [2]–[4]. More recently, Heasman and colleagues provided strong evidence that vegetally localized maternal Wnt11 cooperates with Wnt5A to activate the canonical Wnt pathway and is required for dorsal axis formation [5]–[7]. However, despite extensive studies on dorsal specification, some observations cannot be fully explained. For example, although the cortex is rotated only 30° toward the dorsal side, activated nuclear β-catenin is observed in all dorsal cells, including dorsal cells near the animal pole [8]. Previous studies suggested that Wnt pathway components may be transferred beyond 30° to the dorsal animal region [8],[9]. However, it remains unknown whether such movements can fully account for Wnt activation in dorsal animal cells, and it is also unclear how these movements precisely regulate the earliest steps of neuroectoderm formation in the blastula.
In addition to canonical Wnt signaling, the BMP pathway has also been implicated in neuroectoderm specification and patterning. During early gastrulation, Noggin, Chordin, and Follistatin expressed in the Spemann organizer bind to BMPs in the extracellular space and antagonize their epidermal-promoting effects [10]–[12]. These results support a “default model” for neural induction in which ectoderm cells are predisposed to become neurons if they receive no BMP signals [13],[14]. Genetic screens in Drosophila and zebrafish have yielded mutants that affect dorsal–ventral patterning. Interestingly, most of these mutants show defects in the BMP signaling pathway, indicating that BMP signaling has a conserved role in dorsal–ventral patterning [1].
On the other hand, dorsal animal cells in the Xenopus blastula can develop into dorsal and neural tissues cell-autonomously when cultured in a saline solution [15],[16]. De Robertis and colleagues found that a subset of the dorsal ectoderm cells in the late blastula expressed Chordin, Noggin, Siamois, and Xnr3 prior to Spemann organizer functioning and referred to these cells as the blastula Chordin- and Noggin-expressing center (BCNE center) [16]. Early Chordin and Noggin transcription is activated by maternal β-catenin, but the precise mechanism underlying this activation remains to be uncovered [16].
We report here that maternal Xenopus Norrin (xNorrin) is required for β-catenin activation in dorsal animal cells in the Xenopus blastula and in early neuroectoderm development. Norrin is a non-Wnt ligand that was previously shown to activate β-catenin through LRP5 and Frizzled4 or TSPAN12 during retina vascular development [17]–[19]. In humans, mutations in Norrin cause Norrie disease [20]. We further show that xNorrin can directly antagonize TGF-β/BMP signaling. Our results not only identify an endogenous maternal factor required for early neuroectoderm specification, but may also add TGF-β inhibition to the increasingly complex regulatory activities of Norrin in retinal vascular development [17],[19].
Results
xNorrin Promotes Dorsal and Anterior Neural Formation
We sought to identify additional secreted molecules that are involved in neuroectoderm formation. Neuroectoderm is derived from dorsal animal regions in early Xenopus embryos. Therefore, we used early neural markers to search for molecules that may be responsible for early neural specification. In Xenopus, ultraviolet (UV) irradiation of the vegetal pole in embryos causes severe dorsal axis development defects [21] (Figure 1A–1F) in otherwise normal embryos (Figure 1A). We selected a set of candidate genes that were previously shown to activate Wnt/β-catenin pathways and tested their ability to reorganize the dorsal axis or anterior neural tissues by injecting them individually into UV-irradiated embryos. Among the maternally expressed Wnt genes (Wnt5a, Wnt8b, and Wnt11) tested, Wnt11 and Wnt8b were able to induce some dorsal axis structures (Figure 1C and data not shown) [22],[23]. However, none of these molecules triggered the formation of anterior neural tissues (data not shown).
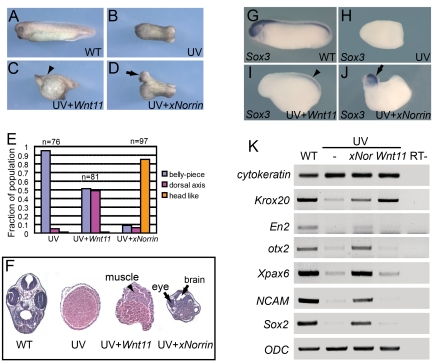
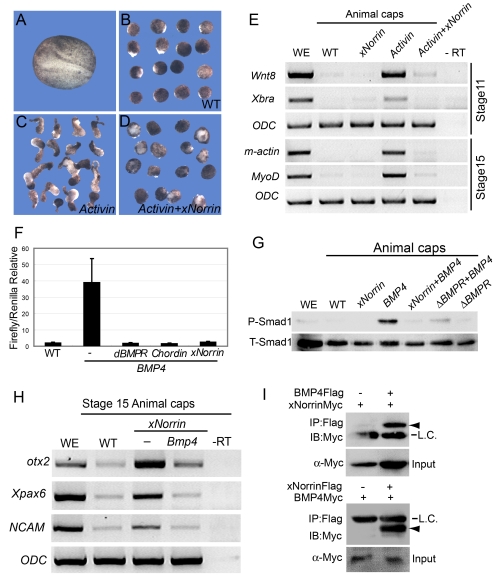
Figure 1. xNorrin induces anterior CNS formation in ventralized embryos.
(A–D) xNorrin mRNA induces anterior neural tissues, while Wnt11 mRNA restores only a partial dorsal axis (without anterior structures) in UV-irradiated embryos. (A) A wild-type embryo (stage 33); (B) an embryo UV-irradiated (50 µJ) at the vegetal pole; (C) a UV-irradiated embryo injected with Wnt11 mRNA (500 pg) into one cell at the four-cell stage (arrowhead: partial dorsal axis); (D) a UV-irradiated embryo injected with xNorrin mRNA (500 pg) as in (C) (arrow: head). (E) Summary of (A–D). Fraction of the population within each group is indicated. (F) Histological analysis of stage 40 embryos. Arrowhead: muscle; arrows: brain and eye. (G–J) Whole-mount in situ hybridizations to Sox3. (G) A wild-type (WT) embryo (100%, n = 65); (H) a UV-treated embryo (4% Sox3 positive, n = 70); (I) a UV+Wnt11 (500 pg) rescued embryo (45% Sox3 positive, n = 77); (J) a UV+xNorrin (500 pg) rescued embryo (83% Sox3 positive, n = 69). All embryos are shown with the anterior pole to the left. Arrowhead: posterior neural structure; arrow: anterior neural structure. (K) Neural marker expression detected by RT-PCR. xNorrin induced expression of anterior neural and pan-neural markers (En2, otx2, Xpax6, NCAM, and Sox2) in UV-irradiated embryos. Wnt11 induced only the hindbrain marker Krox20 in UV-irradiated embryos.
We also cloned X. laevis xNorrin (GenBank accession number: EU528658) from unfertilized eggs. This gene encodes a homolog of human Norrin that can activate β-catenin [18]. The injection of xNorrin mRNA into UV-ventralized embryos produced a well-defined head-like structure (Figure 1), including cement gland, eye, and brain-like tissues (85%, n = 97). In contrast, only 5% of UV-ventralized embryos (n = 76) developed any dorsal axial structures (such as notochord and neural tube), and 49% of Wnt11-injected, UV-irradiated embryos (n = 81) developed dorsal ridges without notochords and neural tubes (Figure 1C and 1F). Gene expression analysis showed that xNorrin induced not only pan-neural markers, such as Sox3, Sox2, and NCAM, but also anterior neural markers, such as otx2, Xpax6, and En2, in stage 20 embryos (Figure 1J and 1K). In contrast, Wnt11 induced the expression of the rhombomere marker Krox20 (Figure 1K) and only weakly induced the expression of the pan-neural marker Sox3 (Figure 1I). These results indicate that xNorrin can promote anterior neural tissue formation in an otherwise non-neural background. The neural formation triggered by xNorrin expression in UV-ventralized embryos may perhaps be attributable to early neuroectoderm induction by the injected xNorrin.
We reasoned that for maternal xNorrin to act in specifying the neuroectoderm in a cell-autonomous fashion, it should meet two criteria. First, it should be expressed in the dorsal ectoderm of the blastula [16],[24]. Second, it should be able to activate canonical Wnt signaling [16],[25]. Indeed, we confirmed that xNorrin mRNA is expressed in the animal pole of stage 6 oocytes and early cleavage embryos (Figures 2A, 2C, and S2A). In addition, much more xNorrin mRNA was detected in dorsal blastomeres than in ventral blastomeres in 16-cell-stage embryos (Figure 2B).
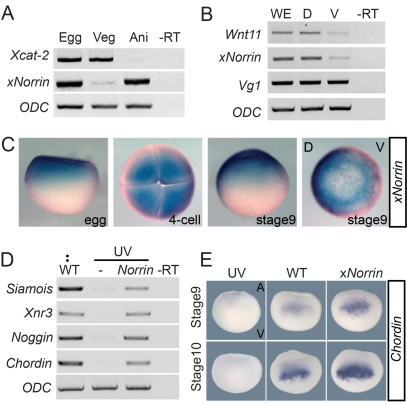
Figure 2. Maternal xNorrin activates the canonical Wnt signaling pathway.
(A) RT-PCR analysis of mRNAs from equatorially bisected oocytes (Egg). xNorrin mRNA is present in the animal half (Ani) of fully grown oocytes, while Xcat-2 mRNA is present in the vegetal half (Veg). –RT: no reverse transcription. (B) Both xNorrin mRNA and Wnt11 mRNA are enriched in the dorsal cells of 16-cell embryos. Embryos are evenly bisected into dorsal and ventral halves. D, dorsal half; V, ventral half; WE, whole embryo. (C) Spatial and temporal expression patterns of xNorrin mRNA from fertilized eggs to the late blastula stage (stage 9) revealed by whole-mount in situ hybridization. (D) xNorrin mRNA (500 pg) injection into the animal region of UV-ventralized embryos at one-cell stage reactivates the expression of Siamois, Chordin, Noggin, and Xnr3 at the late blastula stage. (E) xNorrin injection enhanced Chordin expression (detected by in situ hybridization) at stage 9 (81%, n = 36) and stage 10 (80%, n = 35) compared to wild-type embryos. UV, UV-irradiated embryos; WT, wild-type embryos; xNorrin, wild-type embryos injected with xNorrin (500 pg) into the dorsal-animal region at the four- to eight-cell stage.
