Abstract
Background
A growing body of evidence has suggested that metformin potentially reduces the risk of cancer. Our objective was to enhance the precision of estimates of the effect of metformin on the risk of any-site and site-specific cancers in patients with diabetes.
Methods/Principal Findings
We performed a search of MEDLINE, EMBASE, ISI Web of Science, Cochrane Library, and ClinicalTrials.gov for pertinent articles published as of October 12, 2011, and included them in a systematic review and meta-analysis. We calculated pooled risk ratios (RRs) for overall cancer mortality and cancer incidence. Of the 21,195 diabetic patients reported in 6 studies (4 cohort studies, 2 RCTs), 991 (4.5%) cases of death from cancer were reported. A total of 11,117 (5.3%) cases of incident cancer at any site were reported among 210,892 patients in 10 studies (2 RCTs, 6 cohort studies, 2 case-control studies). The risks of cancer among metformin users were significantly lower than those among non-metformin users: the pooled RRs (95% confidence interval) were 0.66 (0.49–0.88) for cancer mortality, 0.67 (0.53–0.85) for all-cancer incidence, 0.68 (0.53–0.88) for colorectal cancer (n = 6), 0.20 (0.07–0.59) for hepatocellular cancer (n = 4), 0.67 (0.45–0.99) for lung cancer (n = 3).
Conclusion/Significance
The use of metformin in diabetic patients was associated with significantly lower risks of cancer mortality and incidence. However, this analysis is mainly based on observational studies and our findings underscore the more need for long-term RCTs to confirm this potential benefit for individuals with diabetes.
Introduction
Hyperinsulinemia and hyperglycemia are thought to promote carcinogenesis in patients with diabetes mellitus. Several meta-analyses have demonstrated that diabetes is associated with increased risks of site-specific cancers of the breast (1.2) [1], endometrium (2.1) [2], bladder (1.2) [3], liver (2.5) [4], colorectum (1.3) [5], and pancreas (1.8–2.1) [6], [7], and also a decreased risk of prostate cancer (0.8–0.9) [8], [9]. The evidence for non-Hodgkin's lymphoma remains inconclusive [10], [11]. Our previous meta-analyses showed that patients with diabetes have an inscreased risk of total cancer (relative risk, 1.1–1.7) [12]–[14], whereas more recent studies did not [15], [16]. Metformin is an insulin sensitizer that is the drug of first choice in the management of type 2 diabetes [17], given its safety profile and lower cost. Metformin reportedly has a potential anti-cancer effect by activating adenosine 5′-mono-phosphate-activated protein kinase (AMPK) in addition to alleviating hyperinsulinemia and hyperglycemia. Although other mechanisms for this risk reduction have been hypothesized, none have been elucidated entirely. Previous meta-analyses have suggested that metformin is associated with a reduced risk of cancer in diabetic subjects [18], [19]. However, those analyses were based solely on a few observational studies and additional reports have been published recently.
In light of the worldwide diabetes epidemic and the higher mortalities in cancer patients with diabetes [20], [21], explorations of effective cancer prevention are of clinical importance for the targeted management of diabetes in daily practice. Moreover, they are crucial in the areas of public health, since a modest increase in the risk of cancer translates into a substantial social burden. These circumstances prompted us to investigate, with greater precision, the preventive effect of metformin on cancer mortality and incidence by scrutinizing pertinent original reports including randomized controlled trials (RCTs), and combining their data in an attempt to obtain meaningful clues for the prevention of cancer in patients with diabetes [13].
Methods
Search
Searches of MEDLINE, EMBASE, ISI Web of Science, Cochrane Library, and ClinicalTrials.gov from their inception until October 12, 2011, were performed. Studies evaluating the risks of cancer mortality or incidence among diabetic patients taking metformin, compared with those not taking metformin, were identified using a combination of the following medical subject heading terms: ‘diabetes’, ‘metformin’, ‘cancer’ or ‘neoplasms’, and ‘risk’ or ‘risk factors’. The reference lists of the pertinent articles were also inspected.
Selection/Study Characteristics
We assessed all the identified RCTs, cohort studies, case-control studies, and cross-sectional studies on the risk of cancer based on original data analyses to determine their eligibility for inclusion in a qualitative analysis. The inclusion criteria in the meta-analysis are as follows: published full-text report in English-language, RCTs with parallel-design of metformin as a treatment of type 2 diabetes at least one year's follow-up period, observational studies of any duration in patients with type 2 diabetes, reporting relative risks, i.e. hazard ratios (HRs), RRs, or odds ratios, adjusted for possible confounders with confidence intervals (CIs). The comparators were defined as any treatment not including metformin.
Validity assessment
To ascertain the validity of the eligible studies, the quality of each report was appraised in reference to the CONSORT statement [22] and the STROBE statement [23].
Data abstraction
We reviewed each full-text report to determine its eligibility and extracted and tabulated all the relevant data independently. The extracted data included the characteristics of the subjects (including age, sex, and other treatment), study design, published year, follow-up period, and the methods used for ascertaining the diagnosis of cancer. Study authors were contacted as needed to obtain detailed data. Any disagreement was resolved by a consensus among the investigators.
Quantitative data synthesis
If more than one study was published for the same cohort, the report containing the most comprehensive information on the population was included to avoid overlapping populations. The reports were summarized both qualitatively and quantitatively. Three articles that did not specify the case numbers were not included in the calculation of the mortality and incidence. If the metformin comparator included more than one treatment, the oral monotherapy groups were included in the analysis because these groups were deemed to be at an equivalent stage of diabetes. If an article provided the relative risks for all cancer and site-specific cancers, the all cancer data were included in the primary qualitative and quantitative analyses and the site-specific data were used in the secondary analyses performed according to cancer site. The risks for site-specific cancers were appraised if three or more qualified reports were identified for a given cancer site. Response to metformin exposure was evaluated by using linear-regression analysis.