Norrin proteins are highly conserved among vertebrates (Figure S1A and S1B). xNorrin, like its mouse ortholog, can activate Wnt-responsive reporters (data not shown) and induce LRP6 phosphorylation in HEK293T cells (Figure S2C). Next, we examined whether xNorrin could activate early Wnt target gene expression in vivo. The injection of xNorrin into UV-irradiated embryos robustly induced the expression of the known Wnt targets Chordin, Noggin, Xnr3, and Siamois (Figure 2D). Further, animal caps injected with xNorrin plus Xenopus Frizzled4 plus human Lrp5 mRNA (NFL) also expressed Xnr3 and Siamois, but not Xbra (Figure S2B). We noted that xNorrin injection alone did not induce Xnr3 or Siamois expression in animal caps (Figure S2B), suggesting that some components of the xNorrin pathway may not be expressed in the caps (see Discussion). However, the injection of xNorrin into dorsal animal cells enhanced Chordin expression during the late blastula and early gastrula stages (Figure 2E). These results suggest that maternal xNorrin may promote neuroectoderm specification by activating canonical Wnt signaling.
xNorrin Is Required for Neuroectoderm Specification
To address whether maternal xNorrin is required for neuroectoderm specification and hence anterior CNS formation at a later stage, we used an xNorrin antisense morpholino (MO) oligonucleotide (xNor-MO) to inhibit xNorrin translation (Figure 3A). The inhibition of xNorrin mRNA translation by xNor-MO was both specific and dose-dependent (Figure 3B). We injected xNor-MO into the animal region of the two dorsal blastomeres in the four- to eight-cell embryo stage to suppress endogenous xNorrin translation. The majority of xNor-MO-injected embryos (61%, n = 64) displayed anterior head truncations, and another 15% of the embryos lacked morphological eye structures and other anterior neural structures at tadpole stages (Figure 3D). The injection of a mismatched MO (misMO), xNor-misMO, that failed to block xNorrin-Myc translation (data not shown) produced no discernible phenotype compared to uninjected controls (Figure 3C and 3E). The specificity of xNor-MO was further tested by the co-injection of a wild-type xNorrin mRNA lacking the xNor-MO target sequence. The injection of 25 pg of xNorrin mRNA significantly rescued the anterior neural development defects in xNor-MO-injected embryos (Figure 3F) (n = 81, 77% rescued). Furthermore, the injection of xNor-MO into one dorsal animal cell in eight-cell-stage embryos, while leaving the other side intact, resulted in severe defects in eye development at later stages (compare Figure 3G and 3H). Because xNorrin is also expressed zygotically at later stages, we designed a splicing MO (spMO) to specifically block its splicing (Figure S3A and S3B). While xNor-MO inhibited anterior development, xNor-spMO had almost no effect on axis development (Figure S3C–S3G). We further confirmed that xNor-MO preferentially inhibited XBF-1 (an anterior neural marker [26]) expression in the injected side, while xNor-spMO had a much weaker effect (Figure S3H). Neither MO had a significant role in regulating the expression of HoxB9 (a posterior marker [27]) (Figure S3H). These results suggest that maternal xNorrin signaling is required for anterior CNS formation.
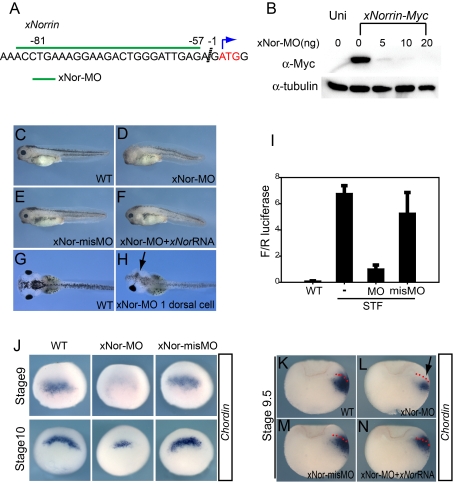
Figure 3. Maternal xNorrin is required for dorsal ectoderm specification.
(A) The xNor-MO target sequence (green line) in xNorrin mRNA. (B) xNor-MO dose-dependently suppresses xNorrin-Myc (1.5 ng) mRNA translation in Xenopus embryos. xNorrin-Myc was detected using an anti-c-Myc monoclonal antibody. Uni, no xNorrin-Myc injected. (C–F) xNorrin is required for head formation. (C) A wild-type (WT) stage 35 tadpole. (D) xNor-MO (20 ng) caused anterior truncation (61%, n = 64) when injected into the animal regions of two dorsal cells in four- to eight-cell-stage embryos. (E) xNor-misMO-injected embryos are generally normal (88%, n = 66). (F) The anterior defects caused by NorMO were rescued by xNorrin (25 pg) mRNA (77%, n = 81). (G) Dorsal view of a wild-type tadpole at stage 45. (H) Anterior defects on only one side (arrow) were generated by injecting xNor-MO (10 ng) into one dorsal cell at the four- to eight-cell stage (63% of injected embryos showed defects in the injected side, n = 30). The other side shows normal morphology. The anterior end is to the left. (I) xNor-MO inhibits Wnt signaling in dorsal animal cells. xNor-MO and SuperTopFlash (STF) reporter plasmids were co-injected into the dorsal animal cells of eight-cell embryos. F/R luciferase: ratio of firefly luciferase reading to renilla luciferase reading. (J) Whole-mount in situ hybridization shows that Chordin expression is reduced at stage 9 (53% of injected embryos, n = 80) and stage 10 (61% of injected embryos, n = 79) in xNor-MO-injected embryos, compared to xNor-misMO-injected embryos or uninjected embryos. (K–N) Whole-mount in situ hybridization for Chordin in bisected xNor-MO-injected embryos (stage 9.5) showing that xNor-MO inhibits Chordin expression in neuroectoderm precursors (arrow) (reduction in 66% of injected embryos, n = 104) (L) compared to wild-type embryos (K) and embryos with xNor-misMO injected into dorsal animal cells at the eight- to 16-cell stage (reduction in 13% of injected embryos, n = 78) (M). Note that xNorrin mRNA (100 pg) rescues Chordin expression in the dorsal ectoderm (80% of co-injected embryos showed expression comparable to wild-type embryos, n = 50) (N). Embryos are oriented such that their dorsal side is on the right. Dotted lines indicate the boundaries between the deep mesoderm and the superficial ectoderm.
The loss of anterior head development may be an indirect effect due to a lack of early neuroectoderm specification. Thus, we tried to address whether β-catenin activation in the ectoderm, which is indispensable for full dorsal axis formation [3], depends on xNorrin activity. First, we used a SuperTopFlash Wnt reporter, which can be activated by injection into the dorsal animal blastomeres of eight-cell-stage embryos [5] (Figure 3I). The co-injection of xNor-MO with the reporter plasmid largely blocked reporter activity compared to co-injection with xNor-misMO (Figure 3I). In a separate assay, we examined whether maternal xNorrin was required for the expression of Chordin, Noggin, Xnr3, and Siamois in dorsal animal cells, which is one of the earliest indications of β-catenin activation [16]. We found that xNor-MO reduced the expression of these genes in late blastula embryos (Figures 3J, S4A and S4B) but did not interfere with the expression of gsc or Xnr1 (Figure S4A and S4B) at the early gastrula stage. The reductions in the expression of these genes in xNor-MO embryos can be rescued by the co-expression of xNorrin (Figure S4B). In late blastula xNor-MO embryos (stage 9.5), the reduction of Chordin expression was mostly restricted to the ectoderm, while deep dorsal mesoderm cells retained weak expression (Figure 3L). The ectoderm expression of Chordin in the later blastula was fully restored by the co-injection of wild-type xNorrin mRNA lacking the MO target sequence (Figure 3N). In the blastula ectoderm, Chordin expression is controlled by maternal β-catenin [16],[28]. Thus, the control of early Chordin expression by xNorrin should partially reflect how xNorrin functions in neuroectoderm precursors. Together, these results indicate that, besides vegetally localized Wnt11 activity, maternal xNorrin is required to activate the canonical Wnt pathway in the dorsal ectoderm and is essential for the proper expression of early zygotic neural inducers before gastrulation.
xNorrin-Activated Wnt Signaling Fails to Dorsalize Ventral Mesoderm
Mouse Norrin is a secreted protein that is tightly associated with the extracellular matrix [29]. However, we found that xNorrin was secreted into culture medium when expressed in HEK293 cells and Xenopus embryo explants (data not shown). The secretion of xNorrin in the culture cells and its potent activity in early embryos prompted us to speculate that other mechanisms may be required to restrict xNorrin activity in early embryos.
We first tested whether xNorrin was active when expressed ectopically in embryos. Previous studies indicated that the ectopic activation of the canonical Wnt pathway in the ventral side of early embryos is sufficient for secondary dorsal axis formation [30]–[32]. In addition, the co-expression of NFL was shown to activate canonical Wnt signaling in tissue culture cells and in animal cap explants (Figure S2B and S2C). Thus, we examined whether NFL could mimic canonical Wnt proteins and induce secondary axes in early embryos. Surprisingly, when injected into the ventral vegetal cells of early embryos, NFL failed to generate any complete secondary axes (Figure 4C), while Wnt8 was able to generate secondary axes, as shown previously [31],[32] (Figure 4B). However, NFL was able to weakly induce partial secondary axes in which the neural marker Sox3 was detected (Figure 4F). NFL-injected embryos had neural tubes but not notochords in their secondary axes (Figure 4I, 4L, and 4L'), while Wnt8-injected embryos had complete secondary axes containing both neural tubes and notochords (Figure 4E, H, K, and K') [31],[32].
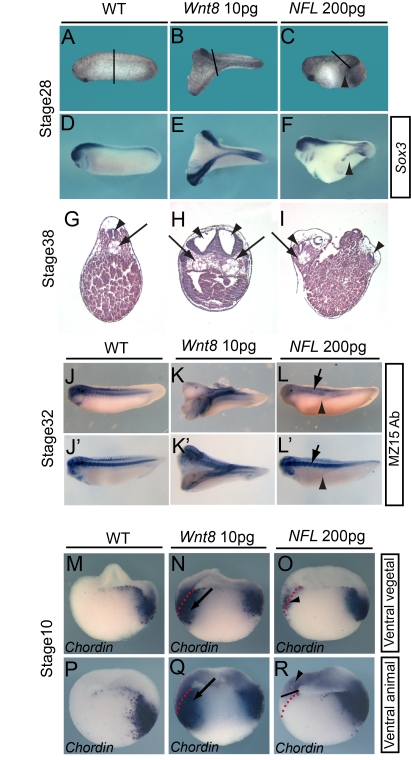
Figure 4. xNorrin activity is restricted to the ectoderm.