In the meta-analysis, each adjusted relative risk was combined and the pooled RRs with the 95% CI was calculated using the random-effects model with inverse-variance weighting. Heterogeneity among the studies was evaluated using I2 statistics. The possibility of a publication bias, which can result from the non-publication of small studies with negative findings, was assessed visually using a funnel plot for asymmetry. RevMan (version 5.1) was used for these calculations. A sensitivity analysis was performed by separating the RCTs and the observational cohort / case-control studies and the equality of RRs between RCTs and observational studies were assessed by using z-statistic tests. All the procedures were in accordance with the guidelines for the Quality of Reporting of Meta-analyses [24], the meta-analysis of observational studies in epidemiology [25] and the PRISMA statement [26].
Results
Search Results
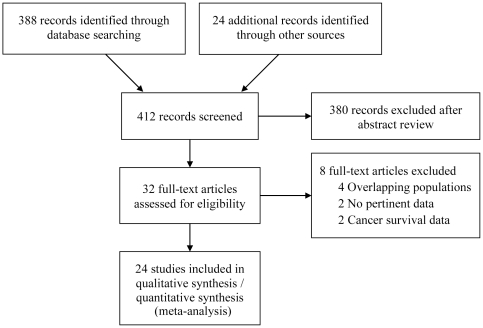
A total of 412 articles were identified during our search; of these, 32 were assessed with respect to their eligibility for inclusion in our review, which was aimed at determining the influence of metformin on cancer mortality and incidence in patients with diabetes ( Fig. 1 ). Four articles [27]–[30] were excluded from the systematic review because of population overlapping and four other reports were excluded because they investigated the overall survival rate [31], [32], cancer incidence exclusively in patients with hepatitis C [33], and biochemical recurrence [34]. Out of these 32 articles, a total of 24 (11 observational cohort studies [35]–[45], 3 randomized controlled trials [46]–[49], and 10 case-control studies [29], [50]–[58]) were included in the systematic review and meta-analysis. The UK Prospective Diabetes Study (UKPDS) 34 [49] involved two independent investigational trials (metformin vs. conventional therapy and sulfonylurea vs. sulfonylurea plus metformin), and these trials were included in the meta-analysis as two separate data.
Figure 1. Flow diagram of study selection.
Table S1 shows the characteristics of each included study according to the study design. The 24 selected articles included in the systematic review were moderately heterogeneous in terms of population demographics, study design, and the assessment of confounding factors. The diabetes sample size in these studies ranged from 361 to 998,947 patients. Of the 21,195 diabetic patients in 6 studies, 991 (4.5%) cases of cancer death were reported. A total of 11,117 (5.3%) cases of incident cancer at any site were reported among 210,892 patients in 10 studies. Major confounding factors such as cigarette smoking, alcohol intake, and hyperglycemia were not reported in several studies.
The risk of bias and the adjustment factors among the studies are summarized in Table S2. Diabetes was diagnosed using blood tests (n = 8), prescription databases (n = 6), medical records (n = 4), self-reports (n = 3), and health insurance database (n = 4). All the diagnoses of cancer were confirmed using valid records or registries. All the studies, except for the RCTs, adjusted the estimates for potential confounding factors. The analysis of dose-response was performed in 3 studies [38]–[40]. Some studies excluded the data for metformin exposure less than 1 year [50], [52] or 2 years [58] to minimize bias. The effect on the total cancer risk over the follow-up period was inspected in 3 studies [40], [55], [58]. Direct comparison of the effect between metformin and other specific medications were reported in 2 RCTs [46]–[48].
Qualitative Summary
The majority of the studies included were methodologically fair in quality. Among 10 case-control studies, six were nested ones [50]–[52], [55], [56], [58]. All the four cohort studies [35], [38], [40], [41] on cancer mortality revealed a significant decrease (range, 23%–75%), and the two RCTs showed no significant effect of metformin [49]. There was no study that directly compared the risk associated with metformin vs other medications or analyzed the correlation between the follow-up length and the effect of metformin on cancer mortality. The overall correlation of the follow-up period with the mortality was nonsignificant (r = −0.04, p = 0.9). One study revealed that the HR (95% CI) for cancer mortality with every increase of 1 g metformin was 0.58 (0.36–0.93) [38].
Five studies (3 cohort studies [36], [39], [40] and 2 case-control studies [55], [56]) reported a significant decrease (range, 26%–88%), the two RCTs showed no significant effect of association [46]–[48] and none demonstrated a statistically significant increase in the risk of all-cancer incidence among metformin users. The cancer risk for metformin users was not significantly different from that for rosiglitazone or sulfonylurea users in RCTs [46]–[48]. One cohort study showed a trend for metformin users to have a higher risk of cancer in the first 2 years of follow-up. The beneficial effect of metformin on the risk of total cancer incidence was exposure-dependent in 2 case-control studies [55], [56]. The overall correlation of the follow-up period with the incidence was nonsignificant (r = −0.32, p = 0.4). One study reported that its effect on cancer incidence was dose-dependent (p for trend <0.05) [39] suggesting that the minimal effective dose can be 500 mg /day, while the other showed no significant differences among doses [40].
Among the studies evaluating the risks of site-specific incident cancers in patients with diabetes who were taking metformin, more than two studies (including subgroup analyses) recognized significantly reduced risks for cancers of the pancreas [36], [39], [54], colorectum [36], [39], [40], and liver [29], [39], [53], and none showed a significantly increased risk of a site-specific cancer. All these risk decrements were moderate (RR range, 0.06–0.60). Of note, no significant increases or decreases in the risk of cancers of the breast, prostate or stomach were reported, except for a significant decrease in the risk of prostate cancer in one report [42] and breast cancer in another [52]. The number of studies examining other cancer sites was two or fewer, and these studies were not reviewed in the present analysis.
Quantitative Summary (Meta-analysis)
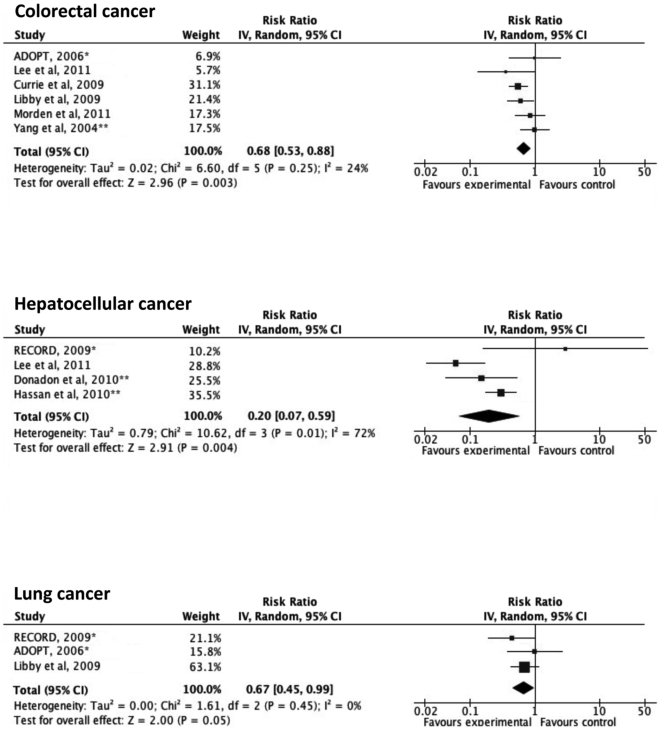
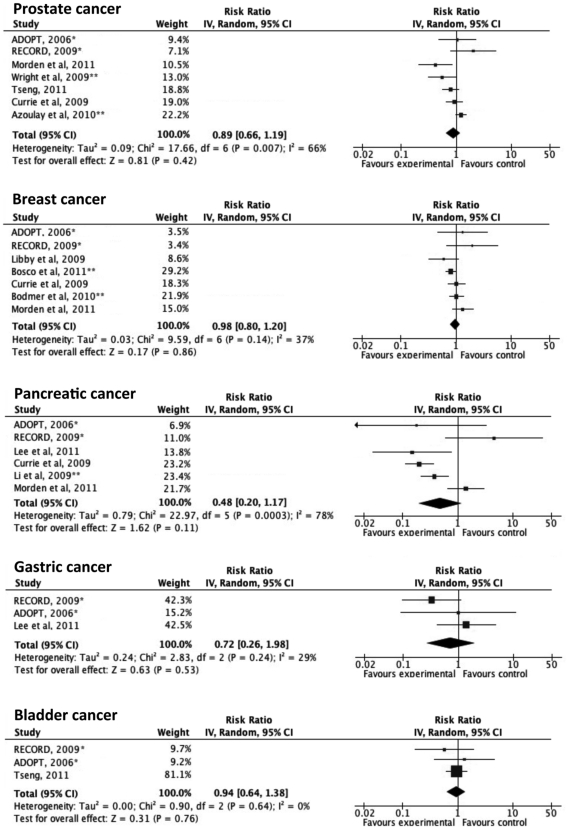
Based on the quality appraisal in our systematic review, a total of 24 articles that provided sufficient information were included in the meta-analysis ( Fig. 1 ). Fig. 2 illustrates the significantly decreased risks of all-cancer mortality and incidence in metformin-users, compared with non-metformin users. In a sensitivity analysis, the pooled estimate (95% CI) for all-cancer mortality among the observational cohort studies was 0.62 (0.46–0.82), I2 = 56%, p = 0.08 and the estimate among the RCTs was 1.22 (0.36–4.11), I2 = 60%, p = 0.12. The difference in the RRs between the observational studies and the RCTs was not statistically significant (p = 0.35). The pooled RR (95% CI) for all-cancer incidence among the observational cohort studies was 0.66 (0.49–0.88), I2 = 96%, p<0.00001, the pooled RR among the case-control studies was 0.38 (0.23–0.61), I2 = 3%, p = 0.31 and the estimate among the RCTs was 1.03 (0.82–1.31), I2 = 30%, p = 0.23. The difference in the RRs between the observational studies and the RCTs was statistically significant (p = 0.019). As summarized in Fig. 3 and Fig. 4 , the incident cancer risks were also significantly decreased for cancers of the colorectum, liver and lung. The RRs of prostate cancer, breast cancer, pancreatic cancer and gastric cancer were not statistically significant. Significant heterogeneity was observed in the majority of these analyses. No apparent publication bias was apparent, as assessed using a funnel plot (Fig. S1).
Figure 2. Adjusted risk ratios for all-cancer mortality and incidence among subjects with diabetes taking metformin.