(A–F) Injection of NFL into ventral vegetal cells at the eight-cell stage induced partial secondary axes. Wild-type (WT) embryos (stage 28) (A and D). Injection of Wnt8 mRNA (10 pg) into ventral vegetal cells in eight-cell embryos induces complete secondary axis formation (stage 28) (69% of injected embryos showed secondary axes, n = 85) (B and E). NFL mRNA (600 pg total; 200 pg each) co-injection of ventral vegetal cells in eight-cell embryos induces partial secondary axis formation (stage 28) (45% of co-injected embryos showed secondary axes, n = 105) (C and F). Whole-mount in situ hybridization for Sox3 in stage 28 embryos (D–F). Note that NFL induces Sox3 expression in the partial secondary axis (arrowhead in [F]). The black lines in (A–C) indicate the section planes in (G–I), respectively. (G–I) Histological sections of embryos at stage 30. A wild-type embryo (G). Wnt8 mRNA injection induced both secondary neural tube and notochord (H). NFL co-injection induced secondary neural tube but not notochord formation (I). Arrowhead: neural tube; arrow: notochord. (J–L) Immunostaining of notochords with the monoclonal antibody MZ15. Wild-type embryo with single notochord (all examined embryos) (J and J'). An embryo injected ventrally with Wnt8 mRNA (10 pg) showed two notochords (induced secondary notochord and the primary notochord) (all examined embryos with secondary axes) (K and K'). An embryo injected ventrally with NFL (200 pg each) showed only the primary notochord (arrows), with notochord tissue absent in the partial secondary axis (arrowheads) (all examined embryos with partial secondary axes) (L and L'). Embryos are at stage 30. (M–R) NFL and Wnt8 induced Chordin expression in different domains in the ventral cells. Whole-mount in situ hybridization was used to evaluate Chordin expression in stage 10 embryos. Chordin expression in wild-type early gastrula (M and P). Wnt8 mRNA (10 pg) injection into ventral vegetal cells induced Chordin expression both in the superficial layer and in the deep layer of the marginal zone (87.5% of injected embryos showed the expression in both layers, n = 24) (N). Injection of NFL mRNAs (200 pg each) into the same domain induced Chordin expression mainly in the superficial layer (76% of injected embryos showed the expression, n = 30) (O). Injection of Wnt8 into the ventral-animal cells of eight-cell embryos induced Chordin expression both in the ectoderm and in the mesoderm (84% of injected embryos showed the expression in both layers, n = 25) (Q). Injection of NFL mRNAs (200 pg each) into the same domain of eight-cell embryos induced Chordin expression only in the ectoderm (72% of injected embryos showed the expression, n = 32) (R). Arrowheads: superficial layer (O) or ventral ectoderm (R); arrows: deep layers on the ventral side. All embryos in (M–R) are shown with their dorsal sides to the right. Dotted lines delineate the superficial layer and deeper layer on the ventral side.
The failure of NFL-injected embryos to form complete secondary axes was not due to a lack of activation of Wnt signaling by NFL, because Chordin, Siamois, and Xnr3 expression could be detected in the ventral side of the early gastrula (stage 10) (Figure S4C and S4D). However, Chordin expression was mostly induced in the superficial layer and not in the deep ventral mesoderm (Figure 4O). The much lower expression of Chordin in the deep layer was considered unlikely to be a staining artifact because strong Chordin signal was readily detected in the dorsal mesoderm (Figure 4O). Embryos injected ventrally with Wnt8 strongly induced Chordin expression in both germ layers, as expected (Figure 4N and 4Q). Similarly, the injection of NFL into ventral animal cells induced Chordin transcription only in the ventral ectoderm and not in the mesoderm (Figure 4R).
These results suggest that an intrinsic mechanism may exist to restrict endogenous xNorrin activity to the prospective neuroectoderm. Alternatively, injected NFL may alter the cell fate of endomesoderm, making it incompetent to form dorsal endomesoderm, even in the presence of canonical Wnt signaling.
xNorrin Inhibits Activin/Nodal-Related Induced Mesoderm Formation
Because NFL injection failed to activate Wnt target genes in the endomesoderm (Figure 4O and 4R), we initially proposed that an xNorrin-specific inhibitor might exist in the endomesoderm. However, after extensive investigation, we were not able to identify any molecule that could fulfill the proposed criteria for the inhibitor, i.e., that it should be expressed specifically in the endomesoderm and exert its antagonizing activity on xNorrin but not Wnt8. We thus turned to an alternative possibility, that NFL may influence the fate of endomesoderm precursor cells, making the germ layer incapable of conversion into dorsal endomesoderm. Previously, TGF-β family members, such as Xnr1, -2, -4, -5, and -6 and derriere were shown to be essential for mesoderm induction in Xenopus embryos [33]. Zygotic transcription of Xnr genes is activated by maternal transcription factor VegT and β-catenin. The Nodal-related molecules form a dorsal–ventral gradient that induces dose-dependent endomesoderm formation. Higher concentration of Nodal-related molecules results in dorsal specification [34],[35]. In a mesoderm induction assay, we used Activin, in lieu of Nodal-related molecules, to induce strong axial mesoderm and convergent extension in animal cap cells (Figure 5A–5C) [33],[36]. When co-expressed in animal cap cells, xNorrin completely blocked the Activin-induced elongation of animal cap explants (Figure 5D; compare to Figure 5B and 5C). The inhibition of mesoderm formation was confirmed by the lack of expression of the mesoderm markers Xbra, Xwnt8, MyoD, and m-actin in the co-expressing explants (Figure 5E). In whole embryos, xNorrin injection into the vegetal pole also blocked Xbra expression (Figure S5A–S5D). These results suggest that xNorrin may negatively regulate mesoderm induction in vivo. Next, we tested whether Xnr1 and xNorrin could be directly associated extracellularly. We combined and incubated conditioned medium from Xnr1-transfected HEK293 cells and from xNorrin-transfected cells and used the medium for immunoprecipitation. Indeed, we detected an association between Xnr1 and xNorrin (Figure S6B), suggesting that maternal xNorrin may restrict Nodal-related activity from extending into the animal pole.
Figure 5. Reciprocal inhibition between xNorrin and TGF-β.
(A–E) xNorrin inhibits Activin-B-mRNA-induced mesoderm formation. A wild-type (WT) embryo at a neurula stage (A). Wild-type animal caps with elongation (5% of the caps showed elongation, n = 60) (B). Elongated animal caps induced by Activin-B mRNA (25 pg) injection (82% of the injected caps showed elongation, n = 55) (C). Animal cap elongation was blocked in animal caps injected with Activin-B (25 pg) and xNorrin (200 pg) mRNAs (10% of the co-injected caps showed elongation, n = 58) (D). The Activin-B-mRNA-induced expression of mesoderm markers (Wnt8, Xbra, m-actin, and MyoD) was inhibited by xNorrin (E). RNAs were injected into the animal pole of one-cell embryos, and animal caps were cut around stage 8 and cultured in 1× MMR until the sibling embryos reached neurula stage. (F and G) xNorrin inhibits BMP4 signaling. xNorrin mRNA (500 pg), like ΔBMPR mRNA (200 pg) and Chordin mRNA (100 pg), inhibited BRE-Luc reporter activity in Xenopus embryos (F). xNorrin mRNA (500 pg) inhibited BMP4-induced Smad1 phosphorylation in animal caps (G). P-Smad1, phosphorylated Smad1; T-Smad1, total Smad1; WE, whole embryo. (H) BMP4 inhibited xNorrin-induced otx2, Xpax6, and NCAM RNA expression in animal caps of stage 15 embryos. –RT, no reverse transcription; WE, whole embryo; WT, wild-type animal caps. (I) xNorrin interacts with BMP4. BMP4-Flag and xNorrin-Myc mRNAs or xNorrin-Flag and BMP4-Myc mRNAs were injected into adjacent cells of four-cell embryos. FLAG-tagged proteins were immunoprecipitated (IP) from later gastrula embryos with a FLAG antibody. The proteins were PAGE separated and immunoblotted (IB) with an anti-c-Myc antibody. Arrowheads indicate xNorrin-Myc (top) or BMP4-Myc (bottom). L.C., IgG light chain.
Reciprocal Inhibition between xNorrin and BMP4
Because xNorrin can inhibit Activin/Nodal-related activity, we hypothesized that it may also antagonize other members of the TGF-β superfamily. Indeed, we found that xNorrin also strongly inhibited the activity of a BMP4 reporter (BRE-Luc) (Figure 5F). As expected, xNorrin also inhibited Smad1 phosphorylation induced by BMP4 (Figure 5G). One possible mechanism for inhibition between proteins is through direct binding. We examined this possibility between BMP4 and xNorrin. To this end, we injected differently tagged BMP4 and xNorrin mRNAs into adjacent blastomeres in advanced four-cell-stage embryos to allow secretion of the respective proteins into the extracellular space. At late gastrula, protein extract was immunoprecipitated with one tag antibody and blotted with the other tag antibody. Results showed that BMP4 was indeed associated with xNorrin extracellularly. Thus, the inhibition by xNorrin is likely through direct binding to BMP4 (Figure 5I).
The direct interactions between xNorrin and BMP4 led us to investigate whether xNorrin activity was regulated by BMP4. We showed that xNorrin induced neural marker expression in animal caps (Figure 5H). In an animal cap assay, BMP4 significantly inhibited the otx2, Xpax6, and NCAM expression induced by xNorrin (Figure 5H). Thus, reciprocal inhibition between xNorrin and BMP4 may also be implicated in dorsal–ventral ectoderm development.