Boxes, estimated risk ratios (RRs); bars, 95% confidence intervals (CIs). Diamonds, random-effects model RRs; width of diamonds; pooled CIs. The size of each box is proportional to the weight of each study in the meta-analysis. *, randomized controlled trials; **, case-control studies; IV, inverse-variance.
Figure 3. Adjusted risk ratios for site-specific cancer incidence among subjects with diabetes taking metformin.
Boxes, estimated risk ratios (RRs); bars, 95% confidence intervals (CIs). Diamonds, random-effects model RRs; width of diamonds; pooled CIs. The size of each box is proportional to the weight of each study in the meta-analysis. *, randomized controlled trials; **, case-control studies; IV, inverse-variance.
Figure 4. Adjusted risk ratios for other site-specific cancer incidence among subjects with diabetes taking metformin.
Boxes, estimated risk ratios (RRs); bars, 95% confidence intervals (CIs). Diamonds, random-effects model RRs; width of diamonds; pooled CIs. The size of each box is proportional to the weight of each study in the meta-analysis. *, randomized controlled trials; **, case-control studies; IV, inverse-variance.
Discussion
Our systematic review and meta-analyses of worldwide reports demonstrated that metformin is associated with a substantially lower risk of all-cancer mortality and incidence, compared with other treatments for diabetes. They also showed that metformin significantly reduced the risks of cancers of the colorectum, liver and lung. These findings support the hypothesis that metformin potentially has an anti-cancer effect. In light of the fact that cancer is the second and diabetes the twelfth leading cause of death worldwide [59] and that the number of people with diabetes is rapidly increasing, our findings have substantial clinical and public implications on a global scale and point to the need for the further investigation of the anti-cancer mechanism of metformin and for long-term RCTs to confirm this clinical benefit.
The strength of our present study is that the analysis was mainly based on large population-based data originating from multiple nations and was performed with a high level of precision. Compared with recently published studies [18], [19], our updated study is novel in that data from RCTs were incorporated and cancer risks for substantially more sites were analyzed. Although the significantly decreased pooled RRs for all-cancer mortality / incidence and cancer at most sites were robust, the results of the component studies were statistically heterogeneous. Of note, all the individual and pooled results of the RCTs were neutral. It seems that each follow-up period in these RCTs is similar to many others in the observational studies and they have power enough to detect the differences in cancer risk. In the analysis of cancer mortality, there was no significant difference in RR between the RCTs and the observational studies. For cancer incidence, on the other hand, the overall RR was significantly reduced but the difference was statistically significant. This discordance may imply that the apparent anti-cancer effect of metformin in observational studies was affected by confounding biases and thus more RCTs are awaited to clarify the effect of metformin on cancer incidence. The large I2 values indicated that the range of the plausible risk estimates was wide but no evidence in our analysis suggested that metformin may increase the risk of cancer. These findings may reflect the different mechanisms of cancer prevention at different sites and / or different epidemiological characteristics among the diverse populations included in our study.
Evidence has been accumulating to suggest that diabetic patients have a higher risk of cancer than non-diabetic people [12], [13]. While the mechanisms are yet to be investigated, insulin resistance with secondary hyperinsulinemia is the most frequently proposed hypothesis, as insulin may have a possible mitogenic effect via its binding to the insulin-like growth factor-1 receptor [60]–[70]. In addition, hyperglycemia itself may promote carcinogenesis directly [71], [72] or indirectly by increasing oxidative stress [73]–[79]. However, these speculations are derived from retrospective observational studies and may not necessarily demonstrate causality because of possible biases and confounders, such as co-existing obesity and age [15], [80], [81]. In fact, more recent studies demonstrated no or minimal increments in cancer risk [15], [16] and the data from insulin-treated patients are inconclusive [82]. Of interest, diabetes reportedly protects against the development of prostate cancer [8], [9], since it is testosterone-dependent and testosterone deficiency is common among men with diabetes secondary to low levels of sex hormone-binding globulin (SHBG) and partially because of insulin resistance [83]–[85]. Low SHBG levels may facilitate the conversion of testosterone to estradiol, which in turn may result in an increased risk of hormone-dependent breast cancer.
Several mechanisms for the anti-cancer effect of metformin have been postulated, and several prospective clinical trials to evaluate its safety and efficacy are ongoing [82], [86]. Indirect pathways include the prevention of weight gain and the amelioration of hyperinsulinemia, both of which may promote carcinogenesis. In addition, metformin activates AMPK through LKB-1, a tumor suppressor protein kinase. AMPK inhibits protein synthesis and gluconeogenesis during cellular stress and inhibits mammalian target of rapamycin (mTOR), a downstream effector of growth factor signaling, which is frequently activated in malignant cells. In human breast cancer cells, it reduces HER-2 protein expression by inhibiting mTOR. Metformin also induces cell cycle arrest and apoptosis and reduces growth factor signaling. Supporting the idea of these direct effects, metformin reportedly potentiated the effect of neoadjuvant chemotherapy in early-stage breast cancer [87], decreased the risk of colorectal cancer in a small randomized trial involving non-diabetic subjects [88], and was associated with a decreased cancer risk while another insulin-sensitizer, thiazolidinedione, were not [18], [54], [89], [90].