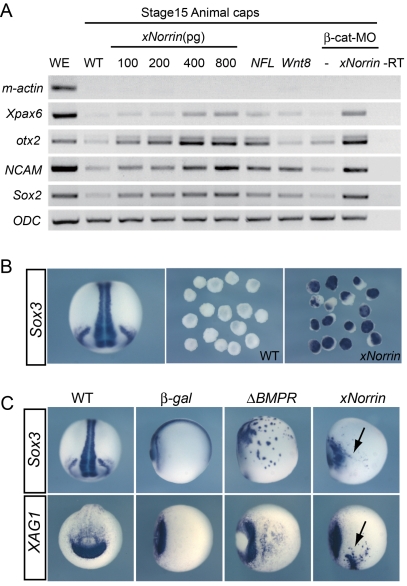
Previous studies indicated that the dorsally expressed BMP4 inhibitors Chordin, Noggin, and Follistatin could induce neural formation through direct binding [10]–[12]. Because xNorrin can also inhibit BMP4, we investigated xNorrin neural induction activity. Indeed, we found that xNorrin alone can induce the expression of neural-specific genes in animal cap cells in a dose-dependent manner (Figures 6A and S5A). The neural promoting activity of xNorrin in ectodermal cells was confirmed by the Sox3 (a neural marker) expression in xNorrin-injected animal caps (Figure 6B). Further, xNorrin, like the truncated BMP receptor ΔBMPR, can induce ectopic Sox3 and XAG1 (an anterior marker) expression when injected into one ventral blastomere of 32-cell embryos (Figure 6C). More importantly, neural induction was observed when a β-catenin-specific MO was co-injected, indicating that canonical Wnt pathway activation is not required for neural formation in this setting (Figure 6A, compare the two β-catenin-MO-injected lanes). Furthermore, we did not observe activation of Xnr3 or Siamois in xNorrin-injected animal caps, confirming the lack of canonical Wnt activation (Figure S2B). We conclude that xNorrin and BMP4 are reciprocally inhibited and that xNorrin may promote neural development independent of Wnt signaling activation.
Figure 6. xNorrin induces neural formation independent of β-catenin signaling.
(A) xNorrin dose-dependently induced neural marker (Xpax6, otx2, NCAM, and Sox2) expression. The induction is independent of mesoderm formation (m-actin: muscle actin) and could occur in the presence of a β-catenin-MO. RNA and MO were injected at the one-cell stage, and the caps were dissected around stage 8 and cultured until they reached stage 15. –RT, no reverse transcription; WE, whole embryo; WT, wild-type. (B) xNorrin induced Sox3 expression in animal caps (89% of xNorrin injected caps showed the expression, n = 45). xNorrin mRNA (300 pg) was injected into the animal pole of two-cell embryos. Sox3 expression in stage 15 animal caps was analyzed with in situ hybridization. WT, wild-type animal caps; xNorrin, xNorrin-mRNA-injected caps. (C) xNorrin induced the expression of ectopic neural and anterior markers in whole embryos. xNorrin mRNA (300 pg) was injected into the ventral animal tier cells of 32-cell embryos. Expression of Sox3 (by ΔBMPR: 80%, n = 30; by xNorrin: 73%, n = 30) and XAG1 (by ΔBMPR: 69%, n = 35; by xNorrin: 79%, n = 34) were induced at ectopic sites. β-gal, β-gal-mRNA-injected embryos; ΔBMPR: ΔBMPR-mRNA-injected embryos; WT, wild-type uninjected embryos; xNorrin: xNorrin-mRNA-injected embryos. Arrows: ectopically induced Sox3 or XAG1. Sox3 expression in wild type is shown in a dorsal view, while XAG1 expression in wild type is shown in an anterior view. All other embryos are shown in a ventral view, except the embryo in the β-gal/Sox3 panel, which is in a lateral view.
Loss of TGF-β Inhibition in a Subset of Norrin Mutants
Norrin mutations are responsible for both X-linked familial exudative vitreoretinopathy (FEVR) and Norrie disease (Online Mendelian Inheritance of Man MIM#310620) in humans [37],[38]. The finding that Norrin can inhibit BMP/TGF-β activity prompted us to test whether this regulation is involved in the disease development. We noticed that some previously identified Norrin mutants isolated from human patients did not significantly affect Wnt pathway activation [18],[39]. We hypothesized that these human Norrin mutants might instead be compromised in their ability to antagonize BMP/TGF-β activities in vivo.
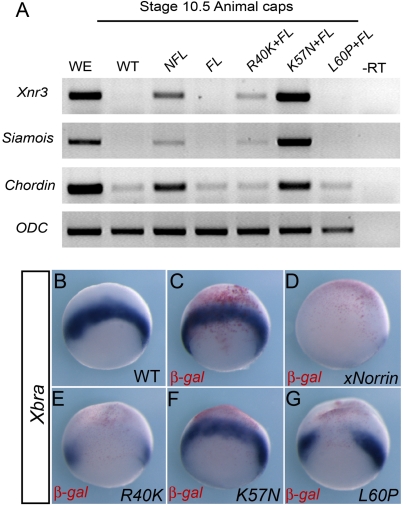
The ectopic expression of xNorrin or mouse Norrin in the vegetal cells of whole embryos potently inhibited the expression of the mesoderm-specific marker Xbra, which is dependent on Nodal-related activity in vivo (Figure 7D compared to 7B and 7C; Figure S5B–S5D). We thus used this assay to examine the activity of various xNorrin mutants on Xbra expression. We constructed three xNorrin point mutants (R40K, L60P, and K57N) based on mutations identified from human patients. Compared to wild-type xNorrin, the xNorrin R40K and L60P mutants showed decreased Xnr3, Siamois, and Chordin expression when co-expressed with Lrp5 and Frizzled4 in animal caps, while xNorrin K57N strongly activated these Wnt target genes (Figure 7A). This is consistent with previous findings using cell culture assays [18],[39]. In a whole-embryo assay, the xNorrin R40K mutant largely inhibited Xbra expression, while the xNorrin L60P mutant showed only slight inhibitory activity (Figure 7E and 7G compared to Figure 7B and 7D). In an extreme case, the xNorrin K57N mutant completely failed to suppress Xbra expression (Figure 7F). A lack of BMP4 binding ability might explain this loss of TGF-β inhibition. However, only a minor reduction in BMP4 binding was observed for the xNorrin K57N mutant compared to wild-type xNorrin. The xNorrin R40K mutant also did not show significantly reduced binding to BMP4 (Figure S6).
Figure 7. TGF-β inhibition is implicated in Norrie disease.
(A) xNorrin point mutants showed various levels of Wnt activation activity. Wild-type (WT) or mutant xNorrin and Fizzled4 and Lrp5 (FL) were injected into animal poles. The expression of Xnr3, Saimois, and Chordin in animal caps was analyzed by RT-PCR. xNorrin R40K and xNorrin L60P showed slightly decreased and no Wnt activation, respectively. xNorrin K57N moderately increased Wnt activation. –RT, no reverse transcription; WE, whole embryo. (B–G) xNorrin point mutants showed various levels of mesoderm inhibition activity. Individual xNorrin point mutant mRNAs and β-gal mRNA were co-injected into the vegetal halves of two-cell-stage embryos. The expression of Xbra was analyzed at stage 10.5 by whole-mount in situ hybridization. While wild-type xNorrin inhibited Xbra expression (83% of the injected embryos showed very low or no Xbra expression, n = 35) (D), xNorrin K57N failed to inhibit Xbra expression (13% of the injected embryos showed reduced Xbra expression, n = 39) (F). xNorrin R40K (61% of the injected embryos, n = 33) and L60P (41% of the injected embryos, n = 32) also showed decreased Xbra expression (E and G). Uninjected (B) and β-gal-injected embryos (C). β-gal is stained in red. The embryos are in vegetal views, but slightly tilted toward marginal zones to show Xbra signal.
Next, we examined whether a lack of TGF-β inhibition by xNorrin compromised its neural induction function in a loss-of-function background. Because we could not directly study the K57N mutation through a knock-in experiment in Xenopus, we tested K57N mutant function in xNor-MO-injected embryos. In contrast to wild-type xNorrin, which was able to significantly rescue the anterior defects of the morphants (including eyes in 23% of the embryos) (Figure S7A–S7D), the K57N mutant was far less efficient, often producing phenotypes similar to those of xNor-MO-injected embryos (Figure S7E). We did not observe normal eye formation in any K57N-mutant-injected embryos (Figure S7F), suggesting that TGF-β inhibition is crucial for the full activity of xNorrin.
Together, these results indicate that Wnt activation and TGF-β inhibition activities are encoded by distinct domains in Norrin proteins and that the loss of TGF-β inhibition in Norrin mutants may be a novel mechanism implicated in the development of Norrie disease in humans (see Discussion).
Discussion
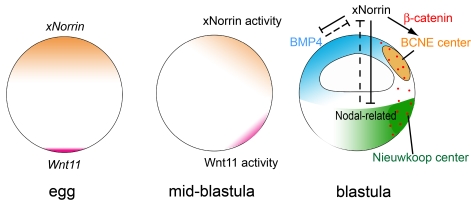
The present work addresses the molecular nature and mechanism of a maternal signal that specifies the early neuroectoderm. Our findings reveal an essential coordination of canonical Wnt signaling activation and extracellular BMP/TGF-β inhibition by maternal xNorrin and further highlight the integration of the two major signaling pathways during early neuroectoderm specification (Figure 8). Our results also point to the de-repression of BMP/TGF-β as a new molecular mechanism in Norrie disease.
Figure 8. A model of dorsal specification in Xenopus.
During oogenesis, maternal xNorrin and Wnt11 are localized to the animal and vegetal poles, respectively. After fertilization, both mRNAs are enriched at the dorsal side, leading to two localized activity domains: BCNE center and Nieuwkoop center. xNorrin in the dorsal animal cells helps to specify neuroectoderm fate by activating a Wnt/β-catenin signaling domain, the BCNE center, and also participates in antagonizing the Nodal-related signal from the vegetal half and the BMP signal from the ventral side. Wnt11 in the dorsal vegetal domain is required for β-catenin activation in all dorsal regions, including in the BCNE center. Yellow: xNorrin and BCNE center. Purple: Wnt11. Red dots indicate stabilized β-catenin. Green: Nieuwkoop center.
Wnt Signaling Induction by xNorrin and Early Neuroectoderm Specification
Canonical Wnt signaling activation in early embryos is essential for the initial dorsal specification [2],[25]. Heasman and colleagues previously provided strong evidence that Wnt11 and Wnt5A are endogenous ligands required for β-catenin signaling in all dorsal cells, including dorsal animal cells [5]–[7]. These important findings seem to indicate that any additional Wnt agonists specifically required for β-catenin activation in dorsal animal cells would be redundant. However, previous studies suggested that in Xenopus, an animal-to-vegetal signal was implicated in promoting neural fate before gastrulation, and dorsal animal cells from the blastula are able to develop into neural tissues cell-autonomously in culture [18],[28],[40]. Noggin and Chordin were discovered to act as neural inducers prior to gastrulation. We demonstrated a lack of β-catenin activation in xNor-MO-injected embryos, which strongly indicated that Wnt11 activity was not sufficient to compensate for the loss of xNorrin activity in vivo (Figure 3I). The severe neural tissue formation defect in xNor-MO embryos is likely due to a failure in the specification of the early neuroectoderm. The significant down-regulation of the dorsal marker Chordin supports this hypothesis (Figure 3J–3N).
If both Wnt11 and xNorrin are involved in dorsal specification, then why does maternal xNorrin, which is likely retained in Wnt11-depleted embryos, fail to compensate for the loss of Wnt11 RNA in generating anterior dorsal formation [5]? It is possible that additional molecules are required for xNorrin function in dorsal animal cells. For example, cortical rotation may play a role in the activation of xNorrin signaling. In fact, we found that the dorsal enrichment of xNorrin was lost in UV-irradiated embryos (Figure 2B and 2C and data not shown). One possibility is that a vegetal signal, such as Wnt11, may be required to fully activate xNorrin signaling in the dorsal ectoderm during cortical rotation. Candidate targets of this vegetal signal may include Xenopus Frizzled4 and Xenopus LRP5, two known receptors for xNorrin [18]. Similarly, the absence of Xnr3 and Siamois expression in xNorrin-injected animal caps can be attributed to the lack of functional xNorrin receptors, which are required for xNorrin signaling (Figure S2B).
Reciprocal Inhibition between xNorrin and TGF-β
In early embryos, balanced signaling activities from opposite domains are critical for patterning the dorsal–ventral, anterior–posterior, and animal–vegetal axes. For example, in the Xenopus gastrula, ventral BMP molecules antagonize Chordin and Noggin from dorsal cells through direct binding in the extracellular space [1]. Similarly, mesoderm-promoting Nodal activity in the vegetal pole is negatively regulated by maternal TGF-β signaling inhibitors, such as Coco and Ectodermin, from the animal half [41],[42]. In addition, the competence of blastomeres to form neural and retinal progeny is repressed by endomesoderm-promoting factors in the vegetal pole [43].
Previously, Coco expressed at the animal pole was proposed as a competence factor to block Nodal signaling and ensure the correct patterning of the ectoderm [41]. Our results indicate that xNorrin also directly inhibits BMP/TGF-β signaling, likely through direct extracellular binding without the activation of Wnt signaling (Figures 5 and 6). It is possible that this BMP antagonizing activity is required to predispose the dorsal ectoderm toward neural fates before zygotic BMP inhibitors are expressed. Both maternal Coco and xNorrin are expressed in overlapping domains in the animal pole of Xenopus oocytes [41]. It would be interesting to investigate how distinct TGF-β antagonists are coordinated to modulate multiple TGF-β signaling pathways in vivo. Although both Coco and xNorrin are TGF-β antagonists, there is a clear difference in that Coco also functions as a Wnt inhibitor by some unknown mechanism, while xNorrin is a Wnt agonist.
We also showed that the ectopic expression of xNorrin in the vegetal-marginal regions inhibited mesoderm formation and blocked gastrulation (Figure 7D and data not shown), underscoring the importance of restricting xNorrin activity to the dorsal animal pole. This result seems to suggest that the xNorrin-mediated inhibition of mesoderm formation may account for the unexpected failure of NFL to induce complete secondary axes on the ventral side (Figure 4). However, we observed that the combination of the xNorrin K57N mutant, Frizzled4, and Lrp5 also failed to induce secondary axes (data not shown), suggesting that an alternative mechanism must prevent NFL from inducing secondary axes (Figure 4) [18]. A putative Norrin-specific inhibitor in the endomesoderm other than TGF-β cannot be excluded.
The BMP/TGF-β inhibition function of xNorrin may be attributed to a predicted cysteine-knot domain in the carboxyl terminal (Figure S1) [44]. A previous bioinformatics study classified the putative Norrin cysteine-knot domain as a mucin protein, along with secretory mucin and von Willebrand factor [45]. Other members of this subgroup may be tested for their potential ability to negatively regulate TGF-β family members.
Conversely, BMP4 was shown to repress Norrin-induced neural formation (Figure 5H). In addition to xNorrin RNA localization in the dorsal animal region, ventrally expressed BMP4 and vegetally expressed mesoderm inducers, such as Nodal, may further restrict xNorrin activity to the prospective neuroectoderm. Thus, reciprocal inhibition between BMP/TGF-β and xNorrin are equally important for appropriate embryonic patterning.
A Link between TGF-β Signaling and Norrie Disease
Norrin has been identified as an activator of the canonical Wnt signaling pathway through two separate receptor complexes, Frizzled4/Lrp5 and Frizzled4/TSPAN12 [17]–[19]. Given the direct link between Norrin mutations and Norrie disease, and the roles of TGF-β signaling in multiple human diseases, it is important to recognize that Norrin also functions as a potent inhibitor of TGF-β family members. Two lines of evidence indicate that canonical Wnt signaling and TGF-β inhibition are induced separately by Norrins. First, xNorrin can induce neural formation in the absence of Wnt target activation (Figure 6A). Second, selected xNorrin point mutants (e.g., K57N) potently suppress endogenous TGF-β target gene expression but maintain robust Wnt activation capability (Figure 7).
One of the major defects caused by Norrie disease is abnormal vascular development in the retina and inner ear [18]. The development of the elaborate vascular structure in the retina is strongly influenced by VEGF, which in turn can be positively regulated by TGF-β/ALK5 signaling [46]. Although Norrin mutations cause retinal hypovascularization, a previous study showed that the numbers of blood vessels in the ganglion cell layer and the nerve fiber layer are actually increased in the Norrin knock-out mice [47], suggesting a pro-angiogenesis activity that may be enhanced in these cell layers. Because Norrin hemizygous mutant mice also have severe defects in ear and brain development [18],[48], further investigation of local TGF-β regulation in these organs is also warranted.
Finally, the finding that two independent activities are encoded in the small Norrin protein (mature human Norrin has only 109 amino acid residues) raises the question of how Wnt pathway activation and TGF-β signal tuning are coordinated by Norrin in vivo. Solving the three-dimensional structures of the wild-type Norrin protein and selected point mutants may help answer this question and may even help elucidate the molecular mechanisms of Wnt agonist signaling through its receptors.
Materials and Methods
Plasmid and mRNA
The initial xNorrin cDNA clone was first amplified from a X. laevis cDNA library using Pyrobest DNA polymerase (TaKaRa) and PCR primers partially based on a predicted X. tropicalis Norrin gene sequence. (http://genome.jgi-psf.org/cgi-bin/dispGeneModel?db=Xentr4&id=158316): xNorrin Up: 5′-AGACGAATTCACCTGAGAGGAAGACTGGG-3′, xNorrin Down: 5′-AGACCTCGAGAGCAACGCAAGCGAATGG-3′. The cDNA for the coding region was amplified using xNorrin Up: 5′-AATCGGATCCATGGGAAATCGTGTCCTTC-3′ and xNorrin Down: 5′-ATATCTCGAGCTATGAATTGCACTCTTC-3′. The xNorrin cDNA was then cloned between the BamHI and XhoI sites of pCS2+. The xNorrin5′-Myc plasmid was generated by inserting xNorrin cDNA, including the 5′ UTR, between the BamHI and ClaI sites of pCS2+MT, thereby introducing a C-terminal Myc-tag. The pCS2+ xNorrin-FLAG plasmid was generated by inserting xNorrin cDNA between the BamHI and XbaI sites of pCS2+-FLAG-C4. The xNorrin single point mutants (R40K, K57N, and L60P), which mimic human Norrie disease mutants, were generated by site-direct mutagenesis (Fast Mutagenesis System, TransGen) [18].
The mRNA for Xenopus injections was prepared using the RiboMax Large Scale RNA Production System (Ambion) according to the manufacturer's instructions. The pCS2+-xNorrin, pCS2+-xNorrin (R40K, K57N, or L60P), xNorrin-Myc, pCS2+-xFrizzled4 [49], pCS2+-hLrp5 [50], and Wnt8 [31] plasmids were all linearized with NotI; BMP4 was linearized with EcoRI; and Wnt11 [51] was linearized with BamHI. All plasmids were transcribed with SP6 RNA polymerase. RNA microinjections were carried out as described [52].
Embryo Manipulation and Injection
All animal studies in this report were approved by the Institutional Review Board of the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences. X. laevis eggs were isolated in 1× MBS plus high salt solution and fertilized using sperm suspensions in 1× MMR. Embryos were cultured in 0.1× MMR. Embryo dissection was performed as previously described [52]. Briefly, mid-blastula embryos were transferred into 1× Steinberg's solution, the vitelline membrane was removed, and 3×3 mm2 animal caps were cut. Explants were cultured in 1× Steinberg's solution until they reached the indicated stages [53].
For MO oligonucleotides and mRNA injections, embryos were transferred into 1× MMR containing 2% Ficoll (Amersham Biosciences). Pigment intensity was used to differentiate the dorsal and ventral sides. After injections, embryos were washed thoroughly and returned to 0.1× MMR during the blastula stage.
For UV treatment, embryos were irradiated by placing them in a quartz colorimetric cup oriented with the animal pole upwards and UV-irradiated at 50 µJ using the Stratagene Crosslinker 1800. Immediately after irradiation, the embryos were transferred into 1× MMR containing 2% Ficoll. For rescue experiments, four- to eight-cell-stage embryos were injected with 500 pg of xNorrin mRNA or 500 pg of Wnt11 mRNA [23].
Antisense Morpholinos
xNorrin antisense MOs were purchased from Genetools. The MO sequences used were: xNor-MO: CTCAATCCCAGTCTTCCTTTCAGGT, xNor-misMO: CTGAATCCGAGTGTTCGTTTCACGT, and xNor-spMO: TTAAAGTGGACTGTACCTTGGCAGT. MOs were dissolved in sterile, filtered water at a concentration of 5 ng/nl and injected at the doses described in the text.
Reverse Transcription PCR
Total RNA was prepared using the Proteinase K method and treated with 10 µg of yeast tRNA and RNase-free DNase (Promega) before cDNA synthesis [52]. cDNA was synthesized by reverse transcription, and the reactions were performed in a volume of 20 µl using 200 ng of random primer (Promega), 5× first-strand buffer, 0.01 M DTT, 40 U RNase inhibitor (TaKaRa), 1 mM each dNTP, and 200 U M-MLV RT (Invitrogen) at 37°C for 50 min. Reactions were then heat-inactivated at 70°C for 10 min and stored at −20°C. One-tenth of the mixture was used as a template for PCR. PCR was carried out in a volume of 25 µl containing 100 mM dNTPs, 0.2 µM each primer, and 1 U of rTaq DNA polymerase (TaKaRa). The PCR parameters and DNA primers are described in Table S1. PCR cycles were determined such that no amplification saturation was reached in semi-quantitative assays.