Our research revealed that metformin use is associated with reduced mortality and incidence of cancer at any site, supporting the general applicability of the proposed anti-cancer mechanisms. The anti-cancer effect of metformin may also be applicable to diabetic Asians, who are generally lean and insulinopenic [12], given the fact that they have a higher cancer risk than non-diabetic Asians [12]–[14] and the data for Asians [39] were in line with the results of our meta-analyses. On the other hand, the magnitude of the risk reduction varies among site-specific cancers. This variance in efficacy may result from differences in carcinogenesis at certain sites. For instance, elevated levels of insulin and glucose may exert an important influence in the development or growth of epithelial malignant tumors of the colon [91]–[93], pancreas [94], [95], and breast [96], and metformin may prevent incident colon cancer in non-diabetic subjects [88]. An animal study suggested that metformin prevented smoking-related lung cancer in mice, probably by inducing some hormone from the liver [97]. With regard to sex hormone-dependent cancers, the effect of metformin on the development of prostate cancer and breast cancer in our analysis was neutral. Metformin improves insulin sensitivity, thereby possibly raising the testosterone level. This may have promoted prostate cancer development and may have diluted the beneficial effect of metformin. In fact, one cohort study reported no benefit of metformin in terms of the biochemical recurrence rate after radical prostatectomy in diabetic patients [34]. The nonsignificant pooled RR for breast cancer may have resulted from the diversity in confounder adjustments and follow-up periods: some analyses were not fully adjusted for risk factors, including the menopause status, and one study suggested that only long-term exposure to metformin reduced the risk of breast cancer [51]. The fact that one preliminary study suggested a promising effect of metformin on pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer [87] may point to the possibility that metformin simply augmented the efficacy of chemotherapy for breast cancer [18], [86]. Further detailed studies to analyze the interaction between carcinogenesis and the action of metformin, and to evaluate its effect for nondiabetic people are eagerly awaited.
Limitations
Our analysis should be interpreted in the context of the following limitations. First, the relation may not necessarily be causal, particularly in the observational studies [80], because of possible confounding factors and biases that may not have been fully adjusted for in this study: some risk factors such as cigarette smoking, alcohol intake, and hyperglycemia were not specified in several studies, which may have rendered the results less valid. Few studies demonstrated the dose-response to support biological plausibility. Confounding by treatment indication [98], which may have been minimizes by using propensity-score matching analysis, might overestimate the effect of metformin: the presence of such pre-existing conditions as older age and liver disease precludes metformin usages and thus, metformin users may be generally younger and at lower risk of cancer than in those in comparator groups. Only a few observational studies analyzed the effects over time and thus protopathic bias (i.e. early cancer leading to unstable diabetes and hyperglycemia, with patients switching diabetes treatment) [15] may remain moderate. In fact, the individual and pooled estimates from the RCTs were all neutral; the estimates comparing with other medication were neutral, as well. For all these limitations, however, observational studies provide the good available evidence regarding potential treatment effects / harms and the overall pooled estimates were robust. Moreover, evidence has been accumulating to support causality, both clinically and biochemically, as discussed earlier. Secondly, it is also important to realize that the populations of the studies were heterogeneous, most likely because of the diversity of the study designs and ethnicities, and that the sensitivity of each site-specific cancer to metformin may vary. Lack of the standardized treatment protocol in the descriptive studies might explain the observed associations: the possibility that other diabetes treatments may increase the risk of cancer may have resulted in an overestimation of the effect of metformin. Lack of the standardized diagnostic procedures for cancer may have caused detection bias in some cases. Even with these limitations, our analysis supports oncogenic safety of metformin and it should provide physicians with an additional incentive to pay integrated clinical attention and elucidate the complex interactions between diabetes treatment and cancer.
Conclusions
Our meta-analysis favors the oncogenic benefit of metformin for diabetic patients. However, observational studies were moderately heterogeneous and biased, and RCTs did not show a significant effect. Our findings underscore the need for long-term randomized prospective studies to confirm this potential benefit.
Supporting Information
Funnel plot of the included studies.
(TIFF)
Study characteristics.
(DOC)
Quality assessments of the included studies.
(DOC)
PRISMA Checklist.
(PDF)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by a Health Sciences Research Grant (Comprehensive Research on Diabetes/Cardiovascular and Life-Style Related Diseases H22-019) from the Ministry of Health, Labour and Welfare of Japan and by a grant from Japan Diabetes Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121:856–862. doi: 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- 2.Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia. 2007;50:1365–1374. doi: 10.1007/s00125-007-0681-5. [DOI] [PubMed] [Google Scholar]
- 3.Larsson SC, Orsini N, Brismar K, Wolk A. Diabetes mellitus and risk of bladder cancer: a meta-analysis. Diabetologia. 2006;49:2819–2823. doi: 10.1007/s00125-006-0468-0. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–1687. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 6.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA. 1995;273:1605–1609. [PubMed] [Google Scholar]
- 8.Bonovas S, Filioussi K, Tsantes A. Diabetes mellitus and risk of prostate cancer: a meta-analysis. Diabetologia. 2004;47:1071–1078. doi: 10.1007/s00125-004-1415-6. [DOI] [PubMed] [Google Scholar]
- 9.Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2056–2062. doi: 10.1158/1055-9965.EPI-06-0410. [DOI] [PubMed] [Google Scholar]
- 10.Mitri J, Castillo J, Pittas AG. Diabetes and risk of Non-Hodgkin's lymphoma: a meta-analysis of observational studies. Diabetes Care. 2008;31:2391–2397. doi: 10.2337/dc08-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao C, Page JH. Type 2 diabetes mellitus and risk of non-Hodgkin lymphoma: a systematic review and meta-analysis. Am J Epidemiol. 2008;168:471–480. doi: 10.1093/aje/kwn160. [DOI] [PubMed] [Google Scholar]
- 12.Noto H, Osame K, Sasazuki T, Noda M. Substantially increased risk of cancer in patients with diabetes mellitus: a systematic review and meta-analysis of epidemiologic evidence in Japan. J Diabetes Complications. 2010;24:345–353. doi: 10.1016/j.jdiacomp.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Noto H, Tsujimoto T, Sasazuki T, Noda M. Significantly Increased Risk of Cancer in Patients with Diabetes Mellitus. Endocrine Practice. 2011;17:616–628. doi: 10.4158/EP10357.RA. [DOI] [PubMed] [Google Scholar]
- 14.Noto H, Tsujimoto T, Noda M. Significantly Increased Risk of Cancer in Diabetes Mellitus Patients: A meta-analysis of epidemiologic evidence in Asians and non-Asians. Journal of Diabetes Investigation. in press doi: 10.1111/j.2040-1124.2011.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Staa TP, Patel D, Gallagher AM, de Bruin ML. Glucose-lowering agents and the patterns of risk for cancer: a study with the General Practice Research Database and secondary care data. Diabetologia. 2011 doi: 10.1007/s00125-011-2390-3. Nov 30. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Wotton CJ, Yeates DG, Goldacre MJ. Cancer in patients admitted to hospital with diabetes mellitus aged 30 years and over: record linkage studies. Diabetologia. 2011;54:527–534. doi: 10.1007/s00125-010-1987-2. [DOI] [PubMed] [Google Scholar]
- 17.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–1461. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 19.Zhang ZJ, Zheng ZJ, Kan H, Song Y, Cui W, et al. Reduced Risk of Colorectal Cancer With Metformin Therapy in Patients With Type 2 Diabetes: A meta-analysis. Diabetes Care. 2011;34:2323–2328. doi: 10.2337/dc11-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300:2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, et al. Postoperative mortality in cancer patients with preexisting diabetes: systematic review and meta-analysis. Diabetes Care. 2010;33:931–939. doi: 10.2337/dc09-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 23.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 25.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 26.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 27.Bowker SL, Yasui Y, Veugelers P, Johnson JA. Glucose-lowering agents and cancer mortality rates in type 2 diabetes: assessing effects of time-varying exposure. Diabetologia. 2010;53:1631–1637. doi: 10.1007/s00125-010-1750-8. [DOI] [PubMed] [Google Scholar]
- 28.Donadon V, Balbi M, Ghersetti M, Grazioli S, Perciaccante A, et al. Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease. World journal of gastroenterology : WJG. 2009;15:2506–2511. doi: 10.3748/wjg.15.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver International. 2010;30:750–758. doi: 10.1111/j.1478-3231.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- 30.Yang X, So WY, Ma RC, Kong AP, Lee HM, et al. Low HDL cholesterol, metformin use, and cancer risk in type 2 diabetes: the Hong Kong Diabetes Registry. Diabetes Care. 2011;34:375–380. doi: 10.2337/dc10-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He XX, Tu SM, Lee MH, Yeung SC. Thiazolidinediones and metformin associated with improved survival of diabetic prostate cancer patients. Ann Oncol. 2011;22:2640–2645. doi: 10.1093/annonc/mdr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen TM, Lin CC, Huang PT, Wen CF. Metformin associated with lower mortality in diabetic patients with early stage hepatocellular carcinoma after radiofrequency ablation. Journal of gastroenterology and hepatology. 2011;26:858–865. doi: 10.1111/j.1440-1746.2011.06664.x. [DOI] [PubMed] [Google Scholar]
- 33.Nkontchou G, Cosson E, Aout M, Mahmoudi A, Bourcier V, et al. Impact of metformin on the prognosis of cirrhosis induced by viral hepatitis C in diabetic patients. The Journal of clinical endocrinology and metabolism. 2011;96:2601–2608. doi: 10.1210/jc.2010-2415. [DOI] [PubMed] [Google Scholar]
- 34.Patel T, Hruby G, Badani K, Abate-Shen C, McKiernan JM. Clinical outcomes after radical prostatectomy in diabetic patients treated with metformin. Urology. 2010;76:1240–1244. doi: 10.1016/j.urology.2010.03.059. [DOI] [PubMed] [Google Scholar]
- 35.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 36.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 37.Hense HW, Kajuter H, Wellmann J, Batzler WU. Cancer incidence in type 2 diabetes patients - first results from a feasibility study of the D2C cohort. Diabetology & metabolic syndrome. 2011;3:15. doi: 10.1186/1758-5996-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, et al. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care. 2010;33:322–326. doi: 10.2337/dc09-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, et al. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC cancer. 2011;11:20. doi: 10.1186/1471-2407-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, et al. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mellbin LG, Malmberg K, Norhammar A, Wedel H, Ryden L. Prognostic implications of glucose-lowering treatment in patients with acute myocardial infarction and diabetes: experiences from an extended follow-up of the Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) 2 Study. Diabetologia. 2011;54:1308–1317. doi: 10.1007/s00125-011-2084-x. [DOI] [PubMed] [Google Scholar]