Luciferase Assays
SuperTopFlash DNA (20 pg), containing eight copies of the TCF-binding site upstream of a minimal TK promoter and the luciferase open reading frame, and pRL-TK DNA (10 pg) (Renilla luciferase was used as an internal control) [5] were co-injected into two dorsal animal cells at the eight-cell stage of wild-type, xNor-MO (20 ng)–injected, or xNor-misMO (20 ng)–injected embryos Three replicate samples for each of the three embryo types were frozen at the late blastula stage, and luciferase assays were carried out using a Promega Luciferase Assay system.
Western Blot
To test for xNor-MO activity, one-cell-stage embryos were injected with 5 ng, 10 ng, or 20 ng of xNor-MO at the marginal zone and then injected four times with a total of 1.5 ng of xNorrin-Myc mRNA into the marginal zone at the four-cell stage. A total of five blastula embryos were homogenized in 100 µl of ice-cold lysis buffer [54]. Protein lysates were spun for 15 min at high speed at 4°C. Protein detection by Western blot was performed using anti-c-Myc (9E10) primary (Santa Cruz Biotechnology) and HRP-conjugated secondary (Pierce) antibodies with Pierce Western blot detection reagents.
For co-immunoprecipitation assays, 500 pg of xNorrin-Myc and 500 pg of BMP4 mRNA were injected into different cells of four-cell-stage embryos. The injected embryos were frozen at stage 10 in batches of five and lysed with 500 µl of ice-cold lysis buffer. The cleared lysate was mixed with anti-FLAG-M2 agarose beads and incubated overnight at 4°C. The beads were pelleted, washed four times with lysis buffer, mixed with SDS-PAGE sample buffer, and processed in a standard electrophoresis and Western blot protocol.
Whole-Mount In Situ Hybridization
Whole-mount in situ hybridization was performed according to a standard protocol as described previously [55], with minor modifications for dissected embryos. For dissected embryos, whole pigmented embryos were fixed for 1 h in MEMFA, bisected along the dorsal–ventral axis with a scalpel blade, fixed for two additional hours in MEMFA, and washed and stored in 100% methanol. The embryos were hybridized at 65°C overnight. BM Purple was used as a substrate (Roche). Pigment was then bleached. The RNA probes were labeled with digoxigenin-UTP (Roche) with the appropriate RNA polymerase using linearized plasmids.
Histological Analysis
For histological analysis, embryos were fixed overnight in Bouin's solution and then dehydrated and embedded in paraffin. Sections of 10-mm thickness were prepared and stained with hematoxylin and eosin as previously described [56].
Supporting Information
Norrins are highly conserved in vertebrates. (A) An alignment of Norrin protein sequences from selected vertebrate species. Prefixes used for Norrins from different species: human (h), mouse (m), chicken (c), X. laevis (xl), X. tropicalis (xt), and zebrafish (z). Conserved cysteine residues are highlighted in red. (B) Percentages of identical amino acid residues between Norrin proteins from different species.
(TIF)
Maternal xNorrin activates the canonical Wnt pathway. (A) xNorrin expression during early Xenopus development detected by reverse transcription PCR (RT-PCR). ODC (ornithine decarboxylase) served as a loading control. RT–: no reverse transcription. Embryos were staged according to Nieuwkoop and Faber [53]. (B) Expression of Siamois and Xnr3 (Wnt target genes) and Xbra (mesoderm marker) in isolated animal caps from embryos co-injected with NFL (200 pg each), xNorrin (200 pg), or Wnt8 (10 pg) mRNAs. Nor, Norrin; WE, whole wild-type embryo; WT, wild type. ODC served as a loading control. (C) Norrin can lead to phosphorylation of its receptor, LRP6. LRP6 phosphorylation at three specific threonine (T) and serine (S) sites (T1479, S1490, and T1493) was analyzed in HEK293 cells transfected with Lrp6/Axin/mFz4, with or without mouse Norrin or xNorrin, using site-specific antibodies. Total LRP was detected using a general LRP antibody.
(TIF)
Maternal xNorrin is required for anterior neural formation. (A) The genomic sequence of the first exon and the first intron boundary of xNorrin. The first two presumptive nucleotides “gt” of the intron are labeled in red. The splicing site sequence targeted by xNor-spMO is indicated by a green line. See Materials and Methods for sequence information for all MOs. (B) RT-PCR to detect xNorrin mRNA expression in stage 15 embryos. xNor-spMO (20 ng) inhibited zygotic xNorrin transcription, while xNor-MO (20 ng) or xNor-misMO (20 ng) (a four-nucleotide mismatched MO compared to xNor-MO) did not. (C–F) Representative MO-injected tadpole at stage 34. xNo-spMO (20 ng) did not cause severe anterior defects, unlike xNor-MO. (G) Summary of (C–F). Uninjected: n = 30; MO: n = 24; misMO: n = 30; spMO: n = 40. (H) xNor-MO injection inhibited anterior neural formation. Whole-mount in situ hybridization was performed for XBF-1 mRNA (anterior neural marker) and HoxB9 mRNA (posterior neural marker) in stage 15 embryos. Dorsal animal cell injection of xNor-MO (10 ng) at the four- to eight-cell stage greatly reduced the expression of the anterior neural marker XBF-1 (63%, n = 32), while injection of xNor-misMO (10 ng) or xNor-spMO (10 ng) (27%, n = 29) was far less effective to reduce the expression. Neither xNor-MO (n = 25) nor xNor-spMO (n = 25) injection affected HoxB9 expression. MOs were co-injected with β-gal mRNA (100 pg). β-gal staining is shown in red. XBF-1 staining embryos are shown in anterior view, and HoxB9 staining embryos are shown in dorsal view, with the anterior pole at the top. misMO, xNor-misMO; MO, xNor-MO; spMO, xNor-spMO.
(TIF)
xNorrin is essential for early dorsal-specific gene expression. (A) The injection of xNor-MO reduced Xnr3 (stage 9: 70% reduced, n = 17; stage 10: 70% reduced, n = 20) but not gsc (14% reduced, n = 22) expression, as assayed by whole-mount in situ hybridization. (B) RT-PCR analysis showed that xNor-MO injected into dorsal animal cells reduced the expression of early dorsal-specific genes in stage 9 embryos. This reduction could be rescued by the injection of xNorrin mRNA (50 pg) lacking the xNor-MO target sequence. Note that xNor-MO did not change Xnr1 expression. ODC served as a loading control. (C) The overexpression of NFL in ventral vegetal blastomeres induced Wnt target gene expression. Upon injection into the ventral blastomeres of early eight-cell embryos, both NFL and Wnt8 induced Xnr3 (NFL: 56%, n = 25; Wnt8: 83%, n = 46) and Chordin (NFL: 72%, n = 29; Wnt8: 88%, n = 60) expression. However, NFL only weakly induced the Spemann organizer marker gsc (NFL: 7%, n = 28; Wnt8: 84%, n = 50). All embryos are in vegetal views. Arrowheads indicate the injection sites. (D) RT-PCR results (not quantitative) showed that NFL injection into ventral-vegetal cells ectopically activated Wnt target genes (Chordin, Siamois, and Xnr3) at the ventral side of the embryos. ODC served as a loading control.
(TIF)
xNorrin induces neural formation and inhibits mesoderm formation. (A) RT-PCR analysis of neural gene expression in stage 15 animal caps from embryos injected with Wnt8 (20 pg), Wnt11 (200 pg), xNorrin (xNor) (200 pg), and NFL (200 pg each). The expression of otx2, Sox2, and NCAM (all neural markers) was analyzed. ODC served as a loading control. (B–D) Xbra expression detected in whole-mount in situ hybridization. A wild-type embryo at stage 10.5 (B); reduced Xbra expression in stage 10.5 embryos injected with xNorrin RNA (200 pg) at the vegetal pole at the two-cell stage (82% reduced, n = 34) (C); reduced Xbra expression in stage 10.5 embryos injected with mouse Norrin RNA (200 pg) at the vegetal pole at the two-cell stage (66% reduced, n = 36) (D).
(TIF)
xNorrin interacts with TGF-β family members. (A) xNorrin binds to BMP4. Epitope-tagged wild-type xNorrin or xNorrin point mutants (R40K or K57N) and BMP4 were separately expressed in HEK293 cells. The conditioned medium from cells expressing individual xNorrin and BMP4 were mixed and incubated. The FLAG-tagged protein complexes were immunoprecipitated using an anti-FLAG antibody and separated in SDS-PAGE and blotted. An anti-c-Myc antibody was used to detect Myc-tagged BMP4. The expression of FLAG-tagged proteins was detected using an anti-FLAG antibody. xNorrin was shown to bind to BMP4. The R40K mutant retained this binding activity, while the K57N mutant showed slightly reduced BMP4 binding. Conditioned medium of the parent FLAG-plasmid-transfected cells was used as a control (ctrl). Arrowhead, BMP4-Myc; arrow, immunoglobulin light chains (LC). α-FLAG, anti-FLAG tag monoclonal antibody; α-Myc, anti-c-Myc-tag monoclonal antibody. (B) xNorrin binds to Xnr1 but not DKK-1. The assay performed was similar to that described in (A). Conditioned medium (ctrl) was the same as in (A). Arrowheads indicate FLAG-tagged protein. Arrows point to immunoglobulin heavy chain (HC, top) and light chain (LC, bottom).
(TIF)
The xNorrin K57N mutant failed to efficiently rescue anterior defects in xNorrin morphants. (A–E) An uninjected embryo (A). A xNor-MO (20 ng)–injected embryo (B). Note the lack of eye pigment. A xNor-misMO (20 ng)–injected embryo (C). A xNor-MO and wild-type Norrin RNA (50 pg) co-injected embryo (D). A xNor-MO and xNorrin K57N RNA (50 pg) co-injected embryo (E). (F) Summary of anterior defect frequency in (A–E). Uninjected: n = 40; MO: n = 24; xNor rescue: n = 20; K57N rescue: n = 19; misMO: n = 15. RNAs and MO were injected into the dorsal animal region at the four-cell stage. All embryos shown are around stage 36.