- 42.Morden NE, Liu SK, Smith J, Mackenzie TA, Skinner J, et al. Further exploration of the relationship between insulin glargine and incident cancer: a retrospective cohort study of older Medicare patients. Diabetes Care. 2011;34:1965–1971. doi: 10.2337/dc11-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tseng CH. Diabetes and risk of prostate cancer: a study using the National Health Insurance. Diabetes Care. 2011;34:616–621. doi: 10.2337/dc10-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tseng CH. Diabetes and risk of bladder cancer: a study using the National Health Insurance database in Taiwan. Diabetologia. 2011;54:2009–2015. doi: 10.1007/s00125-011-2171-z. [DOI] [PubMed] [Google Scholar]
- 45.Yang X, So WY, Ma RC, Yu LW, Ko GT, et al. Use of sulphonylurea and cancer in type 2 diabetes-The Hong Kong Diabetes Registry. Diabetes research and clinical practice. 2010;90:343–351. doi: 10.1016/j.diabres.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 46.Home PD, Kahn SE, Jones NP, Noronha D, Beck-Nielsen H, et al. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia. 2010;53:1838–1845. doi: 10.1007/s00125-010-1804-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 48.Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 49.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 50.Azoulay L, Dell'Aniello S, Gagnon B, Pollak M, Suissa S. Metformin and the incidence of prostate cancer in patients with type 2 diabetes. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20:337–344. doi: 10.1158/1055-9965.EPI-10-0940. [DOI] [PubMed] [Google Scholar]
- 51.Bodmer M, Meier C, Krahenbuhl S, Jick SS, Meier CR. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes Care. 2010;33:1304–1308. doi: 10.2337/dc09-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bosco JL, Antonsen S, Sorensen HT, Pedersen L, Lash TL. Metformin and incident breast cancer among diabetic women: a population-based case-control study in Denmark. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20:101–111. doi: 10.1158/1055-9965.EPI-10-0817. [DOI] [PubMed] [Google Scholar]
- 53.Hassan MM, Curley SA, Li D, Kaseb A, Davila M, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938–1946. doi: 10.1002/cncr.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–488. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monami M, Lamanna C, Balzi D, Marchionni N, Mannucci E. Sulphonylureas and cancer: a case-control study. Acta diabetologica. 2009;46:279–284. doi: 10.1007/s00592-008-0083-2. [DOI] [PubMed] [Google Scholar]
- 56.Monami M, Colombi C, Balzi D, Dicembrini I, Giannini S, et al. Metformin and cancer occurrence in insulin-treated type 2 diabetic patients. Diabetes Care. 2011;34:129–131. doi: 10.2337/dc10-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wright JL, Stanford JL. Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer causes & control : CCC. 2009;20:1617–1622. doi: 10.1007/s10552-009-9407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 2004;127:1044–1050. doi: 10.1053/j.gastro.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 59.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 60.White MF. The insulin signalling system and the IRS proteins. Diabetologia. 1997;40(Suppl 2):S2–17. doi: 10.1007/s001250051387. [DOI] [PubMed] [Google Scholar]
- 61.Kim YI. Diet, lifestyle, and colorectal cancer: is hyperinsulinemia the missing link? Nutr Rev. 1998;56:275–279. doi: 10.1111/j.1753-4887.1998.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 62.Kaaks R. Nutrition, hormones, and breast cancer: is insulin the missing link? Cancer Causes Control. 1996;7:605–625. doi: 10.1007/BF00051703. [DOI] [PubMed] [Google Scholar]
- 63.Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995;6:164–179. doi: 10.1007/BF00052777. [DOI] [PubMed] [Google Scholar]
- 64.Yu H, Berkel H. Insulin-like growth factors and cancer. J La State Med Soc. 1999;151:218–223. [PubMed] [Google Scholar]
- 65.Zhang W, Thornton WH, MacDonald RS. Insulin-like growth factor-I and II receptor expression in rat colon mucosa are affected by dietary lipid intake. J Nutr. 1998;128:158–165. doi: 10.1093/jn/128.2.158. [DOI] [PubMed] [Google Scholar]
- 66.Bruning PF, Bonfrer JM, van Noord PA, Hart AA, de Jong-Bakker M, et al. Insulin resistance and breast-cancer risk. Int J Cancer. 1992;52:511–516. doi: 10.1002/ijc.2910520402. [DOI] [PubMed] [Google Scholar]
- 67.Hu FB, Manson JE, Liu S, Hunter D, Colditz GA, et al. Prospective study of adult onset diabetes mellitus (type 2) and risk of colorectal cancer in women. J Natl Cancer Inst. 1999;91:542–547. doi: 10.1093/jnci/91.6.542. [DOI] [PubMed] [Google Scholar]
- 68.Silverman DT, Schiffman M, Everhart J, Goldstein A, Lillemoe KD, et al. Diabetes mellitus, other medical conditions and familial history of cancer as risk factors for pancreatic cancer. Br J Cancer. 1999;80:1830–1837. doi: 10.1038/sj.bjc.6690607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolf I, Sadetzki S, Catane R, Karasik A, Kaufman B. Diabetes mellitus and breast cancer. Lancet Oncol. 2005;6:103–111. doi: 10.1016/S1470-2045(05)01736-5. [DOI] [PubMed] [Google Scholar]
- 70.Le Roith D. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N Engl J Med. 1997;336:633–640. doi: 10.1056/NEJM199702273360907. [DOI] [PubMed] [Google Scholar]
- 71.Richardson LC, Pollack LA. Therapy insight: Influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nat Clin Pract Oncol. 2005;2:48–53. doi: 10.1038/ncponc0062. [DOI] [PubMed] [Google Scholar]
- 72.Morss AS, Edelman ER. Glucose modulates basement membrane fibroblast growth factor-2 via alterations in endothelial cell permeability. J Biol Chem. 2007;282:14635–14644. doi: 10.1074/jbc.M608565200. [DOI] [PubMed] [Google Scholar]
- 73.Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, et al. Glycemic index, glycemic load, and chronic disease risk–a meta-analysis of observational studies. Am J Clin Nutr. 2008;87:627–637. doi: 10.1093/ajcn/87.3.627. [DOI] [PubMed] [Google Scholar]
- 74.Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L, et al. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA. 2000;283:2552–2558. doi: 10.1001/jama.283.19.2552. [DOI] [PubMed] [Google Scholar]
- 75.Seow A, Yuan JM, Koh WP, Lee HP, Yu MC. Diabetes mellitus and risk of colorectal cancer in the Singapore Chinese Health Study. J Natl Cancer Inst. 2006;98:135–138. doi: 10.1093/jnci/djj015. [DOI] [PubMed] [Google Scholar]
- 76.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, et al. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 77.Stocks T, Rapp K, Bjorge T, Manjer J, Ulmer H, et al. Blood glucose and risk of incident and fatal cancer in the metabolic syndrome and cancer project (me-can): analysis of six prospective cohorts. PLoS Med. 2009;6:e1000201. doi: 10.1371/journal.pmed.1000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abe R, Yamagishi S. AGE-RAGE system and carcinogenesis. Curr Pharm Des. 2008;14:940–945. doi: 10.2174/138161208784139765. [DOI] [PubMed] [Google Scholar]
- 79.Inoue M, Kurahashi N, Iwasaki M, Tanaka Y, Mizokami M, et al. Metabolic factors and subsequent risk of hepatocellular carcinoma by hepatitis virus infection status: a large-scale population-based cohort study of Japanese men and women (JPHC Study Cohort II). Cancer Causes Control. 2009;20:741–750. doi: 10.1007/s10552-008-9287-6. [DOI] [PubMed] [Google Scholar]
- 80.Johnson JA, Gale EA. Diabetes, insulin use, and cancer risk: are observational studies part of the solution-or part of the problem? Diabetes. 2010;59:1129–1131. doi: 10.2337/db10-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pocock SJ, Elbourne DR. Randomized trials or observational tribulations? N Engl J Med. 2000;342:1907–1909. doi: 10.1056/NEJM200006223422511. [DOI] [PubMed] [Google Scholar]
- 82.McFarland MS, Cripps R. Diabetes mellitus and increased risk of cancer: focus on metformin and the insulin analogs. Pharmacotherapy. 2010;30:1159–1178. doi: 10.1592/phco.30.11.1159. [DOI] [PubMed] [Google Scholar]
- 83.Grossmann M, Thomas MC, Panagiotopoulos S, Sharpe K, Macisaac RJ, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab. 2008;93:1834–1840. doi: 10.1210/jc.2007-2177. [DOI] [PubMed] [Google Scholar]
- 84.Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, et al. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:5462–5468. doi: 10.1210/jc.2004-0804. [DOI] [PubMed] [Google Scholar]
- 85.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 86.Jalving M, Gietema JA, Lefrandt JD, de Jong S, Reyners AK, et al. Metformin: taking away the candy for cancer? European journal of cancer. 2010;46:2369–2380. doi: 10.1016/j.ejca.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 87.Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:3297–3302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hosono K, Endo H, Takahashi H, Sugiyama M, Uchiyama T, et al. Metformin suppresses azoxymethane-induced colorectal aberrant crypt foci by activating AMP-activated protein kinase. Molecular carcinogenesis. 2010;49:662–671. doi: 10.1002/mc.20637. [DOI] [PubMed] [Google Scholar]
- 89.Piccinni C, Motola D, Marchesini G, Poluzzi E. Assessing the Association of Pioglitazone Use and Bladder Cancer Through Drug Adverse Event Reporting. Diabetes Care. 2011 doi: 10.2337/dc10-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes are. 2011;34:916–922. doi: 10.2337/dc10-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. The American journal of clinical nutrition. 2007;86:s836–842. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 92.Zakikhani M, Dowling RJ, Sonenberg N, Pollak MN. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer prevention research. 2008;1:369–375. doi: 10.1158/1940-6207.CAPR-08-0081. [DOI] [PubMed] [Google Scholar]
- 93.Algire C, Amrein L, Zakikhani M, Panasci L, Pollak M. Metformin blocks the stimulative effect of a high-energy diet on colon carcinoma growth in vivo and is associated with reduced expression of fatty acid synthase. Endocrine-related cancer. 2010;17:351–360. doi: 10.1677/ERC-09-0252. [DOI] [PubMed] [Google Scholar]
- 94.Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nature reviews Gastroenterology & hepatology. 2009;6:699–708. doi: 10.1038/nrgastro.2009.177. [DOI] [PubMed] [Google Scholar]
- 95.Schneider MB, Matsuzaki H, Haorah J, Ulrich A, Standop J, et al. Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology. 2001;120:1263–1270. doi: 10.1053/gast.2001.23258. [DOI] [PubMed] [Google Scholar]
- 96.Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. The American journal of clinical nutrition. 2007;86:s823–835. doi: 10.1093/ajcn/86.3.823S. [DOI] [PubMed] [Google Scholar]
- 97.Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, et al. Metformin prevents tobacco carcinogen–induced lung tumorigenesis. Cancer prevention research. 2010;3:1066–1076. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang YX. Do diabetes drugs modify the risk of pancreatic cancer? Gastroenterology. 2009;137:412–415. doi: 10.1053/j.gastro.2009.06.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Funnel plot of the included studies.
(TIFF)
Study characteristics.
(DOC)
Quality assessments of the included studies.
(DOC)
PRISMA Checklist.
(PDF)