(TIF)
Acknowledgments
We appreciate Drs. Mary Lou King, Anming Meng, Yi Rao, and Qinghua Tao for critically reading the manuscript. We thank members of the Zhang laboratory for their help during the study.
Abbreviations
- BCNE center
blastula Chordin- and Noggin-expressing center
- CNS
central nervous system
- misMO
mismatched morpholino
- MO
morpholino
- NFL
Xenopus Norrin plus Xenopus Frizzled4 plus human Lrp5
- RT-PCR
reverse transcription PCR
- spMO
splicing morpholino
- UV
ultraviolet, xNorrin, Xenopus Norrin
Footnotes
The authors have declared that no competing interests exist.
This work was supported by grants from MOST China (2011CB943800 to J.Z. and 2006CB943402 to J.Z. and W.W.), CAS (XDA01010108) and NSFC (90408001 and 30425013 to J.Z.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.De Robertis E. M, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, et al. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 3.Heasman J, Kofron M, Wylie C. Beta-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev Biol. 2000;222:124–134. doi: 10.1006/dbio.2000.9720. [DOI] [PubMed] [Google Scholar]
- 4.Wylie C, Kofron M, Payne C, Anderson R, Hosobuchi M, et al. Maternal beta-catenin establishes a ‘dorsal signal’ in early Xenopus embryos. Development. 1996;122:2987–2996. doi: 10.1242/dev.122.10.2987. [DOI] [PubMed] [Google Scholar]
- 5.Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, et al. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Cha S. W, Tadjuidje E, Tao Q, Wylie C, Heasman J. Wnt5a and Wnt11 interact in a maternal Dkk1-regulated fashion to activate both canonical and non-canonical signaling in Xenopus axis formation. Development. 2008;135:3719–3729. doi: 10.1242/dev.029025. [DOI] [PubMed] [Google Scholar]
- 7.Cha S. W, Tadjuidje E, White J, Wells J, Mayhew C, et al. Wnt11/5a complex formation caused by tyrosine sulfation increases canonical signaling activity. Curr Biol. 2009;19:1573–1580. doi: 10.1016/j.cub.2009.07.062. [DOI] [PubMed] [Google Scholar]
- 8.Larabell C. A, Torres M, Rowning B. A, Yost C, Miller J. R, et al. Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in beta-catenin that are modulated by the Wnt signaling pathway. J Cell Biol. 1997;136:1123–1136. doi: 10.1083/jcb.136.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowning B. A, Wells J, Wu M, Gerhart J. C, Moon R. T, et al. Microtubule-mediated transport of organelles and localization of beta-catenin to the future dorsal side of Xenopus eggs. Proc Natl Acad Sci U S A. 1997;94:1224–1229. doi: 10.1073/pnas.94.4.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fainsod A, Deissler K, Yelin R, Marom K, Epstein M, et al. The dorsalizing and neural inducing gene follistatin is an antagonist of BMP-4. Mech Dev. 1997;63:39–50. doi: 10.1016/s0925-4773(97)00673-4. [DOI] [PubMed] [Google Scholar]
- 11.Piccolo S, Sasai Y, Lu B, De Robertis E. M. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmerman L. B, De Jesus-Escobar J. M, Harland R. M. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 13.Hemmati-Brivanlou A, Kelly O. G, Melton D. A. Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell. 1994;77:283–295. doi: 10.1016/0092-8674(94)90320-4. [DOI] [PubMed] [Google Scholar]
- 14.Hemmati-Brivanlou A, Melton D. Vertebrate neural induction. Annu Rev Neurosci. 1997;20:43–60. doi: 10.1146/annurev.neuro.20.1.43. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher B. C, Hainski A. M, Moody S. A. Autonomous differentiation of dorsal axial structures from an animal cap cleavage stage blastomere in Xenopus. Development. 1991;112:1103–1114. doi: 10.1242/dev.112.4.1103. [DOI] [PubMed] [Google Scholar]
- 16.Kuroda H, Wessely O, De Robertis E. M. Neural induction in Xenopus: requirement for ectodermal and endomesodermal signals via Chordin, Noggin, beta-Catenin, and Cerberus. PLoS Biol. 2004;2:e92. doi: 10.1371/journal.pbio.0020092. doi: 10.1371/journal.pbio.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Junge H. J, Yang S, Burton J. B, Paes K, Shu X, et al. TSPAN12 regulates retinal vascular development by promoting Norrin- but not Wnt-induced FZD4/beta-catenin signaling. Cell. 2009;139:299–311. doi: 10.1016/j.cell.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 18.Xu Q, Wang Y, Dabdoub A, Smallwood P. M, Williams J, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 19.Ye X, Wang Y, Cahill H, Yu M, Badea T. C, et al. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139:285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z. Y, Battinelli E. M, Fielder A, Bundey S, Sims K, et al. A mutation in the Norrie disease gene (NDP) associated with X-linked familial exudative vitreoretinopathy. Nat Genet. 1993;5:180–183. doi: 10.1038/ng1093-180. [DOI] [PubMed] [Google Scholar]
- 21.Harland R, Gerhart J. Formation and function of Spemann's organizer. Annu Rev Cell Dev Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- 22.Cui Y, Brown J. D, Moon R. T, Christian J. L. Xwnt-8b: a maternally expressed Xenopus Wnt gene with a potential role in establishing the dorsoventral axis. Development. 1995;121:2177–2186. doi: 10.1242/dev.121.7.2177. [DOI] [PubMed] [Google Scholar]
- 23.Ku M, Melton D. A. Xwnt-11: a maternally expressed Xenopus wnt gene. Development. 1993;119:1161–1173. doi: 10.1242/dev.119.4.1161. [DOI] [PubMed] [Google Scholar]
- 24.Hainski A. M, Moody S. A. Xenopus maternal RNAs from a dorsal animal blastomere induce a secondary axis in host embryos. Development. 1992;116:347–355. doi: 10.1242/dev.116.2.347. [DOI] [PubMed] [Google Scholar]
- 25.Weaver C, Kimelman D. Move it or lose it: axis specification in Xenopus. Development. 2004;131:3491–3499. doi: 10.1242/dev.01284. [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Skarmeta J. L, de la Calle-Mustienes E, Modolell J, Mayor R. Xenopus brain factor-2 controls mesoderm, forebrain and neural crest development. Mech Dev. 1999;80:15–27. doi: 10.1016/s0925-4773(98)00190-7. [DOI] [PubMed] [Google Scholar]
- 27.Itoh K, Sokol S. Y. Graded amounts of Xenopus dishevelled specify discrete anteroposterior cell fates in prospective ectoderm. Mech Dev. 1997;61:113–125. doi: 10.1016/s0925-4773(96)00627-2. [DOI] [PubMed] [Google Scholar]
- 28.Wessely O, Agius E, Oelgeschlager M, Pera E. M, De Robertis E. M. Neural induction in the absence of mesoderm: beta-catenin-dependent expression of secreted BMP antagonists at the blastula stage in Xenopus. Dev Biol. 2001;234:161–173. doi: 10.1006/dbio.2001.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez-Vilar J, Hill R. L. Norrie disease protein (norrin) forms disulfide-linked oligomers associated with the extracellular matrix. J Biol Chem. 1997;272:33410–33415. doi: 10.1074/jbc.272.52.33410. [DOI] [PubMed] [Google Scholar]
- 30.McMahon A. P, Moon R. T. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989;58:1075–1084. doi: 10.1016/0092-8674(89)90506-0. [DOI] [PubMed] [Google Scholar]
- 31.Smith W. C, Harland R. M. Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell. 1991;67:753–765. doi: 10.1016/0092-8674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- 32.Sokol S, Christian J. L, Moon R. T, Melton D. A. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell. 1991;67:741–752. doi: 10.1016/0092-8674(91)90069-b. [DOI] [PubMed] [Google Scholar]
- 33.Smith J. C, Price B. M, Van Nimmen K, Huylebroeck D. Identification of a potent Xenopus mesoderm-inducing factor as a homologue of activin A. Nature. 1990;345:729–731. doi: 10.1038/345729a0. [DOI] [PubMed] [Google Scholar]
- 34.Agius E, Oelgeschlager M, Wessely O, Kemp C, De Robertis E. M. Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development. 2000;127:1173–1183. doi: 10.1242/dev.127.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimelman D. Mesoderm induction: from caps to chips. Nat Rev Genet. 2006;7:360–372. doi: 10.1038/nrg1837. [DOI] [PubMed] [Google Scholar]
- 36.Thomsen G, Woolf T, Whitman M, Sokol S, Vaughan J, et al. Activins are expressed early in Xenopus embryogenesis and can induce axial mesoderm and anterior structures. Cell. 1990;63:485–493. doi: 10.1016/0092-8674(90)90445-k. [DOI] [PubMed] [Google Scholar]
- 37.Schuback D. E, Chen Z. Y, Craig I. W, Breakefield X. O, Sims K. B. Mutations in the Norrie disease gene. Hum Mutat. 1995;5:285–292. doi: 10.1002/humu.1380050403. [DOI] [PubMed] [Google Scholar]
- 38.Shastry B. S, Hejtmancik J. F, Trese M. T. Identification of novel missense mutations in the Norrie disease gene associated with one X-linked and four sporadic cases of familial exudative vitreoretinopathy. Hum Mutat. 1997;9:396–401. doi: 10.1002/(SICI)1098-1004(1997)9:5<396::AID-HUMU3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 39.Smallwood P. M, Williams J, Xu Q, Leahy D. J, Nathans J. Mutational analysis of Norrin-Frizzled4 recognition. J Biol Chem. 2007;282:4057–4068. doi: 10.1074/jbc.M609618200. [DOI] [PubMed] [Google Scholar]
- 40.Huang S, Yan B, Sullivan S. A, Moody S. A. Noggin signaling from Xenopus animal blastomere lineages promotes a neural fate in neighboring vegetal blastomere lineages. Dev Dyn. 2007;236:171–183. doi: 10.1002/dvdy.20944. [DOI] [PubMed] [Google Scholar]
- 41.Bell E, Munoz-Sanjuan I, Altmann C. R, Vonica A, Brivanlou A. H. Cell fate specification and competence by Coco, a maternal BMP, TGFbeta and Wnt inhibitor. Development. 2003;130:1381–1389. doi: 10.1242/dev.00344. [DOI] [PubMed] [Google Scholar]
- 42.Dupont S, Zacchigna L, Cordenonsi M, Soligo S, Adorno M, et al. Germ-layer specification and control of cell growth by Ectodermin, a Smad4 ubiquitin ligase. Cell. 2005;121:87–99. doi: 10.1016/j.cell.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 43.Yan B, Moody S. A. The competence of Xenopus blastomeres to produce neural and retinal progeny is repressed by two endo-mesoderm promoting pathways. Dev Biol. 2007;305:103–119. doi: 10.1016/j.ydbio.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meitinger T, Meindl A, Bork P, Rost B, Sander C, et al. Molecular modelling of the Norrie disease protein predicts a cystine knot growth factor tertiary structure. Nat Genet. 1993;5:376–380. doi: 10.1038/ng1293-376. [DOI] [PubMed] [Google Scholar]
- 45.Vitt U. A, Hsu S. Y, Hsueh A. J. Evolution and classification of cystine knot-containing hormones and related extracellular signaling molecules. Mol Endocrinol. 2001;15:681–694. doi: 10.1210/mend.15.5.0639. [DOI] [PubMed] [Google Scholar]
- 46.Goumans M. J, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, et al. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol Cell. 2003;12:817–828. doi: 10.1016/s1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- 47.Richter M, Gottanka J, May C. A, Welge-Lussen U, Berger W, et al. Retinal vasculature changes in Norrie disease mice. Invest Ophthalmol Vis Sci. 1998;39:2450–2457. [PubMed] [Google Scholar]
- 48.Rehm H. L, Zhang D. S, Brown M. C, Burgess B, Halpin C, et al. Vascular defects and sensorineural deafness in a mouse model of Norrie disease. J Neurosci. 2002;22:4286–4292. doi: 10.1523/JNEUROSCI.22-11-04286.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi D. L, Boucaut J. C. Xenopus frizzled 4 is a maternal mRNA and its zygotic expression is localized to the neuroectoderm and trunk lateral plate mesoderm. Mech Dev. 2000;94:243–245. doi: 10.1016/s0925-4773(00)00294-x. [DOI] [PubMed] [Google Scholar]
- 50.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 51.Tada M, Smith J. C. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- 52.Zhang J, Houston D. W, King M. L, Payne C, Wylie C, et al. The role of maternal VegT in establishing the primary germ layers in Xenopus embryos. Cell. 1998;94:515–524. doi: 10.1016/s0092-8674(00)81592-5. [DOI] [PubMed] [Google Scholar]
- 53.Nieuwkoop P. D, Faber J. Normal table of Xenopus laevis (Daudin): a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. New York: Garland; 1994. 252 [Google Scholar]
- 54.Messenger N. J, Kabitschke C, Andrews R, Grimmer D, Nunez Miguel R, et al. Functional specificity of the Xenopus T-domain protein Brachyury is conferred by its ability to interact with Smad1. Dev Cell. 2005;8:599–610. doi: 10.1016/j.devcel.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 55.Harland R. M. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- 56.Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, et al. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Norrins are highly conserved in vertebrates. (A) An alignment of Norrin protein sequences from selected vertebrate species. Prefixes used for Norrins from different species: human (h), mouse (m), chicken (c), X. laevis (xl), X. tropicalis (xt), and zebrafish (z). Conserved cysteine residues are highlighted in red. (B) Percentages of identical amino acid residues between Norrin proteins from different species.
(TIF)
Maternal xNorrin activates the canonical Wnt pathway. (A) xNorrin expression during early Xenopus development detected by reverse transcription PCR (RT-PCR). ODC (ornithine decarboxylase) served as a loading control. RT–: no reverse transcription. Embryos were staged according to Nieuwkoop and Faber [53]. (B) Expression of Siamois and Xnr3 (Wnt target genes) and Xbra (mesoderm marker) in isolated animal caps from embryos co-injected with NFL (200 pg each), xNorrin (200 pg), or Wnt8 (10 pg) mRNAs. Nor, Norrin; WE, whole wild-type embryo; WT, wild type. ODC served as a loading control. (C) Norrin can lead to phosphorylation of its receptor, LRP6. LRP6 phosphorylation at three specific threonine (T) and serine (S) sites (T1479, S1490, and T1493) was analyzed in HEK293 cells transfected with Lrp6/Axin/mFz4, with or without mouse Norrin or xNorrin, using site-specific antibodies. Total LRP was detected using a general LRP antibody.
(TIF)
Maternal xNorrin is required for anterior neural formation. (A) The genomic sequence of the first exon and the first intron boundary of xNorrin. The first two presumptive nucleotides “gt” of the intron are labeled in red. The splicing site sequence targeted by xNor-spMO is indicated by a green line. See Materials and Methods for sequence information for all MOs. (B) RT-PCR to detect xNorrin mRNA expression in stage 15 embryos. xNor-spMO (20 ng) inhibited zygotic xNorrin transcription, while xNor-MO (20 ng) or xNor-misMO (20 ng) (a four-nucleotide mismatched MO compared to xNor-MO) did not. (C–F) Representative MO-injected tadpole at stage 34. xNo-spMO (20 ng) did not cause severe anterior defects, unlike xNor-MO. (G) Summary of (C–F). Uninjected: n = 30; MO: n = 24; misMO: n = 30; spMO: n = 40. (H) xNor-MO injection inhibited anterior neural formation. Whole-mount in situ hybridization was performed for XBF-1 mRNA (anterior neural marker) and HoxB9 mRNA (posterior neural marker) in stage 15 embryos. Dorsal animal cell injection of xNor-MO (10 ng) at the four- to eight-cell stage greatly reduced the expression of the anterior neural marker XBF-1 (63%, n = 32), while injection of xNor-misMO (10 ng) or xNor-spMO (10 ng) (27%, n = 29) was far less effective to reduce the expression. Neither xNor-MO (n = 25) nor xNor-spMO (n = 25) injection affected HoxB9 expression. MOs were co-injected with β-gal mRNA (100 pg). β-gal staining is shown in red. XBF-1 staining embryos are shown in anterior view, and HoxB9 staining embryos are shown in dorsal view, with the anterior pole at the top. misMO, xNor-misMO; MO, xNor-MO; spMO, xNor-spMO.
(TIF)
xNorrin is essential for early dorsal-specific gene expression. (A) The injection of xNor-MO reduced Xnr3 (stage 9: 70% reduced, n = 17; stage 10: 70% reduced, n = 20) but not gsc (14% reduced, n = 22) expression, as assayed by whole-mount in situ hybridization. (B) RT-PCR analysis showed that xNor-MO injected into dorsal animal cells reduced the expression of early dorsal-specific genes in stage 9 embryos. This reduction could be rescued by the injection of xNorrin mRNA (50 pg) lacking the xNor-MO target sequence. Note that xNor-MO did not change Xnr1 expression. ODC served as a loading control. (C) The overexpression of NFL in ventral vegetal blastomeres induced Wnt target gene expression. Upon injection into the ventral blastomeres of early eight-cell embryos, both NFL and Wnt8 induced Xnr3 (NFL: 56%, n = 25; Wnt8: 83%, n = 46) and Chordin (NFL: 72%, n = 29; Wnt8: 88%, n = 60) expression. However, NFL only weakly induced the Spemann organizer marker gsc (NFL: 7%, n = 28; Wnt8: 84%, n = 50). All embryos are in vegetal views. Arrowheads indicate the injection sites. (D) RT-PCR results (not quantitative) showed that NFL injection into ventral-vegetal cells ectopically activated Wnt target genes (Chordin, Siamois, and Xnr3) at the ventral side of the embryos. ODC served as a loading control.
(TIF)
xNorrin induces neural formation and inhibits mesoderm formation. (A) RT-PCR analysis of neural gene expression in stage 15 animal caps from embryos injected with Wnt8 (20 pg), Wnt11 (200 pg), xNorrin (xNor) (200 pg), and NFL (200 pg each). The expression of otx2, Sox2, and NCAM (all neural markers) was analyzed. ODC served as a loading control. (B–D) Xbra expression detected in whole-mount in situ hybridization. A wild-type embryo at stage 10.5 (B); reduced Xbra expression in stage 10.5 embryos injected with xNorrin RNA (200 pg) at the vegetal pole at the two-cell stage (82% reduced, n = 34) (C); reduced Xbra expression in stage 10.5 embryos injected with mouse Norrin RNA (200 pg) at the vegetal pole at the two-cell stage (66% reduced, n = 36) (D).
(TIF)
xNorrin interacts with TGF-β family members. (A) xNorrin binds to BMP4. Epitope-tagged wild-type xNorrin or xNorrin point mutants (R40K or K57N) and BMP4 were separately expressed in HEK293 cells. The conditioned medium from cells expressing individual xNorrin and BMP4 were mixed and incubated. The FLAG-tagged protein complexes were immunoprecipitated using an anti-FLAG antibody and separated in SDS-PAGE and blotted. An anti-c-Myc antibody was used to detect Myc-tagged BMP4. The expression of FLAG-tagged proteins was detected using an anti-FLAG antibody. xNorrin was shown to bind to BMP4. The R40K mutant retained this binding activity, while the K57N mutant showed slightly reduced BMP4 binding. Conditioned medium of the parent FLAG-plasmid-transfected cells was used as a control (ctrl). Arrowhead, BMP4-Myc; arrow, immunoglobulin light chains (LC). α-FLAG, anti-FLAG tag monoclonal antibody; α-Myc, anti-c-Myc-tag monoclonal antibody. (B) xNorrin binds to Xnr1 but not DKK-1. The assay performed was similar to that described in (A). Conditioned medium (ctrl) was the same as in (A). Arrowheads indicate FLAG-tagged protein. Arrows point to immunoglobulin heavy chain (HC, top) and light chain (LC, bottom).
(TIF)
The xNorrin K57N mutant failed to efficiently rescue anterior defects in xNorrin morphants. (A–E) An uninjected embryo (A). A xNor-MO (20 ng)–injected embryo (B). Note the lack of eye pigment. A xNor-misMO (20 ng)–injected embryo (C). A xNor-MO and wild-type Norrin RNA (50 pg) co-injected embryo (D). A xNor-MO and xNorrin K57N RNA (50 pg) co-injected embryo (E). (F) Summary of anterior defect frequency in (A–E). Uninjected: n = 40; MO: n = 24; xNor rescue: n = 20; K57N rescue: n = 19; misMO: n = 15. RNAs and MO were injected into the dorsal animal region at the four-cell stage. All embryos shown are around stage 36.
(TIF)